Summary
Inflammatory fibroid polyps (IFP) are an extremely rare entity that arise within the submucosa of the gastrointestinal tract, and represent less than 0.1% of all gastric polyps. They are most commonly localized to the gastric antrum, small intestines and recto-sigmoid colon. IFPs are most commonly found incidentally upon endoscopic evaluation in the absence of symptoms. Presenting symptoms depend on the location of the tumor, although polyps located in the stomach most commonly present with epigastric pain and early satiety. Classic histologic features include perivascular onion skinning of spindle cells with an abundance of eosinophilic infiltration. The prompt diagnosis and management of IFP is essential due to its underlying risk for intussusception, outlet obstruction and acute hemorrhage. In addition, recent evidence has shown that IFP is driven by an activating mutation in the platelet derived growth factor receptor alpha (PDGFRA) gene, suggesting a neoplastic etiology. Herein, we discuss a case of a 65-year-old woman with an inflammatory fibroid polyp of the gastric antrum who initially presented with early hypovolemic shock and melena. Diagnosis was made by endoscopic visualization, biopsy and immunohistochemical analysis.
Keywords: Inflammatory fibroid polyp, vanek tumor, gastric polyp, platelet derived growth factor receptor alpha, benign neoplasms of the gastrointestinal tract
1. Introduction
Inflammatory fibroid polyps (IFP) are an extremely rare entity that arise within the gastrointestinal tract, and represent less than 0.1% of all gastric polyps (1). These lesions most commonly affect older adults, especially those localizing to the gastric antrum. In children, IFPs more commonly occur in the small intestines and present as intussusception (2). IFPs arise from the submucosa and penetrate through the lamina propria leading to bulging of the mucosal layer. Rarely, they can ulcerate through the mucosa causing hemorrhage, leading to progressive blood loss and symptoms related to hypovolemic shock. Herein, we discuss a case of an adult woman who initially presented with a recent history of orthostatic hypotension and melena who was found to have an IFP of the gastric antrum evaluated by endoscopic visualization, biopsy and immunohistochemical analysis. A discussion involving the pathological characterization and management of IFP, followed by a brief review of the literature, is included in this article.
2. Case Report
A 65-year-old Puerto Rican woman with a past medical history of hypertension and asthma first presented to the emergency department with a one-day history of lightheadedness and a recent fall at home. The patient endorsed feeling generally unwell for several days, associated with two weeks of diarrhea, and was recovering with bed rest at home. On the day of presentation, while attempting to stand up from bed, the patient immediately felt dizzy and eventually lost consciousness. She awoke on the floor, denying convulsive limb movements, tongue biting, or post-event confusion, and came to the hospital for further evaluation.
The patient was initially found to be hypotensive and tachycardic. While taking a urine sample, the patient noticed black, tarry stools. She denied any abdominal pain, nausea, vomiting, dysphagia, early satiety or use of iron supplementation. The patient denied having an upper endoscopy or colonoscopy in the past and was not currently taking anticoagulation or antiplatelet therapy. Family history was significant for a maternal grandmother and niece with stomach cancer. Physical examination revealed dry mucous membranes, reduced skin turgor and guaiac positive stool. Initial laboratory studies revealed a hemoglobin of 8.3 mg/dL and mean corpuscular volume of 91.6 fL with normal electrolytes and kidney function.
The patient was admitted to the hospital and given aggressive intravenous (IV) fluid resuscitation. A chest radiograph and computed tomography (CT) scan of the head were both negative for acute changes. CT abdomen/ pelvis revealed an antral mass, measuring 1.82 × 2.08 cm in greatest diameter, protruding within the gastric lumen with associated sub-centimeter perigastric lymph nodes (Figure 1). No other sources of acute hemorrhage were identified. Upper endoscopy revealed a 3 cm ulcerated, semi-sessile mass in the gastric antrum with evidence of recent hemorrhage (Figure 2). An endoscopic ultrasound of the antral mass showed it to be derived from the submucosa and extending through the mucosa, with no evidence of invasion to surrounding organs. A repeat upper endoscopy was completed for tumor resection using a snare technique.
Figure 1.
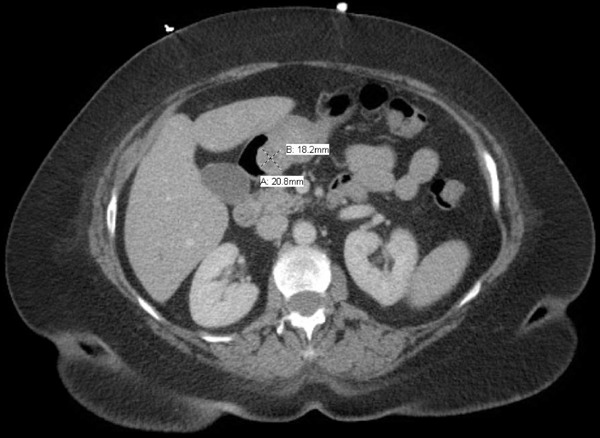
CT Abdomen with IV Contrast depicting a 1.82 × 2.08 cm mass of the antrum of the stomach. There are small subcentimeter perigastric nodes identified.
Figure 2.
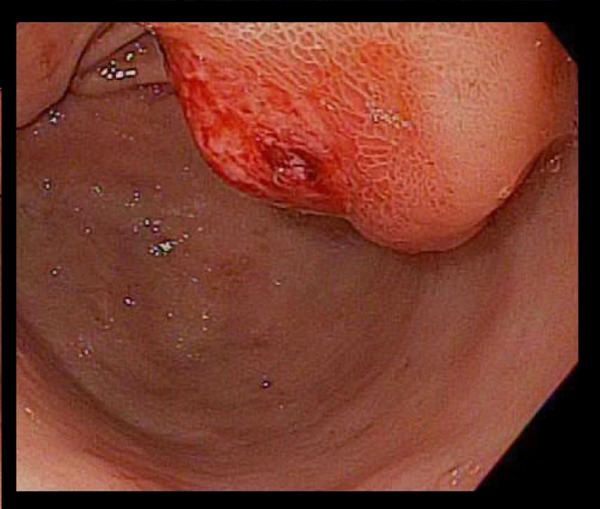
Upper endoscopy identifying a 3 cm large, submucosal, ulcerated, semi-sessile mass located in the gastric antrum.
Pathological evaluation showed a nodular proliferation of bland spindle cells, small vessels, and an eosinophil-rich inflammatory infiltrate localized primarily to the submucosa with extension into the mucosa of the gastric antrum (Figure 3A–D). Immunohistochemistry analysis revealed positive staining for CD34 and negative staining for CD117, supporting the diagnosis of an inflammatory fibroid polyp. The patient tolerated the procedure and recovered without complications. Upon two-week follow-up evaluation, the patient's symptoms had resolved completely. An outpatient colonoscopy revealed no abnormalities.
Figure 3:
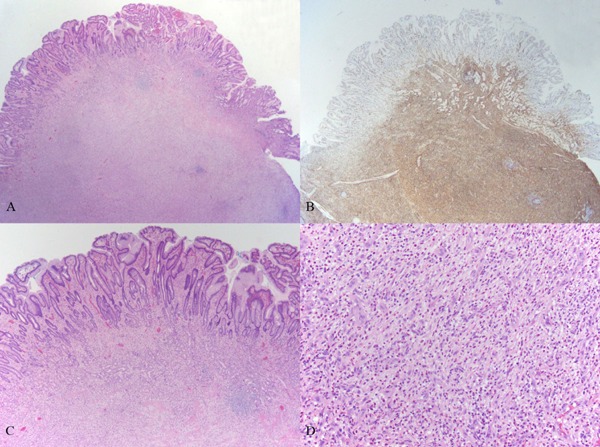
Pathological examination of case. (A), Low-power image of the inflammatory fibroid polyp showing a predominantly submucosal, nodular proliferation with extension into the mucosa (H&E, 20×); (B), Immunohistochemical stain for CD34 shows characteristic diffuse positivity in the lesional cells (20×); (C), Higher power image of the lesion infiltrating into and effacing mucosal architecture (H&E, 40×); (D), High-power image showing the primary constituents of the lesion: bland spindled cells, small vessels, and eosinophil-rich mixed inflammatory infiltrate (H&E, 200×).
3. Discussion
The inflammatory fibroid polyp, also known as a Vanek tumor/polyp, was first reported in 1949 by Vanek (3). The term inflammatory fibroid polyp was first proposed in 1953 by Helwig and Ranier (4). As a result, IFP has now been defined as a benign lesion of the GI tract derived from the submucosa. It is characterized pathologically by the presence of CD34 staining spindle cells, a prominent network of blood vessels, fibromyxoid stroma and an inflammatory infiltrate, typically dominated by eosinophils. Perivascular spindle cell “onion skinning” is present in about half of the lesions (5,6).
IFP must be differentiated from other benign and malignant spindle cell lesions including gastrointestinal stromal tumor (GIST), leiomyoma, leiomyosarcoma, schwannoma, and inflammatory myofibroblastic tumors (6,7). Definite diagnosis is made by immunohistochemical analysis. IFPs are most commonly positive for vimentin and CD34, and negative for S100, CD117 and desmin (5,7,8). The most common site of IFP was first reported at the gastric antrum (70%) followed by the small intestines (20%) in 1986 (9). However, the largest series of IFP to date (83 specimens) found the most commonly involved site to be the large intestines (37%), followed by the gastric antrum (23%) and small intestines (20%) (5).
The true pathogenesis of IFP is currently unknown. Historically, the etiology was thought to be the result of an inflammatory response to an underlying granuloma. The development of a submucosal granuloma is often associated with an irritating stimulus (e.g. trauma, tuberculosis, helicobacter pylori, Crohn disease, sarcoidosis) (10). However, IFP has been showed to occur more frequently in patients with a family history of gastrointestinal polyps (11). More recently, in the first genetic study involving IFP, activating mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene were present in 70% of cases (12). This evidence suggests instead that IFP may represent a true neoplastic lesion driven by activating mutations in PDGFRA. However, no evidence of invasive growth or aggressive metastatic spread has been reported to our knowledge.
The therapeutic strategy of IFP can be viewed similar to that of other benign gastrointestinal polyps. More than 90% of gastrointestinal polyps discovered by routine endoscopic evaluation are present in asymptomatic patients and have no malignant potential (13). However, these polyps cannot be distinguished from those that require further intervention by gross examination alone. Therefore, histological analysis is always indicated in determining the polyp type and presence of underlying dysplasia (14). Additional diagnostic modalities including immunohistochemistry staining, tandem biopsies, and endoscopic ultrasound (EUS) may also be necessary to correctly identify the polyp type (13).
Once the diagnosis of IFP is made, the decision to resect the polyp should be based on both the clinical presentation of the patient and the expertise of the gastroenterologist. Patients presenting with early satiety, chronic epigastric pain, or obstruction should undergo resection. Those who present with an acutely bleeding polyp should be treated swiftly by localizing the lesion and achieving adequate hemostasis. Once the patient is stabilized, eventual polyp resection is suggested to avoid similar adverse events in the future. Following IFP resection, pathological analysis should be initiated to confirm the diagnosis and rule out any potential for malignancy. Finally, because IFPs have no documented malignant potential, routine endoscopic follow-up is not recommended for both post-operative and asymptomatic patients (13).
A MEDLINE review of the literature published from 2000–2017 was conducted using the search terms “(Stomach neoplasms [MeSH Terms]) AND inflammatory fibroid polyp” to evaluate the characteristics of IFPs localized to the stomach. A total of 37 cases were identified (Table 1), with ages ranging from 19–93 year-old, averaging 64.3, including 19 women (51.4%) and 18 men (48.6%) (8,15–37). The gastric antrum was the most commonly involved site, n = 22 (59.5%), followed by the pyloric and pre-pyloric area, n = 5 (13.5%), gastric cardia, n = 2 (5.4%), gastric body, n = 1 (2.7%), and the stomach not otherwise specified, n = 7 (18.9%). Gross morphology, when available, was most commonly described as polypoid or pedunculated, n = 14 (66.7%), followed by sessile, protruding, or rounded, n = 7 (33.3%). Associated ulceration or erosion of the tumor was often present, n = 18 (48.6%). Tumor size ranged from 0.15 to 6.2 cm in greatest diameter, with an average of 2.33 cm. Helicobacter pylori testing was positive in seven cases (18.9%). The most common presenting symptom was epigastric pain, n = 7 (18.9%) followed by anemia, n = 6 (16.2%). The most common treatment modality was endoscopic polypectomy, n = 10 (47.6%), followed by surgical gastrectomy, n = 9 (42.9%), and endoscopic submucosal dissection (ESD) (4.8%). One case regressed after helicobacter pylori eradication. Complications were rarely reported, with three cases (8.1%) developing gastric adenocarcinoma in close proximity to the IFP and two cases resulting in gastric outlet obstruction (5.4%). Only one polyp (2.7%) was described as having recurred after treatment.
Table 1. Review of inflammatory fibroid polyp cases.
| Reference, Year, (Ref.) | Age, Gender | Location | Largest diameter (cm) | Gross Appearance | Presenting Symptoms | Associated Findings | Treatment |
|---|---|---|---|---|---|---|---|
| Shalom, 2000 (31) | 57, F | Antrum | 5.5 × 3 | Polypoid, two ulcers | Epigastric pain | H. Pylori + | Antrectomy with truncal vagotomy |
| Damn, 2003 (8) | 45–93, M (9) F (5) | Antrum (9) Stomach NOS (5) |
0.4–5.0 | (9) surface erosions or ulcerations | N/A | H. pylori + (2) | N/A |
| Fuke, 2003 (19) | 46, F | Antrum | 2 × 1.3 × 1.2 | Polypoid | Asymptomatic | Anemia | Endoscopic polypectomy |
| Hirasaki, 2003 (21) | 66, M | Antrum | 0.9 × 0.6 | Protruding lesion | Found incidentally | Gastric adenocarcinoma close to IFP, H. pylori + | Endoscopic polypectomy |
| Nishiyama, 2003 (26) | 70, F | Antrum | N/A | Broad based pyramidal with ulceration at apex | Found incidentally | Gastric adenocarcinoma, H. pylori +, anemia | Endoscopic polypectomy |
| Shigeno, 2003 (32) | 74, F | Body | 6.2 × 3.1 | Pedunculated with ulcer | Melena, pallor, tachycardia | Anemia | Endoscopic polypectomy |
| Aydin, 2004 (15) | 71, F | Antrum | 0.2 | Broad based polypoid with normal mucosa | Dyspepsia | N/A | Endoscopic polypectomy |
| Matsuhashi, 2004 (23) | 43, F | Pre-pyloric | 2.0 | Elevated lesion with central reddish depression | N/A | H. pylori + | Regressed with H pylori eradication |
| Zinkiewicz, 2004 (37) | 48, M | Cardia | 0.25 × 0.39 | Polypoid with central ulcer with necrotic tissue | Dysphagia, epigastric pain | Recurred after endoscopic excision one year ago | Proximal gastrectomy, local lymphadenectomy |
| Hirasaki, 2005 (22) | 61, M | Pyloric | 0.2 | Protruding lesion | Found during yearly checkup on radiograph | H. pylori + | ESD |
| Paikos, 2007 (27) | 65, F | Pre-pyloric | 3.0 × 5.0 | Polypoid | Epigastric f with projectile vomiting | Tumor was obstructing duodenal bulb | Endoscopic polypectomy |
| Bhatti, 2008 (16) | 60, M | Antrum | N/A | N/A | Abdominal pain, tachycardia, epigastric tenderness | Perforated viscus | Billroth II gastrectomy |
| Ramachandra, 2008 (28) | 50, M | Cardia | 5 | Polypoid with small ulcer | Epigastric pain | N/A | Laparoscopic transgastric excision |
| Tanaka, 2008 (33) | 73, F | Antrum | 0.1 × 0.2 | Pedunculated | Presented for Anemia evaluation | N/A | Endoscopic polypectomy |
| Yen, 2010 (36) | 61, F | Stomach NOS | 1.0 | N/A | Found incidentally EGD | N/A | Endoscopic submucosal dissection |
| Saritas, 2011 (30) | 60, F | Antrum | 6 × 4 | Pendunculated mass | Epigastric pain and vomiting | Anemia | Endoscopic polypectomy |
| Woodward, 2011 (34) | 64, F | Antrum | 1 × 1 × 0.8 | Pedunculated round polyp | Chest discomfort | History of GERD | N/A |
| Yamashita, 2011 (35) | 73, M | Antrum | N/A | Smooth, flat elevation, with ulceration | Found incidentally on EGD | N/A | N/A |
| Ergun, 2012 (18) | 51, F | Antrum | 3 | Polypoid | Epigastric pain and weight loss | N/A | Endoscopic polypectomy |
| Mucientes, 2012 (25) | 69, M | Antrum | 0.9 | Polypoid | Epigastric pain | Superficial early gastric carcinoma in submucosa | Subtotal gastrectomy |
| Rossi, 2012 (29) | 71, F | Pre pyloric | 6 | Rounded with central ulcer | Anorexia, nausea, early satiety | N/A | Gastric resection with Roux-en-Y reconstruction |
| He, 2013 (20) | 19, M | Pre-pyloric | 6 | Sessile | Anemia and hyperpyrecia | N/A | Partial gastrectomy |
| Mitsui, 2015 (24) | 60, M | Antrum | 0.15 | Pedunculated with erosion | Found on EGD | H pylori positive | Partial gastrectomy |
| Bilgin, 2016 (17) | 65, F | Stomach NOS | N/A | Pedunculated tumor | Post prandial abdominal pain, projectile vomiting | Increased 18F-FDG uptake, gastric outlet obstruction | Total resection |
EGD, esophagogastroduodenoscopy; ESD, endoscopic submucosal dissection; F, female; H, Pylori +, Helicobacter Pylori Positive; M, Male; N/A, not available; NOS, not otherwise specified.
4. Conclusion
The case herein represents a rare entity leading to the common presentation of an acute upper gastrointestinal hemorrhage. IFP remains one of the rarest benign tumors of the gastrointestinal tract, and appears most commonly in the gastric antrum and the rectosigmoid colon. Its pathogenesis is not completely understood. However, it is now widely viewed as a PDGFRA-driven benign neoplasm. Patients rarely present with symptoms, but may endorse epigastric pain, early satiety or symptoms related to obstruction. Diagnosis is made primarily with endoscopic biopsy, however additional studies including immunohistochemistry analysis may be required. Therapeutic management is based on the patient's clinical presentation and the expertise of the procedural team. Resection is generally recommended for symptomatic patients and for bleeding IFP lesions. Routine endoscopic evaluation is not indicated due to the lack of evidence for malignant transformation and recurrence.
References
- 1. Roseau G, Ducreux M, Molas G, Ponsot P, Amouyal P, Palazzo L, Amouyal G, Paolaggi JA. [Epithelial gastric polyps in a series of 13000 gastroscopies]. Presse Med. 1990; 19:650-654. (in French) [PubMed] [Google Scholar]
- 2. Huss S, Wardelmann E, Goltz D, Binot E, Hartmann W, Merkelbach-Bruse S, Buttner R, Schildhaus HU. Activating PDGFRA mutations in inflammatory fibroid polyps occur in exons 12, 14 and 18 and are associated with tumour localization. Histopathology. 2012; 61:59-68. [DOI] [PubMed] [Google Scholar]
- 3. Vanek J. Gastric submucosal granuloma with eosinophilic infiltration. Am J Pathol. 1949; 25:397-411. [PMC free article] [PubMed] [Google Scholar]
- 4. Helwig EB, Ranier A. Inflammatory fibroid polyps of the stomach. Surg Gynecol Obstet. 1953; 96:335-367. [PubMed] [Google Scholar]
- 5. Liu TC, Lin MT, Montgomery EA, Singhi AD. Inflammatory fibroid polyps of the gastrointestinal tract: Spectrum of clinical, morphologic, and immunohistochemistry features. Am J Surg Pathol. 2013; 37:586-592. [DOI] [PubMed] [Google Scholar]
- 6. Makhlouf HR, Sobin LH. Inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the gastrointestinal tract: How closely are they related to inflammatory fibroid polyps? Hum Pathol. 2002; 33:307-315. [DOI] [PubMed] [Google Scholar]
- 7. Ozolek JA, Sasatomi E, Swalsky PA, Rao U, Krasinskas A, Finkelstein SD. Inflammatory fibroid polyps of the gastrointestinal tract: Clinical, pathologic, and molecular characteristics. Appl Immunohistochem Mol Morphol. 2004; 12:59-66. [DOI] [PubMed] [Google Scholar]
- 8. Daum O, Hes O, Vanecek T, Benes Z, Sima R, Zamecnik M, Mukensnabl P, Hadravska S, Curik R, Michal M. Vanek's tumor (inflammatory fibroid polyp). Report of 18 cases and comparison with three cases of original Vanek's series. Ann Diagn Pathol. 2003; 7:337-347. [DOI] [PubMed] [Google Scholar]
- 9. Blackshaw AJ, Levison DA. Eosinophilic infiltrates of the gastrointestinal tract. J Clin Pathol. 1986; 39:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calderon MG, Caivano VC, Bagnaresi S, Jr, de Oliveira Lira JO, Raimundo RD, de Abreu LC, Correa JA. A unique case of inflammatory fibroid polyp in the duodenum of a female adolescent: Case report and literature review. Medicine (Baltimore). 2017; 96:e6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allibone RO, Nanson JK, Anthony PP. Multiple and recurrent inflammatory fibroid polyps in a Devon family (‘Devon polyposis syndrome’): An update. Gut. 1992; 33:1004-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schildhaus HU, Cavlar T, Binot E, Buttner R, Wardelmann E, Merkelbach-Bruse S. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J Pathol. 2008; 216:176-182. [DOI] [PubMed] [Google Scholar]
- 13. Islam RS, Patel NC, Lam-Himlin D, Nguyen CC. Gastric polyps: A review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol (N Y). 2013; 9:640-651. [PMC free article] [PubMed] [Google Scholar]
- 14. Park DY, Lauwers GY. Gastric polyps: Classification and management. Arch Pathol Lab Med. 2008; 132:633-640. [DOI] [PubMed] [Google Scholar]
- 15. Aydin A, Tekin F, Gunsar F, Tuncyurek M. Gastric inflammatory fibroid polyp. Gastrointest Endosc. 2004; 60:802-803. [DOI] [PubMed] [Google Scholar]
- 16. Bhatti I, Melhado R, Leeder P, Semeraro D, Tierney G. Clinical challenges and images in GI. Image 3: Inflammatory fibroid polyp. Gastroenterology. 2008; 135:1465, 1808. [DOI] [PubMed] [Google Scholar]
- 17. Bilgin SS, Bilgin M, Savas R, Erdogan EB. Inflammatory fibroid polyp of the stomach mimics malignancy on 18F FDG PET/CT imaging. Clin Nucl Med. 2016; 41:712-713. [DOI] [PubMed] [Google Scholar]
- 18. Ergun M, Zengin N, Kayacetin E. Loop observe and snare technique for endoscopic resection of a gastric inflammatory fibroid polyp. Endoscopy. 2012; 44 Suppl 2 UCTN:E86-87. [DOI] [PubMed] [Google Scholar]
- 19. Fuke H, Hashimoto A, Shimizu A, Yoshimura H, Nakano T, Shiraki K. Computed tomographic image of an inflammatory fibroid polyp of the stomach. Clin Imaging. 2003; 27:400-402. [DOI] [PubMed] [Google Scholar]
- 20. He HY, Shen ZB, Fang Y, Sun YH, Qin XY. Bleeding and hyperpyrexia in an adult with gastric inflammatory fibroid polyp. Chin Med J (Engl). 2013; 126:2594. [PubMed] [Google Scholar]
- 21. Hirasaki S, Endo H, Nishina T, Masumoto T, Tanimizu M, Hyodo I. Gastric cancer concomitant with inflammatory fibroid polyp treated with endoscopic mucosal resection using an insulation-tip diathermic knife. Intern Med. 2003; 42:259-262. [DOI] [PubMed] [Google Scholar]
- 22. Hirasaki S, Tanimizu M, Tsubouchi E, Nasu J, Masumoto T. Gastritis cystica polyposa concomitant with gastric inflammatory fibroid polyp occurring in an unoperated stomach. Intern Med. 2005; 44:46-49. [DOI] [PubMed] [Google Scholar]
- 23. Matsuhashi N, Nakajima A, Nomura S, Kaminishi M. Inflammatory fibroid polyps of the stomach and Helicobacter pylori. J Gastroenterol Hepatol. 2004; 19:346-347. [DOI] [PubMed] [Google Scholar]
- 24. Mitsui Y, Kagemoto K, Itagaki T, Inoue S, Naruse K, Muguruma N, Takayama T. Gastric inflammatory fibroid polyp morphologically changed by Helicobacter pylori eradication. Clin J Gastroenterol. 2015; 8:77-81. [DOI] [PubMed] [Google Scholar]
- 25. Mucientes P, Mucientes F, Klaassen R. Inflammatory fibroid polyp associated with early gastric carcinoma: A case report. Ann Diagn Pathol. 2012; 16:148-151. [DOI] [PubMed] [Google Scholar]
- 26. Nishiyama Y, Koyama S, Andoh A, Kishi Y, Yoshikawa K, Ishizuka I, Yokono T, Fujiyama Y. Gastric inflammatory fibroid polyp treated with Helicobacter pylori eradication therapy. Intern Med. 2003; 42:263-267. [DOI] [PubMed] [Google Scholar]
- 27. Paikos D, Moschos J, Tzilves D, Koulaouzidis A, Kouklakis G, Patakiouta F, Kontodimou K, Tarpagos A, Katsos I. Inflammatory fibroid polyp or Vanek's tumour. Dig Surg. 2007; 24:231-233. [DOI] [PubMed] [Google Scholar]
- 28. Ramachandra S, Lapsia S, Latifaj B, Ghai S. A rare cause of anaemia (2008: 3b). Eur Radiol. 2008; 18:1300-1302. [DOI] [PubMed] [Google Scholar]
- 29. Rossi P, Montuori M, Balassone V, Ricciardi E, Anemona L, Manzelli A, Petrella G. Inflammatory fibroid polyp. A case report and review of the literature. Ann Ital Chir. 2012; 83:347-351. [PubMed] [Google Scholar]
- 30. Saritas U, Ustundag Y, Gedikoglu G. Successful endoscopic treatment of huge gastric inflammatory fibroid polyp. Turk J Gastroenterol. 2011; 22:224-226. [DOI] [PubMed] [Google Scholar]
- 31. Shalom A, Wasserman I, Segal M, Orda R. Inflammatory fibroid polyp and Helicobacter pylori. Aetiology or coincidence? Eur J Surg. 2000; 166:54-57. [DOI] [PubMed] [Google Scholar]
- 32. Shigeno T, Fujimori K, Nakatsuji Y, Kaneko Y, Maejima T. Gastric inflammatory fibroid polyp manifesting massive bleeding and marked morphological changes for a short period. J Gastroenterol. 2003; 38:611-612. [PubMed] [Google Scholar]
- 33. Tanaka K, Toyoda H, Imoto I, Hamada Y, Aoki M, Kosaka R, Noda T, Takei Y. Anemia caused by a gastric inflammatory fibroid polyp. Gastrointest Endosc. 2008; 67:345-346. [DOI] [PubMed] [Google Scholar]
- 34. Woodward K, Gangarosa LM, Hunt HV. Gastric inflammatory fibroid polyp. Indian J Pathol Microbiol. 2011; 54:622-623. [DOI] [PubMed] [Google Scholar]
- 35. Yamashita K, Arimura Y, Tanuma T, Endo T, Hasegawa T, Shinomura Y. Pattern of growth of a gastric inflammatory fibroid polyp with PDGFRA overexpression. Endoscopy. 2011; 43 Suppl 2 UCTN:E171-172. [DOI] [PubMed] [Google Scholar]
- 36. Yen HH, Chen C J. Education and Imaging. Gastrointestinal: Endoscopic submucosal dissection for gastric inflammatory fibroid polyp. J Gastroenterol Hepatol. 2010; 25:1465. [DOI] [PubMed] [Google Scholar]
- 37. Zinkiewicz K, Zgodzinski W, Dabrowski A, Szumilo J, Cwik G, Wallner G. Recurrent inflammatory fibroid polyp of cardia: A case report. World J Gastroenterol. 2004; 10:767-768. [DOI] [PMC free article] [PubMed] [Google Scholar]


