Summary
Lung cancer remains one of the leading causes of death worldwide, and lung cancers have often already metastasized when diagnosed. Numerous studies have noted the infiltration of immune cells in the lung cancer microenvironment, but these cells play a dualistic role, i.e. they suppress and/or promote tumor development and growth based on tumor progression and different cytokines in the microenvironment. These tumor-infiltrating immune cells create different microenvironments depending on their type and interaction. Chemokines act as a bridge in this process by recruiting immune cells to the tumor site and they regulate the phenotypes and functions of those cells. The current review summarizes current knowledge about the tumor-infiltrating immune cells in lung cancer as well as the mechanisms involved in suppression and promotion of tumor development and growth.
Keywords: Lung cancer, tumor microenvironment, infiltrating immune cells, chemokine
1. Introduction
Lung carcinoma is a major cause of death throughout the world; more than 1.82 million people are diagnosed with lung carcinoma annually (13% of all cancers) and lung carcinoma causes 1.59 million deaths (19.4% of all cancers) (1,2). By the time the tumor is diagnosed, it has often already metastasized (3). Despite advances, treatments such as surgery, radiotherapy, chemotherapy, and multimodal therapies can rarely control metastatic disease and they are seldom curative. Patients with lung cancer have a limited long-term survival (4) because treated lung tumors will ultimately relapse since a number of tumor clones or “cancer-initiating cells” have escaped the initial treatment. These escaped cells will be more resistant to therapeutic modalities. Therefore, an adjuvant therapy that could effectively destroy these remaining tumor cells would have a substantial impact on tumor treatment.
Immunotherapy has a demonstrable efficacy in patients with cancer (5). These cells or cytokines can infiltrate the tumor site by activating the host immune cells, thus inducing a specific antitumor immune response. Therefore, the effect of the treatment depends, to a great extent, on the tumor microenvironment. Tumor-infiltrating immune cells play critical roles in the tumor microenvironment by promoting or suppressing tumor development and progression depending on their type and functional interactions (6).
There is an obvious infiltration of different types of immune cells in lung cancer. These immune cells typically include natural killer (NK) cells, T lymphocytes, macrophages, dendritic cells (DC), myeloid-derived suppressor cells (MDSCs), and B cells (7). These cells serve different functions and combine or cancel out each other, thus creating the microenvironment for lung cancer. Cancer progression and survival are significantly associated with these cell types and functions and their localization in tissue.
2. Diagnostic subtypes of lung cancer
Lung carcinoma is a solid tumor with low antigenicity and a heterogenic phenotype that evades the host's immune defense. Lung cancers usually arise from basal epithelial cells and have two main histological types: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). NSCLC accounts for approximately 85% of lung cancer cases. Since their cells differ in appearance, NSCLCs are classified into 3 major groups (squamous, adenocarcinomas, and large cell cancers) depending on the origin of their cells and their pathological characteristics. Adenocarcinoma is the most frequent type, accounting for 40% of all NSCLCs while squamous cancer accounts for 30% of NSCLCs. The remaining NSCLCs (5–10%) are large cell carcinoma. The other type of lung cancer, SCLCs, consists of smaller than normal undifferentiated cells (1).
3. The role of T-lymphocytes in lung cancer
In a variety of human solid tumors, tumor-infiltrating T-lymphocytes (TILs) are considered to play important roles in immunosurveillance in a tumor-bearing host. CD4+ and CD8+ T cells are the two main subsets of T-lymphocytes and have different effects on tumor immunity within the tumor microenvironment (8).
3.1. CD8+ T lymphocytes
Most CD8+ T cells are cytotoxic T lymphocytes. Cytotoxic T cells are a major subset of T cells that constructively mediates an effective antitumor response: these cells can recognize particular tumor-associated antigens (TAA) presenting on major histocompatibility complex (MHC) class I molecules on the surface of cancer cells and they have the ability to kill cancer cells directly (9). Tumor-infiltrating CD8+ T cells can be classified into two groups by their location: CD8+ T cells within cancer stroma adjacent to cancer cell nests, or CD8+ T cells within the cancer cell nests themselves (9). Naito and colleagues (10) found that the presence of large numbers of CD8+ T cells in cancer cell nests was a favorable independent prognostic factor in colorectal carcinomas. A similar result was noted in breast cancer (11). Moreover, studies have indicated the prognostic significance of infiltrating CD8+ T cells in breast cancer (12), melanoma (13), anal squamous cell carcinoma (14), and oropharyngeal squamous carcinoma (15). However, the role of TILs in the survival of patients with an NSCLC is still debated. Wakabayashi and colleagues (16) contended that CD4+ T cells in the cancer stroma, and not CD8+ T cells in cancer cell nests, are associated with a favorable prognosis in human NSCLC. Another study indicated that neither CD8+ T cells within cancer cell nests nor those in the cancer stroma had a significant impact on patient survival (9). That study found that infiltrating CD8+ T cells and CD4+ T cells in NSCLC may cooperate to suppress cancer progression and their presence together appears to be an independent favorable prognostic factor for this disease.
3.2. CD4+ helper T cells
Various studies have indicated that CD4+ helper T cells are present in the lungs of patients with NSCLC, but their role in antitumor activity is dualistic: some CD4+ T cells seemingly hinder the function of CD8+ T cells and therefore indirectly promote tumor growth, whereas others might help with the activation of CD8+ T cells (17), which are key to removing cancerous tissue or cells. CD4+ helper T cells can be functionally classified into Th1, Th2, Th17, and regulatory T cells (Tregs) based on the secretion of cytokines, and each cell subset has its own unique function (18). As shown in Figure 1, these CD4+ T cell subsets play different biological functions in antitumor immunity and tumor immune evasion. Th1 and Th2 play an important role in the tumor microenvironment and Th17 and Tregs play an important role in immune homeostasis (19).
Figure 1.
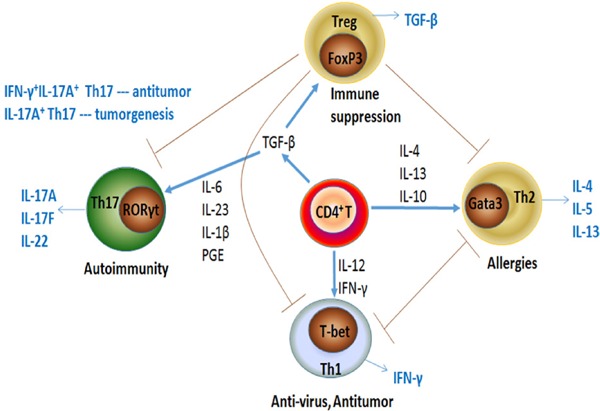
Interaction among CD4+ T-cell subsets. When stimulated with different cytokines, naive CD4+ T cells become polarized toward four different cell subsets: Th1, Th2, Th17, and Treg cells. Each of these CD4+ T-cell subsets serves a different biological role in antitumor immunity by secreting identical cytokines. Th1 and Th2 cells play an important role in the tumor microenvironment and Th17 and Treg cells play an important role in immune homeostasis. The grey arrows indicate the effector functions of the various CD4+ T cell subsets.
3.3. Th1/Th2/Th17/Treg paradigm
When stimulated with different cytokines, different transcription factors select precursor T cells in response to various cytokines or other mediators and differentiate into different subtypes of CD4+ T cells. Th1 cells differentiate when stimulated with interleukin (IL)-12 and interferon-gamma (IFN-γ) through the transcription factor T-bet. Activated Th1 cells release other cytokines like IL-2, IFN-γ, and tumor necrosis factor alpha (TNF-α), which can activate some effector cells such as macrophages, NK cells, and cytotoxic T-lymphocytes (CTLs). These effector cells effectively eliminate tumor cells. The GATA3 transcription factor becomes active and Th2 cells become polarized towards this phenotype in response to IL-4 and IL-6. Activated Th2 cells, which produce IL-4, IL-5, IL-6, IL-10, and IL-13, are key to allergic responses and protection against infection by helminthic parasites (20,21). It is widely believed that cytokines that are secreted by Th1 cells can facilitate CTLs generation, whereas a Th2 cytokine profile might favor antibody response and be detrimental to the induction of CTLs (22,23). A study (24) examined the expression of Th1 and Th2 cells in lung cancer and found that the proportion of Th2 cells was significantly lower than that of Th1 cells, indicating that Th1 cells are dominant tumor infiltrators.
Th17 cel ls become act ivated through the transcription factor retinoid-related orphan receptor gamma t (RORγT) when stimulated with transforming growth factor β (TGF-β), IL-6, IL-23, IL-1β (in humans), and prostaglandin E (PGE) (25,26). Th17 cells have been found to play a contradictory role in tumorigenesis. Numerous studies have identified the tumor-promoting effects of Th17 cells. However, IL-17A+IFN-γ+ Th17 cells have tumor-protective characteristics in tumor immunity (27). The varying functions of the Th17 subset in tumor immunity are largely due to the cytokines they secrete, such as IL-17A, IL-17F, IL-21, IL-22, IFN-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (28). In addition, the antitumor functions of Th17 cells are related to effector lymphocytes, including Th1, Tc1, and NK cells (29).
Treg cells are formed as a result of the transcription factor forkhead box P3 (Foxp3), which is the dominant transcription factor of Treg cells. Developmental pathways of Th17 and Treg cells are closely related. TGF-β alone induces expression of FoxP3 (30,31) and RORγT (31) in TCR-stimulated naive CD4+ T cells. Thus TGF-β is a critical factor for Th17 and Treg cells and is essential for inducing both RORγT and Foxp3. Despite the induction of these transcription factors, TGF-β is unable to initiate Th17 differentiation in vitro unless pro-inflammatory cytokines, such as IL-6 or IL-21, are present. When these cytokines are present, the TGF-β-induced Foxp3 expression is down-regulated and RORγT expression is up-regulated (30,32). In the absence of significant inflammation, TGF-β promotes the differentiation of Treg cells, which maintain immune tolerance. This is because of Foxp3-mediated inhibition of the activity of RORγT, resulting in silencing of IL-17 and IL-23 expression (33).
Th17 and Treg cells have opposite roles in the development of autoimmune and inflammatory diseases. Th17 cells promote autoimmunity while Treg cells seem to control it and therefore play an important role in autoimmune pathogenesis by maintaining self-tolerance and by controlling the growth and activation of autoreactive CD4+ effector T cells (34). Tregs inhibit the recruitment of CD8+ T lymphocytes in tumors and their effector functions are critical to inhibiting lung tumor growth (35). Tregs inhibit the immune system partly by utilizing membrane TGF-β and are thought to be key to downregulating Th1, Th2, and Th17 cells, possibly preventing autoimmunity (36). Thus, the abundance of Tregs are within lung tumor tissue is not surprising (37).
Accordingly, the Th1/Th2/Th17/Treg paradigm is essential for maintaining immune homeostasis based on cytokine production, and the cytokine environment they create determines the fate of the host's antitumor immunity.
4. The role of MDSCs in lung cancer
4.1. Functional and molecular characterization of MDSCs
MDSCs were first identified as immunosuppressive CD11b+ Gr-1+ myeloid cells in cancer patients in the 1980s (38). MDSCs represent a heterogenic population of immature myeloid cells that consists of myeloid progenitors and precursors of macrophages, granulocytes, and DCs they are characterized by a potent ability to suppress various T cell functions (39).
In mice, MDSCs are cells that simultaneously express the two markers CD11b and Gr-1 (38). Gr-1 includes the macrophage and neutrophil markers Ly6C and Ly6G, while CD11b is characteristic of macrophages (40). More recently, MDSCs have been subdivided into different subtypes based on their expression of Ly6C and Ly6G. CD11b+Ly6G+Ly6Clo cells with a granulocytic-like morphology and multilobed nuclei are called granulocytic MDSCs, whereas CD11b+Ly6G−Ly6Chi cells with a monocytic-like morphology are referred to as monocytic MDSCs (41). In cancer patients, MDSCs are defined as cells that express the common myeloid marker CD33 but that lack expression of markers of mature myeloid and lymphoid cells (42). MDSCs are typically CD11b+CD33+CD34+CD14− cells that vary in the expression of CD15, CD124, CD66, and MHC-II, along with that of other markers (43).
4.2. Cellular and molecular mechanisms of MDSC-mediated immune suppression
Lung cancer escapes the host's immune surveillance by dysregulating inflammation. Tumors and their surrounding stroma can produce growth factors, cytokines, and chemokines that recruit, expand, and activate MDSCs. Teixeira and colleagues (44) noted an increased number of MDSCs infiltrating lung cancer in mice. Another study confirmed the hypothesis that activating immune cells through disruption of MDSC-mediated immune suppression would promote antitumor immunity in a murine model of lung cancer (45). MDSCs can suppress immune responses to newly displayed tumor antigens and promote tumor progression; they may even contribute to the metastasis of the tumor. There are several mechanisms by which MDSCs suppress antitumor responses. MDSCs restrain T cell functions in tumor tissues and draining lymph nodes by using two enzymes involved in L-arginine metabolism: arginase-1(ARG-1), which depletes the milieu of L-arginine, and induced nitric oxide synthase 2 (iNOS2), which generates nitric oxide (NO) (46,47).
Reactive oxygen species (ROS) are physiologically produced by activated neutrophils and macrophages as mediators of innate immunity. In chronic inflammation, ROS can weaken T cell responses by affecting CD3-ζ expression. H2O2 produced either by tumor-associated macrophages or by neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis has been found to substantially decrease T cell proliferation in vitro (48,49). ROS can also affect the affinity of Ag-specific TCR, which may explain the specificity of the tolerance induced by Gr-1+CD11b+ MDSCs. In addition to the effect of ARG-1 and ROS, MDSCs may affect CD8+ T cells via the inhibitory molecules B7-H1 and B7-H4 (50). In addition, MDSCs can enhance immune suppression by directly inducing Tregs through the production of IL-10 and TGF-β or ARG (51,52). Tregs actively down-regulate the activation and expansion of antitumor-reactive T cells (53) and NK cells (54). Cysteine, another essential amino acid for T-cell activation, is depleted by MDSCs (55).
5. The role of macrophages in lung cancer
Macrophages are among the most abundant normal cells in the tumor microenvironment and play a central role in tissue repair and remodeling during homeostasis and stress response. They are the first line of defense against pathogens. These cells originate from bone marrow precursors and extravasate into normal tissues, where they acquire distinct morphological and functional properties directed by local tissue and the immunological microenvironment (56).
Tumor-associated macrophages (TAMs) have complex dual functions in their interaction with neoplastic cells, and evidence suggests that they are part of inflammatory circuits that promote tumor progression (57). This is evidence that macrophages, rather than being tumoricidal as suggested after their activation in vitro (58), have a pro-tumoral phenotype in vivo both at the primary site and at metastases (59). Indeed, macrophages are polarized into a pro-tumoral phenotype when lung cancer develops (60).
Macrophages are exceptionally diverse in their functions, reflecting their different origins, differing local environments, and different responses to challenges (61). Given the function of macrophages in immunity, two classes of macrophages have been proposed: i) activated macrophages responding to IFN-γ, TNF-a, and Toll-like receptor 4 (TLR4) activation are involved in Th1-type responses since Th1 cells are capable of killing pathogens via mechanisms just like iNOS, and ii) alternatively activated macrophages that differentiate in response to IL-4 and IL-13 are involved in Th2-type responses, including humoral immunity and wound healing (62). Mills and colleagues called these states M1 (activated) and M2 (alternatively activated). M1 activity inhibits cell proliferation and causes tissue damage, while M2 activity promotes cell proliferation and tissue repair (63). These descriptions suggest that TAMs could either suppress (M1) or promote (M2) tumor development and progression (64). However, such definitions are limited and may not be applicable to the complex tumor microenvironment (65). In contrast to this dualistic M1/ M2 definition, TAMs include several distinct populations that often share characteristics of both types but with greater overall similarity to macrophages involved in developmental processes (66,67).
6. The role of NK cells in lung cancer
NK cells can mediate several effector functions: i) direct cytotoxicity, including exocytosis of cytotoxic granules containing perforin and granzyme B, ii) up-regulation of death receptor ligand expression and the engagement of cognate death receptors on target cells, which can lead to apoptosis of target cells, iii) NK cells produce an array of immune-active cytokines, including IFN-γ, TNF-α, and GM-CSF (68), which places them at the crossroads of innate and adaptive immunity. They also augment monoclonal antibody activity through antibody-mediated cellular cytotoxicity and can be transfected with chimeric antigen receptors (69), and iv) NK cells can release other soluble mediators, such as PGE2, that shape the responses of the immune system. A clear correlation between NK cell activity in the lungs and tumor cell clearance from the lungs has been reported in both mouse and human studies (70). Manuela and colleagues found that depletion of NK cells resulted in an increase in the number of tumor nodes in lung cancer, suggesting that NK cells are key to the control of the growth of lung cancer. A study has found that the activity of NK cells is key to the development or rejection of MHC-I-deficient lymphomas (71) while another study found that depletion of NK cells promoted the growth of fibrosarcomas (72). In addition, a recent study found that the development of lung cancer depends on the function of NK cells. Kreisel and colleagues found that depletion of NK cells promoted urethane-induced lung tumor growth in a mouse strain that is normally not susceptible to lung cancer (73).
7. The role of dendritic cells in lung cancer
Antigen-presenting cells can deliver TAA and prime TAA-specific T cells. DCs are professional antigen-presenting cells and can express MHC-I and MHC-II molecules and costimulatory molecules on their cell surface, all of which assist in T-cell activation (74). There are several cell types capable of antigen presentation in the lung, including resident DCs (75).
Evidence indicates that DCs play a significant role in induction of antitumour immunity (76). Immunotherapy with DCs co-cultured with cytokine-induced killer cells (DC-CIK) has been widely studied and might be a new strategy for treatment of NSCLC. Studies have indicated the efficacy and the safety of DC-CIK immunotherapy (77). When immature in peripheral tissue, DCs express different chemokine receptors, such as CC chemokine receptor 1 (CCR1), CCR2, CCR4, CCR5, CCR6, CCR8, and CXC chemokine receptor 4 (CXCR4) (76). When they encounter exogenous and endogenous antigens including tumor cell-derived antigens around a tumor, DCs can capture those antigens and they can mature in response to inflammatory stimuli such as toll-like receptor-mediated signals. Mature DCs start to express chemokine receptors such as CCR7 and CXCR4. Guided by chemokines, DCs enter the T-cell areas of regional lymph nodes (78).
8. The important role of chemokines in lung carcinoma
When considering the role of tumor-infiltrating immune cells in the lung cancer microenvironment, one cannot ignore the importance of chemokines, which recruit immune cells to the tumor and also regulate their phenotypes and functions. Relatively recent studies have revealed that chemokines regulate the movement of a wide variety of immune cells including lymphocytes, NK cells, and DCs in both physiological and pathological conditions (79). These features mean that chemokines play crucial roles in immune responses. Chemokines function biologically by linking to their corresponding receptors, which are 7-transmembrane G protein-coupled receptors (GPCR) (80). Chemokines are structurally divided into 4 subgroups: CXC, CC, CX3C, and C (81).
TAMs are derived mostly from circulating monocytes that are attracted to tumor sites by locally produced chemotactic factors, such as CCL2, CCL5, CCL7, CCL8, and CXCL12, and macrophage colony stimulating factor (M-CSF). CCL2 is presumed to play an important role in TAM recruitment (82). MDSCs can be recruited by CCL2 in several types of mouse cancers, such as Lewis lung cancer, meth A sarcoma, melanoma, and lymphoma (83). MDSCs express CXCR2 and are detected in abundance in NSCLC (84). CXCR2 ligands, including CXCL1, CXCL2, and CXCL5, are abundant in lung cancer tissue and tumorigenesis can be markedly inhibited by a deficiency in CXCR2 as a result of inhibiting infiltration of MDSCs (Figure 2). NK cells migrate to lymph nodes mainly by utilizing CXCR3, while their migration to inflamed tissues, including tumor sites, involves CCR1, CCR2, CCR5, CXCR3, and CX3CR1 (85). Thus, the ligands for these receptors can regulate the migration of NK cells and may enhance their functions. Treg cells express CCR4, and its ligand, CCL22, regulates intratumoral infiltration of Tregs in various tumors (86).
Figure 2.
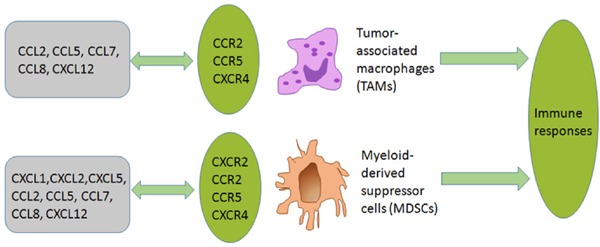
Effects of chemokines on TAMs and MDSCs. TAMs are derived mostly from circulating monocytes which are attracted to tumor sites by locally produced chemotactic factors including CCL2, CCL5, CCL7, CCL8, and CXCL12. MDSCs express CXCR2 and can be recruited to a tumor site by CXCR2 ligands like CXCL1, CXCL2, and CXCL5. MDSCs can also be recruited by CCL2, CCL5, CCL7, CCL7, CCL8, and CXCL12 in lung cancer.
Lung stroma cells can produce abundant chemokines, such as CXCL1, CXCL2, CXCL5, CXCL9, CXCL10, and CXCL11. CXCL10 is an important chemokine responsible for the recruitment and localization of inflammatory cells to sites of tissue damage or infection. It is bound by CXCR3, a receptor shared with CXCL4. CXCL9 and CXCL11, which are expressed on CD4+ T cells, CD8+ T cells, NK cells, B cells, and DCs (87,88), are responsible for their recruitment and localization to the lungs.
9. Conclusion
Studies have indicated the importance of tumor-infiltrating immune cells in tumor development and control and studies have sought to characterize the infiltration of a tumor by those immune cells, providing insight into the effects of tumor-infiltrating immune cells on tumor behavior. Different tumors have different immune profiles, and the current review has focused on lung cancer. There is an obvious infiltration of different types of immune cells, including NK cells, T lymphocytes, macrophages, DCs, MDSCs, and B cells, in lung cancer. A distinctive lung cancer microenvironment is created by immune cells with various functions and interactions, and this microenvironment can ultimately affect tumor progression and survival. In this process, chemokines act as a bridge since they can recruit immune cells to a tumor and they can also regulate the phenotypes and functions of those cells. Although the role of lung cancer-infiltrated immune cells has been reviewed here, the paradoxical role of tumor-infiltrating cells in lung carcinoma still needs to be studied further.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (No. 81573728) and the Natural Science Foundation of Shandong Province (No. ZR2015HM007).
References
- 1. Mendes F, Antunes C, Abrantes AM, Gonçalves AC, Nobre-Gois I, Sarmento AB, Botelho MF, Rosa MS. Lung cancer: The immune system and radiation. Br J Biomed Sci. 2015; 72:78-84. [DOI] [PubMed] [Google Scholar]
- 2. Chapman AM, Sun KY, Ruestow P, Cowan DM, Madl AK. Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer. 2016; 102:122-134. [DOI] [PubMed] [Google Scholar]
- 3. Tyl M, Domagała-Kulawik J. Lung cancer and COPD - Growing clinical problem. Pol Merkur Lekarski. 2017; 43:5-9. [PubMed] [Google Scholar]
- 4. Skowronek J. Brachytherapy in the treatment of lung cancer - A valuable solution. J Contemp Brachytherapy. 2015; 7:297-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bustamante Alvarez JG, González-Cao M, Karachaliou N, Santarpia M, Viteri S, Teixidó C, Rosell R. Advances in immunotherapy for treatment of lung cancer. Cancer Biol Med. 2015; 12:209-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohue Y, Kurose K, Nozawa R, Isobe M, Nishio Y, Tanaka T, Doki Y, Hori T, Fukuoka J, Oka M, Nakayama E. Survival of lung adenocarcinoma patients predicted from expression of PD-L1, galectin-9, and XAGE1 (GAGED2a) on tumor cells and tumor-infiltrating T cells. Cancer Immunol Res. 2016; 4:1049-1060. [DOI] [PubMed] [Google Scholar]
- 7. Domingues P, González-Tablas M, Otero Á, Pascual D, Miranda D, Ruiz L, Sousa P, Ciudad J, Gonçalves JM, Lopes MC, Orfao A, Tabernero MD. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav Immun. 2016; 53:1-15. [DOI] [PubMed] [Google Scholar]
- 8. Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, Wu C, Jiang J. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: A meta-analysis. Cell Physiol Biochem. 2015; 37:1560-1571. [DOI] [PubMed] [Google Scholar]
- 9. Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006; 94:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998; 58:3491-3494. [PubMed] [Google Scholar]
- 11. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: A meta-analysis. Breast Cancer Res Treat. 2014; 148:467-476. [DOI] [PubMed] [Google Scholar]
- 12. Rathore AS, Kumar S, Konwar R, Makker A, Negi MP, Goel MM. CD3+, CD4+ & CD8+ tumour infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J Med Res. 2014; 140:361-369. [PMC free article] [PubMed] [Google Scholar]
- 13. Rahbar M, Naraghi ZS, Mardanpour M, Mardanpour N. Tumor-infiltrating CD8+ lymphocytes effect on clinical outcome of muco-cutaneous melanoma. Indian J Dermatol. 2015; 60:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu WH, Miyai K, Cajas-Monson LC, Luo L, Liu L, Ramamoorthy SL. Tumor-infiltrating CD8+ T lymphocytes associated with clinical outcome in anal squamous cell carcinoma. J Surg Oncol. 2015; 112:421-426. [DOI] [PubMed] [Google Scholar]
- 15. Oguejiofor K, Hall J, Slater C, Betts G, Hall G, Slevin N, Dovedi S, Stern PL, West CM. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. 2015; 113:886-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sznurkowski JJ, Zawrocki A, Emerich J, Biernat W. Prognostic significance of CD4+ and CD8+ T cell infiltration within cancer cell nests in vulvar squamous cell carcinoma. Int J Gynecol Cancer. 2011; 21:717-721. [DOI] [PubMed] [Google Scholar]
- 17. Wang L, Song Y, Men X. Variance of TNFAIP8 expression between tumor tissues and tumor-infiltrating CD4+ and CD8+ T cells in non-small cell lung cancer. Tumour Biol. 2014; 35:2319-2325. [DOI] [PubMed] [Google Scholar]
- 18. Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: A (co-)evolutionary perspective. Nat Rev Immunol. 2009; 9:883-889. [DOI] [PubMed] [Google Scholar]
- 19. Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur J Immunol. 2009; 39:2076-2082. [DOI] [PubMed] [Google Scholar]
- 20. Meng M, Li C, Yang F, Chen H, Li X, Yang Y, Chen D. Novel immunostimulators with a thiazolidin-4-one ring promote the immunostimulatory effect of human iNKT cells on the stimulation of Th2-like immune responsiveness via GATA3 activation in vitro. Int Immunopharmacol. 2016; 39:353-358. [DOI] [PubMed] [Google Scholar]
- 21. Bredo G, Storie J, Shrestha Palikhe N, Davidson C, Adams A, Vliagoftis H, Cameron L. Interleukin-25 initiates Th2 differentiation of human CD4+ T cells and influences expression of its own receptor. Immun Inflamm Dis. 2015; 3:455-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sadeghi S, Sanati MH, Taghizadeh M, Mansouri P, Jadali Z. Study of Th1/Th2 balance in peripheral blood mononuclear cells of patients with alopecia areata. Acta Microbiol Immunol Hung. 2015; 62:275-285. [DOI] [PubMed] [Google Scholar]
- 23. Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Jr, Paul WE. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015; 16:1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito N, Nakamura H, Tanaka Y, Ohgi S. Lung carcinoma: analysis of T helper type 1 and 2 cells and T cytotoxic type 1 and 2 cells by intracellular cytokine detection with flow cytometry. Cancer. 1999; 85:2359-2367. [PubMed] [Google Scholar]
- 25. Quintana FJ. Old dog, new tricks: IL-6 cluster signaling promotes pathogenic TH17 cell differentiation. Nat Immunol. 2016; 18:8-10. [DOI] [PubMed] [Google Scholar]
- 26. Peters A, Fowler KD, Chalmin F, Merkler D, Kuchroo VK, Pot C. IL-27 induces Th17 differentiation in the absence of STAT1 signaling. J Immunol. 2015; 195:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009; 31:787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Llosa NJ, Geis AL, Thiele Orberg E, Housseau F. Interleukin-17 and type 17 helper T cells in cancer management and research. Immunotargets Ther. 2014; 3:39-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones SA, Sutton CE, Cua D, Mills KH. Therapeutic potential of targeting IL-17. Nat Immunol. 2012; 13:1022-1025. [DOI] [PubMed] [Google Scholar]
- 30. Li S, Fan Q, He S, Tang T, Liao Y, Xie J. MicroRNA-21 negatively regulates Treg cells through a TGF-β1/Smad-independent pathway in patients with coronary heart disease. Cell Physiol Biochem. 2015; 37:866-878. [DOI] [PubMed] [Google Scholar]
- 31. Lee YJ, Hyung KE, Yoo JS, Jang YW, Kim SJ, Lee DI, Lee SJ, Park SY, Jeong JH, Hwang KW. Effects of exposure to extremely low-frequency electromagnetic fields on the differentiation of Th17 T cells and regulatory T cells. Gen Physiol Biophys. 2016; 35:487-495. [DOI] [PubMed] [Google Scholar]
- 32. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007; 8:967-974. [DOI] [PubMed] [Google Scholar]
- 33. Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008; 453:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014; 13:668-677. [DOI] [PubMed] [Google Scholar]
- 35. Ganesan AP, Johansson M, Ruffell B, Yagui-Beltrán A, Lau J, Jablons DM, Coussens LM. Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. J Immunol. 2013; 191:2009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arellano B, Graber DJ, Sentman CL. Regulatory T cell-based therapies for autoimmunity. Discov Med. 2016; 22:73-80. [PMC free article] [PubMed] [Google Scholar]
- 37. Wei T, Zhang J, Qin Y, Wu Y, Zhu L, Lu L, Tang G, Shen Q. Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. Am J Cancer Res. 2015; 5:2190-2201. [PMC free article] [PubMed] [Google Scholar]
- 38. Atretkhany KN, Drutskaya MS. Myeloid-derived suppressor cells and proinflammatory cytokines as targets for cancer therapy. Biochemistry (Mosc). 2016; 81:1274-1283. [DOI] [PubMed] [Google Scholar]
- 39. Ballbach M, Hall T, Brand A, Neri D, Singh A, Schaefer I, Herrmann E, Hansmann S, Handgretinger R, Kuemmerle-Deschner J, Hartl D, Rieber N. Induction of myeloid-derived suppressor cells in cryopyrin-associated periodic syndromes. J Innate Immun. 2016; 8:493-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Srivastava MK, Andersson Å, Zhu L, Harris-White M, Lee JM, Dubinett S, Sharma S. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy. 2012; 4:291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011; 32:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qu P, Wang LZ, Lin PC. Expansion and functions of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Lett. 2016; 380:253-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meirow Y, Kanterman J, Baniyash M. Paving the road to tumor development and spreading: Myeloid-derived suppressor cells are ruling the fate. Front Immunol. 2015; 6:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teixeira D, Almeida JS, Visniauskas B, Gomes GN, Hirata AE, Bueno V. Myeloid-derived suppressor cells and associated events in urethane-induced lung cancer. Clinics (Sao Paulo). 2013; 68:858-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012; 7:e40677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gato-Cañas M, Martinez de Morentin X, Blanco-Luquin I, Fernandez-Irigoyen J, Zudaire I, Liechtenstein T, Arasanz H, Lozano T, Casares N, Chaikuad A, Knapp S, Guerrero-Setas D, Escors D, Kochan G, Santamaría E. A core of kinase-regulated interactomes defines the neoplastic MDSC lineage. Oncotarget. 2015; 6:27160-27175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Otsubo D, Yamashita K, Fujita M, Nishi M, Kimura Y, Hasegawa H, Suzuki S, Kakeji Y. Early-phase treatment by low-dose 5-fluorouracil or primary tumor resection inhibits MDSC-mediated lung metastasis formation. Anticancer Res. 2015; 35:4425-4431. [PubMed] [Google Scholar]
- 48. Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci U S A. 1996; 93:13119-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu YK, Yang HW, Wang MH, Wang W, Liu F, Yang HL. N-acetylcysteine attenuates cobalt nanoparticle-induced cytotoxic effects through Inhibition of cell death, reactive oxygen species-related signaling and cytokines expression. Orthop Surg. 2016; 8:496-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chou HS, Hsieh CC, Charles R, Wang L, Wagner T, Fung JJ, Qian S, Lu LL. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012; 93:272-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rastad JL, Green WR. Myeloid-derived suppressor cells in murine AIDS inhibit B-cell responses in part via soluble mediators including reactive oxygen and nitrogen species, and TGF-β. Virology. 2016; 499:9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Enokida T, Nishikawa H. Regulatory T cells, as a target in anticancer immunotherapy. Immunotherapy. 2017; 9:623-627. [DOI] [PubMed] [Google Scholar]
- 54. Fan R, Xiang Y, Yang L, Liu Y, Chen P, Wang L, Feng W, Yin K, Fu M, Xu Y, Wu J. Impaired NK cells' activity and increased numbers of CD4 + CD25+ regulatory T cells in multidrug-resistant Mycobacterium tuberculosis patients. Tuberculosis (Edinb). 2016; 98:13-20. [DOI] [PubMed] [Google Scholar]
- 55. Edgington-Mitchell LE, Rautela J, Duivenvoorden HM, Jayatilleke KM, van der Linden WA, Verdoes M, Bogyo M, Parker BS. Cysteine cathepsin activity suppresses osteoclastogenesis of myeloid-derived suppressor cells in breast cancer. Oncotarget. 2015; 6:27008-27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Panni RZ, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy. 2013; 5:1075-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol. 2015; 12:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fidler IJ. Macrophage therapy of cancer metastasis. Ciba Found Symp. 1988; 141:211-222. [DOI] [PubMed] [Google Scholar]
- 59. Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013; 35:585-600. [DOI] [PubMed] [Google Scholar]
- 60. Huang WC, Chan ML, Chen MJ, Tsai TH, Chen YJ. Modulation of macrophage polarization and lung cancer cell stemness by MUC1 and development of a related small-molecule inhibitor pterostilbene. Oncotarget. 2016; 7:39363-39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013; 496:445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roy S, Schmeier S, Arner E, et al. Redefining the transcriptional regulatory dynamics of classically and alternatively activated macrophages by deepCAGE transcriptomics. Nucleic Acids Res. 2015; 43:6969-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mills CD. M1 and M2 macrophages: Oracles of health and disease. Crit Rev Immunol. 2012; 32:463-488. [DOI] [PubMed] [Google Scholar]
- 64. Almendros I, Gileles-Hillel A, Khalyfa A, Wang Y, Zhang SX, Carreras A, Farré R, Gozal D. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: Effect of tumor microenvironment. Cancer Lett. 2015; 361:233-239. [DOI] [PubMed] [Google Scholar]
- 65. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010; 141:39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang ZM, Yang Z, Zhang Z. Distribution and characterization of tumor-associated macrophages/ microglia in rat C6 glioma. Oncol Lett. 2015; 10:2442-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang J, Yao H, Song G, Liao X, Xian Y, Li W. Regulation of epithelial-mesenchymal transition by tumor-associated macrophages in cancer. Am J Transl Res. 2015; 7:1699-1711. [PMC free article] [PubMed] [Google Scholar]
- 68. Li J, Dong X, Zhao L, Wang X, Wang Y, Yang X, Wang H, Zhao W. Natural killer cells regulate Th1/Treg and Th17/ Treg balance in chlamydial lung infection. J Cell Mol Med. 2016; 20:1339-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Klingemann HG. Cellular therapy of cancer with natural killer cells-Where do we stand? Cytotherapy. 2013; 15:1185-1194. [DOI] [PubMed] [Google Scholar]
- 70. Al Omar SY, Marshall E, Middleton D, Christmas SE. Increased killer immunoglobulin-like receptor expression and functional defects in natural killer cells in lung cancer. Immunology. 2011; 133:94-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. J Immunol. 2005; 174:6566-6569. [PubMed] [Google Scholar]
- 72. Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001; 13:459-463. [DOI] [PubMed] [Google Scholar]
- 73. Kreisel D, Gelman AE, Higashikubo R, Lin X, Vikis HG, White JM, Toth KA, Deshpande C, Carreno BM, You M, Taffner SM, Yokoyama WM, Bui JD, Schreiber RD, Krupnick AS. Strain-specific variation in murine natural killer gene complex contributes to differences in immunosurveillance for urethane-induced lung cancer. Cancer Res. 2012; 72:4311-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017; 27:74-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Desch AN, Henson PM, Jakubzick CV. Pulmonary dendritic cell development and antigen acquisition. Immunol Res. 2013; 55:178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012; 12:265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang S, Wang Z. Efficacy and safety of dendritic cells co-cultured with cytokine-induced killer cells immunotherapy for non-small-cell lung cancer. Int Immunopharmacol. 2015; 28:22-28. [DOI] [PubMed] [Google Scholar]
- 78. Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev. 2005; 16:581-592. [DOI] [PubMed] [Google Scholar]
- 79. Liu J, Li F, Ping Y, Wang L, Chen X, Wang D, Cao L, Zhao S, Li B, Kalinski P, Thorne SH, Zhang B, Zhang Y. Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget. 2015; 6:24978-24989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moser B, Wolf M, Walz A, Loetscher P. Chemokines: Multiple levels of leukocyte migration control. Trends Immunol. 2004; 25:75-84. [DOI] [PubMed] [Google Scholar]
- 81. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012; 36:705-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett. 2008; 267:204-215. [DOI] [PubMed] [Google Scholar]
- 83. Lazennec G, Richmond A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol Med. 2010; 16:133-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Spaks A. Role of CXC group chemokines in lung cancer development and progression. J Thorac Dis. 2017; 9:S164-S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Walzer T, Vivier E. G-protein-coupled receptors in control of natural killer cell migration. Trends Immunol. 2011; 32:486-492. [DOI] [PubMed] [Google Scholar]
- 86. Smyth MJ, Ngiow SF, Teng MW. Targeting regulatory T cells in tumor immunotherapy. Immunol Cell Biol. 2014; 92:473-474. [DOI] [PubMed] [Google Scholar]
- 87. Kohrgruber N, Gröger M, Meraner P, Kriehuber E, Petzelbauer P, Brandt S, Stingl G, Rot A, Maurer D. Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol. 2004; 173:6592-6602. [DOI] [PubMed] [Google Scholar]
- 88. Basset L, Chevalier S, Danger Y, Arshad MI, Piquet-Pellorce C, Gascan H, Samson M. Interleukin-27 and IFNγ regulate the expression of CXCL9, CXCL10, and CXCL11 in hepatitis. J Mol Med (Berl). 2015; 93:1355-1367. [DOI] [PubMed] [Google Scholar]


