Graphical abstract
Keywords: Disaccharidase activity, Hemagglutination assay, Jejunum, Phytohemagglutinin (PHA), Serologic assay, White kidney bean (Phaseolus vulgaris L.)
Highlights
-
•
Impact of bean exposure on disaccharidase activity in rat jejunum was investigated.
-
•
Raw white beans depressed the jejunal maltase and sucrase activities in Wistar rats.
-
•
No lectins were found in the blood and feces of rats after 10 days of bean feeding.
-
•
White beans may pose a risk to the consumer when eaten raw or undercooked.
Abstract
The chronic ingestion of raw or undercooked kidney beans (Phaseolus vulgaris L.) is involved in the pathogenesis of multiple organ dysfunction; the underlying mechanisms are still poorly understood. The objective of this study was to assess the gavage effects of a raw Beldia bean variety on the brush border disaccharidase activities in the jejunal mucosa of Wistar rats. Twenty young adult male rats were randomly assigned into 2 groups of 10 rats each: Control, rats were gavaged with 300 mg of a rodent pellet flour suspension (RPFS); Experimental, rats were orogastrically fed a dose of 300 mg Beldia bean flour suspension (BBFS). Prior to determining the disaccharidase activity by Dahlqvist method, the blood and stool specimens were collected on day 10. The sera and feces were screened for the presence of lectins by serologic and hemagglutination assays. The results showed that the brush border maltase and sucrase activities were significantly diminished but lactase activity did not undergo any change in BBFS-gavaged animals as compared with control. Preliminary immunobiochemical assays revealed the absence of lectins in the systemic circulation and feces of rats, but further work is required to prove this. Overall, the dietary administration of BBFS caused depression of the activity of the small intestinal enzymes maltase and sucrase.
1. Introduction
It is well established that the incorporation of raw kidney bean (Phaseolus vulgaris L.) in animal diets reduces food intake, depresses growth, and may cause death [[1], [2], [3], [4]]. Similarly, improperly-prepared beans are known to be toxic for human beings [5,6]. Earlier, toxic effects were also shown to occur in insects [7], birds [8], and also ruminants [9].
This was attributed to the presence in the seeds of potentially bioactive compounds usually referred as phytohemagglutinins (PHAs); known also as Phaseolus vulgaris lectins. PHAs are carbohydrate-binding proteins and can interact with most differentiated mammalian cells which express membrane glycoconjugates containing complex oligosaccharide chains. They have been reported from different species of kidney beans and have been found to be responsible for most of the toxicological manifestations. Among beans varieties, red kidney beans and white kidney beans (i.e., also known as cannellini) in particular are rich in phytohemagglutinins [10].
PHA is resistant to breakdown by both digestive enzymes and bacteria in the consumer’s gut and therefore most phytohemagglutinins survive, at least in part, the passage through the digestive tract in immunologically and functionally intact form. This allows the PHAs to bind specifically to the glycoconjugates located on the luminal surface of the gut and to partially endocytose into the circulation system [11]. Once bound to digestive tract, P. vulgaris lectin can cause dramatic changes in the cellular morphology and metabolism of the small intestine and activate a cascade of signals which alters the intermediary metabolism. Thus, PHAs may induce changes in some, or all, of the digestive, absorptive, protective or secretory functions of the digestive system and affect cellular proliferation and turnover [[12], [13], [14]]. It has been demonstrated that high intakes of PHA can seriously damage the intestinal wall, result in an overgrowth of coliform bacteria in the gut lumen [15], and can significantly affect the internal organs, such as the small intestine, pancreas, liver, and thymus [16]. It is also evident that PHA can affect the immune system since a powerful humoral anti-lectin IgG-antibody response has been shown to occur after inclusion of the lectin in the diet [17].
Considering the vast and varied biological activities that phytohemagglutinins can cause, it is surprising that there exists a paucity of studies related to the nutritional implications of dietary lectins in Tunisia. The reasons for the experimental gap are unclear, in part, be due to a lack of awareness within the health and medical communities of which variety of beans in our diet contain lectin activity and the extent of exposure.
Incidents of foodborne illness associated with undercooked red kidney beans have been reported in Canada [18], Australia [19], and in the United Kingdom [6]. Recently, the importation of dry red kidney beans (a variety of the species Phaseolus vulgaris L.) for cultivation or consumption in South Africa is prohibited because of their potential toxicity to humans [20]. It has been established that the hemagglutinating lectins (e.g., phytohemagglutinin, PHA) in kidney beans are responsible for this toxicity [20].
Illness is usually related to the ingestion of raw (e.g., fresh, soaked, or sprouted form as a part of salad), flour (e.g., porridge for infant food), or little processed (e.g., steamed or prepared in dehydrators, slow cookers, or crockpots) beans [[26],[21], [22], [23], [24], [25]]. Symptoms generally appear within 1–3 h after consumption of improperly prepared beans. Onset is usually marked by extreme nausea, followed by vomiting and diarrhea. Some individuals also report abdominal pain. Those affected rarely require hospitalization or treatment, and adverse symptoms generally subside within a short period of time. Outbreaks of poisoning have been associated with cooking kidney beans in slow cookers. However, it is important to note that PHA can be deactivated by boiling beans for 10 min at 100 °C and the U.S. Food and Drug Administration recommends an initial soak of at least 5 h in water which should then be discarded. If the beans are cooked at a temperature below boiling (i.e., without a preliminary boil), as in a slow cooker, the toxic effect of hemagglutinin is increased. It was therefore found that beans cooked at 80 °C are five times as toxic as raw beans. In studies of casseroles cooked in slow cookers, internal temperatures often did not exceed 75 °C [26].
In Tunisia, dry bean production has rallied since 2011, reaching 170 tons in 2013 [27]. According to the National Agricultural Research Institute of Tunisia (INRAT, Tunis, Tunisia), Twila, Coco, and Beldia beans are, by far, the most commonly consumed white dry varieties. Bean consumption may be on the upswing due to increased public recognition of possible health benefits of beans and to increase in Tunisian population.
To ensure the beans safety, Tunisia government ordered that a Food Toxicology Department be set up in order to coordinate the work concerning beans safety and take effective measures to ensure beans safety for consumers. At the present, this food safety department composed of the Department of Animal Resources, Fisheries, and Food Technology, National Institute of Agronomy of Tunisia, and the Intestinal Immunophysiology-Research Unit, Faculty of Medicine of Tunis has started the work and commenced the supervision in an all-round way, and has subjected the phytohemagglutinin (PHA), present in white dry beans, to an extensive investigation in the fields of hematology, cytology, immunology, biotechnology, microbiology, and clinical use.
In a preliminary study, the phytohemagglutinin present in white kidney beans (Phaseolus vulgaris L.) variety Beldia, frequently consumed by Tunisian population (INRAT, Tunis, Tunisia), was characterized and some of its properties were described [28]. Biochemical and immunological evidence indicated that raw Beldia seeds contain elevated levels of bioactive lectins, approximately 9.20 g/kg [28]. The raw Beldia beans demonstrated when orally administered to growing rats for 10 days at dose of 300 mg induced substantial modification of the morphology and physiological functions of the small intestine (i.e., disruption of absorption process) [29]. Furthermore, serological data revealed effectively the presence of biologically active PHA in the rat jejunal lumen, 3 h and 30 min after oral challenge with 300 mg of Beldia bean flour suspension [30].
Kidney bean poisoning is known to affect animals as well as human beings. The mechanisms proposed for the toxicity of ingested Phaseolus vulgaris lectins always call for more answers. Therefore, this work was undertaken to highlight the impact of a raw Beldia variety gavage on the disaccharidases activities in the jejunal brush border of Wistar rats and to fill the gaps that exist currently.
2. Materials and methods
2.1. Plant material
The common white beans (Phaseolus vulgaris L. var. Beldia) were purchased in August 2016 from a local market in Ariana city, Tunis, Tunisia. The seeds were free of dust, foreign material, and broken and small kernels. The authentication of plant material was performed by the Department of Botany, National Agricultural Research Institute of Tunisia (INRAT), Ariana, Tunis, Tunisia. Bean seeds were aseptically ground using a mortar and pestle, and then passed through a U.S. No. 200 sieve. The powder was packed, sealed in polyethylene bags, and stored in a cold room at 4 °C until use.
2.2. Extraction of seed proteins
The extraction of proteins from seed flour was done following the method of Itoh et al. [31] with slight modifications. Briefly, one hundred grams (100 g) of white kidney beans (Phaseolus vulgaris L., var. Beldia) were soaked overnight in 1 L of distilled water at room temperature (ca. 25 °C), which will help in softening them. These soften seeds then crushed with Tris-NaCl (Tris-HCl 50 mM, 50 mM NaCl, at pH 8 and 4 °C) in a mortar and pestle. The prepared mixture then centrifuged at 3500 rpm at 4 °C for 30 min and filtered through Whatman No. 1 filter paper. Finally, the liquid supernatant (i.e., referred to as the total crude extract) was precipitated using ammonium sulfate. The protein fractions were collected by centrifugation at 3500 rpm for 30 min and stored frozen in aliquots.
2.3. Protein concentration determination
Protein concentration in the white Beldia bean extracts was determined following the Bradford method [32] using BSA (i.e., bovine serum albumin) as a standard. Absorbance was measured at 280 nm. Lectin specific activities were expressed as titer over milligrams of protein as hemagglutination units (HU) per mg of protein.
2.4. Animals, diets, and management
Twenty (20) young Wistar male rats (Rattus norvegicus albinus), weighting 60–80 g were initially acclimatized for 7 days, were used in this study. Rattus albinus were purchased from the Pasteur Institute of Tunis, Tunis, Tunisia, kept singly in polypropylene cages on chopped aspen wood bedding, and maintained in a well-ventilated, thermostatically controlled room (25 ± 1.4 °C) with 12:12 h light-dark cycle. They were fed with standard pellet diet from Cereal Office, Tunis, Tunisia, and water ad libitum. The proximate composition of the daily feed offered to rats was determined by the Food Technology Service (STA), National Institute of Nutrition and Food Technology (INNTA), Tunis, Tunisia, according to AOAC [33]. One hundred grams (100 g) dry rodent pellets, deprived of lectins, contained approximately 12.77% protein, 2.52% lipid, 69.18% carbohydrate, and 350 Kcal energy. Rats were handled daily by the same investigator and were fasted overnight (15 h) with free access to water before the experiment. Animals were monitored during the study for clinical signs, mortality, and stool consistency (e.g., watery, moderate, or normal). The rats were allotted at random to 2 groups each of 10 rats and were treated daily for 10 days as follows: Group 1- was daily administered with a single dose of a 300 mg rodent pellet flour (lectin-free), suspended in 3 mL distilled water (RPFS), by oral intubations using gavage needle and served as control. Group 2- was orally intubated (by gavage needle) with a single dose of a 300 mg Beldia bean flour (22.8% protein, 2.44% lipid, 59.51% carbohydrate, and 351.2 Kcal energy/100 g) mixed with 3 mL distilled water (BBFS, ∼0.92 mg/mL of PHA in seed flour) and served as experimental [13,34,35]. This research has the approval of the Ethics Committee for the Use of Laboratory Animals of the Faculty of Medicine of Tunis and followed the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.5. Collection of blood samples
At the end of the experiment (i.e., day 10), the rats were anaesthetized by diethyl ether (PARAPHARM, Athens, Greece) prior to sacrifice by exsanguination. The abdomen and thorax were then opened immediately and blood (0.5–1.0 mL) was collected via cardiac puncture using sterile syringes and needles and emptied into plain tubes, allowed to clot for about 2 h. The clotted blood was thereafter centrifuged at 3500 rpm for 30 min to recover serum from clotted blood. Serum was separated with sterile syringes and needles and stored at −70 °C until used.
2.6. Collection of fecal samples
The fecal materials collected from rats fed with raw Beldia beans were screened for the presence of lectins by hemagglutination assay, Ouchterlony double diffusion, immunoelectrophoresis, counterimmunoelectrophoresis, and CI-ELISA techniques. On day ten and after 3 h of gavage with BBFS, the feces were collected and 1 g (wet weight) was dissolved in 10 mL of physiological saline solution (0.9% NaCl). After vigorous shaking by a vortex for 5 min, the resulting mixture was filtered through cheese cloth and centrifuged at 1400 rpm for 30 min at 4 °C to remove the feces debris. The clarified extract was immediately subjected to immunobiochemical analyses.
2.7. Hemagglutination assay (HA)
The main objective of hemagglutination study was to determine whether the fecal extracts and sera of BBFS-fed rats contain the P. vulgaris lectins on day 10. In a 96-well microtiter U-plate (Greiner Bio-One, Monroe, North California, USA), a serial 2-fold dilution of the test sample (50 μL; crude Beldia bean extract (CBBE, 100 mg/mL), fecal extract (100 mg/mL), and sera) in phosphate-buffered saline (PBS) (pH 7.2) was performed. A 2% A red blood cell suspension (50 μL) (Blood banks, Hospital Charles Nicolle, Tunis, Tunisia) in PBS was added to the sample. The mixture was gently shaken and then incubated at room temperature (ca. 25 °C) for 1 h until the red blood cells in the blank (PBS, negative control, no protein sample) had fully sedimented and appeared as a red spot at the bottom of the well. Presence of red-carpet layer in the wells indicated hemagglutination activity. Positive wells containing only purified PHA (1 mg/mL, Sigma-Aldrich, Lesquin, France; equivalent to 0.92 mg/mL of PHA obtained from mixing of 300 mg Beldia flour beans with 3 mL distilled water) and red cells were always included. One hemagglutination unit (hemagglutination titer, HU) is the reciprocal of the highest dilution of the lectin sample inducing hemagglutination. Specific activity is the number of hemagglutination units (HU) per mg protein [36]. The hemagglutination was assessed visually and microscopically. The assay was reproducible to ±1 dilution. Values given in this investigation are the means from three separate measurements.
2.8. Production of rabbit anti-PHA antibodies
Antisera to PHA were raised in rabbits as previously described [37]. Young adult male New Zealand White (NZW, n = 6) rabbits (Pasteur Institute of Tunis, Tunis, Tunisia), each weighting 3–4 kg, were used in this study, which was approved by the Faculty of Medicine of Tunis Animal Care Committee and followed the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The rabbits were housed individually in stainless-steel hanging cages in a single room. They had free access to high-fiber rabbit chow (Cereal Office, Tunis, Tunisia) and municipal tap water. The room was maintained at 18–21 °C, at 40–60% relative humidity, and 14:10 h light: dark cycle. The phytohemagglutinin-P (PHA-P) was purchased from Sigma-Aldrich, Lesquin, France. Experimental rabbits (n = 3) were injected subcutaneously in five sites on their back at 1st, 10th, 15th, and 30th days with 1 mL of an emulsion composed of 100 μL of PHA solution (1 mg/mL; 100 μg PHA), 500 μL complete Freund’s adjuvant (CFA, Merck), and 400 μL distilled water. Control rabbits (n = 3) received the same emulsion but without PHA. The production of antibodies and an increase in their titer were assessed using ELISA, Ouchterlony double immunodiffusion, and electrophoresis techniques by taking blood samples from the marginal vessels of rabbit’s ear on 35th day. The blood was collected in sterile centrifuge tubes and was allowed to stand for 2 h at room temperature (ca. 25 °C) for clot formation to occur. After storage overnight in a refrigerator to permit clot retraction, the serum was separated by centrifugation at 3500 rpm for 30 min. Serum was stored at −20 °C by adding 0.1% sodium azide (Merck).
2.9. Ouchterlony double immunodiffusion (ODI)
To screen for the presence or absence of lectins in the sera and feces of experimental rats, the radial double immunodiffusion was carried out as previously described by Ouchterlony [38], with slight modifications. Briefly, a 1.5% agarose (Bio-Rad, Marnes-la-Coquette, France) solution in veronal buffer (0.02 M, pH 8.6) was poured onto clean microscope slides. After gel solidification, cylindrical wells 5 mm in diameter were cut with a 7-well cutter (Gel Punch, E-C Apparatus Corp; Savant Instruments, Inc., New York, USA). The wells were filled with samples (e.g., rabbit anti-PHA serum (10 μL, neat), purified PHA (10 μL, 1 mg/mL, Sigma-Aldrich, Lesquin, France), crude Beldia bean extract (CBBE, 10 μL, 100 mg/mL), sera (10 μL, neat), and fecal extracts (10 μL, 100 mg/mL) of rats) and the slides were incubated in a wet chamber at 24 °C for 24 h. The slides were then washed out by two changes in 0.9% NaCl and two changes in distilled water. The slides were stained for 3 min with 0.5% (w/v) Coomassie Brilliant Blue R 250 (Merck, Darmstadt, Germany) in a mixture of ethanol, acetic acid, and distilled water (4:1:5, v/v/v). Excess stain was washed out by repeated changes in the same mixture (4:1:5, v/v/v). The ODI experiments were repeated three times and with different dilutions of samples to avoid false results and to assure accuracy, reliability, and reproducibility.
2.10. Immunoelectrophoresis (IEP)
Immunoelectrophoresis (IEP) was performed in veronal buffer (pH 8.6, ionic force μ = 0.05) with 1.5% agarose on microscope glass slides (7.5 cm × 2.5 cm) according to the method of Scheidegger [39]. For this type of electrophoresis, the troughs were 1.3 mm wide and 18-gauge needle connected with a syringe for suction was used to create wells in the gel on the slide. A prerun of 30 min was given and after that the wells were loaded with the purified PHA (10 μL, 1 mg/mL, Sigma-Aldrich, Lesquin, France), CBBE (10 μL, 100 mg/mL) or the rat biological fluids (e.g. fecal extract (10 μL, 100 mg/mL) and sera (10 μL, neat)). After loading, the slide was placed in the Buchler electrophoresis chamber and the process was started at 400 V constant current for 50 min (panel meter), about 1 mA per slide at room temperature (ca. 25 °C) along with Whatman 3MM paper wicks. Then, the trough was separated by cutting and they were filled with rabbit anti-PHA immunoserum (10 μL, neat), and then the slides were incubated at 36 °C in a humid chamber for 20 h. Precipitation can be observed by using a down-up highlight source. Washing was given to the troughs with 0.9% PBS for 20 h and with distilled water for 3 h. After washing, the slides were dried in the incubator at 37 °C and then stained. Staining was applied with 0.1% (0.1 g/100 mL) amido black (i.e., mixture of 45% methanol and 10% acetic acid). All the IEP experiments were performed three times to ensure reproducibility and with different dilutions of specimens to assure accuracy and reliability of results.
2.11. Counterimmunoelectrophoresis (CIE)
All stool and serum specimens from BBFS-gavaged rats were screened for the presence of Phaseolus vulgaris agglutinins using the modified method of Mackenzie and Philpot [40]. In this test, the glass slides (2.5 cm × 7.5 cm) were coated to a depth 1.5 mm with 3 mL 1% Agarose GP 60 dissolved in veronal buffer (pH 8.6, ionic strength μ = 0.05). Wells of 4 mm diameter, set 2 mm apart, were cut in the gel. Neat rabbit immunoserum and antigen solutions (e.g., purified PHA, 1 mg/mL, Sigma-Aldrich, Lesquin, France; CBBE, 100 mg/mL; rat fecal extract, 100 mg/mL; neat rat serum), twenty microliters (20 μL) each were added into the wells on two opposite side, at anode and at cathode, respectively. Veronal buffer (pH 8.6, ionic strength μ = 0.05) was used as a reservoir buffer for the electrophoresis procedure. Continuous supply of current was provided for 30 min at 5 V/cm. The slides were washed for 2 days in 0.9% saline water and then dried, and later stained with Coomassie brilliant blue R. For destaining process, again the slide was washed with distilled water and then with 7% acetic acid. To assure accuracy, reliability, and reproducibility of results, the CIE experiments were replicated at least three times and were performed with different dilutions of samples.
2.12. Competitive inhibition enzyme-linked immunosorbent assays (CI-ELISA)
Competitive Inhibition ELISA (CI-ELISA) with little modification in the protocol presented by Hajós et al. [41] was implemented for the detecting the presence of PHAs in feces and sera of BBFS-challenged rats on day 10. Microtitration polystyrene plates were used for performing ELISAs (MaxiSorp, Nunc., Denmark). Briefly, the microtiter plates were sensitized with 100 μL (1 mg/mL protein concentration) of Phaseolus vulgaris agglutinins (purified PHA, Sigma-Aldrich, Lesquin, France) in 0.01 M PBS (phosphate-buffered saline, pH 7.2) overnight at 4 °C. The plates were then washed four times with 0.5% Tween 20 in PBS (PBS-T). Remaining plastic reactive sites were blocked by incubation (2 h, 37 °C) with 100 μL of bovine serum albumin (BSA) diluted at 3% with PBS and washed with PBS-T. Afterwards, each well was filled with 50 μL of rabbit anti-PHA serum diluted to 1:1500 and 50 μL of inhibitor (e.g., feces extract or serum), incubated at 37 °C for 1 h, and washed six times with PBS-T. One hundred microliters (100 μL) of peroxidase conjugated goat anti-rabbit IgG (Sigma-Aldrich, Lesquin, France) diluted 1:500 in PBS was loaded and incubated for a further 1 h at 37 °C. Upon removal of the soluble secondary antibody, the plates were washed twice with PBS-T and twice with PBS. The reaction was revealed in the dark at room temperature (ca. 25 °C) for 10 min by addition of 100 μL of o-phenylenediamine (Sigma-Aldrich, Lesquin, France) dissolved to a concentration of 0.4 mg/mL in 0.1 M citric acid phosphate buffer (pH 5.0) containing 0.0120% H2O2. Then, for stopping the reaction, 50 μL 2 N H2SO4 was added to the plate. Finally, the absorbance at 492 nm of the resulting solution was measured by an ELISA microplate reader (Axia Microreader, Biomérieux, France). For better validation of results, the samples were analyzed in triplicates and the mean of the three values was considered as result. The inhibition percentage in competitive ELISA was calculated using the following formula:
| (1) |
| OD inhibitor(i = x), absorbance measured at μg/mL inhibitor concentration. |
| OD inhibitor(i = 0), absorbance measured at zero inhibitor concentration. |
2.13. Assay of jejunal mucosal disaccharidase activities
2.13.1. Sample preparation
At the end of the experimental period (i.e., 10 days), the rats were starved for 15 h, and then anaesthetized with halothane. A 20 cm proximal jejunum loop was removed and rinsed with ice-cold Ringer’s solution. The intestinal segment was opened longitudinally on an ice-glass plate and the mucosa was then scraped off completely with a piece of glass slide in a uniform fashion. The scrapings were immediately dropped into liquid nitrogen and frozen at −80 °C until analyzed for disaccharidase activities and protein content.
2.13.2. Analysis of samples and calculation of results
Disaccharidase activities were determined by the modified method of Dahlqvist [42], using a substrate concentration of 28 mM. Briefly, the principle of this method is the following: an intestinal homogenate is incubated with the appropriate disaccharide. The disaccharidase activity is then interrupted by the addition of Tris, and the glucose liberated is measured with a glucose-oxidase peroxidase reagent. The mean weight of the mucosa was approximately 4 mg. Tissue dispersion was carried out by a Potter-Elvehjem homogenizer with a Teflon pestle (Bioblock Scientific, Illkirch-Graffenstaden, France) for 2–3 min under ice-cold conditions. This mucosal homogenate was diluted with cold distilled water to a final volume of 0.5 mL. For disaccharidase assays, 100 μL of homogenate was taken to determine lactase activity. Measurements of maltase and sucrase activities were performed by dilution of 20 μL of homogenate to 1:21 to obtain maltase activity and to 1:3 to get access to the sucrase activity. Thereafter, 25 μL of samples were mixed by vortexing and incubated with substrate-buffer solution containing 25 μL lactose, 25 μL maltose, and 25 μL sucrose at 37 °C for 60 min. The reaction was stopped by adding Tris-glucose oxidase reagent, mixed and incubated at 37 °C again for 1 h for development of color. The blanks were a mixture of diluted mucosal homogenate, Tris-glucose oxidase reagent and substrate-buffer solution, in the order mentioned, and incubated at 37 °C for 1 h. Standards were a series of glucose solutions. Samples, blanks, and standards were all read at the wavelength 450 nm on a Multiscan spectrophotometer. All assays were performed in duplicate and results represent the mean of two determinations.
The amount of glucose formed (μg) (i.e., sample minus blank) was determined from the standard curve of glucose. Thus, the activities can be calculated as follows:
| (2) |
Where μg of glucose = amount of glucose (μg) liberated in 60 min (sample-blank); F = dilution factor of the homogenate; 180 = molecular weight of the glucose; 60 = incubation time in min; N = number of molecules of glucose liberated by hydrolysis in each sugar. (The specific activities of disaccharidases were expressed as μmoles of substrate hydrolyzed per minute per mg of protein or per mg of mucosa, at 37 °C; one unit of enzymatic activity (U) hydrolyzed 1 μmoL of disaccharide per minute at 37 °C).
Protein content was determined by the method of Lowry et al. [43]. The standard curves were made with freshly dissolved bovine serum albumin (BSA). Thus, the specific disaccharidase activity was calculated as follows:
| (3) |
2.14. Statistical analysis
Results are expressed as means ± SEM. Student’s t-test was used to determine the significance of the differences between the means of the values obtained from the experiment rat group and those obtained from the control rat group. Statistical significance was established at P < 0.05.
3. Results
3.1. Hemagglutination activity (HA)
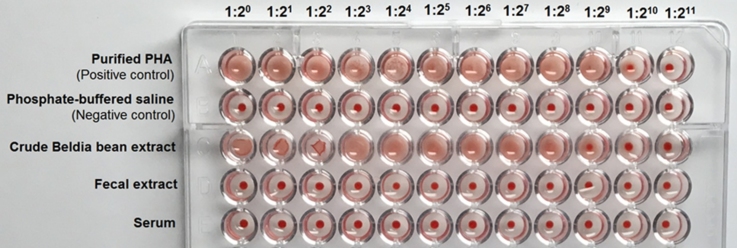
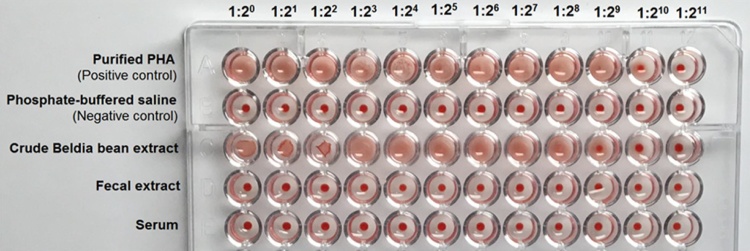
The hemagglutination assay (HA) obtained with human red cells type A at various dilutions (i.e., 1:20, 1:21, 1:22, 1:23, 1:24, 1:25, 1:26, 1:27, 1:28, 1:29, 1:210, and 1:211) of fecal extract and serum samples collected from experimental rats on day 10, are portrayed in Fig. 1. Results of agglutination test obviously indicate that none of fecal and serum dilutions showed reactivity towards the red blood cells (10 out of 10 cases). Whilst, these latter, were clumped by the purified PHA (Sigma-Aldrich, Lesquin, France) and the crude Beldia bean extract (CBBE). The data outlined in Table 1 indicates that the soluble protein content and specific activity of the CBBE were 1.5 mg/mL and 170.66 HU/mg protein, respectively. For the purified P. vulgaris lectin, the values were 1 mg/mL and 512 HU/mg protein, respectively.
Fig. 1.
Hemagglutination assays of fecal extracts (100 mg/mL) and sera obtained from experimental rats on day 10, 3 h after their gavage with Beldia bean flour suspension (BBFS), and the crude Beldia bean extract (CBBE, 100 mg/mL). Purified PHA (1 mg/mL) from Sigma-Aldrich, Lesquin, France, was used as the positive control. Phosphate-buffered saline (PBS) was used as the negative control. Each well of the microtiter U-plate contained 50 μL of sample or its dilution and 50 μL of 2% suspension of human A erythrocytes. The agglutinated red blood cells (RBCs) form a carpet that covers the whole well; where no agglutination occurs, RBCs form a button at the bottom of the well. Picture was taken 2 h after incubation at room temperature (ca. 25 °C).
Table 1.
Hemagglutination activities of the crude Beldia bean extract (CBBE) and purified PHA.
| Sample | Total proteina(mg/mL) | Hemagglutination activities |
|
|---|---|---|---|
| Titerb(HU) | Specific activityc(HU/mg) | ||
| CBBEd | 1.5 | 28 | 170.66 |
| Purified PHA | 1 | 29 | 512 |
Protein concentration was determined by Bradford assay [32], using BSA (i.e., bovine serum albumin) as a standard.
Titer (Hemagglutination unit, HU) was the reciprocal of the maximal dilution of the sample that gave visible agglutination.
Specific activity (HU/mg) was the number of titer/mg of protein [36].
Extract obtained after homogenization of 300 mg Beldia bean flour with 3 mL distilled water, centrifugation, and dilution.
3.2. Ouchterlony double immunodiffusion (ODI)
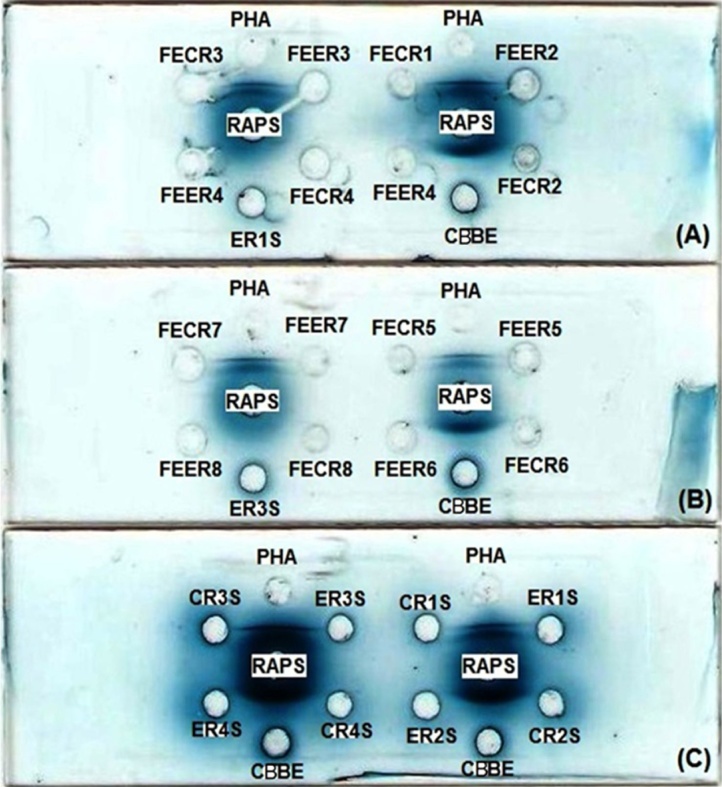
Fig. 2 pinpoints a typical gel diffusion experiments in which the neat rabbit anti-PHA serum (RAPS) was challenged against different preparations of purified PHA, crude Beldia bean extract (CBBE), neat sera, and fecal extracts obtained from control and experimental rats (CRS, ERS, FECR, and FEER, respectively). As can be seen, the commercially available phytohemagglutinins derived from red kidney beans Phaseolus vulgaris L. and the crude Beldia bean extract (CBBE) yielded precipitation lines with the rabbit anti-PHA serum (RAPS) (slides A, B, and C/Fig. 2). This cross-reaction indicates the presence of identical antigenic determinants in the reactant under study (PHAs). However, no precipitin lines were encountered in the sera and stool extracts (10 out of 10 cases).
Fig. 2.
Ouchterlony double immunodiffusion test showing the reaction between the crude Beldia bean extract (CBBE) and purified PHA (Sigma-Aldrich, Lesquin, France) with the rabbit anti-PHA serum (RAPS). The peripheral wells contained: purified PHA (10 μL, 1 mg/mL); CBBE: crude Beldia bean extract (10 μL, 100 mg/mL); FECR: fecal extract of control rat (10 μL, 100 mg/mL); FEER: fecal extract of experimental rat (10 μL, 100 mg/mL); CRS: control rat serum (10 μL, neat); ERS: experimental rat serum (10 μL, neat); whereas, the center wells charged with rabbit anti-PHA serum (RAPS, 10 μL, neat); Diffusion allowed for 1 day in a wet chamber at 24 °C; n: number of the tested rat. A, B, and C denote the slide.
3.3. Immunoelectrophoresis (IEP)
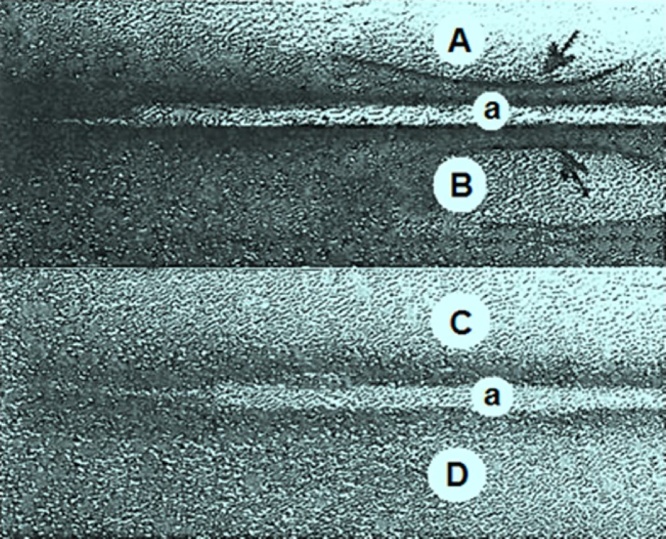
As evident from Fig. 3, a reaction of identity in the immunoelectrophoresis test was demonstrated by the occurrence of a single continuous precipitin arc between the rabbit serum and the commercial purified PHA (i.e., homogeneity of the antiserum to the antigen). The same results were observed with the CBBE. There were no reactions with the serum of BBFS-gavaged rats (10 out of 10 cases). These results indicate that the anti-PHA antibodies have been successfully produced in the PHA-immunized rabbits after the booster injection, but no or with a very low concentration in the BBFS-challenged animals. Furthermore, the feces extract failed to exhibit any cross-reactivity on immunoelectrophoresis with the anti-PHA serum (10 out of 10 cases), suggesting thereby the absence of lectins in the raw Beldia fed-rats’ stools. The results of the IEP studies were confirmed in ODI tests.
Fig. 3.
Immunoprecipitin studies. Electrophoresis was performed with a 1.5% agarose in veronal buffer, pH 8.6 at 400 V constant current for a period of 50 min. Antibody diffusion was allowed to continue for 20 h at room temperature (ca. 25 °C). Troughs contained the rabbit anti-PHA serum (a, RAPS, 10 μL, neat). Antigens wells contained the following: A. Purified PHA (10 μL, 1 mg/mL, Sigma-Aldrich, Lesquin, France); B. crude Beldia bean extract (CBBE, 10 μL, 100 mg/mL); C. Fecal extract of experimental rat (FEER, 10 μL, 100 mg/mL); D. experimental rat serum (ERS, 10 μL, neat); Characteristic immunoprecipitin lines were seen (arrows) in each case.
3.4. Counterimmunoelectrophoresis (CIE)
Fig. 4 shows the precipitin lines of identity observed immediately after electrophoresis for 30 min between the rabbits’ immunosera and the purified PHA or the CBBE. No precipitin arcs were detected between BBFS-gavaged rats’ sera and the purified PHA (10 out of 10 cases). In the same way, none of the fecal extracts developed precipitin lines in CIE against the anti-PHA rabbit immunosera (10 out of 10 cases). This reflects the existence of an antigenic determinant (phytohemagglutinin, PHA) in the CBBE and its absence in the BBFS-challenged rats’ sera and feces. The results of CIE are in consonance with those of HA, IEP, and ODI.
Fig. 4.
Counterimmunoelectrophoresis (CIE) test illustrating the precipitin lines obtained with the rabbit anti-PHA serum in the anodal wells and the purified PHA and the crude Beldia bean extract in the cathodal wells. After electrophoresis for 30 min, the precipitin lines appeared immediately. A. Purified PHA (20 μL, 1 mg/mL, Sigma-Aldrich, Lesquin, France); B. crude Beldia bean extract (CBBE, 20 μL, 100 mg/mL); C. Fecal extract of experimental rat (FEER, 20 μL, 100 mg/mL); D. experimental rat serum (ERS, 20 μL, neat); a. rabbit anti-PHA serum (RAPS, 20 μL, neat). Characteristic immunoprecipitin lines were seen (arrows) in each case.
3.5. Competitive inhibition enzyme-linked immunosorbent assay (CI-ELISA)
The CI-ELISA on microtiter plates coated with purified PHA against rabbit anti-Phaseolus vulgaris agglutinin as the primary antibody and different inhibitors (e.g., fecal extracts or sera of rats) indicated intriguing findings. The sera with titers 1:1, 1:2, and 1:4, from BBFS-gavaged rats inhibited significantly the rabbit anti-PHA antibodies binding to the purified PHA by 23.98% (P < .05), 12.99% (P < .05), and 6% (P < .05), respectively; indicating a very weak systemic immune response (possibly involving IgG) on day 10. On the other hand, the pool fecal extracts (titers 1:1, 1:2, and 1:4) did not produce any significant inhibition (P > .05). The percentage inhibition values were less than 5%. Together, these data strongly suggest the presence of anti-PHA antibodies or PHAs in the sera of BBFS-fed rats and their absence in the stools. On the basis of hemagglutination and cross-reaction studies (e.g., Ouchterlony immunodiffusion, immunoelectrophoresis, and counterimmunoelectrophoresis), it can be argued that the inhibition observed on CI-ELISA with rats’ sera was presumably caused by the anti-PHA antibodies and not by the PHAs.
3.6. Brush border disaccharidases enzymes activities
Table 2 reports the data for specific activity (U/mg of protein; U/mg of mucosa) of jejunum brush border disaccharidases after daily gavage of rats with RPFS (control group) and with BBFS (experimental group) for a period of 10 days. BBFS treatment resulted in a highly significant decrease in maltase and sucrase activities as compared with control values (P < .05). However, for lactase activity, a fairly decrease was recorded, which was not statistically significant (P > .05). It seems that lactase was less susceptible than sucrase and maltase to the adverse effect of raw Beldia bean variety.
Table 2.
Changes in specific enzymatic activities of lactase, maltase, and sucrase after 10 days of oral treatment of rats with BBFS.
| Specific enzymatic activities | Control rats (RPFS) | Experimental rats (BBFS) |
|---|---|---|
| U/mg of protein | ||
| Lactase | 1.85 ± 0.71 | 1.21 ± 0.68b |
| Maltase | 73.33 ± 24.00 | 32.00 ± 17.33a |
| Sucrase | 12.87 ± 3.58 | 4.97 ± 1.02a |
| U/mg of mucosa | ||
| Lactase | 2.75 ± 0.87 | 1.93 ± 1.06b |
| Maltase | 10.66 ± 5.33 | 5.11 ± 2.22a |
| Sucrase | 17.65 ± 2.81 | 8.12 ± 3.75a |
Values are mean ± SEM of 10 rats; Unpaired Student’s t test.
Statistically significant when compared to control, at P < .05.
Not statistically significant when compared to control, at P > .05. The specific activities of disaccharidases are expressed as μmoles of substrate hydrolyzed per minute per mg of protein or per mg of mucosa, at 37 °C. U – One unit of enzymatic activity hydrolyzed 1 μmoL of disaccharide per minute at 37 °C. RPFS, Rodent pellet flour suspension. BBFS, Beldia bean flour suspension.
4. Discussion
The ultimate goal of this physiological research was to ascertain whether the ingestion of a raw Beldia bean variety (Phaseolus vulgaris L.) that is widely consumed in Tunisia, would affect the digestion process in Wistar rat small intestine. In this investigation, no mortality or clinical signs of disease of any rats of experimental group were recorded. The Beldia bean flour suspension (BBFS), prepared at the Intestinal Immunophysiology-Research Unit, Faculty of Medicine of Tunis, is revealed to be somehow nontoxic to rats.
The results of hemagglutination test and serological assays (e.g., Ouchterlony double diffusion, immunoelectrophoresis, counterimmunoelectrophoresis, and CI-ELISA) initially highlighted that the sera as well as the fecal specimens from rats treated with BBFS may not contain any trace of PHA. This suggests that the administration of 300 mg daily upon ten days seemed inadequate for PHA to reach the systemic circulation or the fecal materials of rats.
Here, it must be stressed that these results are only preliminary and were obtained within the limits of sensitivity and specificity of each technique used. Therefore, further in-depth investigations should be conducted to bolster them. Additional research may include radio-immunoassay, immuno-PCR, mass balance, Western blot (immunoblot), etc.
Looking for lectins, the systemic circulation has been examined by a number of researchers. For instances, Pusztai and colleagues [44,45] have shown that an appreciable amounts of intact and fully reactive lectin rapidly appeared in the systemic circulation and in the liver and kidneys after oral exposure of rats to kidney bean lectin. Wang et al. [46] have announced that when humans ate 200 g whole raw peanuts, intact peanut agglutinin (PNA) was detectable in the systemic circulation within 1–4 h. Dietary lectins including both WGA (i.e., wheat germ agglutinin) and PHA have been shown to cross the gastrointestinal barrier rapidly and enter the peripheral circulation [47].
The complete missing of PHA in the systemic circulation of BBFS-gavaged rats implied that the rate of uptake of this lectin in the gastrointestinal tract was negligible than other lectins assayed in the literature. It is generally known that lectin uptake after oral administration has two major components: uptake rate and bioavailability. Uptake rate is controlled partially by the physicochemical properties of the lectin but in many cases it is modified by the diet formulation. Bioavailability is the term used to describe the fraction of the dose that is absorbed into the systemic circulation. It can range from 0 to 100% and depends on a number of physicochemical and clinic factors. Low availability may occur if the lectin has low solubility or concentration (i.e., concentration of phytohemagglutinins (i.e., macromolecules) in contact with the apical membrane of the epithelial cells) [48,49]. Changing the diet formulation can affect the bioavailability of a lectin and it can also be altered by feed (e.g., standard rodent pellet diet) or the co-administration of other components. For instance, particular sugars can reduce the uptake of lectins, such as N-acetylglucosamine, galactose, and mannose residues, by binding PHAs in the gut [50,51]. Other factors influencing bioavailability include metabolism by gut flora, the intestinal wall or the liver.
Hemagglutination assay of the current study showed that the fecal extracts of rats fed by gavage with BBFS for 10 days were devoid of any agglutinating activity towards red blood cells group A. This signifies that the feces of these animals may not include the P. vulgaris agglutinins. The results obtained from the present set of experiments, did not support previous findings that the hemagglutinating activity could be detected in the feces of rats after they had been fed raw black beans or kidney beans [52]. In some experiments, feces from rats fed on known amounts of pure lectin were found by rocket immunoelectrophoresis to contain up to 90% of the ingested lectin still fully reactive towards rabbit antilectin antibodies [53]. Using other different kinds of lectins, the studies by Brady et al. [54] showed that biologically intact wheat germ agglutinin (WGA) was detected in ileostomy effluent and fecal collections from human subjects consuming a diet containing wheat germ. Experiments by Kilpatrick and co-investigators [55] demonstrated that rats fed a tomato lectin (LEA)-rich diet passed feces containing serologically detectable tomato lectin.
Since feces reported elsewhere were characterized upon their immuno-hemagglutination reactivities, it is very likely that the phytohemagglutinins (PHAs) were eliminated into the stools, but before day 10 (i.e., material collection day). Our preliminary immunobiochemical studies substantially evidenced the presence of bioactive PHAs in the jejunum and ileum of raw Beldia beans-fed rats [30]. However in the present study, no lectins were found in the feces and sera of animals on day 10 after 3 h of oral intubation. These results, when taken together suggest very strongly that the P. vulgaris lectins are still bound to the glycosylated receptors occurring on the surface of the epithelial cells and have not yet reached neither the systemic circulation nor the fecal material. Hara and his co-investigators offered a similar interpretation and postulated that considerable amounts of the Kintoki bean lectins were bound to the small intestinal mucosa of rat and remained there for a relatively long period [56].
The behavior of ingested PHA in the gastrointestinal tract can be illustrated by the following scenario: After consecutive oral gavages of the rats with the Beldia bean flour suspension (BBFS), 1. The ingested PHA inertly passes through the esophagus to the stomach lumen. 2. The PHA enters the small intestine and binds to the luminal surface, thereby causing morphological and functional disturbances of the brush border membrane [29]. 3. As a result of the continuous turnover of the epithelium, the attached PHA is shed off together with mature epithelial cells into the lumen. 4. The free PHA is transferred functionally unaltered to the large intestine, and it avidly binds to different components of intestinal tissues (i.e., the next receptor with an appropriate carbohydrate moiety) [57]. 5. Then, the PHA is directly excreted in the feces before day 10.
It is noteworthy that most of phytolectins require a well-defined period of time to occur in the feces, depending on their concentration, type (i.e., different isolectins may exist), and nature (i.e., pure or crude), bean variety or cultivar, status of binding sites in the gut lumen (e.g., vacant or occupied), glycosylation state of the gut, pH and temperature in the gastrointestinal tract, etc. For examples, the lectins of Green peas (Pisum sativum) and Kintoki bean (Phaseolus vulgaris) can be recovered from feces only over a period of 24 h of intubation [56,58]. In the case of Jack bean (Canavalia ensiformis), appreciable amounts of ingested concanavalin A (ConA) could be recovered intact and biologically active from the feces (ca. 90% recovery), 4 days after exposure [59]. Following oral intake in rats, the concentration of lectins from Tepary bean seeds (Phaseolus acutifolius) increased slightly in the feces on day 1 and then sharply on day 3. Afterwards, it declined on day 4 and became insignificant on day 5 [60]. On the basis of these observations and the available scientific literature, it can be logically assumed that the lectins from raw Beldia beans were released into the stools before day 10 and will be ejected again after further feedings (i.e., after day 10). However, in order to ascertain this hypothesis, further careful works remain to be undertaken.
In general, disaccharidase deficiency is clinically characterized by the development of diarrhea, abdominal cramps, and flatulence after the ingestion of diet. The stools are usually frothy or watery in appearance [61]. In this study, the BBFS-treated animals appeared healthy and active, and their fecal pellets were normal, except they were suffering from hydroelectrolytic losses (e.g., water, sodium, and potassium) into the jejunal lumen [29].
The most striking finding of this investigation is that a chronic treatment of growing rats with 300 mg of raw BBFS for 10 subsequent days was associated with decrease in the specific and total activity of brush border disaccharidases. The specific maltase and sucrase activities (U/mg of protein; U/mg of mucosa) decreased significantly in the jejunum of orogastrically administered-ground raw beans rats (P < .05). However, the specific lactase activity in the jejunum remained virtually unchanged after gavage (P > .05). These results coincide somewhat with the data reported by Roe and his group [62] who stated when male adult rats fed crude red kidney beans (RKB) for 15 days, their intestinal sucrase activities were significantly decreased than those in the control group in all small intestinal segments (e.g., proximal, middle, and distal). However, their lactase activities were declined significantly only in the proximal and middle segments compared with the control group values. Also, Song and coworkers [63] found that the enzymes activities in the small intestinal mucosa such as; sucrase, lactase, aminopeptidase, and alkaline phosphatase were depressed in male adult rats, after receiving red kidney bean (RKB) diet for 2 weeks. On the other hand, the studies by Rådberg et al. [64] clearly showed that the gavage of suckling pigs, by stomach tube a crude red kidney bean lectin preparation at 10, 11, 12 day of life induced a change in small intestinal disaccharidase activities, with increased maltase and sucrase activities. Whereas, the activity of lactase was not significantly changed.
Several general factors, most likely acting in concert, may well be responsible for the variability in the activity of disaccharide hydrolases; such as, the concentration and the nature or type of lectins present in the kidney bean dietary, bean variety, dose or route of administration of lectins, animal species, duration of treatment, and physiological factors (e.g., T °C, pH, etc.).
Different mechanisms may enter into the disaccharidase deficiency encountered in BBFS-challenged animals. The precise mechanism responsible for the impairment in the digestive capacity of gut is still unclear and poorly understood, but seems to be linked with profound ultrastructural and morphological changes in the small intestine and even the intraluminal proliferation of bacteria induced by the ingestion of raw kidney beans.
In connection with our ultrastructural investigations [29], it was found that when rats were subjected to oral gavage treatment using a 300 mg raw BBFS for 10 days resulted in an extensive disruption of the intestinal microvilli included disorganization of the terminal web. The microvilli were abnormally short and dense or long and thin accompanied with shedding of vesicles derived from the apical brush border membrane. Such disruption would interfere undoubtedly with the disturbances of brush border enzymes activities.
Furthermore, it was evident from our histological and morphometric data [29] that BBFS supplementation has significantly increased the villous height, crypt depth, and crypt depth/villus height ratio (i.e., mucosal hyperplasia) in the jejunum that would increase in turn the surface area for nutrient absorption and digestion. Unexpectedly, it was observed that the water and electrolytes (e.g., sodium and potassium) uptakes were significantly depressed in BBFS-treated rats compared with those of controls. Simultaneously, a depression of jejunal disaccharidases activities was recorded in this in vitro physiological study. Since no obvious injuries were detected in the intestinal mucosa, the pronounced drop in maltase and sucrase activities and the stability of lactase activity could be interpreted as follows: the fast increased turnover of cells in the crypts and the migration of immature cells to the villi could abnormally disrupt the intestinal brush border enzymes synthesis [65] and the process of absorption as well [66].
Regarding the bacteriological factor, it is well recognized that the inclusion of raw kidney beans (P. vulgaris L.) containing considerable amount of lectins in the diet of rat could lead in a dramatic overgrowth of coliform bacteria mainly type 1-mannose sensitive fimbriated Escherichia coli in the small intestine. This factor plays an essential role in the manifestation of kidney bean toxicity in rats [67]. Indeed, it can adversely affect the digestion and absorption of nutrients [13]. This concurs very well with the results obtained from the current and our previous studies [29,68]. Bacterial overgrowth of the small intestine appears to involve adherence of sugar binding proteins (lectins) of the microorganism to specific oligosaccharides of the microvillus membrane [69,70]. Brush border enzyme activities may then be reduced by the action of bacterial proteases and toxins and this may contribute to the malabsorption.
An additional toxic effect of ingested raw Beldia bean variety, which may further reduce the efficiency of sugar digestion, is anti-nutritional factors (ANF). Poor acceptability and digestibility, enzymes inhibitors (e.g., trypsin, chymotrypsin, and α-amylase), and lectins [71] could be responsible, but there is overwhelming evidence that the latter are at least one of the major causes. Since, an extensive mucosal hyperplasia was revealed along the jejunum and ileum of the rat small intestine [29], the damage noted on the disaccharidases activities could be attributed largely to the potent mitogens (i.e., growth factors); phytohemagglutinins (PHAs) [11], which were detected in immunologically intact form in the jejunal lumen, 3 h and 30 min after the orogastric gavage with BBFS [30].
Despite their toxicological drawbacks, kidney beans (Phaseolus vulgaris) containing lectins have several health benefits, if taken carefully. Live human population research and within the laboratory setting investigations demonstrate that the phytohemagglutinins (PHAs) have protective effects against DNA damage [[72], [73], [74]], and are potent modulators of immune response [75], cell growth [76,77], and mitosis [78,79], and can cause cancer regression [[80], [81], [82]]. These results further confirm the importance of bean as a functional food due, in part, to the presence of bioactive lectins [83,84].
5. Conclusion
The present investigation provides valuable insights into the impact of chronic ingestion of raw Beldia beans (Phaseolus vulgaris L.) on the brush border disaccharidase activities in Wistar rats, with an in-depth evaluation of underling mechanisms as well as factors of pathogenicity on a molecular and cellular level. After 10 days of dietary exposure, there were no apparent clinical signs of infection. Orogastric intubation studies clearly showed that a significant decrease in maltase and sucrase activities of the jejunal mucosa was generated, but no effect on lactase activity was noted. Serological techniques and hemagglutination assay initially confirmed the absence of phytohemagglutinins (PHAs) in the systemic circulation and feces of rats. However, further experiments are necessary to definitively prove this. The main key finding from this research is when raw or undercooked Beldia bean consumed excessively by animals or humans may induce a staggered loss of disaccharidase activities beginning with the loss of maltase and sucrase activities in the small intestine.
Acknowledgments
Financial support from the Intestinal Immunophysiology-Research Unit (02/RU/09-02), Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia, is gratefully recognized.
Contributor Information
Nader Nciri, Email: nader.nciri@koreatech.ac.kr.
Namjun Cho, Email: njuncho@koreatech.ac.kr.
References
- 1.Evans R.J., Pusztai A., Watt W.B., Bauer D.H. Isolation and properties of protein fractions from navy beans (Phaseolus vulgaris) which inhibit growth of rats. Biochim. Biophys. Acta. 1973;303:175–184. doi: 10.1016/0005-2795(73)90159-1. [DOI] [PubMed] [Google Scholar]
- 2.Jayne-Williams D.J., Burgess C.D. Further observations on the toxicity of navy beans (Phaseolus vulgaris) for japanese quail (Coturnix coturnix japonica) J. App. Bacteriol. 1974;37:149–169. doi: 10.1111/j.1365-2672.1974.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 3.Jaffé W.J. Hemagglutinins (lectins) In: Liener I.E., editor. Toxic Constituents of Plant Foodstuffs. 2nd ed. Academic Press; New York: 1980. pp. 73–102. [Google Scholar]
- 4.King T.P., Begbie R., Cadenhead A. Nutritional toxicity of raw kidney beans in pigs. Immunocytochemical and cytopathological studies on the gut and the pancreas. J. Sci. Food Agric. 1983;34:1404–1412. doi: 10.1002/jsfa.2740341214. [DOI] [PubMed] [Google Scholar]
- 5.Haidvogl M., Fritsh G., Grubauer H. Acute poisoning with green beans (phaseolus vulgaris and phaseolus coccineus) Padiatr. Padol. 1979;14:293–296. [PubMed] [Google Scholar]
- 6.Rodhouse J.C., Haugh C.A., Roberts D., Gilbert R.J. Red kidney bean poisoning in the UK: an analysis of 50 suspected incidents between 1976 and 1989. Epidemiol. Infect. 1990;105:485–491. doi: 10.1017/s095026880004810x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janzen D.H., Juster H.B., Liener I.E. Insecticidal action of the phytohemagglutinin in black beans on the bruchid beetle. Science. 1976;192:795–796. doi: 10.1126/science.1265481. [DOI] [PubMed] [Google Scholar]
- 8.Williams P.E.W., Pusztai A., MacDearmid A., Innes G.M. The use of kidney beans (Phaseolus vulgaris) as protein supplements in diets for rapidly growing beef steers. Anim. Feed Sci. Techol. 1984;12:1–10. [Google Scholar]
- 9.Andrews A.T., Jayne-Williams D.J. The identification of a phytohemagglutinin in raw navy beans (Phaseolus vulgaris L.) toxic for the Japanese quail (Coturnix coturnix japonica) Br. J. Nutr. 1974;32:181–188. doi: 10.1079/bjn19740068. [DOI] [PubMed] [Google Scholar]
- 10.Patel R.K., Roy A. Comparative evaluation of the effect of various lectins on short term lymphocyte culture in cattle and buffalo. J. Bio. Innov. 2013;2:1–4. [Google Scholar]
- 11.Pusztai A. Cambridge University Press; Cambridge, UK: 1991. Plant Lectins; p. 272. [Google Scholar]
- 12.Pusztai A., Clarke E.M.W., King T.P. The nutritional toxicity of Phaseolus vulgaris lectins. Proc. Nutr. Soc. 1979;38:115–120. doi: 10.1079/pns19790015. [DOI] [PubMed] [Google Scholar]
- 13.King T.P., Pusztai A., Clarke E.M.W. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in rat small intestine: 1. Light microscope studies. J. Comp. Path. 1980;90:585–595. doi: 10.1016/0021-9975(80)90107-3. [DOI] [PubMed] [Google Scholar]
- 14.King T.P., Pusztai A., Clarke E.M.W. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in rat small intestine: 3. Ultrastructural studies. J. Comp. Path. 1982;92:357–373. doi: 10.1016/0021-9975(82)90021-4. [DOI] [PubMed] [Google Scholar]
- 15.Pusztai A., Grant G., Spencer R.J., Duguid T.J., Brown D.S., Ewen S.W., Peumans W.J., Van Damme E.J., Bardocz S. Kidney bean lectin-induced Escherichia coli overgrowth in the small intestine is blocked by GNA, a mannose-specific lectin. J. Appl. Bacteriol. 1993;75:360–368. doi: 10.1111/j.1365-2672.1993.tb02788.x. [DOI] [PubMed] [Google Scholar]
- 16.De Oliveira J.T.A., Pusztai A., Grant G. Changes in organs and tissues induced by feeding of purified kidney bean (Phaseolus vulgaris) lectins. Nutr. Res. 1988;8:943–947. [Google Scholar]
- 17.Pusztai A., Ewen S.W.B., Grant G., Peumans W.J., Van Damme E.J.M., Rubio L.M., Bardocz S. Plant food lectins as molecular signal molecules. In: Kilpatrick D.C., Driessche van E., Bog-Hasen T.C., editors. vol. 1. Sigma Library; St. Louis, Missouri, USA: 1992. pp. 1–15. (Effects on the Morphology and Bacterial Ecology of the Small Intestine, Lectin Reviews). [Google Scholar]
- 18.Todd E., Pivnick H., Pivnick K. Illness from kidney beans. Can. Dis. Wkly. Rep. 1980;6:101–103. [Google Scholar]
- 19.Tan A. Non-bacterial food poisoning. Commun. Dis. Intell. 1982;82:7. [Google Scholar]
- 20.Venter F.S., Thiel P.G. Red kidney beans: to eat or not to eat? S. Afr. Med. J. 1995;85:250–252. [PubMed] [Google Scholar]
- 21.Griebel C. Erkrankungen durch Bohnenflocken (Phaseolus vulgaris L.) und Platterbsen (Lathyrus tingitanus Z.) [Illness caused by bean flakes (Phaseolus vulgaris L.) and flat peas (Lathyrus tingitanus Z.)] Zeitschrift für Lebensmittel-Untersuchung und Forschung. 1950;90:191–197. [Google Scholar]
- 22.Korte R. Heat resistance of phytohemagglutinins in weaning food mixtures containing beans (Phaseolus vulgaris) Ecol. Food Nutr. 1972;1:303–307. [Google Scholar]
- 23.Noah N.D., Bender A.E., Reaidi G.B., Gilbert R.J. Food poisoning from raw red kidney beans. Br. Med. J. 1980;281:236–237. [PMC free article] [PubMed] [Google Scholar]
- 24.Bender A.E., Reaidi G.B. Toxicity of kidney beans (Phaseolus vulgaris) with particular reference to lectins. J. Plant Foods. 1982;4:15–22. [Google Scholar]
- 25.Bender A.E. Hemagglutinins (lectins) in beans. J. Food Chem. 1983;4:309–320. [Google Scholar]
- 26.Foodborne Pathogenic Microorganisms and Natural Toxins Handbook (Bad Bug Book) Center for Food Safety & Applied Nutrition, U.S. Food & Drug Administration; 1992. CFSAN: phytohemagglutinin. http://www.cfsan.fda.gov/∼mow/chap43.html. (Accessed 15 November 2016) [Google Scholar]
- 27.Food and Agriculture Organization of the United Nations (FAOSTAT). http://www.faostat3.fao.org (Accessed 15 November 2016).
- 28.Nciri N., Cho N., El Mhamdi F., Ben Ismail H., Ben Mansour A., Haj Sassi F., Ben Aissa-Fennira F. Toxicity assessment of common beans (Phaseolus vulgaris L.) widely consumed by tunisian population. J. Med. Food. 2015;18:1049–1064. doi: 10.1089/jmf.2014.0120. [DOI] [PubMed] [Google Scholar]
- 29.Nciri N., Cho N., Bergaoui N., El Mhamdi F., Ben Ammar A., Trabelsi N., Zekri S., Guémira F., Ben Mansour A., Haj Sassi F., Ben Aissa-Fennira F. Effect of white kidney beans (Phaseolus vulgaris L. var. beldia) on small intestine morphology and function in wistar rats. J. Med. Food. 2015;18:1387–1399. doi: 10.1089/jmf.2014.0193. [DOI] [PubMed] [Google Scholar]
- 30.Nciri N., Cho N., El Mhamdi F., Ben Mansour A., Haj Sassi F., Ben Aissa-Fennira F. Identification and characterization of phytohemagglutinins from white kidney beans (Phaseolus vulgaris L., var beldia) in the rat small intestine. J. Med. Food. 2016;19:85–97. doi: 10.1089/jmf.2014.0194. [DOI] [PubMed] [Google Scholar]
- 31.Itoh M., Kondo K., Komada H., Izutsu K., Shimabayashi Y., Takahashi T. Purification and characterization of a lectin from Phaseolus vulgaris seed. Agric. Biol. Chem. Tokyo. 1980;44:125–133. [Google Scholar]
- 32.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Helrich K. vol. 1. Association of Official Analytical Chemists, Inc.; Arlington, Virginia, USA: 1990. (AOAC: Official Methods of Analytical Chemists). p. 771. [Google Scholar]
- 34.Weinman M.D., Allan C.H., Trier J.S., Hagen S.J. Repair of microvilli in the rat small intestine after damage with lectins contained in the red kidney bean. Gastroenterology. 1989;97:1193–1204. doi: 10.1016/0016-5085(89)91690-9. [DOI] [PubMed] [Google Scholar]
- 35.Hagen S.J., Trier J.S., Dambrauskas R. Exposure of the rat small intestine to raw kidney beans results in reorganization of absorptive cell microvilli. Gastroenterology. 1994;106:73–84. doi: 10.1016/s0016-5085(94)94465-2. [DOI] [PubMed] [Google Scholar]
- 36.Wong J.H., Ng T.B. Purification of a trypsin-stable lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activity. Biochem. Biophys. Res. Commun. 2003;301:545–550. doi: 10.1016/s0006-291x(02)03080-2. [DOI] [PubMed] [Google Scholar]
- 37.Osunkoya B.O., Williams A.I., Alder W.H., Smith R.T. Studies on the interaction of phytomitogens with lymphoid cells. I. Binding of phytohemagglutinin to cell membrane receptors of cultured Burkitt’s lymphoma and infectious mononucleosis cells. Afr. J. Med. Med. Sci. 1970;1:3–16. [PubMed] [Google Scholar]
- 38.Ouchterlony O. Antigen-antibody reactions in gels. Acta Pathol. Microbiol. Scand. 1949;26:507–515. doi: 10.1111/j.1699-0463.1949.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 39.Scheidegger J.J. A micro-method of immuno-electrophoresis [Une micro-méthode de l’immuno-életrophorèse] Int. Arch. Allergy. 1955;7:103–110. [PubMed] [Google Scholar]
- 40.Mackenzie D.W.R., Philpot C.M. Counterimmunoelectrophoresis as a routine mycoserological procedure. Mycopathologia. 1975;57:1–7. doi: 10.1007/BF00431169. [DOI] [PubMed] [Google Scholar]
- 41.Hajós G., Gelencsér E., Pusztai A., Grant G., Sakhri M., Bardocz S. Biological effects and survival of trypsin inhibitors and the agglutinin from soybean in the small intestine of the rat. J. Agric. Food Chem. 1995;43:165–170. [Google Scholar]
- 42.Dahlqvist A. Assay of intestinal disaccharidases. Anal. Biochem. 1968;22:99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- 43.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 44.Pusztai A., Clarke E.M.W., Grant G., King T.P. The toxicity of Phaseolus vulgaris lectins. Nitrogen balance and immunochemical studies. J. Sci. Food Agric. 1981;32:1037–1046. doi: 10.1002/jsfa.2740321014. [DOI] [PubMed] [Google Scholar]
- 45.Pusztai A. Transport of proteins through the membranes of the adult gastro-intestinal tract-a potential for drug delivery? Adv. Drug. Deliv. Rev. 1989;3:215–228. [Google Scholar]
- 46.Wang Q., Yu L.G., Campbell B.J., Milton J.D., Rhodes J.M. Identification of intact peanut lectin in peripheral venous blood. Lancet. 1998;352:1831–1832. doi: 10.1016/S0140-6736(05)79894-9. [DOI] [PubMed] [Google Scholar]
- 47.Pusztai A., Greer F., Grant G. Specific uptake of dietary lectins into the circulation of rats. Biochem. Soc. Trans. 1989;17:481–482. [Google Scholar]
- 48.Vellenga L., Mouwen J.M., Van Dijk J.E., Breukink H.J. Biological and pathological aspects of the mammalian small intestinal permeability to macromolecules. Vet. Q. 1985;7:322–332. doi: 10.1080/01652176.1985.9694006. [DOI] [PubMed] [Google Scholar]
- 49.Kik J.M., Rojer J.M.V.M., Koninkx J.F.J.G., van Dijk J.E., van der Hage M.H. The interaction between plant lectins and the small intestinal epithelium: a primary cause of intestinal disturbance. Vet. Q. 1989;11:108–115. doi: 10.1080/01652176.1989.9694207. [DOI] [PubMed] [Google Scholar]
- 50.Hamelryck T.W., Dao-Thi M.H., Poortmans F., Chrispeels M.J., Wyns L., Loris R. The crystallographic structure of phytohemagglutinin-L. J. Biol. Chem. 1996;271:20479–20485. doi: 10.1074/jbc.271.34.20479. [DOI] [PubMed] [Google Scholar]
- 51.Hamid R., Masood A. Dietary lectins as disease causing toxicants. Pak. J. Nutr. 2009;8:293–303. [Google Scholar]
- 52.Jaffé W.G., Vega Lette C.L. Heat-labile growth-inhibiting factors in beans (Phaseolus vulgaris) J. Nutr. 1968;94:203–210. doi: 10.1093/jn/94.2.203. [DOI] [PubMed] [Google Scholar]
- 53.Pusztai A. Nutritional toxicity of the kidney bean (Phaseolus vulgaris), Rowett. Res. Inst. Annu. Rep. 1980;36:110–118. [Google Scholar]
- 54.Brady P.G., Vannier A.M., Banwell J.G. Identification of the dietary lectin, wheat germ agglutinin, in human intestinal contents. Gastroenterology. 1978;75:236–239. [PubMed] [Google Scholar]
- 55.Kilpatrick D.C., Pusztai A., Grant G., Graham C., Ewen S.W. Tomato lectin resists digestion in the mammalian alimentary canal and binds to intestinal villi without deleterious effects. FEBS Lett. 1985;185:299–305. doi: 10.1016/0014-5793(85)80927-3. [DOI] [PubMed] [Google Scholar]
- 56.Hara T., Mukunoki Y., Tsukamoto I., Miyoshi M., Hasegawa K. Susceptibility of Kintoki bean lectin to digestive enzymes in vitro and its behavior in the digestive organs of mouse in vivo. J. Nutr. Sci. Vitaminol. 1984;30:381–394. doi: 10.3177/jnsv.30.381. [DOI] [PubMed] [Google Scholar]
- 57.Pusztai A., Ewen S.W.B., Grant G., Peumans W.J., Van Damme E.J.M., Rubio L., Bardocz S. Relationship between survival and binding of plant lectins during small intestine passage and their effectiveness as growth factors. Digestion. 1990;46:308–316. doi: 10.1159/000200402. [DOI] [PubMed] [Google Scholar]
- 58.Le Gall M., Quillien L., Sève B., Guéguen J., Lallès J.P. Weaned piglets display low gastrointestinal digestion of pea (Pisum sativum L.) lectin and pea albumin 2. J. Anim. Sci. 2007;85 doi: 10.2527/jas.2006-795. 2972–2781. [DOI] [PubMed] [Google Scholar]
- 59.Nakata S., Kimura T. Effect of ingested toxic bean lectins on the gastrointestinal tract in the rat. J. Nutr. 1985;115:621–1629. doi: 10.1093/jn/115.12.1621. [DOI] [PubMed] [Google Scholar]
- 60.Ferriz-Martínez R., García-García K., Torres-Arteaga I., Rodriguez-Mendez A.J., Guerrero-Carrillo M.D.J., Moreno-Celis U., Ángeles-Zaragoza M.V., Blanco-Labra A., Gallegos-Corona M.A., Robles-Álvarez J.P., Mendiola-Olaya E., Andrade-Montemayor H.M., Garcia O.P., Garcia-Gasca T. Tolerability assessment of a lectin fraction from Tepary bean seeds (Phaseolus acutifolius) orally administered to rats. Toxicol. Rep. 2015;2:63–69. doi: 10.1016/j.toxrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irmiter T.F. Diarrhea caused by disaccharidase deficiency. Nutr. Rev. 1964;22:43–45. doi: 10.1111/j.1753-4887.1964.tb07748.x. [DOI] [PubMed] [Google Scholar]
- 62.Roe I.H., Kim N.Y., Yoo K., Jung H.C., Lee H.S., Yoon Y.B., Song I.S., Choi K.W., Kim C.Y. The effect of red kidney bean on the small intestinal mucosa of rat. Korean J. Intern. Med. 1989;37:747–758. [Google Scholar]
- 63.Song I.S., Roe I.H., Kim C.Y. The reversibility of the effect of red kidney bean on the small intestinal mucosa of rat. Korean J. Intern. Med. 1991;40:308–317. [Google Scholar]
- 64.Rådberg K., Biernat M., Linderoth A., Zabielski R., Pierzynowski S.G., Weström B.R. Enteral exposure to crude red kidney bean lectin induces maturation of the gut in suckling pigs. J. Anim. Sci. 2001;79:2669–2678. doi: 10.2527/2001.79102669x. [DOI] [PubMed] [Google Scholar]
- 65.Tajiri H., Klein R.M., Lebenthal E., Lee P.C. Oral feeding of isolated lectins from red kidney bean stimulates rat small intestinal mucosal DNA synthesis and crypt cell division. Dig. Dis. Sci. 1988;33:1364–1369. doi: 10.1007/BF01536989. [DOI] [PubMed] [Google Scholar]
- 66.Rossi M.A., Filho J.M., Lajolo F.M. Jejunal ultrastructural changes induced by kidney bean (Phaseolus vulgaris) lectins in rats. Br. J. Exp. Pathol. 1984;65:117–123. [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson A.B., King T.P., Clarke E.M.W., Pusztai A. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in rat small intestine: 2. Microbiological studies. J. Comp. Pathol. 1980;90:597–602. doi: 10.1016/0021-9975(80)90108-5. [DOI] [PubMed] [Google Scholar]
- 68.Nciri N. Faculty of Medicine of Tunis, University of Tunis El Manar; Tunisia: 2010. Effects of White Kidney Beans (Phaseolus Vulgaris L. Var. Beldia) Exposure on Food Intake, Growth Performance, Internal Organs, and Fecal Microflora of Rats, Master Thesis. [Google Scholar]
- 69.Jones G.W., Freter R. Adhesive properties of Vibrio Cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect. Immun. 1976;14:240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheney C.P., Boedeker E.C. Adherence of an entero-toxigenic Escherichia coli strain serotype 078:H11, to purified human intestinal brush borders. Infect. Immun. 1983;39:1280–1284. doi: 10.1128/iai.39.3.1280-1284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyimo M., Mugula J., Elias T. Nutritive composition of broth from selected bean varieties cooked for various periods. J. Sci. Food Agric. 1992;58:535–539. [Google Scholar]
- 72.Bertazzoni U., Stefanini M., Noy G.P., Giulotto E., Nuzzo F., Falaschi A., Spadari S. Variations of DNA polymerases-α and −β during prolonged stimulation of human lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1976;73:785–789. doi: 10.1073/pnas.73.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lavin M.F., Kidson C. Repair of ionizing radiation induced DNA damage in human lymphocytes. Nucleic Acids Res. 1977;4:4015–4022. doi: 10.1093/nar/4.11.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prindull G., Prindull B., Schröter W., Yoffey J.M. Comparison of RNA and DNA synthesis spontaneous and PHA induced, between blood lymphoid cells of newborn infants, older infants, and adults. Eur. J. Pediatr. 1977;126:243–252. doi: 10.1007/BF00477050. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan M.S., Mino F.O., Kummerfled K.B., Lundak R.L. Phytohemagglutinin-stimulated immune response. Assay in colorectal carcinoma patients. Arch. Surg. 1975;110:1217–1220. doi: 10.1001/archsurg.1975.01360160055008. [DOI] [PubMed] [Google Scholar]
- 76.Harris G., Littleton R.J. The effects of antigens and of phytohemagglutinin on rabbit spleen cell suspensions. J. Exp. Med. 1966;124:621–634. doi: 10.1084/jem.124.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L., Wang C., Yang M., Zhang T., Wang M. Effect of phytohemagglutinin (PHA) from Yunnan white kidney bean on development of mouse embryos. Zhongguo Zhong. Yao. Za. Zhi. 2011;36:1665–1669. [PubMed] [Google Scholar]
- 78.Nowell P.C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462–466. [PubMed] [Google Scholar]
- 79.Bishun N.P., Morton W.R.M., McLaverty B. The effect of phytohemagglutinin in vivo on the mitotic activity of the bone marrow cells in young rats. Exprientia. 1965;21:527–528. doi: 10.1007/BF02138975. [DOI] [PubMed] [Google Scholar]
- 80.Okuda M., Sakaguchi K., Tomiyama S., Takahashi M. Use of phytohemagglutinin in the treatment of maxillary cancer. Arch. Otorhinolaryngol. 1980;228:127–134. doi: 10.1007/BF00455340. [DOI] [PubMed] [Google Scholar]
- 81.Pryme I.F., Pusztai A.J., Bardocz S. A diet containing the lectin phytohemagglutinin (PHA) shows down the proliferation of Krebs II cell tumours in mice. Cancer Lett. 1994;76:133–137. doi: 10.1016/0304-3835(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 82.Chen K.Y., Kuo W.T., Ho Y.J., Yao C.H. Cytotoxic effect and apoptosis induction by phytohemagglutinin erythroagglutinating on lung cancer cells. Eur. J. Cancer. 2014;50:S23–S242. [Google Scholar]
- 83.Reynoso-Camacho R., González de Mejía E., Loarca-Piña G. Purification and acute toxicity of a lectin extracted from tepary bean (Phaseolus acutifolius) Food Chem. Toxicol. 2003;41:21–27. doi: 10.1016/s0278-6915(02)00215-6. [DOI] [PubMed] [Google Scholar]
- 84.Azevedo L., Gomes J.C., Stringheta P.C., Á. Gontijo M.M.C., Padovani C.R., Ribeiro L.R., Salvadori D.M.F. Black bean (Phaseolus vulgaris L.) as a protective agent against DNA damage in mice. Food Chem. Toxicol. 2003;41:1671–1676. doi: 10.1016/s0278-6915(03)00173-x. [DOI] [PubMed] [Google Scholar]