Abstract
Berberine, an isoquinoline alkaloid, is a traditional oriental medicine used to treat diarrhea and gastroenteritis. Recently, we reported that it could inhibit the growth of intestinal polyp in animals and in patients with the familial adenomatous polyposis by downregulating β-catenin signaling. However, the intracellular target mediating the effects of berberine remains elusive. Here, we provide evidence that berberine inhibits β-catenin function via directly binding to a unique region comprising residues Gln275, Arg316 and Arg371 in nuclear receptor retinoid X receptor alpha (RXRα), where berberine concomitantly binding to and synergistically activating RXRα with 9-cis-retinoic acid (9-cis-RA), a natural ligand binding to the classical ligand-binding pocket of RXRα. Berberine binding promotes RXRα interaction with nuclear β-catenin, leading to c-Cbl mediated degradation of β-catenin, and consequently inhibits the proliferation of colon cancer cells. Furthermore, berberine suppresses the growth of human colon carcinoma xenograft in nude mice in an RXRα-dependent manner. Together, our study not only identifies RXRα as a direct protein target for berberine but also dissects their binding mode and validates that berberine indeed suppresses β-catenin signaling and cell growth in colon cancer via binding RXRα, which provide new strategies for the design of new RXRα-based antitumor agents and drug combinations.
Introduction
Traditional medicines represent a rich source of therapeutic leads for modern drug development. However, turning traditional medicines into modern drugs depends on our identification and validation of their cellular targets and understanding of their mechanisms of action.1 Berberine, an isoquinoline alkaloid isolated from various medicinal herbs such as Coptis chinensis, is a nonprescription medicine traditionally used to treat diarrhea and gastroenteritis caused by bacterial and intestinal parasitic infections.2 Recent studies have unraveled additional clinical indications and the underlying mechanisms for this drug. Oral administration of berberine in hypercholesterolemic patients significantly reduces their serum cholesterol, triglycerides and low-density lipoprotein cholesterol.3, 4 Berberine functions as an activator of fatty acid receptor GPR40, which might be an important mechanism for the antidiabetic action of berberine.5 In addition, berberine activates thermogenesis in white and brown adipose tissue via AMPK and PGC-1α, implying potential therapeutic applications for the treatment of obesity.6 This drug also showed protective effect on neural damage, through binding to the poly(A) tail on retinoblastoma (Rb) mRNA to suppress its degradation.7 Recently, berberine has generated attention to its antitumor effects in a broad spectrum of cancer cells, and its strong DNA binding ability that probably contributed to epigenetic modifications was considered to be a probable cause of its antineoplastic effect.8 In our study, we found that oral administration of berberine in patients with familial adenomatous polyposis immediately following polypectomy significantly reduced the number and size of polyps and prevented recurrences of colorectal polyp.9 Such an effect was accompanied with dramatic reduction (68%) of cyclin D1 expression,9 implying a mode of berberine action in patients. Overexpression of cyclin D1 is predominantly associated with colon tumorigenesis and metastases, which is mainly attributed to aberrant activation of the Wnt/β-catenin signaling pathway.10 Indeed, studies from our and other groups have demonstrated that berberine could inhibit the β-catenin pathway in vitro and in animals.9, 11, 12 However, intracellular targets mediating the inhibitory effect of berberine on the β-catenin signal transduction pathway remain elusive.
Mounting evidence has revealed an extensive interplay between members of the nuclear receptor superfamily and the canonical Wnt/β-catenin signaling pathway.13, 14 Retinoid X receptor α (RXRα) is a unique member of the nuclear receptor superfamily with capability of heterodimerizing with many members of the superfamily.15, 16, 17 RXRα and its heterodimerization partners, including retinoic acid receptor (RAR), vitamin D receptor (VDR), peroxisome proliferator-activated receptors (PPARs), liver X receptors (LXRs), thyroid hormone receptor (T3R) and Nur77, have been shown to physically interact with β-catenin to modulate the β-catenin signaling.13, 14 Thus, abnormal function of RXRα may contribute to aberrant activation of the β-catenin pathway. Indeed, abnormal modification of RXRα protein is often observed in colon tumor tissues and cancer cells.18, 19, 20 Genetic variation in RXRα is also associated with the risk of colorectal adenoma recurrence.19, 21 Overphosphorylation of RXRα, an event that inhibit its transactivation, occurs in human colon cancer tissues,18 while its expression is downregulated in tumors of adenomatous polyposis coli multiple intestinal neoplasia (ApcMin/+) mice.22 These findings suggest that RXRα plays an important role in colorectal carcinogenesis. Consistently, RXRα agonists greatly enhance the interaction between RXRα and β-catenin and induce adenomatous polyposis coli (APC)-independent β-catenin degradation.23, 24 They also synergistically inhibit the growth in colon cancer cells when used together with PPAR ligands.18, 25 Targretin (LGD1069), a synthetic RXRα agonist approved by Food and Drug Administration for treating cutaneous T-cell lymphoma,26 inhibited the progression of chemically induced colon adenomas to adenocarcinomas in rats.27 Sulindac, one of the early non-steroidal anti-inflammatory drugs, binds RXRα28 and shows promising chemopreventive effect for adenomatous colorectal polyps and colon cancer, especially in patients with familial adenomatous polyposis.29, 30 Another non-steroidal anti-inflammatory drug R-etodolac, which also binds RXRα,31 inhibited β-catenin signaling likely through its modulation of β-catenin interaction with RXRα and PPARγ.31 Thus, RXRα represents an intriguing drug target for colon cancer therapeutics.
In the present study, we provide evidence that berberine binds RXRα through a unique binding mechanism that promotes RXRα interaction with nuclear β-catenin, leading to inhibition of β-catenin signaling in vitro and in animals.
Results
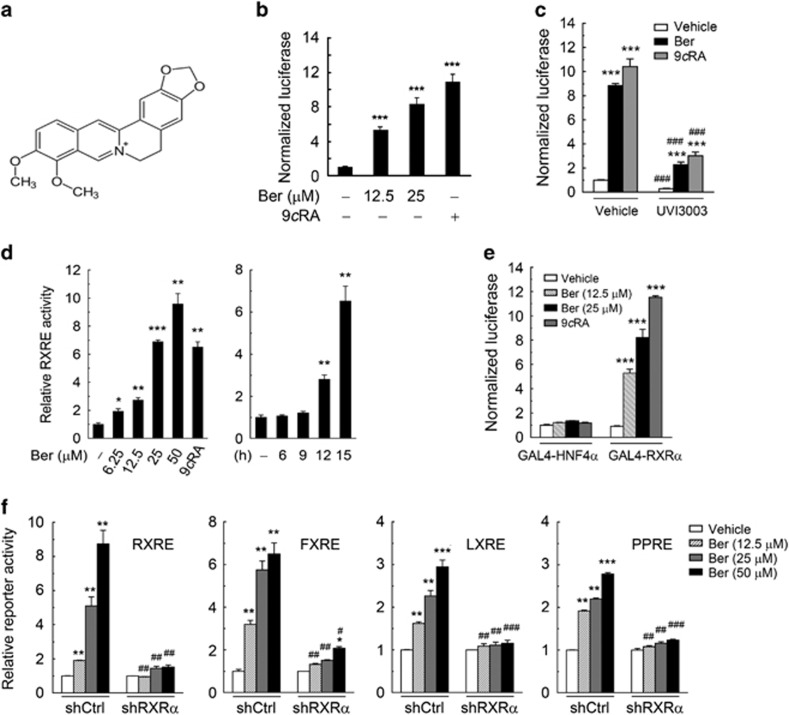
Identification of berberine as a novel RXRα activator
Dysregulated β-catenin turnover is critical for the tumorigenesis and development of colon cancer.32 We recently reported that oral administration of berberine (Figure 1a) significantly inhibited the growth and recurrence of colon polyps in familial adenomatous polyposis patients and Apcmin/+ mice through inhibition of nuclear β-catenin protein level.9 Due to the high frequency of mutations in critical genes constituting the APC pathways in colon cancer, such as APC, Axin, p53 and β-catenin itself, we speculate that the APC-independent RXR agonist/RXR pathway23, 24 may contribute to the regulation of β-Catenin by berberine. To determine whether berberine could exert its inhibitory effect on β-catenin through acting as an RXRα activator, we first used luciferase-based mammalian one-hybrid assay to test the effect of berberine on RXRα transactivation. Our results showed that berberine could strongly induce RXRα-dependent transcriptional activation. Berberine at 25 μm induced RXRα transactivation to an extent comparable to that induced by 0.1 μm 9-cis-retinoic acid (9-cis-RA), the natural RXRα ligand15, 16, 17 (Figure 1b). RXR antagonist UVI3003 could antagonize the stimulatory effects of both berberine and 9-cis-RA on RXRα transactivation (Figure 1c). Berberine treatment also increased the transcriptional activation of RXRα on RXR DNA response element (RXRE) in both dose- and time-dependent manners (Figure 1d). When examined for its effects on RXRα heterodimers, berberine activated RXRα/FXR, RXRα/LXR and RXRα/PPARγ heterodimers on their cognate DNA response elements (Supplementary Figure S1a). An enhanced induction of RXR/LXR transactivational activity on LXRE were observed when cells were co-treated with berberine and LXR ligand GW3965 (Supplementary Figure S1b). In contrast, berberine had no effect on the transcriptional activity of nuclear receptors that could not form heterodimer with RXRα, such as HNF4α (Figure 1e). Besides, berberine treatment induced a dose-dependent moderate decrease in RXRα mRNA and protein levels as many other RXR agonists did, including 9-cis-RA (Supplementary Figure S1c).
Figure 1.
Identification of berberine as an RXRα activator. (a) Chemical structure of berberine. (b) Berberine induced the transactivational activity of RXRα-LBD. KM12C cells were cotransfected with pBIND-RXRα-LBD and pG5luc constructs. After 24 h of transfection, cells were treated with vehicle (DMSO) or berberine (Ber) for 15 h at the indicated concentrations. The basal level of transcriptional activity in the vehicle-treated group was normalized to 1. (c) RXR antagonist UVI3003 antagonized the stimulatory effect of berberine on RXRα transactivation. The transfected cells in Figure 1b were treated with berberine (25 μm) and/or UVI3003 (10 μm) for 15 h. 9-Cis-RA (9cRA, 0.1 μm) was used as a positive control. (d) Berberine induced the transactivational activity of RXRα homodimers on RXRE in both dose- and time-dependent manners. KM12C cells were cotransfected with pGL3-RXRE and an internal control plasmid—renilla luciferase (pRL-TK) plasmid. Twenty-four hours after transfection, cells were treated with vehicle, different doses of berberine or 9-cis-RA (0.1 μm) for 15 h (left panel) or 25 μm berberine for different durations (right panel). (e) Berberine failed to induce transcriptional activity of HNF4α2. KM12C cells cotransfected with pG5luc and pBIND-HNF4α2 or pBIND-RXRα were treated with different doses of berberine for 15 h. (f) Effects of shRNA against RXRα on berberine-induced transactivational activity of RXRα homodimers and heterodimers. KM12C cells expressing control or RXRα shRNA (shCtrl or shRXRα) were cotransfected with reporter vector for RXRE, FXRE, LXRE or PPRE, together with pRL-TK plasmid. Twenty-four hours later, cells were treated with berberine for 15 h at the indicated concentration. The basal levels of transcriptional activity in the vehicle-treated groups of shCtrl and shRXRα cells were normalized to 1. All data were presented as the mean±s.e.m. of three independent experiments. Significant differences compared with vehicle controls were indicated as *P<0.05, **P<0.01 and ***P<0.001; significant differences of UVI3003 vs vehicle control (c) or shRXRα vs shCtrl control (f) at the same dose of berberine or 9-cis-RA were indicated as #P<0.05, ##P<0.01 and ###P<0.001.
We also used a lentiviral vector-based shRNA technique to knock down the expression of endogenous RXRα in KM12C colon cancer cells to determine the role of RXRα (Supplementary Figure S1d). Our results showed that berberine-induced transcriptional activity of RXRα homodimers and heterodimers on their cognate DNA response elements was substantially attenuated in cells expressing RXRα-shRNA (shRXRα) as compared with cells expressing control shRNA (shCtrl) (Figure 1f). Additionally, berberine elevated the transcription activity of several downstream target genes of RXRα homodimer or heterodimers in an RXRα-dependent manner, including FOXO3A,33 APOE,34, 35 ABCA1 (ref. 36) and COX2 (ref. 37) (Supplementary Figure S1e). These results thus indicated that berberine selectively activates the transcriptional activity of RXRα.
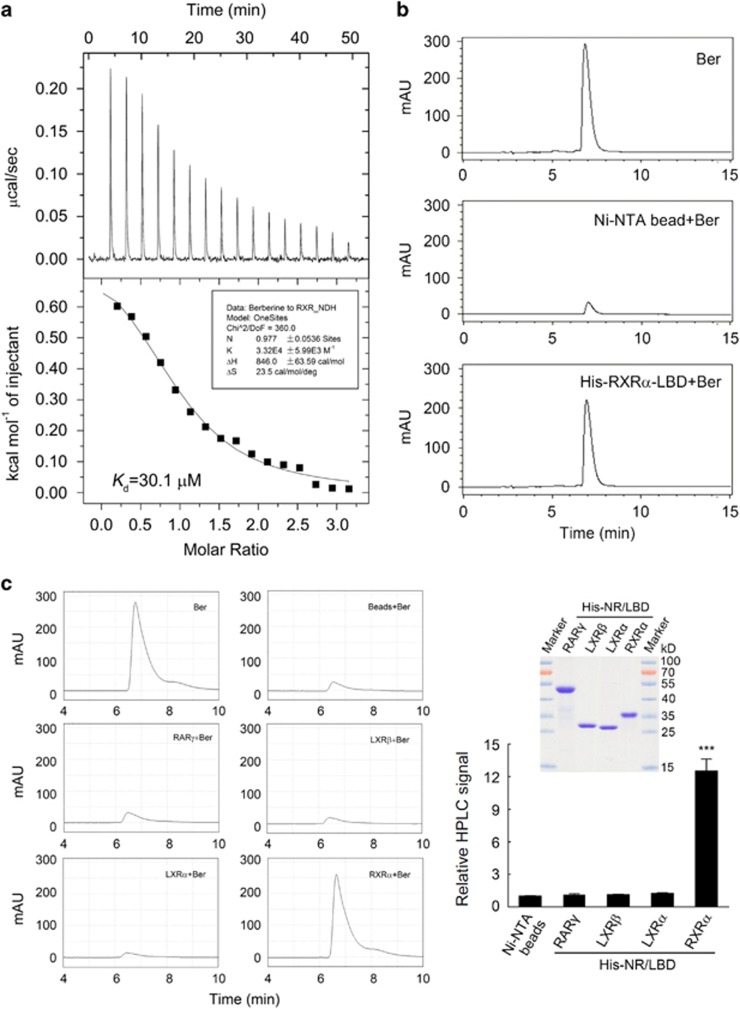
Berberine binds to the ligand-binding domain (LBD) of RXRα
We next studied whether berberine binds directly to RXRα by using isothermal titration calorimetry that determines both binding affinities and stoichiometric ratios. Isothermal titration calorimetry analysis revealed a specific binding of berberine to the purified RXRα-LBD with a Kd value of 30.1 μm at a 1:1 ratio (Figure 2a), which was comparable to the binding stoichiometric ratio of 9-cis-RA and other RXRα agonists.38 We also used high-performance liquid chromatography (HPLC) to confirm the binding of berberine to RXRα (Figure 2b). In this study, berberine was detected as a single peak at a retention time of 7.08 min (Figure 2b, upper panel). Then, berberine was incubated with either Ni-NTA beads alone or with RXRα-LBD/Ni-NTA beads together overnight. After washed with phosphate-buffered saline and dissolved with chloroform–methanol, the supernatants were dried and applied to HPLC. This berberine peak was observed in the precipitate of RXRα-LBD/Ni-NTA beads pre-incubated with berberine (Figure 2b, lower panel), but not in that of Ni-NTA control beads subjected to the same treatment (Figure 2b, middle panel). These results demonstrate that berberine can directly bind to the RXRα-LBD. We further used another two nuclear receptors RAR and LXR as control to check the specificity of the berberine binding to RXRα. Results from HPLC assay showed that berberine bound to RXRα, but not RARγ, LXRα or LXRβ, indicating the specificity of berberine binding to RXRα (Figure 2c).
Figure 2.
Berberine directly bound to RXRα-LBD. (a, b) Analysis of berberine binding to RXRα-LBD by isothermal titration calorimetry (ITC) (a) and HPLC (b). (a) Purified RXRα-LBD protein was dialyzed against phosphate-buffered saline (PBS) buffer containing 4% DMSO and diluted to 0.1 mm with the same buffer. Berberine was dissolved to 1 mm in the same buffer, then the binding affinity and the number of binding sites of berberine to RXRα-LBD was measured by ITC. (b) Ni-NTA beads bound RXRα-LBD proteins or Ni-NTA beads alone were incubated overnight with equal mole of berberine at 4 °C, then the beads were collected after centrifugation and washed with PBS. The beads were dissolved in chloroform–methanol 1:1(v/v) and subjected to HPLC analysis as described in Materials and Methods. The same amount of berberine as mentioned above was used for identification and quantitative calculations. (c) HPLC analysis of berberine binding to the LBD of several nuclear receptors (NRs) including RARγ, LXRα and LXRβ. Ni-NTA beads bound NR-LBD proteins or Ni-NTA beads alone were incubated overnight with equal mole of berberine at 4 °C, then the beads were washed with PBS, dissolved in chloroform–methanol 1:1(v/v) and subjected to HPLC analysis. The HPLC signal indicating the relative binding affinity in all groups were normalized to the Ni-NTA beads group. Inserted right panel showed the expression levels of these NRs-LBD proteins, detected by SDS–PAGE with coomassie blue staining. All data were shown as the mean±s.e.m. of three independent experiments. ***P<0.001 vs Ni-NTA beads control.
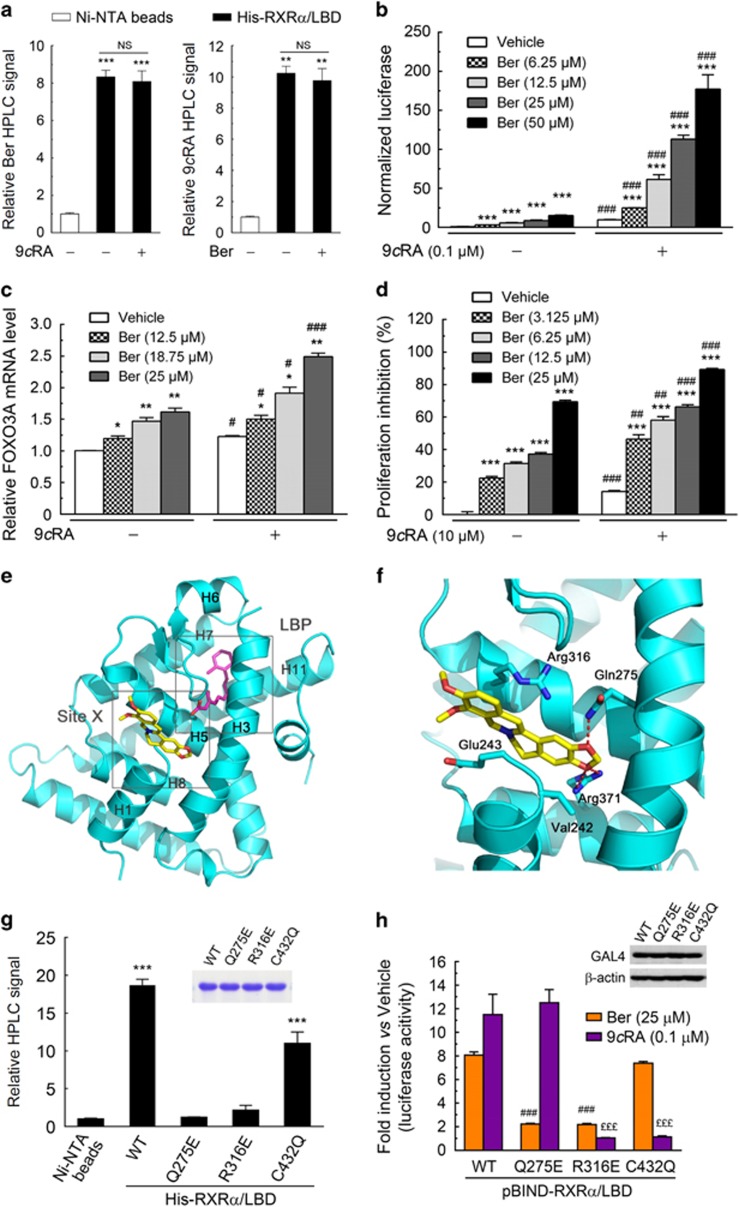
To understand how berberine binds to RXRα, we first analyzed if berberine bound to the ligand-binding pocket (LBP) of RXRα as 9-cis-RA does. If this is the case, berberine and 9-cis-RA would compete for binding to RXRα-LBD, that is, the presence of 9-cis-RA would suppress the binding of berberine with RXRα-LBD and vice versa. However, results from HPLC assay showed that the presence of 9-cis-RA did not decrease the binding of berberine with RXRα-LBD proteins and vice versa (Figure 3a and Supplementary Figure S2a), indicating the concomitant binding of berberine and 9-cis-RA to RXRα-LBD (though not cooperative binding, as the presence of 9-cis-RA did not increase the binding of berberine with RXRα-LBD and vice versa). Moreover, berberine and 9-cis-RA acted synergistically in the activation of RXRα-LBD when they were used together (more than an additive effect) (Figure 3b and Supplementary Figure S2b). They also synergistically elevated the mRNA expression of tumor repressor FOXO3A, a target gene of RXRα,33 and inhibit the growth of colon cancer cells (Figures 3c and d). The synergistic rather than competitive effect of berberine and 9-cis-RA suggested that they might bind to different regions in RXRα. Recent studies have revealed the existence of additional small molecule binding regions in RXRα, including antagonist-binding regions near the opening of LBP39 and at the co-activator binding site.39 Examining the structure surface of RXRα-LBD and taking the shape of the berberine molecule into account, we speculated that a small pocket located adjacent to the LBP and delimited by H1, H3, H5 and H8 could be a potential binding site for berberine (site X, Figure 3e). Molecular docking showed that berberine makes extensive contacts with the protein including H-bonds and Van Der Waals contacts. Residues Val242, Glu243, Gln275, Arg316 and Arg371 make Van Der Waals contacts with berberine, and the oxygen atoms of the dioxolane in berberine form H-bonds with Gln275 and Arg371, respectively (Figure 3f). To confirm our docking results, several of the aforementioned amino acids in the site X and the LBP of RXRα were mutated. As expected, when Gln275 or Arg316 in site X was mutated to Glu, its binding with berberine and its transactivation induced by berberine were almost eliminated; in contrast, mutant of Cys432 in LBP (critical for 9-cis-RA binding)40 to Gln retained 60% of RXRα binding ability with berberine and the transactivation of RXRα in response to berberine (Figures 3g and h and Supplementary Figure S2c). These results together suggested a unique RXRα binding mode by berberine, which is distinct from the binding by 9-cis-RA.
Figure 3.
Mutational analysis of RXRα for berberine binding. (a) Concomitant binding of berberine and 9-cis-RA with RXRα-LBD proteins by HPLC analysis. Ni-NTA beads bound RXRα-LBD proteins or Ni-NTA beads alone were incubated overnight with equal mole of berberine in the absence and presence of equal mole of 9-cis-RA (left) or with equal mole of 9-cis-RA in the absence and presence of equal mole of berberine (right) at 4 °C. The HPLC signal indicating the relative binding affinity in all groups was normalized to the Ni-NTA beads group. (b) Synergistic effect of berberine and 9-cis-RA on RXRα transactivational activity. KM12C cells cotransfected with pBIND-RXRα-LBD and pG5luc were treated berberine and/or 9-cis-RA at indicated concentrations for 15 h. (c) Real-time PCR analysis of FOXO3 mRNA expression in KM12C cells treated with vehicle or different concentrations of compounds as indicated for 15 h (9cRA, 10 μm). (d) Synergistic inhibitory effect of berberine and 9-cis RA on colon cancer cell growth. After treatment with berberine and/or 9-cis RA at indicated doses for 36 h, KM12C cells were subjected to the EdU assay. (e) Overall structure of RXRα-LBP (cyan) (PDB code 3OAP) in complex with berberine (yellow) and 9-cis-RA (magenta). Berberine was docked in a unique pocket of RXRα (Site X) in the presence of 9-cis-RA in the classic binding pocket (LBP). Helices are numbered from N- to C-terminus. H, helix. (f) Detailed view of the interaction between berberine (yellow) and RXRα-LBD (cyan) in the Site X. Key residues involved in berberine binding are shown in sticks, with carbon atoms in cyan, oxygen atoms in red and nitrogen atoms in blue. Potential hydrogen-bonding interaction between berberine and RXRα-LBD residues Arg371 and Gln275 is shown as dashed red line. (g) Determination of berberine binding to wild-type (WT) RXRα-LBD and its mutants by HPLC as described in Materials and Methods. Insets showed the expression levels of RXRα-LBD and its mutants, as detected by SDS–PAGE with coomassie blue staining. (h) Binding to RXRα-LBD was essential for berberine to induce the transactivational activity of RXRα. KM12C cells cotransfected with pBIND-RXRα-LBD or its mutants, together with pG5luc plasmid were treated with berberine (25 μm) or 9-cis-RA (0.1 μm) for 15 h. Results were presented as fold induction by normalized berberine-treated or 9-cis-RA-treated group to the vehicle-treated group of the same mutant. Insets showed the expression levels of GAL4-RXRα-LBD and its mutants, as detected by western blot with anti-GAL4 antibody. All data were shown as the mean±s.e.m. of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 and NS non-significant vs vehicle control (b–d), vs Ni-NTA beads control or as indicated (a, g). Significant differences of 9-cis-RA vs vehicle control at the same dose of berberine were indicated as #P<0.05, ##P<0.01 and ###P<0.001 (b–d). Significant differences of RXRα mutants vs WT upon the same drug treatment were indicated as ###P<0.001 for berberine, and £££P<0.001 for 9-cis-RA (h).
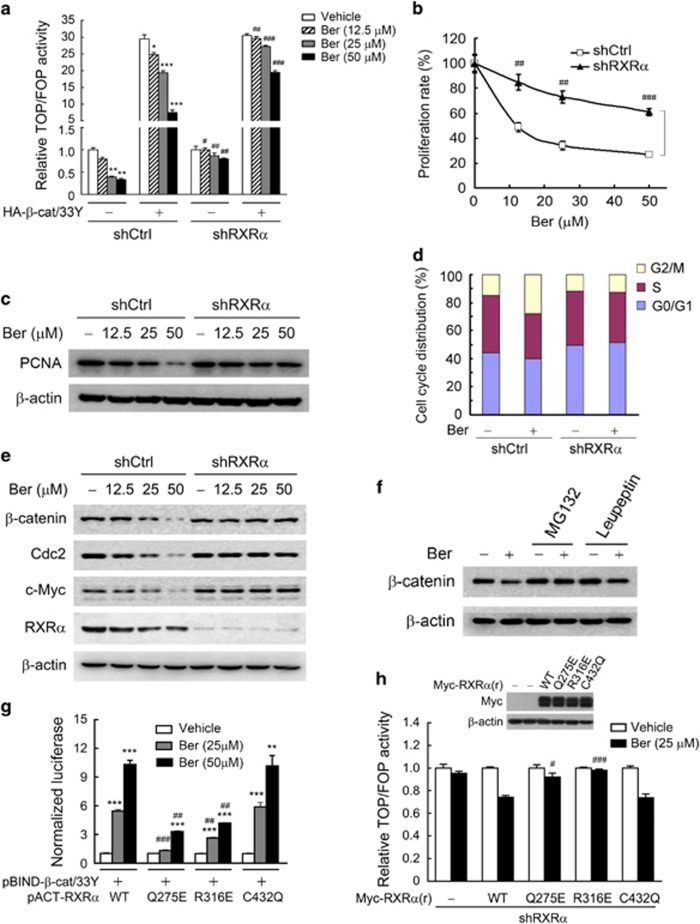
Binding to RXRα is required for berberine to inhibit β-catenin signaling and colon cancer cell growth
Since our above observations indicated berberine as a novel RXRα activator, we further investigated whether binding to RXRα is essential for berberine to suppress the hyperactivated β-catenin signaling and cell growth in APC- and p53-mutated KM12C cells, and the underlying mechanisms were also studied. Endogenous RXRα in KM12C were firstly knocked down to examine the necessity of RXRα in berberine’s functions on β-catenin signaling and cell growth in colon cancer. TOP/FOP luciferase assay indicated that berberine dramatically inhibited β-catenin-dependent transcriptional activity, which was greatly reduced after knocking down RXRα (Figure 4a). Moreover, the growth of KM12C cells and its expression of proliferating cell nuclear antigen, a marker of cell proliferation, were inhibited by berberine via RXRα mediation (Figures 4b and c). These data suggested that RXRα mediated the inhibitory effect of berberine on β-catenin signaling and cell proliferation in colon cancer cells. It was previously shown that the inhibition of β-catenin signaling pathway resulted in a cell cycle arrest at the G2 phase in colorectal cancer cells.41, 42 Consistently, we observed that berberine induced cell cycle arrest of KM12C cell and two other colon cancer cell lines at the G2/M phase (Figure 4d and Supplementary Figure S3a), and the expression of RXRα-shRNA impaired this effect of berberine (Figure 4d). The expression of Cdc2 and c-Myc, two target genes of the β-catenin signaling pathway involved in regulating the G2/M checkpoint,42 were further analyzed. Results showed that the expression of Cdc2, c-Myc, and p21WAF1/CIP1, a downstream effector of c-Myc for G2/M checkpoint regulation,43 were regulated by berberine in an RXRα-dependent manner (Figure 4e and Supplementary Figure S3b). These data suggested that RXRα mediated the inhibitory effect of berberine on β-catenin signaling and cell growth in colon cancer cells.
Figure 4.
Binding RXRα was required for berberine to inhibit β-catenin signaling and cell proliferation in colon cancer cells. (a) RXRα mediated the effect of berberine on β-catenin signaling activity as detected by TOP/FOP luciferase assay. KM12C sublines, shCtrl and shRXRα, were cotransfected with pRL-TK, TOPflash or FOPflash plasmid, and HA-β-catenin/33Y or pCMV5-HA vector, and then treated with berberine for 15 h. The base TOP/FOP ratio in each cell sublines was normalized to 1. (b) RXRα-dependent effect of berberine on cell proliferation evaluated by MTT assay. KM12C sublines, shCtrl and shRXRα, were treated with berberine for 15 h. The base proliferation rate in the vehicle-treated group of each cell subline was normalized to 1. (c) Berberine inhibited the expression of PCNA and β-catenin in an RXRα-dependent manner. KM12C cells expressing control or RXRα shRNA were treated with berberine for 15 h, and then subjected to western blot. (d) RXRα mediated the effects of berberine on cell cycle arrest. ShCtrl and shRXRα KM12C cells treated with 50 μm berberine for 15 h were subjected to cell cycle analysis. (e) Berberine inhibited the expression of β-catenin protein and regulates the expression of downstream target genes of β-catenin signaling in an RXRα-dependent manner. (f) Proteasome-dependent effect of berberine on β-catenin protein expression. KM12C cells were pre-incubated with 10 μm MG132 or 25 μg/ml leupeptin for 2 h and then exposed to 50 μm berberine for another 15 h. (g) Binding RXRα was essential for berberine to promote RXRα interaction with β-catenin as detected by mammalian two-hybrid assay. KM12C cells were cotransfected with pBIND-β-catenin/33Y, pACT-RXRα or its mutants and pG5luc vector, and treated with berberine for 15 h. The basal level of transcriptional activity in the vehicle-treated group of RXRα or its mutants was normalized to 1. (h) Binding RXRα was essential for berberine to inhibit the β-catenin signaling activity. KM12C shRXRα cells were transfected with pRL-TK, TOPflash or FOPflash plasmid, and pCMV5-Myc vector or Myc-RXRα(r) and its mutants (the RXRα rescue construct RXRα(r) did not require silence mutation as shRXRα targeted the 3′-UTR region of RXRα mRNA). Twenty-four hours after transfection, cells were treated with 25 μm berberine for 15 h, and then subjected to TOP/FOP luciferase assay. Insets showed the expression levels of Myc-RXRα(r) and its mutants, detected by western blot with anti-Myc antibody. The basal TOP/FOP ratio in each vehicle-treated group was normalized to 1. All data were presented as the mean±s.e.m. of three independent experiments. Significant differences compared with vehicle control of the same HA-β-catenin/33Y or pCMV5-HA vector (a) or RXRα construct (g) were indicated as *P<0.05, **P<0.01 and ***P<0.001. Significant differences of shRXRα vs shCtrl with the same HA-β-catenin/33Y or pCMV5-HA vector (a) or RXRα mutants vs WT (g, h) at the same dose of berberine were indicated as #P<0.05, ##P<0.01 and ###P<0.001. Abbreviation: PCNA, proliferating cell nuclear antigen.
RXRα agonists are known to promote RXRα binding to β-catenin and further induce the ubiquitination and proteasomal degradation of β-catenin.23, 44 We therefore examined whether berberine could also promote RXRα–β-catenin interaction and lead to β-catenin degradation. Indeed, our mammalian two-hybrid assay showed that the interaction of RXRα with β-catenin was significantly enhanced by berberine dose-dependently (Supplementary Figure S4a). Moreover, the expression level of β-catenin protein, but not its mRNA, was reduced by berberine in an RXRα-dependent manner (Figure 4e and Supplementary Figure S4b). This reduction of β-catenin protein was likely mediated by proteasome-dependent degradation as treatment of cells with the proteasome inhibitor MG132, but not the lysosome inhibitor leupeptin, prevented the inhibitory effect of berberine (Figure 4f). The mutations of RXRα residues critical for berberine binding (Figures 3g and h) were further employed to investigate whether berberine binding to RXRα were necessary for berberine to promote RXRα–β-catenin interaction and β-catenin degradation. Mammalian two-hybrid assay showed that RXRα mutants Q275E or R316E unable to bind berberine (Figures 3g and h) substantially attenuated the effects of berberine on the interaction of RXRα with β-catenin (Figure 4g). Consistently, reintroduction of RXRα and its mutant C432Q capable of binding berberine, rather than Q275E or R316E, restored the inhibitory effect of berberine on β-catenin-dependent transcriptional activity and colon cancer cell growth in RXRα-knockdown colon tumor cells (Figure 4h and Supplementary Figure 4c). Furthermore, in accord with the synergetic inhibitory effect of berberine and 9-cis-RA on colon cancer proliferation, these two agents also synergistically enhanced the interaction of RXRα with β-catenin as indicated by the mammalian two-hybrid assay (Supplementary Figure S4d). Our results thus suggested that binding to RXRα is required for berberine to promote RXRα–β-catenin interaction and inhibit β-catenin signaling and colon cancer cell growth.
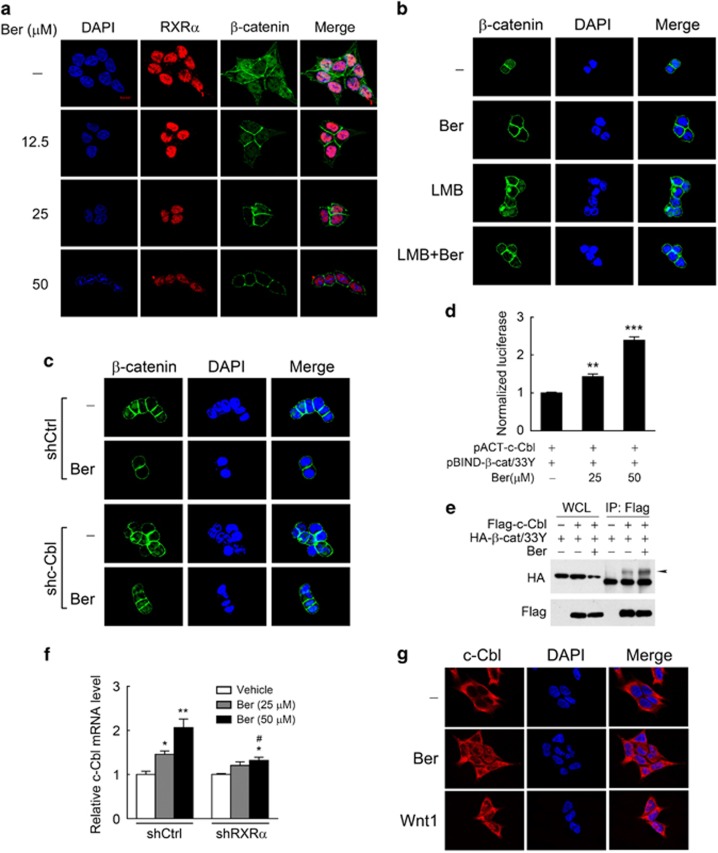
We then considered how β-catenin degradation was promoted by berberine-enhanced interaction of RXRα with β-catenin. Immunostaining revealed that β-catenin was mainly detected in the nucleus and the plasma membrane of KM12C and HCT-116 cells; upon berberine treatment, the nuclear staining of β-catenin disappeared, while β-catenin staining in the cytosolic or plasma membrane remained intact (Figure 5a and Supplementary Figure S4e). This berberine-induced degradation of nuclear β-catenin seemed to happen in the nucleus as pre-treatment of Leptomycin B, an inhibitor of nuclear export, failed to suppress the effect of berberine (Figure 5b). An E3 ubiquitin ligase c-Cbl was found previously to target the active nuclear β-catenin.45 Our results showed that berberine-induced degradation of nuclear β-catenin was substantially attenuated after knocking down endogenous c-Cbl in KM12C colon cancer cells (Figure 5c and Supplementary Figure S4f), suggesting that berberine-induced degradation of nuclear β-catenin was mediated by c-Cbl. Mammalian two-hybrid assay and co-immunoprecipitation further indicated that the interaction between c-Cbl and β-catenin was significantly enhanced by berberine dose-dependently (Figures 5d and e). We also found something unexpected that berberine increased the expression of c-Cbl in an RXRα-dependent manner (Figure 5f), which may also contribute to berberine-induced β-catenin degradation. Notably, c-Cbl was found to translocate from the cytoplasm to the nucleus and subsequently interact with nuclear β-catenin upon Wnt stimulation.46 We further found that the vast majority of c-Cbl proteins were localized in the cytoplasm in untreated KM12C cells, while berberine treatment induced a portion of c-Cbl proteins translocated from the cytoplasm to the nucleus as Wnt1 did (Figure 5g). However, overexpression of RXRα did not influence the subcellular location of c-Cbl (Supplementary Figure S4g), despite that it did induce an increase in both the mRNA and protein expression levels of c-Cbl (Supplementary Figure S4h). Taken together, our results demonstrated that berberine induced an RXRα-independent cytoplasmic-nuclear translocation of c-Cbl, and soon afterwards led to the proteasomal degradation of nuclear β-catenin in colon cancer cells through promoting the interaction of β-catenin with c-Cbl and the expression of c-Cbl via RXRα mediation.
Figure 5.
Berberine induced proteasomal degradation of nuclear β-catenin via RXRα mediation. (a) Berberine decreased the level of nuclear β-catenin. KM12C cells treated with berberine for 15 h were subjected to immunofluorescent staining using anti-β-catenin and anti-RXRα antibodies. Scale bar: 5 μm. (b) Leptomycin B (LMB) could not suppress the effect of berberine on nuclear β-catenin degradation. KM12C cells were pre-incubated with LMB (50 nm, 3 h) before berberine treatment (25 μm, 15 h), and then subjected to immunofluorescent staining. (c) c-Cbl mediated the effect of berberine on nuclear β-catenin in KM12C cells. Endogenous c-Cbl in KM12C cells was stably knocked down by lentivirus-based RNA interference. Cells were treated with berberine (25 μm, 15 h) and then subjected to immunofluorescent staining. (d–e) Berberine enhanced the interaction between c-Cbl and β-catenin in KM12C cells as analyzed by mammalian two-hybrid assay (d) and co-immunoprecipitation (e). Cell were cotransfected with the plasmids as indicated, and then treated with berberine for 15 h. The basal level of transcriptional activity in the vehicle-treated group was normalized to 1. (f) Berberine increased the expression of c-Cbl in an RXRα-dependent manner. ShCtrl and shRXRα KM12C cells were treated with berberine for 15 h, and then subjected to real-time PCR. The base level in each cell sublines was normalized to 1. (g) Berberine induced a portion of c-Cbl proteins translocated from the cytoplasm to nuclear as Wnt1 did. KM12C cells were treated with berberine treatment (25 μm, 15 h) or transfected with Wnt1 expression vector, and then subjected to immunofluorescent staining. All data were presented as the mean±s.e.m. of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs the respective vehicle control. Significant differences of shRXRα vs shCtrl (f) at the same dose of berberine were indicated as #P<0.05.
Berberine inhibits xenograft tumor growth in an RXRα-dependent manner
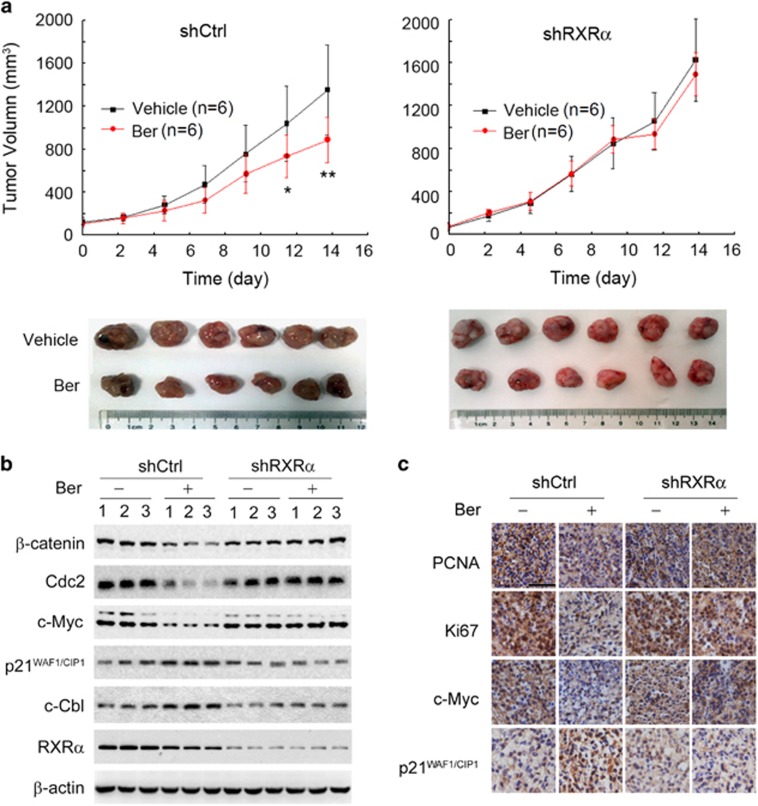
To further determine the role of RXRα in mediating the anti-cancer effect of berberine, we injected subcutaneously two KM12C cell sublines, shCtrl and shRXRα, into BALB/c nude mice. After the xenografts had reached approximately 5 mm in diameter, the mice from each group were randomly divided into two subgroups and administered with either berberine or the vehicle control. As shown in Figure 6a, berberine significantly inhibited the growth of the KM12C/shCtrl tumor, while it had little effect on the growth of the KM12C/shRXRα tumor. Berberine also regulated the expression of proliferating cell nuclear antigen, Ki67, Cdc2, c-Myc, p21WAF1/CIP1 and β-catenin in the xenograft tumors from the KM12C/shCtrl group but not the KM12C/shRXRα group (Figures 6b and c and Supplementary Figure S5). Thus, RXRα mediates the inhibitory effect of berberine on the β-catenin signaling pathway in animals.
Figure 6.
RXRα-dependent effect of berberine in animals. (a) RXRα-dependent inhibitory effect of berberine on colon cancer cell growth in animals. BALB/c nude mice injected subcutaneously with KM12C/shCtrl or KM12C/shRXRα cells were administered with berberine as described in the Materials and methods. Tumor growth curves were plotted (n=6 for each subgroup). Data were presented as the mean±s.d. of three independent experiments. Significant differences compared with vehicle controls were indicated as *P<0.05 and **P<0.01. (b) Expression levels of β-catenin, Cdc2, c-Myc, p21WAF1/CIP1, c-Cbl and RXRα in xenografts were analyzed by western blot. (c) Expression levels of PCNA, Ki67, c-Myc and p21WAF1/CIP1 in the specimens of xenograft tumors were analyzed by immunohistochemistry assays. Scale bar, 30 μm. Abbreviation: PCNA, proliferating cell nuclear antigen.
Discussion
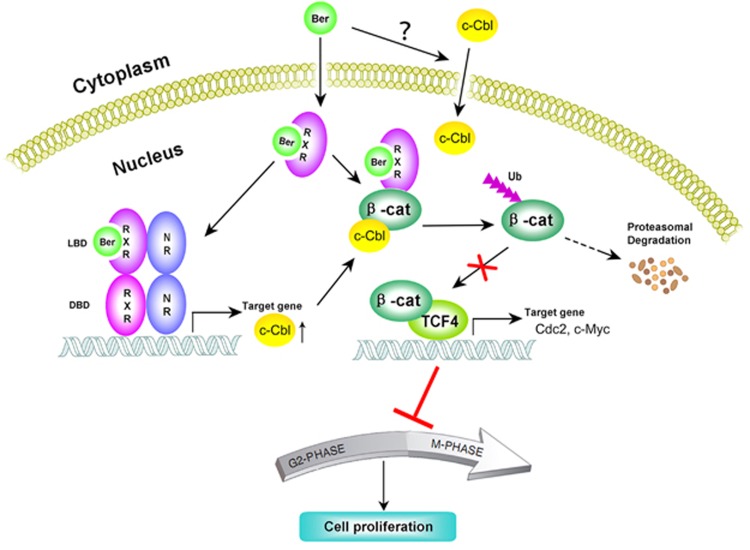
We recently reported that berberine could inhibit the β-catenin signaling pathway in vitro, in animals, and in patients with familial adenomatous polyposis.9 Several lines of evidence presented in this study demonstrate that berberine exerts its inhibitory effect on β-catenin through acting as an RXRα activator. Berberine could strongly activate the transcriptional activity of RXRα homodimers and heterodimers at doses known to inhibit the β-catenin signaling pathway (Figure 1)9, 11, 12 and bind directly to a unique site in the ligand-binding domain of RXRα (Figures 2 and 3). Furthermore, the expression of RXRα shRNA largely impaired the effects of berberine on suppressing the hyperactivated β-catenin signaling pathway (Figures 4a and e), inhibiting the proliferation of colon cancer cells (Figures 4b and c) and inducing their cell cycle arrest (Figure 4d). In addition, binding RXRα is critical for berberine to enhance the interaction of RXRα and β-catenin to induce the proteasomal degradation of β-catenin and thus inhibit the hyperactivated β-catenin signaling in colon cancer cells (Figures 4f–h). The molecular mechanisms underlying berberine-induced proteasomal degradation of β-catenin in the nucleus was further indicated in the present study, that is, through induced an RXRα-independent cytoplasmic-nuclear translocation of c-Cbl and subsequently enhancing the interaction between β-catenin and E3 ubiquitin ligase c-Cbl and elevating the expression of c-Cbl via RXRα mediation (Figures 5a–g). Moreover, we showed that berberine could inhibit the growth of KM12C colon cancer cells by downregulating the hyperactivated β-catenin signaling in nude mice, while it had little effect on KM12C cells expressing RXRα shRNA (Figure 6). These results demonstrate that RXRα represents an important intracellular target mediating the anti-cancer effects of berberine (Figure 7). In addition to its downregulation of β-catenin signaling, berberine has been shown to inhibit EGFR47 and NF-κB signalings,48 and induce AMPK activation49 in colon cancer cells. Whether berberine binding to RXRα accounts for these biological effects is currently unknown, although we found that berberine regulation of p21WAF1/CIP1 (Supplementary Figure S3b) and COX2 expression (Supplementary Figure S1e) was also RXRα dependent. It is also likely that other intracellular targets may contribute to berberine actions in colon cancer cells, which remains to be identified. To be noted, although other protein targets for berberine have been reported before (e.g., GPR40 for the antidiabetic action of berberine5), our study is the first one not only identifies a direct protein target—RXRα—for berberine but also demonstrates their binding mode and validates that berberine indeed functions through binding RXRα (by employing point mutations).
Figure 7.
Proposed model of berberine action.
Berberine binds to RXRα by a unique mode that is distinct from the binding of many natural and synthetic RXRα ligands. The RXRα LBP is highly restrictive to flexible and elongated ligands. The published crystal structures of RXRα bound to natural or synthetic ligands demonstrated that a carboxylate group in these ligands forms salt bridges with basic residue Arg316 at the end of the L-shaped RXRα LBP to establish anchoring ionic interaction for stabilization.40 However, berberine lacks such a carboxylate moiety (Figure 1a) and therefore unlikely binds to the RXRα LBP. This was indeed shown by our computer-assisted docking study (Figures 3e and f), which predicted a distinct binding site (Site X) for berberine. The role of the site X in berberine binding was revealed by mutation analysis showing differential effects of RXRα mutants on transcriptional activity of berberine and 9-cis-RA (Figures 3g and h). Concomitant (though not cooperative) binding of berberine and 9-cis-RA with RXRα-LBD proteins was observed (Figure 3d) and they could synergistically induce RXRα transcriptional activity (Figures 3a and b) and growth inhibition in colon cancer cells (Figure 3c). RXRα has been shown to bind small molecules with diverse structures.15, 17 Recent studies have revealed the existence of small-molecule-binding sites distinct from the classical LBP.38, 39, 50 Crystal structure studies showed that begelovin, a RXRα agonist lacking the carboxylate moiety, binds to RXRα differently from 9-cis-RA binding38 and that sulindac-derived analog K-8008 binds to a site outside of the RXRα LBP.39 Notably, such binding mode may avoid some unwanted side effects associated with binding to the classical LBP.50 Berberine was reported to show great superiority over other anti-cancer agents with its high tumor selectivity,51 which may be attributed to its unique RXRα binding mode.
The mechanism of activation by classical ligands through stabilization of the active conformation of the C-terminal H12 helix in the activate position has been well characterized previously.40 However, ligand binding could also activate RXRα by inducing rearrangement of oligomeric structures of RXRα, from an inactive tetramer to an active dimer.52 Two residues R321 and F318 in mouse RXRα (corresponding to R316 and F313 in human RXRα) were found to be critical for this oligomeric rearrangement of RXRα.53 For example, a mutated mouse RXRα tetramer (R321A) fails to dissociate upon ligand binding and is also defective in ligand-dependent activation; in contrast, RXRα mutant (F318A) fails to form a stable tetramer and shows high levels of transcriptional activity even in the absence of ligand.53 In our present study, we found that berberine binding to a pocket comprising residues Q275, R316 and R371, which is different from the classical ligand-binding site of RXRα. Since R316 plays important roles in the transformation of RXRα between tetramer and dimer as mentioned above, we assume that berberine binding may activate RXRα via inducing the rearrangement of oligomeric structures of RXRα from an inactive tetramer to an active dimer, which were initially proved by our observation that the binding of berberine favors the dissociation of tetramer into dimer (Supplementary Figure S1f).
Although residue C432 located in the classical LBP of RXRα is far away from the berberine binding site (site X), we did observe a weaker binding ability of C432Q mutation with berberine (about half of that of wild-type RXRα) (Figure 3g and Supplementary Figure S2c). The C432Q mutation may alter the conformation of the classical LBP to directly reduce the binding of 9-cis-RA. However, this conformational changes in the LBP may not just happen around the C432 but also at residues adjacent to the site X, which subsequently influence the binding of the berberine to the site X. In spite of the half-reduced binding affinity, berberine exerts a similar effect on the transactivation of C432Q mutation as compared with wild-type RXRα (Figure 3h). We assume that the binding ability of C432Q mutation with berberine, although reduced, may be strong enough to induce the events needed for RXRα transactivation.
The concomitant binding of berberine and 9-cis-RA to RXRα led to a synergistic effect of these two compound on RXRα-dependent transcriptional activity and colon cancer cell growth (Figures 3a–c). A similar situation has been reported recently that the organochlorine pesticide TNC and the synthetic estrogen 17a-ethinylestradiol (EE2) activate the nuclear receptor PXR (pregnane X receptor) in a synergistic fashion via simultaneous binding to PXR.54 However, TNC and EE2 simultaneously bind to a common protein binding site and enhanced the binding affinity of each other;54 while berberine and 9-cis-RA concomitantly (though not cooperatively) bind to two different but adjacent pockets (Figures 3d and e). The synergistic effect of berberine and 9-cis-RA to inhibit colon cancer cell growth has much significance for cancer therapy. Combined treatment with berberine and 9-cis-RA at low concentration exerted a comparable inhibitory effect on colon cancer cell growth to single-drug treatment at high concentrations (i.e., improved efficacy with less toxicity) (Figure 3c), In fact, an clinical example of drug combination for synergistic action can be found in the treatment of leukemia, in which superior efficacy with fewer side effects was achieved with the combination of all-trans retinoic acid and arsenic trioxide, both of which target and degrade the oncogenic fusion gene PML-RARα and consequently induce cancer cell differentiation. Our finding may help to yield novel promising therapies for the treatment of colon cancer.
Together, our results identify berberine as a new RXRα modulator with promising therapeutic effect for colon cancer and provide new strategies for the design of new RXRα-based antitumor agents and drug combinations.
Materials and methods
Transfection and dual-luciferase reporter assays
Cells were seeded in 24-well culture plates and transfected with the different luciferase reporter genes using the Lipofectamine 2000 transfection reagent (Invitrogen, Gaithersburg, MD, USA) according to the manufacturer’s recommendations. Luciferase assays were performed using the Dual-Luciferase Reporter Assay Kit (Promega, Madison, WI, USA). Briefly, cells were lysed using 1 × Passive Lysis Buffer. Then the firefly and renilla luciferase activity was measured using a GloMax 20/20 Luminometer (Promega).
For luciferase-based mammalian one-hybrid assay, cells were cotransfected with pBIND-RXRα-LBD (or pBIND-HNF4α as needed) and pG5luc constructs. For two-hybrid assay, cells were cotransfected with pBIND-β-catenin/33Y, pACT-RXRα or its mutations (or pACT-c-Cbl as needed) and pG5luc vector. These genes were subcloned into the pBIND and pACT vectors to generate fusion proteins with the DNA-binding domain of GAL4 and the activation domain of VP16, respectively. The pG5luc vector contains five GAL4-binding sites upstream of a minimal TATA box, which in turn is upstream of the firefly luciferase gene. The internal control—renilla luciferase—was also included in the pBIND vector. After treatment with berberine for 15 h, firefly luciferase and renilla luciferase signals were measured, and the reporter activity was determined by the firefly luciferase/renilla luciferase ratio.
For the TOP/FOP luciferase assay, cells were transfected with either a β-catenin-dependent reporter gene (TOPflash) or a control reporter gene (FOPflash), together with an internal control plasmid—renilla luciferase (pRL-TK). After treatment with berberine for 15 h, the TOP or FOP reporter activity was determined by normalizing the firefly luciferase signal to the renilla luciferase signal. β-Catenin signaling activity displayed by the TOP/FOP ratio was then calculated by dividing the TOP reporter activity by the FOP reporter activity.
Lentiviral vector-based shRNA technique
Endogenous RXRα or c-Cbl expression in KM12C cells was knocked down using a lentiviral vector-based shRNA technique. The human RXRα shRNA target sequence was 5′-GTGTTGTCACCCTCCTTATTT-3′ (targeting the 3′-UTR region of RXRα mRNA). The human c-Cbl shRNA target sequence was 5′-CTTCATAAAGACAAACCAT-3′. The shRNA control (scramble) sequence was 5′-GGCTACGTCCAGGAGCGCACC-3′. Oligonucleotides (Invitrogen) were annealed and inserted into the pLVshRNA lentiviral vector. Lentiviruses were generated by co-transfecting subconfluent HEK293T cells with the lentivirus and packaging plasmids using polyethylenimine transfection. Viral supernatants were then collected 72 h after transfection, centrifuged at 3000g for 20 min, and filtered through 0.45-μm filters (Millipore, Bedford, MA, USA). Freshly plated KM12C cells were infected with the lentivirus once they had reached 60% confluence. Selection medicine (puromycin) was added 48 h post-infection to screen for stable cell lines.
Isothermal titration calorimetry
The calorimetric experiments with RXRα-LBD protein and berberine were conducted at 25 °C using a MicroCal iTC200 instrument (GE Healthcare, Piscataway, NJ, USA). The RXRα-LBD protein (0.1 mm) was dialyzed against phosphate-buffered saline buffer containing 4% dimethyl sulfoxide, and berberine was dissolved to 1 mm in the same buffer and berberine concentration was determined by weighing. Protein concentrations were determined based on UV 280 nm absorbance. Heat exchanges were monitored throughout titrations consisting of 17 injections (one time 0.5 μl followed by 16 times 2 μl) of berberine solution into the cell containing RXRα-LBD protein solution. Acquired calorimetric titration curves were analyzed with the Origin 7.0 program (OriginLab, Northampton, MA, USA) using the 'One Set of Binding Sites' fitting model.
High-performance liquid chromatography
To determine the binding of berberine with RXRα-LBD or other NR-LBD proteins, Ni-NTA beads bound proteins or Ni-NTA beads alone were incubated overnight with equal mole of berberine at 4 °C. To analyze the concomitant binding of berberine and 9cRA with RXRα-LBD proteins, Ni-NTA beads bound RXRα-LBD proteins or Ni-NTA beads alone were incubated overnight with equal mole of berberine in the presence and absence of equal mole of 9-cis-RA or with equal mole of 9-cis-RA in the presence and absence of equal mole of berberine at 4 °C. After incubation, the beads were collected by centrifugation, and washed with phosphate-buffered saline. Then the chloroform–methanol 1:1 (v/v) was added to the beads and vortexed, and the supernatant were collected and dried at 65 °C for 4 h. Then the dry powder was dissolved in methanol, and subsequently subjected to HPLC (Shimadzu, Tokyo, Japan) as previously described.55 Prior to analysis, a Sepax GP-C18 column was pre-equilibrated with the mobile phase (acetonitrile—0.033 m KH2PO4 9:11(v/v) for berberine, and acetonitrile—0.1% acetic acid 9:1 for 9-cis-RA) for 30 min. Twenty microliters of sample was injected into the column and separated at a flow rate of 1.0 ml/min with the column temperature set at 35 °C and the fluorescence detection set at λex= 345 nm and λem= 498 nm for berberine and at λex= 350 nm and λem= 490 nm for 9-cis-RA. The characteristic peak spectrum and retention time of berberine or 9-cis-RA were used for identification, and the peak areas were measured for quantitative calculations. The HPLC signal indicating the relative binding affinity in all groups was normalized to the Ni-NTA beads group.
Cell cycle analyses
Cells were trypsinized, collected, fixed with ice-cold 70% ethanol for 1 h and then stained with 50 μg/ml of propidium iodide (Sigma Aldrich, St Louis, MO, USA) containing 10 μg/ml RNase A (Roche, Nutley, NJ, USA) for 0.5 h (protected from light). Samples were subsequently analyzed using a flow cytometer (Cyflow Space, Partec, Münster, Germany) and the ModFit LT Tutorial Series software. Values represent the results of three independent experiments.
Tumor xenografts
Nude mice (BALB/c, SPF grade, 16–18 g, 6–7 weeks old) were obtained from Laboratory Animal Center of Xiamen University, and housed in the barrier facility of the Laboratory Animal Center, Xiamen University, as approved by the Animal Ethics Committee of Xiamen University. Two investigators were blinded to the group allocation when doing the experiments and assessing the data. Mice were randomly divided into two groups, and subcutaneously implanted with the cell suspensions of shCtrl and shRXRα KM12C cell sublines, respectively. When the tumors were palpable (approximately 6 days after transplantation), mice in each group were randomly allocated into two subgroups, and then injected intraperitoneally (i.p.) daily with berberine (10 mg/kg) and the vehicle, respectively. Tumor sizes were measured every 2 days using calipers, and the tumor volumes (TV) were calculated according to the following formula: TV=L (length) × W2 (width)/2. After 14 days of drug treatment, mice were killed and the tumor tissues were collected for use.
Statistical analyses
Data are expressed as the mean±s.e.m. or s.d. as indicated. Differences between the two groups were assessed with two-tailed unpaired Student’s t-test using the SPSS (Statistical Package for the Social Sciences) software (Version 13; SPSS Inc., Chicago, IL, USA). P<0.05 was defined as statistically significant.
Other methods
Detailed descriptions of the other methods used in this study, including cell culture and treatment, reagents and antibodies, plasmids, purification of His-tagged fusion proteins, real-time PCR, immunofluorescent staining, co-immunoprecipitation and western blot analysis, molecular modeling, cell proliferation assay and immunohistochemistry, are provided in the Supplementary Methods.
Data availability
All relevant data that support the findings of this study are available within the article and its Supplementary Information files or from the corresponding author on request.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (U1405228, 81472568 and 81572589), the Natural Science Foundation of Fujian grant (2014J07010, 2017J06020, 2015R1036-1, 2017R1036-4, 2016R1034-1 and 2016R1034-4) and the Fundamental Research Funds for the Central Universities (20720150058).
Author contributions
TH, YYZ and XKZ designed the experiments and wrote the manuscript. HR, YYZ, JH, BX, BC, YT, DW and YZ carried out the molecular and cellular experiments. LZ, SZ and XW carried out the structural studies. YZ, XC, PM, HC and WZ carried out the studies on mouse models. HW and HL carried out the ITC experiments. YS was involved in the design of this project.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
The authors declare no conflict of interest.
Supplementary Material
References
- Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell 2007; 130: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res 2008; PTR 22: 999–1012. [DOI] [PubMed] [Google Scholar]
- Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 2004; 10: 1344–1351. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010; 59: 285–292. [DOI] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Sundaram S, Singh W, Kant R, Davis JA et al. Identification of berberine as a novel agonist of fatty acid receptor GPR40. Phytother Res 2010; PTR 24: 1260–1263. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun 2014; 5: 5493. [DOI] [PubMed] [Google Scholar]
- Chai YS, Yuan ZY, Lei F, Wang YG, Hu J, Du F et al. Inhibition of retinoblastoma mRNA degradation through Poly (A) involved in the neuroprotective effect of berberine against cerebral ischemia. PLoS ONE 2014; 9: e90850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha SK, Khuda-Bukhsh AR. Berberine alters epigenetic modifications, disrupts microtubule network, and modulates HPV-18 E6-E7 oncoproteins by targeting p53 in cervical cancer cell HeLa: a mechanistic study including molecular docking. Eur J Pharmacol 2014; 744: 132–146. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cao H, Zhang B, Cao H, Xu X, Ruan H et al. Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signalling. J Cell Mol Med 2013; 17: 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro MC, Velasco-Velazquez M, Aguirre-Alvarado C, Pestell RG. Overview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and present. Expert Opin Investig Drugs 2014; 23: 295–304. [DOI] [PubMed] [Google Scholar]
- Wu K, Yang Q, Mu Y, Zhou L, Liu Y, Zhou Q et al. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/beta-catenin signaling. Int J Oncol 2012; 41: 292–298. [DOI] [PubMed] [Google Scholar]
- Albring KF, Weidemüller J, Mittag S, Weiske J, Friedrich K, Geroni MC et al. Berberine acts as a natural inhibitor of Wnt/beta-catenin signaling—identification of more active 13-arylalkyl derivatives. Biofactors 2013; 39: 652–662. [DOI] [PubMed] [Google Scholar]
- Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Exp Cell Res 2010; 316: 1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev 2005; 26: 898–915. [DOI] [PubMed] [Google Scholar]
- Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Curr Med Chem 2002; 9: 623–637. [DOI] [PubMed] [Google Scholar]
- de Lera AR, Bourguet W, Altucci L, Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 2007; 6: 811–820. [DOI] [PubMed] [Google Scholar]
- Perez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochim Biophys Acta 2012; 1821: 57–69. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Shimizu M, Okuno M, Matsushima-Nishiwaki R, Kanemura N, Araki H et al. Synergistic effects of RXR alpha and PPAR gamma ligands to inhibit growth in human colon cancer cells—phosphorylated RXR alpha is a critical target for colon cancer management. Gut 2007; 56: 1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ET, Martinez ME, Campbell PT, Conti DV, Duggan D, Figueiredo JC et al. Genetic variation in the retinoid X receptor and calcium-sensing receptor and risk of colorectal cancer in the Colon Cancer Family Registry. Carcinogenesis 2010; 31: 1412–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Mirshahidi S, Senthil M, Kazanjian K, Chen CS, Zhang K. Down-regulation of LXR/RXR activation and negative acute phase response pathways in colon adenocarcinoma revealed by proteomics and bioinformatics analysis. Cancer Biomark 2014; 14: 313–324. [DOI] [PubMed] [Google Scholar]
- Egan JB, Thompson PA, Ashbeck EL, Conti DV, Duggan D, Hibler E et al. Genetic polymorphisms in vitamin D receptor VDR/RXRA influence the likelihood of colon adenoma recurrence. Cancer Res 2010; 70: 1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volate SR, Muga SJ, Issa AY, Nitcheva D, Smith T, Wargovich MJ. Epigenetic modulation of the retinoid X receptor alpha by green tea in the azoxymethane-Apc Min/+ mouse model of intestinal cancer. Mol Carcinog 2009; 48: 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao JH, Ghosn C, Hinchman C, Forbes C, Wang J, Snider N et al. Adenomatous polyposis coli (APC)-independent regulation of beta-catenin degradation via a retinoid X receptor-mediated pathway. J Biol Chem 2003; 278: 29954–29962. [DOI] [PubMed] [Google Scholar]
- Dillard AC, Lane MA. Retinol increases beta-catenin-RXRalpha binding leading to the increased proteasomal degradation of beta-catenin and RXRalpha. Nutr Cancer 2008; 60: 97–108. [DOI] [PubMed] [Google Scholar]
- Cesario RM, Stone J, Yen WC, Bissonnette RP, Lamph WW. Differentiation and growth inhibition mediated via the RXR:PPARgamma heterodimer in colon cancer. Cancer Lett 2006; 240: 225–233. [DOI] [PubMed] [Google Scholar]
- Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001; 19: 2456–2471. [DOI] [PubMed] [Google Scholar]
- Janakiram NB, Mohammed A, Zhang Y, Brewer M, Bryant T, Lightfoot S et al. Chemopreventive efficacy of raloxifene, bexarotene, and their combination on the progression of chemically induced colon adenomas to adenocarcinomas in rats. Cancer Prev Res (Phila) 2013; 6: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Liu W, Su Y, Wei Z, Liu J, Kolluri SK et al. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell 2010; 17: 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldenschuh I, Hurlimann R, Muller A, Ammann R, Mullhaupt B, Dobbie Z et al. Relationship between APC genotype, polyp distribution, and oral sulindac treatment in the colon and rectum of patients with familial adenomatous polyposis. Dis Colon Rectum 2001; 44: 1090–1097. [DOI] [PubMed] [Google Scholar]
- Gwyn K, Sinicrope FA. Chemoprevention of colorectal cancer. Am J Gastroenterol 2002; 97: 13–21. [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Corr M, James SY, Bernasconi M, Lu D, Liu W et al. The R-enantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. Proc Natl Acad Sci USA 2005; 102: 2525–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 2005; 19: 877–890. [DOI] [PubMed] [Google Scholar]
- Seo HS, Woo JK, Shin YC, Ko SG. Identification of biomarkers regulated by rexinoids (LGD1069, LG100268 and Ro25-7386) in human breast cells using Affymetrix microarray. Mol Med Rep 2015; 12: 800–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA 2001; 98: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak PA, Kast-Woelbern HR, Anisfeld AM, Edwards PA. Identification of PLTP as an LXR target gene and apoE as an FXR target gene reveals overlapping targets for the two nuclear receptors. J Lipid Res 2002; 43: 2037–2041. [DOI] [PubMed] [Google Scholar]
- Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA 2000; 97: 12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade EA, McIntyre TM, Zimmerman GA, Prescott SM. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem 1999; 274: 8328–8334. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li L, Chen L, Hu L, Jiang H, Shen X. Structure basis of bigelovin as a selective RXR agonist with a distinct binding mode. J Mol Biol 2011; 407: 13–20. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang ZG, Aleshin AE, Chen F, Chen J, Jiang F et al. Sulindac-derived RXRalpha modulators inhibit cancer cell growth by binding to a novel site. Chem Biol 2014; 21: 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea PF, Mitschler A, Rochel N, Ruff M, Chambon P, Moras D. Crystal structure of the human RXRalpha ligand-binding domain bound to its natural ligand: 9-cis retinoic acid. EMBO J 2000; 19: 2592–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T, Nakamura T, Kazuki Y, Oshimura M, Kohu K, Tago K et al. Overexpression of Icat induces G(2) arrest and cell death in tumor cell mutants for adenomatous polyposis coli, beta-catenin, or Axin. Cancer Res 2002; 62: 3322–3326. [PubMed] [Google Scholar]
- Wang W, Liu H, Wang S, Hao X, Li L. A diterpenoid derivative 15-oxospiramilactone inhibits Wnt/beta-catenin signaling and colon cancer cell tumorigenesis. Cell Res 2011; 21: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 2004; 64: 423–428. [DOI] [PubMed] [Google Scholar]
- Han A, Tong C, Hu D, Bi X, Yang W. A direct protein-protein interaction is involved in the suppression of beta-catenin transcription by retinoid X receptor alpha in colorectal cancer cells. Cancer Biol Ther 2008; 7: 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitalia V, Shivanna S, Martorell J, Meyer R, Edelman E, Rahimi N. c-Cbl, a ubiquitin E3 ligase that targets active beta-catenin: a novel layer of Wnt signaling regulation. J Biol Chem 2013; 288: 23505–23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna S, Harrold I, Shashar M, Meyer R, Kiang C, Francis J et al. The c-Cbl ubiquitin ligase regulates nuclear beta-catenin and angiogenesis by its tyrosine phosphorylation mediated through the Wnt signaling pathway. J Biol Chem 2015; 290: 12537–12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao H, Lu N, Liu L, Wang B, Hu T et al. Berberine inhibits proliferation and down-regulates epidermal growth factor receptor through activation of Cbl in colon tumor cells. PLoS ONE 2013; 8: e56666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Tong X, Qi B, Qu H, Dong S, Yu B et al. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NFkappaB. Mol Med Rep 2014; 9: 249–254. [DOI] [PubMed] [Google Scholar]
- Park JJ, Seo SM, Kim EJ, Lee YJ, Ko YG, Ha J et al. Berberine inhibits human colon cancer cell migration via AMP-activated protein kinase-mediated downregulation of integrin beta1 signaling. Biochem Biophys Res Commun 2012; 426: 461–467. [DOI] [PubMed] [Google Scholar]
- Zhang X, Su Y, Chen L, Chen F, Liu J, Zhou H. Regulation of the nongenomic actions of retinoid X receptor-alpha by targeting the coregulator-binding sites. Acta Pharmacol Sin 2015; 36: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu L, Shi Y, Cao H, Chaturvedi R, Calcutt MW et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS ONE 2012; 7: e36418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampe RT Jr, Montana VG, Lambert MH, Wisely GB, Milburn MV, Xu HE. Structural basis for autorepression of retinoid X receptor by tetramer formation and the AF-2 helix. Genes Dev 2000; 14: 2229–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Dong D, Lee W, Reczek PR, Noy N. Auto-silencing by the retinoid X receptor. J Mol Biol 1998; 284: 21–32. [DOI] [PubMed] [Google Scholar]
- Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S et al. Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds. Nat Commun 2015; 6: 8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo F, Nakamura N, Akao T, Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab Dispos 2006; 34: 2064–2072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data that support the findings of this study are available within the article and its Supplementary Information files or from the corresponding author on request.