Summary
Altered global methylation levels revealed LINE-1 methylation in young mothers of Down syndrome (DS) compared to controls suggesting the possibility of impaired DNA methylation causing abnormal segregation of chromosome 21. Methylene Tetrahydrofolate Reductase (MTHFR) is one of the major enzymes of the folate metabolism pathway. MTHFR gene polymorphism has been associated with maternal risk for DS. Studies have revealed that increased MTHFR promoter methylation results in the reduction of MTHFR protein activity further leading to increased risk of various diseases. The aim of this study is to compare the levels of MTHFR promoter methylation in all three study groups. A total of 120 subjects were recruited for the study and was divided into the following three groups: Group I (mothers of DS without Congenital Heart Defects (CHD), n = 40); Group II (mothers of DS with CHD, n = 40); and Group III (age matched control mothers, n = 40). Genomic DNA was isolated from 2 ml peripheral blood and bisulfite treatment was done to convert all unmethylated cytosines into uracil followed by PCR amplification for MTHFR promoter region and Sanger's sequencing. Results showed that there was a two fold increase in methylated promoter region of MTHFR gene in group II compared to other groups. None of the methylation pattern was observed in the control group. MTHFR promoter methylation affects folate metabolism which is known to play a role in chromosomal breakage, abnormal chromosomal segregation and genomic instability and therefore a developmental defect in the form of congenital cardiac anomaly.
Keywords: Down syndrome, congenital heart defects, MTHFR promoter, sequencing
1. Introduction
Aberrant DNA methylation has been associated with several diseases like cancers (1,2), diabetes (3) or neurological diseases including Down syndrome (DS) (4). DNA methylation leads to the addition of a methyl group at 5′ carbon of cytosine, which can bring changes in DNA structure, altering the set patterns of gene expression.
Congenital heart defects (CHD) account for a major portion of life-threatening birth defects. Atrioventricular Septal Defect (AVSD) and Ventricular septal defects (VSD) are common cardiac malformations in DS cases (5,6). The relationship between DS and maternal genetic polymorphism in folate/homocysteine metabolism is well established. Folate is essential for various cellular processes viz., synthesis of DNA, RNA, methylation and embryonic developmental processes including the cardiovascular system (7,8). Folate deficiency may lead to stunted growth, anemia, weight loss, digestive disorders and behavioral issues. Reports of in vivo studies showed a decreased level of folate causes hypomethylation leading to DNA strand breakage and abnormal segregation of chromosomes (9,10). Changes in folate metabolism lead to an increase in DNA hypomethylation due to an altered DNA methylation pattern thereby further increasing the risk of chromosome nondisjunction (11).
Altered global methylation levels reveal LINE-1 methylation in young mothers of DS thus suggesting the possibility of impaired DNA methylation in mothers of DS children causing abnormal segregation of chromosome 21 (12). Also maternal folic acid supplementation has been associated with a decreased risk of congenital heart defects in DS babies (13).
The methylenetetrahydrofolate reductase (MTHFRMTHFR) gene is reported to be associated with the risk of CHD in DS. It was also reported that promoter methylation regulates MTHFR gene and increased MTHFR promoter methylation was seen in DNA isolated from cancer patients, patients with cardiovascular or renal disorders, and placental DNA from women with pre-eclampsia. It was also observed that as the level of promoter methylation in MTHFR gene increases, the MTHFR protein activity is reduced, thereby increasing the risk of various diseases (14,15). In this study, we investigated MTHFR promoter methylation levels in mothers of DS, mothers of DS with CHD and matched healthy control mothers.
2. Materials and Methods
2.1. Study Subjects
A total of 120 subjects recruited in the present study included three groups: Group I comprised of others of DS without CHD (n = 40); Group II had mothers of DS with CHD (n = 40); and Group III included agematched control mothers (n = 40). DS subjects were recruited from outpatient clinics of 2 tertiary care institutes in India, after obtaining informed consent. The DS were enrolled in the study after confirmation of karyotype and echocardiography to confirm the presence or absence of AVSD. Quantitative Fluorescent – Polymerase Chain Reaction (PCR) was also done on DNA samples for confirmation of DS using standard protocols. Ethical clearance was from the ethics committee of the Institute where the work was performed. Peripheral blood samples were available from all hundred and twenty women and the age of all the women ranged from 18 to 44 years.
2.2. Bisulfite treatment and sequencing
Genomic DNA was isolated from 2 mL peripheral blood using standard Phenol Chloroform method and quantified using Nanodrop spectrophotometer (NanoDrop Thermo Scientific). The EpiTect Bisulfite Kit (Qiagen, Italy) was used to convert all unmethylated cytosines into uracil. Bisulfite conversion of genomic DNA is divided into 4 steps: denaturation, sulphonation, hydrolic deamination and alkali desulphonation. Double-stranded genomic DNA was first converted into single stranded before the sulphonation step which proceeds with the addition of bisulphite to cytosine. Later hydrolic deamination of the cytosine-bisulphite derivative was done in order to give a uracil-bisulphite derivative. Finally, alkali desulphonation removed the sulphonate group through alkali treatment which finally gave uracil. Bisulphite treatment deaminated cytosine to uracil in single-stranded DNA. PCR amplification finally amplified uracil to thymine and 5′ Methyl cytosine residues to cytosine, further distinguishing methylated CpGs from unmethylated CpGs by the presence of a cytosine “C” versus thymine “T” residue after sequencing.
PCR amplification was done for the MTHFR promoter region. The CpG islands present in the 5′-untranslated promoter region of MTHFR; from + 30 to 184 from the TSS (transcription start site) was studied. This region contains seven CpG islands and the methylation levels of these CpG islands were found to be associated with gene expression levels in human lung cancer cells (16).
Table 1 shows the details of PCR reaction and primers including: the sequence of primers used, annealing temperature (Ta), amplicon length, the location of promoter region studied and the number of CpG islands it contained. PCR products were visualised by standard ethidium bromide-agarose gel electrophoresis. PCR purified products were directly sequenced using an ABI 310 Automated Sequencer (ABI, Foster City, CA, USA).
Table 1. Sequence of primers, annealing temperature (Ta), amplicon size and number od CpG sites present.
| S.No. | Primer Sequences | Ta | Amplicon length | CpG sites |
|---|---|---|---|---|
| 1 | F: 5′TTTTAATTTTTGTTTGGAGGGTAGT-3′ R: 5′AAAAAAACCACTTATCACCAAATTC-3′ |
55°C | 155bp | 7 |
3. Results and Discussion
The age range of the mothers with DS babies was 18–45 years, with median age of 28.5 years. In the DS babies with cardiac defects, the most common cardiac defect was AVSD, comprising 62.5% cases. Other cardiac defects included VSD, atrial septal defects (ASD) and Tetralogy of Fallot (TOF). Mothers of DS with cardiac defects were in Group II for the study.
The sequencing results of bisulfite converted genomic DNA for all three groups showed that there was a two fold increase in methylated promoter region of MTHFR gene in group mothers of DS having CHD compared to mothers of DS without CHD. However, no methylation was observed in age matched control mothers group (Figure 1). Figure 2 shows the pictorial representation of CpG island frequencies in all three study groups. CpG island methylation frequencies were found to be 24.4% and 75.6% in Group I and II respectively. No methylation was seen in the control group.
Figure 1.
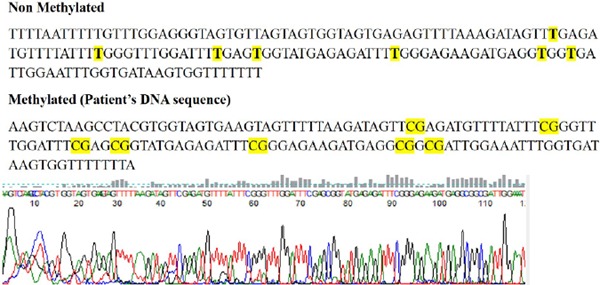
Results of Sanger's sequencing using Bisulfite converted DNA of both non-methylated and methylated (patient's sample). The highlighted text in the non-methylated and methylated sequences shows all seven CpG sites which were methylated in patient's. Given below is the electropherogram of methylated patient's sample.
Figure 2.
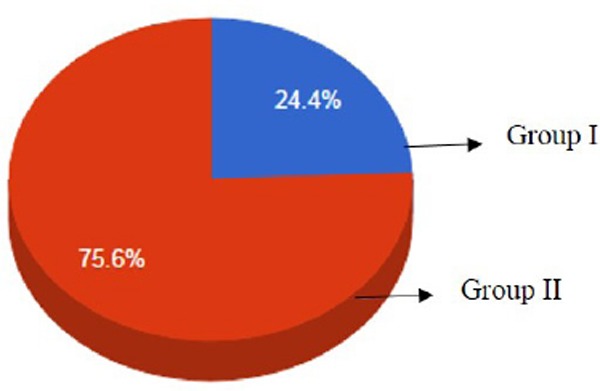
Pictorial representation of CpG island frequencies in all three groups. CpG island methylation frequencies were found to be 24.4% and 75.6% in Group I and II respectively. Note: No methylation was observed in group III.
Decreased folate level has been linked to aberrant cell growth, impaired DNA methylation and chromosomal damage (9,10). The presence of CHD in DS accounts for 40% to 50% in which AVSD is the most common form of CHD followed by VSD. Thyroid abnormalities are also common in DS patients (17). Studies are now focused on why some defects are more common in DS children. The role of epigenetics is considered important in causation of developmental defects.
A case-control study from Spain reported absence of maternal folic acid supplementation was more frequent in DS with atrioventricular septal defects (OR = 1.69, 95% CI = 1.08 − 2.63) or atrial septal defects (OR = 1.69, 95% CI= 1.11 − 2.58) compared to DS without CHD (18). Thus, maternal supplementation with folic acid is likely to be associated with reduced risk of CHD in DS. This study revealed a significant difference in methylation profiles in DNA isolated from blood compared to DNA isolated from heart tissues. Further, 22 samples from the heart showed increased methylation in fetuses having DS compared to fetuses having normal karyotypes (19,20). The present paper focuses on the presence of CHD due to genetic polymorphism present in the genes of folate metabolism to their occurrence in DS offspring. MTHFR is one of the major enzymes involved in the folate/homocysteine metabolism pathway and MTHFR gene polymorphism has been associated with maternal risk for DS progeny (21,22,23). Several congenital complications are reported in an individual with DS. Most of them are affected by maternal carbon metabolism and consequently can lead to epigenetic changes and hence impaired chromosome segregation (24). The promoter methylation of a gene is an epigenetic event which leads to reduced expression of genes.
A similar study was conducted by Coppede et al. in 2015 (7) in Italian mothers of DS along with age-matched controls who conceived before 35 years of age. Authors have investigated MTHFR promoter methylation levels and also searched for correlation between MTHFR promoter methylation and micronucleus frequency and found an increase in the methylation level in mothers of DS when compared to controls (33.3 ± 8.1% vs. 28.3 ± 5.8%; p = 0.001). The frequency of micronucleated lymphocytes was also found to be higher in mothers of DS group than control mothers (16.1 ± 8.6‰ vs. 10.5 ± 4.3‰; p = 0.0004) and it correlated with MTHFR promoter methylation levels r = 0.33; p = 0.006). The study concluded that MTHFR methylation is likely to contribute to the increased genomic instability observed in DNA isolated from mothers of DS, and could play a role in the risk of birth of a child with DS as well as in the onset of age related diseases in those women. We investigated a CpG island in the MTHFR promoter region in three groups (mothers of DS with CHD, mothers of DS without CHD and controls) and the methylation status was found to be elevated in mothers of DS with CHD highlighting the essential role of MTHFR promoter methylation in occurrence of both CHD and DS in women of reproductive age. These studies indicate that MTHFR promoter methylation can be used as a biomarker for screening mothers of DS for the presence of CHD in these populations. However, this is the only study reported in the literature which shows the association of MTHFR promoter methylation with the occurrence of CHD in mothers of DS. Furthermore, these studies can also be conducted in other ethnic groups with a larger sample size to confirm these findings.
In conclusion, MTHFR promoter methylation affects folate metabolism, which is known to play a role in chromosomal breakage, abnormal chromosomal segregation and genomic instability and therefore a developmental defect in the form of congenital cardiac anomaly. The present study thus clearly highlights the association of MTHFR promoter hypermethylation in mothers of DS having DS babies with cardiac defects. We reported increased promoter methylation in mothers of DS with CHD when compared to mothers of DS without CHD and healthy control mothers.
Acknowledgements
This research was supported by the Department of Science and Technology, Ministry of Health, Government of India (DST/Inspire fellowship/2012/499). The study was also supported by Indian Council of Medical Research. We are highly grateful to Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS), Lucknow, UP; for providing the infrastructure for research work.
References
- 1. Heyn H, Esteller M. DNA methylation profiling in the clinic: Applications and challenges. Nat Rev Genet. 2012; 13:679-692. [DOI] [PubMed] [Google Scholar]
- 2. Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004; 22:4632-4642. [DOI] [PubMed] [Google Scholar]
- 3. Fradin D, Le Fur S, Mille C, Naoui N, Groves C, Zelenika D, McCarthy MI, Lathrop M, Bougneres P. Association of the CpG methylation pattern of the proximal insulin gene promoter with type 1 diabetes. PLoS One. 2012; 7:e36278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin S, Lee YK, Lim YC, Zheng Z, Lin XM, Ng DP, Holbrook JD, Law HY, Kwek KY, Yeo GS, Ding C. Global DNA hypermethylation in Down syndrome placenta. Plos Genetics. 2013; 9:e1003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asim A, Kumar A, Muthuswamy S, Jain S, Agarwal S. Down syndrome: An insight of disease. J Biomed Sci. 2015; 22:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39:1890-1900. [DOI] [PubMed] [Google Scholar]
- 7. Coppedè F. The genetics of folate metabolism and maternal risk of birth of a child with Down syndrome and associated congenital heart defects. Front Genet. 2015; 25; 6:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canfield MA, Collins JS, Botto LD, Williams LJ, Mai CT, Kirby RS, Pearson K, Devine O, Mulinare J, National Birth Defect Preventin Network Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: Findings from a multi-state population-based study. Birth Defects Res A Clin Mol Teratol. 2005; 73:679-689. [DOI] [PubMed] [Google Scholar]
- 9. Fenech M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat Res. 2006; 600:58-66. [DOI] [PubMed] [Google Scholar]
- 10. Fenech M. Micronuclei and their association with sperm abnormalities, infertility, pregnancy loss, pre-eclampsia and intra-uterine growth restriction in humans. Mutagenesis. 2011; 26:63-67. [DOI] [PubMed] [Google Scholar]
- 11. James SJ, Pogribna M, Pogribny IP, Melnyk S, Hine RJ, Gibson JB, Yi P, Tafoya DL, Swenson DH, Wilson VL, Gaylor DW. Abnormal folate metabolism and mutation in the methylenetetrahydrofolate reductase gene may be maternal risk factor for Down syndrome. Am J Clin Nutr. 1999; 70:495-501. [DOI] [PubMed] [Google Scholar]
- 12. Božović IB, Stanković A, Živković M, Vraneković J, Kapović M, Brajenović-Milić B. Altered LINE-1 methylation in mothers of children with Down syndrome. PLoS One. 2015; 10:e0127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bean LJ, Allen EG, Tinker SW, Hollis ND, Locke AE, Druschel C, Hobbs CA, Leary O, Romitti PA, Royle MH, Torfs CP, Dooley KJ, Freeman SB, Sherman SL. Lack of maternal folic acid supplementation is associated with heart defects in Down syndrome: A report from the national Down syndrome project. Birth Defects Res A Clin Mol Teratol. 2011; 91:885-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khazamipour N, Noruzinia M, Fatehmanesh P, Keyhanee M, Pujol P. MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: The role of epigenetics in male infertility. Hum Reprod. 2009; 24:2361-2364. [DOI] [PubMed] [Google Scholar]
- 15. Wei LK, Sutherland H, Au A, Camilleri E, Haupt LM, Gan SH, Griffiths LR. Apotential epigenetic marker mediating serum folate and vitamin B12 levels contributes to the risk of ischemic stroke. Biomed Res Int. 2015; 167976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Botezatu A, Socolov D, Iancu IV, Huica I, Plesa A, Ungureanu C, Anton G. Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and promoter methylation in cervical oncogenic lesions and cancer. J Cell Mol Med. 2013; 17:543-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dayal D, Jain P, Panigrahi I, Bhattacharya A, Sachdeva N. Thyroid dysfunction in children with Down syndrome. Indian Pediatr. 2014; 51:751-752. [DOI] [PubMed] [Google Scholar]
- 18. Serra-Juhé C, Cuscó I, Homs A, Flores R, Torán N, Pérez-Jurado LA. DNA methylation abnormalities in congenital heart disease. Epigenetics. 2015; 10:167-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guéant JL, Caillerez-Fofou M, Battaglia-Hsu S, Alberto JM, Freund JN, Dulluc I, Adjalla C, MAurya F, Merle C, Nicolas JP, Namour F, Daval JL. Molecular and cellular effects of vitamin B12 in brain, myocardium and liver through its role as co-factor of methionine synthase. Biochimie. 2013; 95:1033-1040. [DOI] [PubMed] [Google Scholar]
- 20. Rotondo JC, Bosi S, Bazzan E, Di Domenico M, De Mattei M, Selvatici R, Patella A, Marci R, Toqnon M, Martini F. Methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples of infertile couples correlates with recurrent spontaneous abortion. Hum Reprod. 2012; 27:3632-3638. [DOI] [PubMed] [Google Scholar]
- 21. Wu W, Shen O, Qin Y, Niu X, Lu C, Xia Y, Song L, Wang X. Idiopathic male infertility is strongly associated with aberrant promoter methylation of methylenetetrahydrofolate reductase (MTHFR). PLoS One. 2010; 5:e13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaissière T, Hung RJ, Zaridze D, Moukeria A, Cuenin C, Fasolo V, Ferro G, Paliwal A, Hainaut P, Brennan P, Tost J, Boffetta P, Herceq Z. Quantitative analysis of DNA methylation profiles in lung cancer identifies aberrant DNA methylation of specific genes and its association with gender and cancer risk factors. Cancer Res. 2009; 69:243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients. 2013; 5:3481-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism: With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011; 34:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]


