Abstract
Purpose
To provide normative data of foveal avascular zone (FAZ) and thickness.
Methods
In this cross-sectional study both eyes of each normal subject were scanned with optical coherence tomography angiography (OCTA) for foveal superficial and deep avascular zone (FAZ) and central foveal thickness (CFT) and parafoveal thickness (PFT).
Results
Out of a total of 224 eyes of 112 volunteers with a mean age of 37.03 (12–67) years, the mean superficial FAZ area was 0.27 mm2, and deep FAZ area was 0.35 mm2 (P < 0.001), with no difference between both eyes. Females had a larger superficial (0.32 ± 0.11 mm2 versus 0.23 ± 0.09 mm2) and deep FAZ (0.40 ± 0.14 mm2 versus 0.31 ± 0.10 mm2) (P < 0.001) than males. By multivariate linear regression analysis, in normal eyes, superficial FAZ area varied significantly with the gender, CFT, and deep FAZ. Deep FAZ varied with the gender and CFT.
Conclusion
The gender and CFT influence the size of normal superficial and deep FAZ of capillary network.
Keywords: FAZ, Fovea, Foveal avascular zone, Foveal thickness, Normal eye, Optical coherence tomography angiography
Introduction
Foveal avascular zone (FAZ) is a highly specialized region of the human retina for the accurate vision.1, 2 Many imaging modalities have highlighted the variability in FAZ shape and size in normal human eyes.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The changes of shape and size of the FAZ in some diseases, causing the loss of vision, is already documented.16, 17
Introduction of fluorescein angiography (FA) to the ophthalmology as a simple invasive technique facilitated the study of ocular vascular system abnormalities and the FAZ in numerous retinovascular diseases.3, 18, 19
Recent advances in optical coherence tomography (OCT) technology affords a non-invasive optical biopsy, in particular by the increased scanning speed as well as progress in image-processing algorithms, have allowed the development of OCT angiography (OCTA).20, 21, 22, 23, 24, 25, 26
Recently, a few studies have described FAZ sizes in normal population measured with OCTA.4, 5, 10, 11, 16, 17, 27, 28 To understand the effects of retinal diseases on the FAZ, it is essential first to determine the variation of FAZ size and shape and its association with demographics and ocular factors among healthy individuals.29
In this study, we aimed to provide a normative data from a sample of the Iranian population for normal superficial and deep capillary network free zone (FAZ), using XR Avanti AngioVue OCTA in healthy subjects and to ascertain any correlation of FAZ with other measured ocular and demographic factors.
Methods
The Institutional Review Board of Farabi Multi-Specialty Eye Hospital approved the research protocol, and informed consent was obtained from the patients and parents or legal guardians of children. The research conformed to the tenets of the Declaration of Helsinki. Both eyes of ocular and systemically healthy subjects were included. Inclusion criteria were best corrected visual acuity of 20/20 and refractive error between −3 and +1 diopter (D) spherical equivalent. Exclusion criteria were significant media opacity preventing high-quality imaging, motion and blinking artifact, and any ocular and systemic disease.
Two trained readers (F.G., F.B.) from Image Reading Center reviewed and graded all OCT images.
AngioVue retina imaging using optical coherence tomography angiography (OCTA; RTVue XR Avanti; Optovue, USA-pre-release version: 2016.1.0.23- beta) and the split-spectrum amplitude decorrelation angiography (SSADA) algorithm was performed.22 This instrument operated at 840-nm wavelength and performs 70,000 A-scans per second to acquire OCTA of two repeated horizontal and vertical B-scans of 3 × 3 mm (304 × 304 pixels) in the transverse dimension. Low quality scans (i.e., if the subject blinked or the scan had significant motion artifacts) were excluded and repeated until good quality scans were achieved. An internal fixation light was used to center the scanning area.
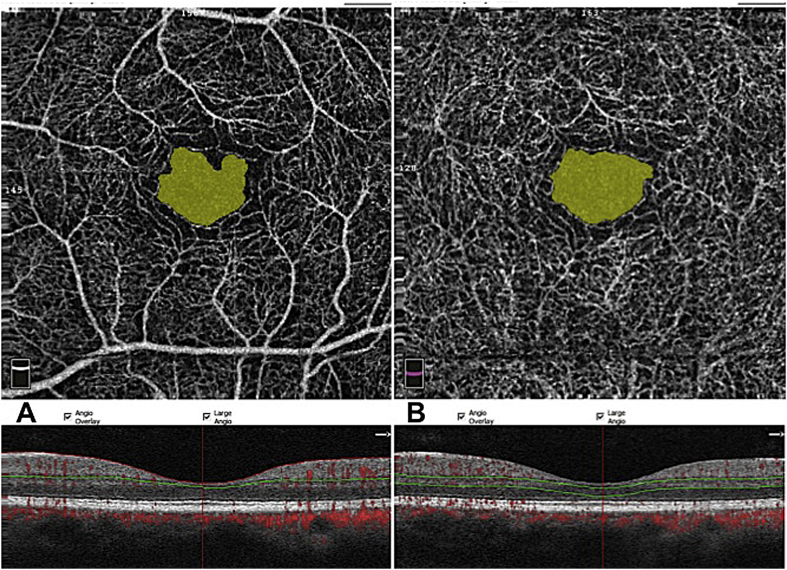
For all measurements, the automated segmentation with the preset settings for the superficial vascular network and deep vascular network was utilized. Hereby, the upper border of the superficial vascular network was defined as 3 μm below the internal limiting membrane (ILM) and the lower border as 15 μm below the inner plexiform layer (IPL) and for the deep vascular network, as 15 μm–71 μm below the IPL, respectively. FAZ area was measured in mm2. Briefly, a non-flow measurement on a superficial reference plane (superficial vascular network) of en-face projection was selected. The user manually fine-tuned the plane to maximize the visualization of the retinal capillary bed by changing the depth of the automatically segmented slab. Via clicking on the center of the FAZ, the software automatically calculated the area of non-perfusion (Fig. 1).22 The user manually fine-tuned the plane to maximize the visualization of the retinal capillary bed.
Fig. 1.
Comparison of foveal avascular zone (FAZ) in the superficial and deep retinal capillary network (A) Superficial FAZ measured as area 0.29 mm2). (B) Deep FAZ (area 0.32 mm2).
These thicknesses were measured in foveal and parafoveal area as a whole and selectively in superior, inferior, nasal, and temporal parafoveal sectors in macular region in superficial and deep capillary networks. In case of erroneous determination by the built-in software, manual correction was performed. The foveal region was outlined as a central circle with a 120-pixel (1.2 mm) diameter, and the parafoveal region was delineated as a ring, by 91 pixels wide, surrounding the foveal region.28
Vertical cup/disc ratio was measured by clinical examination (Lens 90). The disc shaped was classified as round and oval shaped discs in clinical examination (more than 10% difference in vertical and horizontal diameters).
Two trained readers (F.G., F.B.) from our team reviewed and graded all OCT images with each other in each session. The results were the mean amount of values in the same session of reading. If there was any conflict of opinion, the third reader (H.F.) was involved. We categorized the ages of the candidates into 4 groups including: 30 years or less (41 cases), 31–40 years (37 cases), 41–50 years (20 cases), and older than 50 years (14 cases).
Statistical methods
All quantitative variables were reported as mean with standard deviation (SD) after confirming normality of distribution with the Kolmogorov-Smirnov test. All statistical analyses were performed using statistical software (SPSS software Version 21; SPSS, Inc., Chicago, IL, USA). Two-tailed, independent samples t-test was used to compare the FAZ areas with other two tailed variables as laterality and gender. Analysis of variance (ANOVA) was performed among the age groups. Multivariate linear regression analysis was used to determine the correlation between FAZ areas after adjusting for confounding factors including age, gender, and other correlated variables in partial t-test. In this study, collinearity for different variables was checked. P values less than 0.05 were considered statistically significant. Intraclass correlation coefficients (ICCs) were used to assess the intergrader agreement between the graders.
Results
Demographic data
A total of 224 eyes of 112 normal volunteer underwent FAZ area measurement. Mean age of participants was 37.03 ± 11.27 year (range, 12–67 years). The subjects included 59 (52.7%) male with the mean age of 34.98 ± 8.53 and 53 (47.3%) female with the mean age of 40.05 ± 13.87 (P < 0.001). The shapes of optic disc composed of 64 (40.5%) eyes with round and 94 (59.5%) eyes with oval forms. From the age groups, 41 cases (36.3%) were in the 30 years or less, 37 cases (33%) in 31–40 years, 20 cases (17.9%) in the 41–50 years, and 14 cases (12.5%) in more than 50 years group.
The mean ± SD value of superficial and deep FAZ area were 0.27 ± 0.11 mm2 and 0.35 ± 0.12 mm2, respectively (P < 0.001). Central foveal thickness (CFT) was 247 ± 21.1 μm and parafoveal thickness (PFT) was 317 ± 15.6 μm. PFT in superior, temporal, inferior, and nasal quadrants were 321 ± 15.1 μm, 307 ± 15.6 μm, 319 ± 16.5 μm and 319 ± 16.8 μm, respectively (Table 1).
Table 1.
Surface area of superficial and deep foveal avascular zone (FAZ), foveal and parafoveal thicknesses (PFTs), age and cup/disc ratio of normal right eyes.
| FAZ area (mm2)/foveal and parafoveal thicknesses (μm) (Mean ± SD) (ANOVA-Dunnett) | Overall (range) (N = 112) | Male (N = 59) | Female (N = 53) | P value |
|---|---|---|---|---|
| Surface area of superficial FAZ | 0.22 ± 0.08 (0.04–0.63) | 0.22 ± 0.08 | 0.31 ± 0.11 | <0.001 |
| Surface area of deep FAZ | 0.31 ± 0.11 (0.06–0.94) | 0.30 ± 0.10 | 0.39 ± 0.14 | <0.001 |
| Thickness of fovea | 247.12 ± 21.19 (202–313) | 255.54 ± 17.81 | 237.87 ± 21.56 | <0.001 |
| Thickness of parafoveal area | 317.6 ± 15.63 (270–359) | 322.64 ± 14.82 | 311.66 ± 13.56 | <0.001 |
| Thickness of superior hemifield | 317.24 ± 15.06 (271–359) | 321.92 ± 15.03 | 312.09 ± 12.36 | <0.001 |
| Thickness of inferior hemifield | 317.20 ± 16.24 (261–361) | 322.95 ± 15.01 | 311.60 ± 15.23 | <0.001 |
| Thickness of temporal area | 307.70 ± 15.68 (257–350) | 313.78 ± 15.22 | 302.40 ± 13.45 | <0.001 |
| Thickness of superior area | 321.77 ± 15.32 (275–369) | 326.34 ± 15.56 | 316.70 ± 12.54 | 0.001 |
| Thickness of nasal area | 319.74 ± 16.86 (266–365) | 325.51 ± 16.03 | 313.28 ± 14.98 | <0.001 |
| Thickness of inferior area | 319.95 ± 16.57 (262–363) | 325.64 ± 15.00 | 314.58 ± 16.15 | <0.001 |
| Cup/disc ratio (%) | 0.33 ± 0.12 (0.1–0.7) | 0.36 ± 0.15 | 0.31 ± 0.09 | 0.129 |
| Age (year) | 36.39 ± 11.31 (12–67) | 33.42 ± 7.99 | 39.70 ± 13.44 | 0.003 |
FAZ: Foveal avascular zone.
SD: Standard deviation.
No correlation was found between superficial and deep FAZ area and the shape of the disc, and cup/disc ratio in the studied eyes. The areas of superficial and deep FAZ were highly correlated (r = 0.82, P < 0.001). No relation between the age and superficial FAZ area and deep FAZ area was found (P > 0.05) (Fig. 1). A correlation between gender and superficial FAZ (r = 0.45, P < 0.001), deep FAZ (r = 0.33, P < 0.001), and CFT (r = −0.40, P < 0.001) was detected. No significant difference was found between both eyes.
To remove the effect of bilaterality, one eye of each patient was selected sequentially, then all statistics were re-done. In this evaluation, only in the right eye, the difference between deep FAZ in males (9.14 ± 67.91 mm2) and females (0.39 ± 0.14 mm2) was not statistically significant (P = 0.326).
Superficial and deep capillary foveal avascular zone areas
The gender based amounts of superficial FAZ and deep FAZ areas in fovea are summarized in Table 1. The superficial FAZ was 0.27 ± 0.11 (range, 0.04–0.63) and deep FAZ was 0.35 ± 0.12 (range, 0.06–0.94). Comparing all eyes, the deep FAZ was significantly larger than the superficial FAZ (mean difference 0.08 mm2, paired t-test, P < 0.001) (Fig. 1). These FAZ areas were similar in both eyes (P > 0.05). The FAZ areas were both not correlated with cup/disc ratio (r = −0.03, P = 0.82) and age (r = −0.02, P = 0.85) (Fig. 2).
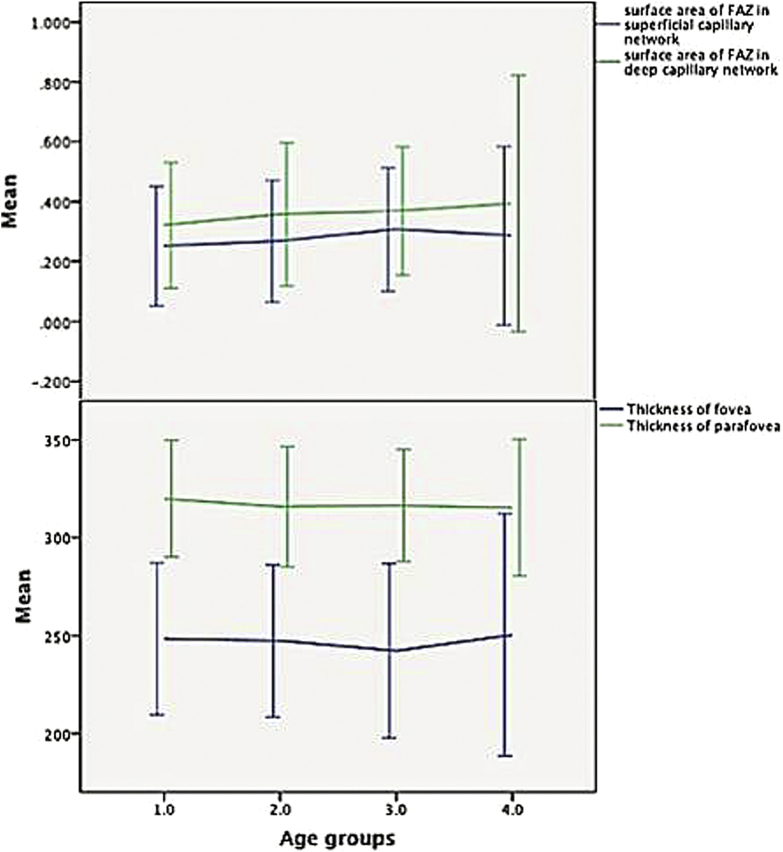
Fig. 2.
The amount of foveal avascular zone (FAZ) areas and foveal and parafoveal thicknesses (PFTs) in different age groups. (Upper) The areas of superficial FAZ area with deep FAZ area. The deep FAZ is consistently larger than the superficial FAZ of the same age. (Lower) The foveal and PFTs in different age groups.
Gender based foveal avascular zone areas and central foveal thickness and parafoveal thickness
The superficial and deep FAZ areas were larger in female volunteers than male (Table 1), and the results persist after adjusting for the age in multivariate linear regression analysis [Beta: 0.40, 95% confidence interval (CI): 0.05–0.13, P < 0.001 for superficial FAZ and Beta: 0.30, 95% CI: 0.03–0.13, P = 0.002 for deep FAZ]. Despite FAZ areas, the mean CFT and PFT are higher in males compared with females (Table 1). The results were established on multivariate linear regression, after adjusting to the age (Beta: −0.46, 95% CI: −27.18 to −11.95, P < 0.001, for CFT and Beta: −0.37, 95% CI: −16.79 to −5.62, P < 0.001 for PFT).
Age grouping-based foveal avascular zone areas and central foveal thickness and parafoveal thickness
The means of FAZ areas and foveal and PFTs, based on age categories, are shown in Table 2. There was no significant difference of these parameters among different groups (by ANOVA test; Dunnet and Tukey, P ≥ 0.05).
Table 2.
Surface area of superficial and deep foveal avascular zone (FAZ) and foveal and parafoveal thicknesses (PFT) of normal right eyes in the age groups ranging from 12 to 67 years.
| FAZ area (mm2)/foveal and parafoveal thicknesses (μm) (Mean ± SD) (ANOVA-Dunnett) | ≤30 years (N = 41) | 31–40 years (N = 37) | 41–50 years (N = 20) | >50 years (N = 14) |
|---|---|---|---|---|
| Surface area of superficial FAZ | 025 ± 0.15 | 0.26 ± 0.16 | 0.30 ± 0.02 | 0.28 ± 0.03 |
| 0.86 | 0.16 | 0.62 | ||
| Surface area of deep FAZ | 0.32 ± 0.16 | 0.35 ± 0.19 | 0.36 ± 0.23 | 0.39 ± 0.05 |
| 0.48 | 0.39 | 0.17 | ||
| Thickness of fovea | 248.37 ± 3.02 | 247.32 ± 3.20 | 242.25 ± 4.96 | 250.36 ± 8.30 |
| 0.99 | 0.63 | 0.98 | ||
| Thickness of parafoveal area | 319.95 ± 2.33 | 315.97 ± 2.53 | 316.45 ± 3.19 | 315.43 ± 4.66 |
| 0.55 | 0.76 | 0.68 | ||
| Thickness of superior hemifield | 318.95 ± 2.40 | 316.76 ± 2.33 | 316.45 ± 3.15 | 314.86 ± 4.02 |
| 0.86 | 0.88 | 0.72 | ||
| Thickness of inferior hemifield | 320.46 ± 2.31 | 315.46 ± 2.79 | 316.60 ± 3.24 | 316.14 ± 5.26 |
| 0.41 | 0.73 | 0.74 | ||
| Thickness of temporal area | 310.05 ± 2.48 | 307.30 ± 2.50 | 308.15 ± 3.17 | 306.79 ± 4.67 |
| 0.80 | 0.95 | 0.86 | ||
| Thickness of superior area | 324.32 ± 2.47 | 321.24 ± 2.38 | 320.25 ± 3.09 | 317.93 ± 4.09 |
| 0.72 | 0.66 | 0.40 | ||
| Thickness of nasal area | 322.88 ± 2.47 | 317.49 ± 2.86 | 318.20 ± 3.47 | 318.57 ± 4.51 |
| 0.37 | 0.63 | 0.76 | ||
| Thickness of inferior area | 323.83 ± 2.32 | 317.78 ± 2.77 | 319.40 ± 3.24 | 318.79 ± 5.78 |
| 0.27 | 0.66 | 0.66 |
FAZ: Foveal avascular zone.
SD: Standard deviation.
Correlation of superficial foveal avascular zone and deep foveal avascular zone areas with other evaluated variables
In the age- and gender-adjusted partial correlation, superficial FAZ area was correlated with deep FAZ area (r = 0.84, P < 0.001), CFT (r = −0.72, P < 0.001), PFT (r = −0.25, P = 0.02), thickness of superior hemifield (r = −0.27, P = 0.02), thickness of inferior hemifield (r = −0.25, P = 0.03), thickness of superior area (r = −0.23, P = 0.05), and thickness of inferior area (r = −0.33, P = 0.003). In multivariate linear regression analysis, after checking co-linearity and adjusting for age and gender, only the correlation of superficial FAZ to deep FAZ area (Beta: 0.60, 95% CI: 0.42–0.59, P < 0.001) and CFT (Beta: −0.49, 95% CI: −0.003 to −0.002, P < 0.001) persisted.
In the partial correlation analysis, as mentioned, deep FAZ area was correlated with superficial FAZ area (r = 0.2, P < 0.001) and CFT (r = −0.50, P < 0.001). In the age- and gender-adjusted multivariate regression analysis, CFT (Beta: −0.519, 95% CI: −0.004 to −0.002, P < 0.001) persisted.
Repeatability of foveal avascular zone measurements
Measurement of superficial and deep FAZ areas showed very good intergrader agreement (ICC, 0.97; 95% CI, 0.95–0.99) and intragrader repeatability for both observers (ICC, 0.98; 95% CI, 0.97–0.99).
Discussion
In our current study of normal OCTA findings, the area of FAZ in superficial and deep capillary network was larger in the female volunteers. Superficial FAZ is highly correlated with deep FAZ. The central fovea and parafovea were thicker in male volunteers. Area of superficial and deep FAZ did not vary significantly within age groups in this study, and their sizes are inversely correlated with the amount of CFT.
According to the FAZ size, similar results were found in other investigations.16, 27 Tan and colleagues reported mean superficial and deep area of FAZ to be 0.24 mm2 and 0.38 mm2 respectively, which is quite similar to our findings29; however, the narrow age range of participants prevented further investigation within age groups. In the study, conducted by Samara et al., FAZ size in superficial network was measured 0.26 ± 0.09 mm2 with a wide range (0.071–0.527 mm2), similar to our results. However, FAZ in deep network was measured 0.49 ± 0.22 mm2, which was quite larger than our findings.10 Shahlaee and colleagues reported FAZ in superficial and deep network to be 0.27 ± 0.1 mm2 and 0.34 ± 0.1 mm2, respectively; however, relative small sample size prevented further analysis of associated factors.28 A consistent finding in these studies was a larger FAZ area in deep network than superficial. Wang and associates measured superficial FAZ in a healthy Chinese population that was 0.35 ± 0.12 mm2, which is quite larger than our results.30 They did not report FAZ area in deep capillary network. The discrepancy emphasizes more attention to ethnic background, the technique of measurement, and artifact removal technology used in newer versions of the software in the interpretation of normative values of the population studies.
Age is another crucial factor that, theoretically, can influence FAZ measurement. In this study, no significant difference in the mean superficial and deep FAZ area was found within age groups. In fact, the association of FAZ area with increasing age is a controversial theme throughout various studies. Yu and colleagues reported an increase in FAZ size annually by 1.48% accompanied by a decrease in vascular density by 0.4%.31 They attributed the enlargement of FAZ to tissue loss and relative reduction on oxygen and nutrient demand that leads to reduce vascular supply.31, 32 As demonstrated in the present study, FAZ area is associated with the thickness of fovea, a factor that is not controlled in the study of Yu et al.31 Therefore different measures of CFT with advancing age could act as a confounder in their results. Coscas and associates investigated 135 eyes of 70 participants divided into three groups: 20–39 years; 40–59 years; and 60 years or older. The superficial capillary network FAZ was smaller in group 3 compared with groups 1 and 2. There was no statistically significant difference for the mean deep capillary network FAZ in the three groups. They found direct association of vascular density with age and gender and a lower mean FAZ area in the third group.33 There are older reports that have measured FAZ area by FA and demonstrated an increase in FAZ size with advancing age.15, 19 On the other hand, Samara et al. and Wang et al. reported no significant association of age with FAZ area.10, 30 Different measurement method and diversity of ethnic background may contribute to these discrepancies.
A relation of FAZ with the gender was documented in our study (Table 1). The areas of both the superficial and the deep FAZ were 33% larger in female compared with the male group. Samara et al. showed no association between FAZ area and gender. In the Chinese population, Yu and associates reported that the capillary free zone area is larger in females than in males (male mean, 0.42 mm2; female mean, 0.52 mm2; P = 0.012).31 Similar to other authors, we think that this could be related to the thinner fovea found in females.7, 19, 28, 31, 32, 33, 34, 35 The possible mechanisms behind these patterns need to be more investigated. Comparable results were found in the study of Tan and colleagues, too.29 Additionally, they reported a direct correlation to spherical equivalent and axial length.
In our study, the morphology of the fovea was not considered in the evaluation. The impact of aging and gender in the dynamicity of foveal morphology is not evaluated so far, as the superficial and deep FAZ were both in the inner nuclear layer. The possible shallow pit in the females could explain the observed differences.
Current investigation is among the rare studies that have evaluated the effect of CFT on FAZ area.10, 28, 36, 37 Based on our findings, superficial and deep capillary network FAZ have an inverse relationship with CFT after adjusting with age and gender, by checking for collinearity. It means that by considering the age and gender, the thinner CFT is correlated with the larger superficial and deep FAZ. In accordance with our results, Samara and associates reported an inverse correlation of both superficial and deep FAZ area with central retinal thickness and volume.10 No relationship was noticed with age and gender in their results. Tan et al.29 documented that the FAZ size in normal eyes was affected by central retinal thickness and gender only. In their study, among eyes with high myopia, in addition to central retinal thickness and gender, central choroidal thickness also affected the superficial and deep FAZ size.
There are several limitations in our study. The unequal sample size in diverse age categories could affect the results and weaken a thorough model of regression. In this study, spherical equivalent of refractive error, axial length, choroidal thickness, foveal morphology, and vascular density of foveal area of the eyes were not included.
We demonstrated FAZ area at superficial and deep capillary level in a healthy Iranian population. It seems that aging has no influence on the size of FAZ areas. FAZ is larger at the deeper level than superficial capillary network. Superficial and deep FAZ areas are correlated with each other and CFT. Our results indicate that research on foveal deep and superficial avascular zone should take into account the gender-related variations.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Jonas J.B., Schneider U., Naumann G.O. Count and density of human retinal photoreceptors. Graefes Arch Clin Exp Ophthalmol. 1992;230:505–510. doi: 10.1007/BF00181769. [DOI] [PubMed] [Google Scholar]
- 2.Yu D.Y., Cringle S.J., Su E.N. Intraretinal oxygen distribution in the monkey retina and the response to systemic hyperoxia. Invest Ophthalmol Vis Sci. 2005;46:4728–4733. doi: 10.1167/iovs.05-0694. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y., Gandhi J.S., Stangos A.N., Campa C., Broadbent D.M., Harding S.P. Automated segmentation of foveal avascular zone in fundus fluorescein angiography. Invest Ophthalmol Vis Sci. 2010;51:3653–3659. doi: 10.1167/iovs.09-4935. [DOI] [PubMed] [Google Scholar]
- 4.Mammo Z., Balaratnasingam C., Yu P. Quantitative noninvasive angiography of the fovea centralis using speckle variance optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56:5074–5086. doi: 10.1167/iovs.15-16773. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.Y., Fingler J., Zawadzki R.J. Noninvasive imaging of the foveal avascular zone with high-speed, phase-variance optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:85–92. doi: 10.1167/iovs.11-8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popovic Z., Knutsson P., Thaung J., Owner-Petersen M., Sjöstrand J. Noninvasive imaging of human foveal capillary network using dual-conjugate adaptive optics. Invest Ophthalmol Vis Sci. 2011;52:2649–2655. doi: 10.1167/iovs.10-6054. [DOI] [PubMed] [Google Scholar]
- 7.Chui T.Y., VanNasdale D.A., Elsner A.E., Burns S.A. The association between the foveal avascular zone and retinal thickness. Invest Ophthalmol Vis Sci. 2014;55 doi: 10.1167/iovs.14-15446. 6870–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinhas A., Razeen M., Dubow M. Assessment of perfused foveal microvascular density and identification of nonperfused capillaries in healthy and vasculopathic eyes. Invest Ophthalmol Vis Sci. 2014;55:8056–8066. doi: 10.1167/iovs.14-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmoll T., Singh A.S., Blatter C. Imaging of the parafoveal capillary network and its integrity analysis using fractal dimension. Biomed Opt Express. 2011;2:1159–1168. doi: 10.1364/BOE2.001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samara W.A., Say E.A., Khoo C.T. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35:2188–2195. doi: 10.1097/IAE.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 11.Kuehlewein L., Tepelus T.C., An L., Durbin M.K., Srinivas S., Sadda S.R. Noninvasive visualization and analysis of the human parafoveal capillary network using swept source OCT optical microangiography. Invest Ophthalmol Vis Sci. 2015;56:3984–3988. doi: 10.1167/iovs.15-16510. [DOI] [PubMed] [Google Scholar]
- 12.Dubis A.M., Hansen B.R., Cooper R.F., Beringer J., Dubra A., Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53:1628–1636. doi: 10.1167/iovs.11-8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam J., Martin J.A., Roorda A. Noninvasive visualization and analysis of parafoveal capillaries in humans. Invest Ophthalmol Vis Sci. 2010;51:1691–1698. doi: 10.1167/iovs.09-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novotny H.R., Alvis D.L. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24:82–86. doi: 10.1161/01.cir.24.1.82. [DOI] [PubMed] [Google Scholar]
- 15.Laatikainen L. The fluorescein angiography revolution: a breakthrough with sustained impact. Acta Ophthalmol Scand. 2004;82:381–392. doi: 10.1111/j.1395-3907.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 16.Takase N., Nozaki M., Kato A., Ozeki H., Yoshida M., Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35:2377–2383. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 17.Freiberg F.J., Pfau M., Wons J., Wirth M.A., Becker M.D., Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1051–1058. doi: 10.1007/s00417-015-3148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ditzel J., Clair R.W. Clinical method of photographing the smaller blood vessels and the circulating blood in the bulbar conjunctiva of human subjects. Circulation. 1954;10(2):277–281. doi: 10.1161/01.cir.10.2.277. [DOI] [PubMed] [Google Scholar]
- 19.Wu L.Z., Huang Z.S., Wu D.Z., Chan E. Characteristics of the capillary-free zone in the normal human macula. Jpn J Ophthalmol. 1985;29:406–411. [PubMed] [Google Scholar]
- 20.Puliafito C.A., Hee M.R., Lin C.P. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–229. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 21.Spaide R.F., Klancnik J.M., Cooney M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 22.Savastano M.C., Lumbroso B., Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 2015;35:2196–2203. doi: 10.1097/IAE.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 23.Mendis K.R., Balaratnasingam C., Yu P. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Invest Ophthalmol Vis Sci. 2010;51:5864–5869. doi: 10.1167/iovs.10-5333. [DOI] [PubMed] [Google Scholar]
- 24.Fingler J., Zawadzki R.J., Werner J.S., Schwartz D., Fraser S.E. Volumetric microvascular imaging of human retina using optical coherence tomography with a novel motion contrast technique. Opt Express. 2009;17:22190–22200. doi: 10.1364/OE.17.022190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motaghiannezam S.M., Koos D., Fraser S.E. Differential phase-contrast, swept-source optical coherence tomography at 1060 nm for in vivo human retinal and choroidal vasculature visualization. J Biomed Opt. 2012;17:026011. doi: 10.1117/1.JBO.17.2.026011. [DOI] [PubMed] [Google Scholar]
- 26.Jia Y., Tan O., Tokayer J. Split-spectrum amplitude decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpineto P., Mastropasqua R., Marchini G., Toto L., Di Nicola M., Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016;100:671–676. doi: 10.1136/bjophthalmol-2015-307330. [DOI] [PubMed] [Google Scholar]
- 28.Shahlaee A., Pefkianaki M., Hsu J., Ho A.C. Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;161 doi: 10.1016/j.ajo.2015.09.026. 50–55 e1. [DOI] [PubMed] [Google Scholar]
- 29.Tan C.S., Lim L.W., Chow V.S. Optical coherence tomography angiography evaluation of the parafoveal vasculature and its relationship with ocular factors. Invest Ophthalmol Vis Sci. 2016;57:224–234. doi: 10.1167/iovs.15-18869. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q., Chan S., Yang J.Y. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol. 2016;168:95–109. doi: 10.1016/j.ajo.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Yu J., Jiang C., Wang X. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci. 2015;56:3212–3217. doi: 10.1167/iovs.14-16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandewalle E., Abegao P.L., Olafsdottir O.B. Oximetry in glaucoma: correlation of metabolic change with structural and functional damage. Acta Ophthalmol. 2014;92:105–110. doi: 10.1111/aos.12011. [DOI] [PubMed] [Google Scholar]
- 33.Coscas F., Sellam A., Glacet-Bernard A. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:211–223. doi: 10.1167/iovs.15-18793. [DOI] [PubMed] [Google Scholar]
- 34.Laatikainen L., Larinkari J. Capillary-free area of the fovea with advancing age. Invest Ophthalmol Vis Sci. 1977;16:1154–1157. [PubMed] [Google Scholar]
- 35.Tick S., Rossant F., Ghorbel I. Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci. 2011;52:5105–5110. doi: 10.1167/iovs.10-7005. [DOI] [PubMed] [Google Scholar]
- 36.Luo H.D., Gazzard G., Fong A. Myopia, axial length, and OCT characteristics of the macula in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:2773–2781. doi: 10.1167/iovs.05-1380. [DOI] [PubMed] [Google Scholar]
- 37.Lam D.S., Leung K.S., Mohamed S. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci. 2007;48:376–382. doi: 10.1167/iovs.06-0426. [DOI] [PubMed] [Google Scholar]