ABSTRACT
Body weight is an important trait to confirm growth and development in humans and animals. In Thoroughbred racehorses, it is measured in the postnatal, training, and racing periods to evaluate growth and training degrees. The body weight of mature Thoroughbred racehorses generally ranges from 400 to 600 kg, and this broad range is likely influenced by environmental and genetic factors. Therefore, a genome-wide association study (GWAS) using the Equine SNP70 BeadChip was performed to identify the genomic regions associated with body weight in Japanese Thoroughbred racehorses using 851 individuals. The average body weight of these horses was 473.9 kg (standard deviation: 28.0) at the age of 3, and GWAS identified statistically significant SNPs on chromosomes 3 (BIEC2_808466, P=2.32E-14), 9 (BIEC2_1105503, P=1.03E-7), 15 (BIEC2_322669, P=9.50E-6), and 18 (BIEC2_417274, P=1.44E-14), which were associated with body weight as a quantitative trait. The genomic regions on chromosomes 3, 9, 15, and 18 included ligand-dependent nuclear receptor compressor-like protein (LCORL), zinc finger and AT hook domain containing (ZFAT), tribbles pseudokinase 2 (TRIB2), and myostatin (MSTN), respectively, as candidate genes. LCORL and ZFAT are associated with withers height in horses, whereas MSTN affects muscle mass. Thus, the genomic regions identified in this study seem to affect the body weight of Thoroughbred racehorses. Although this information is useful for breeding and growth management of the horses, the production of genetically modified animals and gene doping (abuse/misuse of gene therapy) should be prohibited to maintain horse racing integrity.
Keywords: body weight, LCORL, MSTN, Thoroughbred, ZFAT
The Thoroughbred was developed in the early 18th century, and all modern Thoroughbred racehorses can be traced back to a small number of Arabian, Barb, and Turkmen stallions and many native British mares living approximately 300 years ago [1, 4, 31]. At present, about 100,000 foals are born worldwide every year, and almost all are used as racehorses.
Thoroughbred racehorses have been bred for improved racing ability, such as speed and stamina, which has led to variations in morphological traits such as body weight. This characteristic is used for confirming growth in humans and animals, and in Thoroughbred racehorses, it is measured at several occasions including postnatal, training, and racing periods to confirm growth and training degrees [13,14,15,16].
The body weight of mature Thoroughbred racehorses generally ranges from 400 to 600 kg. In Japanese Thoroughbred racehorses, the average body weight at a horse’s first start (897 days) is 455 kg [10], whereas the average body weights of Korean Thoroughbreds are 460, 454, and 441 kg for stallions, geldings, and mares, respectively [2]. Several genetic and environmental factors, such as sire, sex, age, and facility, affect body weight in this breed [10].
Heritability estimates of body weight in no-defined-breed horses belonging to the Brazilian army cavalry and in Japanese Thoroughbred racehorses were 0.40 ± 0.034 and 0.27 ± 0.04, respectively [10, 22]. In Korean Thoroughbred racehorses, heritability estimates of body weight were 0.578, 0.472, and 0.555 for stallions, geldings, and mares, respectively [2]. Thus, genetic factors appear to contribute to the individual differences in body weight in Thoroughbred racehorses. Recently, horse genome mapping has significantly advanced, and with the completion of the Horse Genome Project [29], a single nucleotide polymorphisms (SNPs) map containing more than one million markers is now available. This allows a genome-wide association study (GWAS) that facilitates the discovery of genetic traits that are of interest to breeders, trainers, and owners.
Although body weight is a highly complex trait and its genetic basis is not well elucidated in Thoroughbred racehorses, several genetic factors might contribute to differences among individuals. Therefore, a GWAS using the Equine SNP70 BeadChip (65,156 SNPs) was designed and performed in the present study, aiming to identify the genomic regions associated with body weight in Japanese Thoroughbred racehorses.
Materials and Methods
Body weight of Thoroughbred racehorses
This study was performed according to the ethical standards in animal research. Body weight (kg) is measured when each Thoroughbred horse attends a race in the Japan Racing Association (JRA). The body weights in the final race of each horse at the ages of 2, 3, and 4 years were collected from official JRA race data, and most measurements were taken from September to December. The complete body weight records included 535 horses at the age of 2, 851 horses at the age of 3, and 734 horses at the age of 4 years. In our GWAS, we used only the body weights of 3-year-old horses because more phenotypic information was available for these horses compared with horses of other ages. The 851 three-year-old Thoroughbred racehorses (619 males and 232 females) used in the GWAS were well trained as racehorses and experienced in racing in the JRA.
Genome-wide association study
Blood samples were collected from each animal and stored at −40°C. Genomic DNA was extracted using an MFX-2000 MagExtractor System (Toyobo, Osaka, Japan), according to the manufacturer’s protocol. Almost all blood samples were also used in previous studies [23, 24]. The quantity and purity of the extracted DNA were assessed by spectrophotometric absorbance, and the 260/230 and 260/280 ratios were validated.
Samples were genotyped using the EquineSNP70 Genotyping BeadChips (Illumina, San Diego, CA, U.S.A.). This array contains 65,157 SNPs ascertained from the EquCab2 database of the horse genome [29]. Genotyping was performed at Neogen GeneSeek (Lincoln, NE, U.S.A.). All samples had a genotyping rate above 90%. We omitted SNPs that had a genotyping completion rate below 99%, deviated from Hardy-Weinberg equilibrium (HWE, P<1.0E-6), or were monomorphic or had minor allele frequencies (MAFs) below 5% in our samples. Thus, 44,306 SNPs were used in this study.
GWAS was performed between the 44,306 SNPs and body weight as a quantitative trait of the 851 horses (619 males and 232 females). All statistical analyses for GWAS including quality control were performed using PLINK version 1.07 (http://pngu.mgh.harvard.edu/purcell/plink/) [18]. Manhattan and Q-Q plots were generated with the qqman R package [28].
Statistical analysis
The mean, standard deviation (SD), quartiles, minimum, and maximum body weights at each age were calculated. The differences in body weight among ages were evaluated by analysis of variance (ANOVA). The differences in body weight among genotypes of each candidate gene were evaluated by ANOVA.
The proportion of weight variation explained was estimated using a normal linear model and by comparing the residual variance of a null model with sex only (VN) to a full model (VF) with sex and relevant markers. The proportion of explained variance is defined as 1−(VF/VN).
All statistical analyses were performed using R (http://www.R-project.org/).
Results
Body weight variation
The average body weights and standard deviations of the 2-, 3-, and 4-year-old horses (535, 851, and 734 individuals, respectively) were 468.8 ± 26.1, 473.9 ± 28.0, and 478.8 ± 27.6 kg, respectively (Table 1), and a statistically significant difference was observed between them (ANOVA: P<0.01). Thus, body weight increased with age.
Table 1. Body weight of Japanese Thoroughbred racehorses of three different ages.
| 2 years old | 3 years old | 4 years old | |
|---|---|---|---|
| Sample number (N) | 535 | 851 | 734 |
| Mean (kg) | 468.8 | 473.9 | 478.8 |
| SD (kg) | 26.1 | 28.0 | 27.6 |
| Minimum (kg) | 382 | 390 | 395 |
| First quartile (kg) | 452 | 456 | 460 |
| Second quartile (kg) | 468 | 473 | 478 |
| Third quartile (kg) | 485 | 492 | 496 |
| Maximum (kg) | 568 | 568 | 604 |
Minimum, quartiles, and maximum body weights showed trends similar to that of average body weight. The minimum weight observed in the present study was 382 kg in a 2-year-old racehorse, and the maximum was 604 kg in a 4-year-old racehorse, which had an extreme weight gain (36 kg) at this age.
Genome-wide association study
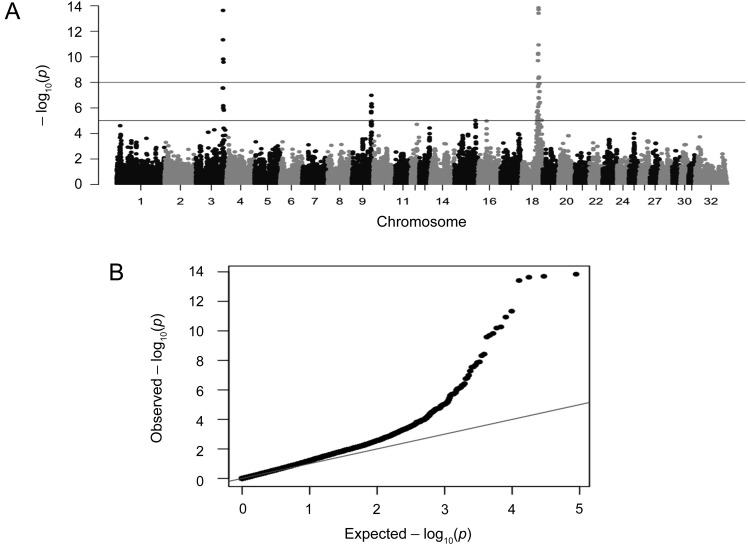
The results from GWAS are represented graphically in a Manhattan plot (Fig. 1a) and a Q-Q plot (Fig. 1b). The nine SNPs located at 104,403,770–108,176,636 bp (spanning 3.77 Mb) on chromosome 3, seven SNPs located at 74,756,685–77,100,429 bp (spanning 2.34 Mb) on chromosome 9, one SNP located at 80,921,871 bp on chromosome 15, and 30 SNPs located at 59,614,257–78,131,099 bp (spanning 18.5 Mb) on chromosome 18 were associated with body weight as a qualitative trait (P<1.00E-8 and/or P<1.00E-5) in our GWAS (Table 2). The SNPs BIEC2_808466 (P=2.32E-14) on chromosome 3, BIEC2_1105503 (P=1.03E-07) on chromosome 9, BIEC2_322669 (P=9.50E-06) on chromosome 15, and BIEC2_417274 on chromosome 18 (P=1.44E-14) revealed the most statistically significant differences for body weight at each genomic region in our GWAS (Table 2).
Fig. 1.
Genome-wide association study (GWAS) for body weight in Japanese Thoroughbred racehorses. A) Manhattan plot of the −log10 (P-values) from a GWAS of body weight in Japanese Thoroughbreds. B) Q-Q plot of observed versus expected −log10 (P-values) from a GWAS in Japanese Thoroughbred racehorses.
Table 2. Results of the genome-wide association study conducted for the body weight of Japanese Thoroughbred racehorses using PLINK.
| Chromosome | SNP | Position (bp) | BETA | SE | R2 | T | P |
|---|---|---|---|---|---|---|---|
| 3 | BIEC2_808344 | 104,403,770 | 8.639 | 1.539 | 0.03577 | 5.612 | 2.71E-08 |
| 3 | BIEC2_808426 | 104,941,985 | 12.180 | 1.735 | 0.05489 | 7.018 | 4.62E-12 |
| 3 | BIEC2_808432 | 104,986,284 | 9.279 | 1.656 | 0.03572 | 5.601 | 2.87E-08 |
| 3 | BIEC2_808466 | 105,163,077 | 17.140 | 2.206 | 0.06633 | 7.766 | 2.32E-14 |
| 3 | BIEC2_808608 | 105,875,809 | −6.841 | 1.389 | 0.02783 | –4.924 | 1.02E-06 |
| 3 | BIEC2_808617 | 105,876,397 | 8.985 | 1.385 | 0.04728 | 6.487 | 1.49E-10 |
| 3 | BIEC2_808640 | 105,947,243 | 7.933 | 1.584 | 0.02873 | 5.009 | 6.67E-07 |
| 3 | BIEC2_808825 | 106,710,385 | 9.541 | 1.491 | 0.04599 | 6.397 | 2.61E-10 |
| 3 | BIEC2_809156 | 108,176,636 | 6.923 | 1.429 | 0.02695 | 4.844 | 1.51E-06 |
| 9 | BIEC2_1165712 | 74,756,685 | 7.002 | 1.474 | 0.02590 | 4.749 | 2.40E-06 |
| 9 | BIEC2_1105370 | 74,795,013 | 6.905 | 1.440 | 0.02639 | 4.794 | 1.93E-06 |
| 9 | BIEC2_1105377 | 74,798,143 | 6.905 | 1.440 | 0.02639 | 4.794 | 1.93E-06 |
| 9 | BIEC2_1165849 | 75,245,697 | 7.284 | 1.460 | 0.02853 | 4.987 | 7.43E-07 |
| 9 | BIEC2_1105503 | 75,374,649 | 7.975 | 1.486 | 0.03282 | 5.368 | 1.03E-07 |
| 9 | BIEC2_1105810 | 76,167,910 | 6.908 | 1.361 | 0.02944 | 5.075 | 4.76E-07 |
| 9 | BIEC2_1105995 | 77,100,429 | 7.948 | 1.601 | 0.02826 | 4.966 | 8.27E-07 |
| 15 | BIEC2_322669 | 80,921,871 | 8.871 | 1.991 | 0.02303 | 4.455 | 9.50E-06 |
| 18 | BIEC2_416419 | 59,614,257 | 8.170 | 1.713 | 0.02634 | 4.770 | 2.17E-06 |
| 18 | BIEC2_416680 | 62,049,912 | 7.233 | 1.500 | 0.02682 | 4.822 | 1.68E-06 |
| 18 | BIEC2_416681 | 62,054,146 | 6.872 | 1.491 | 0.02444 | 4.609 | 4.67E-06 |
| 18 | BIEC2_416683 | 62,115,222 | 6.724 | 1.490 | 0.02351 | 4.513 | 7.28E-06 |
| 18 | BIEC2_416689 | 62,117,055 | 6.686 | 1.504 | 0.02277 | 4.445 | 9.94E-06 |
| 18 | BIEC2_416704 | 62,148,769 | 7.220 | 1.505 | 0.02654 | 4.797 | 1.90E-06 |
| 18 | BIEC2_438369 | 63,193,752 | −6.071 | 1.345 | 0.02344 | –4.514 | 7.24E-06 |
| 18 | BIEC2_416921 | 63,708,318 | 8.945 | 1.389 | 0.04701 | 6.441 | 1.99E-10 |
| 18 | BIEC2_416992 | 64,245,075 | −6.740 | 1.356 | 0.02836 | –4.972 | 8.03E-07 |
| 18 | BIEC2_438541 | 64,252,426 | 7.601 | 1.344 | 0.03645 | 5.657 | 2.10E-08 |
| 18 | BIEC2_417010 | 64,391,698 | 9.084 | 1.372 | 0.04930 | 6.620 | 6.40E-11 |
| 18 | BIEC2_417030 | 64,481,405 | 6.039 | 1.347 | 0.02320 | 4.482 | 8.40E-06 |
| 18 | BIEC2_417075 | 64,725,066 | 8.965 | 1.349 | 0.04946 | 6.646 | 5.37E-11 |
| 18 | BIEC2_417120 | 64,919,213 | −6.439 | 1.412 | 0.02399 | –4.560 | 5.86E-06 |
| 18 | BIEC2_417187 | 65,565,128 | −7.881 | 1.332 | 0.03963 | –5.915 | 4.80E-09 |
| 18 | BIEC2_417274 | 65,868,604 | 10.150 | 1.297 | 0.06737 | 7.831 | 1.44E-14 |
| 18 | UKUL3221 | 65,969,033 | 9.934 | 1.290 | 0.06531 | 7.698 | 3.85E-14 |
| 18 | BIEC2_417291 | 66,010,474 | −8.987 | 1.306 | 0.05281 | –6.880 | 1.16E-11 |
| 18 | BIEC2_417308 | 66,155,365 | −7.025 | 1.328 | 0.03211 | –5.291 | 1.55E-07 |
| 18 | BIEC2_438865 | 66,158,121 | 10.090 | 1.295 | 0.06666 | 7.787 | 2.00E-14 |
| 18 | BIEC2_417372 | 66,539,967 | −7.253 | 1.377 | 0.03180 | –5.268 | 1.75E-07 |
| 18 | BIEC2_417423 | 66,819,091 | −7.847 | 1.370 | 0.03726 | –5.729 | 1.40E-08 |
| 18 | BIEC2_438994 | 66,862,437 | −6.296 | 1.412 | 0.02289 | –4.460 | 9.29E-06 |
| 18 | BIEC2_438997 | 66,892,380 | −6.771 | 1.449 | 0.02508 | –4.673 | 3.45E-06 |
| 18 | BIEC2_417454 | 66,996,889 | 7.087 | 1.399 | 0.02951 | –5.066 | 5.00E-07 |
| 18 | BIEC2_417524 | 67,545,703 | −8.047 | 1.351 | 0.04012 | –5.957 | 3.76E-09 |
| 18 | BIEC2_417704 | 69,258,120 | −7.792 | 1.356 | 0.03750 | –5.748 | 1.26E-08 |
| 18 | BIEC2_417806 | 69,969,173 | −7.272 | 1.324 | 0.03468 | –5.493 | 5.24E-08 |
| 18 | BIEC2_418057 | 71,300,017 | −7.885 | 1.540 | 0.02997 | –5.121 | 3.75E-07 |
| 18 | BIEC2_421048 | 78,131,099 | −6.230 | 1.395 | 0.02297 | –4.465 | 9.11E-06 |
SNP, single nucleotide polymorphism; BETA, regression coefficient; SE, standard error; R2, regression r-squared; T, wald test (based on t-distribtion); P, wald test asymptotic P-value.
Genes annotated on each chromosome
Nineteen protein-coding genes, seven noncoding RNAs (ncRNAs), and one pseudogene have been annotated in the 104,403,770–108,176,636 bp (spanning 3.77 Mb) region of chromosome 3 (Table 3). This genomic region contains the ligand-dependent nuclear receptor compressor-like protein (LCORL) gene.
Table 3. Number of annotated genes in the candidate genomic regions on chromosomes 3, 9, 15, and 18.
| Chromosome 3 | Chromosome 9 | Chromosome 15 | Chromosome 18 | |
|---|---|---|---|---|
| (3.77 Mb) | (2.34 Mb) | (18.52 Mb) | ||
| Protein coding gene | 19 | 2 | 1 | 108 |
| Noncoding RNA | 7 | 2 | 0 | 11 |
| MicroRNA | 0 | 2 | 0 | 1 |
| Pseudogene | 1 | 0 | 0 | 12 |
| Total | 27 | 6 | 1 | 132 |
Two protein-coding genes, two ncRNAs, and two microRNAs have been annotated in the 74,756,685–77,100,429 bp (spanning 2.34 Mb) region of chromosome 9 (Table 3). This genomic region contains the zinc finger and AT hook domain containing (ZFAT) gene.
In the 59,614,257–78,131,099 bp (spanning 18.52 Mb) region of chromosome 18, 108 protein-coding genes, 11 ncRNAs, one microRNA, and 12 pseudogenes have been annotated (Table 3). This genomic region contains the myostatin (MSTN) gene. In addition, tribbles pseudokinase 2 (TRIB2), a protein-coding gene, has been annotated near BIEC2_322669 (80,921,871 bp) on chromosome 15 (Table 3).
The effect of genotypic differences
Table 4 shows the average body weight of each genotype characterized by the most statistically different SNPs. We observed a statistically significant difference in body weight depending on genotypes at BIEC2_808466, BIEC2_1105503, BIEC2_322669, and BIEC2_417274.
Table 4. Body weight of each genotype defined by the single nucleotide polymorphisms found close to candidate genes in each chromosome.
| Chromosome | SNP | Position (bp) | MAF | P-value | Candidate gene | Homozygote (kg) | Heterozygote (kg) | Homozygote (kg) | ANOVA |
|---|---|---|---|---|---|---|---|---|---|
| 3 | BIEC2_808466 | 105,163,077 | 0.112 | 2.32E-14 | LCORL | 470.1 | 487.1 | 510.0 | 0.00024 |
| 9 | BIEC2_1105503 | 75,374,649 | 0.273 | 1.03E-07 | ZFAT | 470.0 | 476.4 | 488.4 | <1.0E-4 |
| 15 | BIEC2_322669 | 80,921,871 | 0.133 | 9.50E-06 | TRIB2 | 471.7 | 480.4 | 490.2 | <1.0E-4 |
| 18 | BIEC2_417274 | 66,158,121 | 0.478 | 1.44E-14 | MSTN | 457.5 | 479.4 | 482.1 | <1.0E-4 |
SNP, single nucleotide polymorphism; MAF, minor allele frequency; ANOVA, analysis of variance.
The four SNPs on chromosomes 3, 9, 15, and 18 explained 17.4% of the weight variance observed in the 851 three-year-old Japanese Thoroughbred racehorses. These four loci together with gender explained 30.0% of the weight variance observed in the Japanese Thoroughbred racehorses.
Discussion
Our GWAS using 851 Japanese Thoroughbred racehorses identified the regions near LCORL on chromosome 3, ZFAT on chromosome 9, TRIB2 on chromosome 15, and MSTN on chromosome 18 as candidate genes for body weights. Similar results were also observed in GWAS performed using body weight information of 2-year-olds (535 horses) and 4-year-olds (734 horses) (data not shown). Therefore, these genomic regions are good candidates for regions associated with body weight as these four loci explained 17.4% of body weight variance in this horse breed. Because the heritability estimate of body weight at a horse’s first start was 0.27 ± 0.04 in Japanese Thoroughbreds [10], these four loci were expected to explain much of the genetic variance in these horses.
In this study, the body weights used were from between September and December of each year. Small differences in age due to birth month and measurement month may influence body weight. However, because Thoroughbred horses are nearly mature by 3 years of age, this influence was excluded from the model in this study.
LCORL encodes a transcription factor that appears to function in spermatogenesis and has been reported to affect withers height in horses. It has been reported that the most significant SNP to withers height is BIEC2_808543 (105,547,002 bp) [8, 9, 19], which is located 63.3 kb upstream of LCORL. BIEC2_808466 (105,163,077 bp), which was identified in the present study, is located 447.3 kb upstream of LCORL. BIEC2_808543 was not identified in the present study due to the quality control performed in our GWAS, which excluded BIEC2_808543. Because no coding genes were found in the genomic region between BIEC2_808466 and LCORL, it is considered that our GWAS identified LCORL as a candidate gene for body weight in Japanese Thoroughbred racehorses.
In a previous study, BIEC2_808543 near LCORL was associated with withers height and cannon circumference in Japanese Thoroughbred horses under training (September to April; 1- to 2-year-olds), which affected their body weights [26]. This could also apply to mature horses. Because LCORL affects skeletal frame and body size in humans and animals [6, 20], changes in the skeletal frame are considered to be associated with body size, reflecting body weight. These findings also suggest that LCORL is the best candidate for body weight in the genomic region of chromosome 3.
ZFAT encodes a protein that likely binds DNA and functions as a transcriptional regulator involved in apoptosis and cell survival, and it has an essential role in hematopoietic differentiation in blood islands [27]. In a previous GWAS, a region from 74,795,013 to 76,254,733 bp including ZFAT was identified as a candidate region for withers height in horses [8]. BIEC2_1105370 and BIEC2_1105377, which were identified in the present study, were also detected in that previous study. Because ZFAT is associated with body height in Japanese and Korean human populations [21] and withers height in several horse breeds [8, 9, 19], it is expected that differences in withers height due to ZFAT affects body weight in a similar fashion as that reported for LCORL. However, because ZFAT and/or its associated SNPs contribute less than LCORL to withers height in Thoroughbred racehorses [8], further detailed analyses are required.
TRIB2, one of the three members of the Tribbles family, encodes a scaffold protein involved in intracellular signal transduction [5], and, interestingly, it is associated with the internal fat area and volume of the pericardial fat [12]. Although there are no reports on the association between morphological traits and fat metabolism in horses, and it is still unclear if internal fat area and volume of pericardial fat affect body weight; it is possible that may contribute to it. While only BIEC2_322669 was identified and located 178.9 kb downstream of TRIB2, BIEC2_323180 (P=2.70E-5) was located 350.3 kb upstream of this gene. Unfortunately, the 10 SNPs between 80,950,328 and 81,428,857 bp, which is the region where TRIB2 is located, had no statistical significance.
MSTN is a member of the TGF-beta (transforming growth factor-beta) superfamily of proteins. This protein negatively regulates skeletal muscle cell proliferation and differentiation. Mutations in this gene are associated with increased skeletal muscle mass in humans and other mammals. In addition, MSTN is associated with optimal race distance in Thoroughbreds by affecting the ratio of muscle fiber types I, IIa, and IIx. Three SNPs, BIEC2_417274, UKUL3221, and BIEC2_438865, were the most significant in this candidate gene region, and BIEC2_438865 was located 332 kb downstream of MSTN. In addition, BIEC2_417274 has been identified as a candidate SNP for racing performance and optimal race distance in Japanese Thoroughbred racehorses [23].
In a previous study, our group also showed that several SNPs near MSTN were associated with muscle mass at withers height in Japanese Thoroughbreds under training (September to April; 1- to 2-year-olds), which also affected body weight [25]. This could also apply to mature horses. Because MSTN affects the muscle mass of animals [11, 17], it is considered that changes in muscle mass may affect body weight. These findings suggest that MSTN is a good candidate gene for body weight on chromosome 18.
The four genes LCORL, ZFAT, TRIB2, and MSTN affected Thoroughbred body weight, which ranged from 470.1 to 510.0 kg, 470.0 to 488.4 kg, 471.7 to 490.2 kg, and 457.5 to 482.1 kg, respectively (Table 4), and LCORL is expected to contribute to the largest weight changes. Because the minor allele frequency (MAF) for LCORL was 0.0125 (Table 4), selective breeding to increase less-frequent genotypes will contribute to higher weights in Thoroughbreds.
High-mobility group AT-hook 2 (HMGA2) on chromosome 6 and LIM and SH3 domain protein 1 (LASP1) on chromosome 11 were not identified as impacting body weight in our study, although these genes, in addition to LCORL and ZFAT, are candidates for withers height or size variation in horses [19]. The architectural transcription factor HMGA2 regulates gene expression and directs cellular growth, proliferation, and differentiation [3], and it is associated with human height [30]. Knockout of this gene in mice resulted in individuals with only 40% of the body weight of control individuals [32]. LASP1 mediates cell migration and survival, and its expression is induced by insulin-like growth factor-1 (IGF1) [7]; its misexpression in mice disrupts chondrocyte differentiation. Therefore, HMGA2 and LASP1 were also expected candidate genes for body weight in Thoroughbred racehorses. However, because they were not identified in our GWAS, it is considered that variants of these genes are not present in Thoroughbreds or that these genes contribute less to Thoroughbred body weight than LCORL and ZFAT.
Although the genes and SNPs identified in this study are useful for effective feeding management, training, and breeding, there is also concern about their use for the production of genetically modified racehorses or possible gene doping in mature individuals. For the purpose of fair horse racing enforcement, the International Federation of Horseracing Authorities (IFHA) prohibits genetic modification and gene doping. Therefore, misuse of these genes needs to be monitored, or an inspection method for gene doping needed to be created in order to maintain horse racing integrity.
Conflict of Interest
The Laboratory of Racing Chemistry (LRC) is licensed by Plusvital (NovaUCD, Belfield, Dublin, Ireland) to perform the Speed Gene Test for genotyping MSTN (Japanese Patent No. 5667057) and provides genotyping services to clients in Japan. The LRC also conducts the withers height test in Thoroughbred racehorses by genotyping LCORL.
Acknowledgments
We would like to thank Dr. M. Kurosawa for useful discussions and Ms. H. Sato for her helpful assistance during this study. We would like to thank the JRA Equine Department for approving and supporting this study through a grant-in-aid (2014–2016).
References
- 1.Bower M.A., Campana M.G., Whitten M., Edwards C.J., Jones H., Barrett E., Cassidy R., Nisbet R.E., Hill E.W., Howe C.J., Binns M. 2011. The cosmopolitan maternal heritage of the Thoroughbred racehorse breed shows a significant contribution from British and Irish native mares. Biol. Lett. 7: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho K.H., Son S.K., Cho B.W., Lee H.K., Kong H.S., Jeon G.J., Park K.D. 2008. Effects of change of body weight on racing time in thoroughbred racehorses. J. Anim. Sci. Technol. Korea 50: 741–746. [Google Scholar]
- 3.Cleynen I., Van de Ven W.J. 2008. The HMGA proteins: a myriad of functions (Review). Int. J. Oncol. 32: 289–305. [PubMed] [Google Scholar]
- 4.Cunningham E.P., Dooley J.J., Splan R.K., Bradley D.G. 2001. Microsatellite diversity, pedigree relatedness and the contributions of founder lineages to thoroughbred horses. Anim. Genet. 32: 360–364. [DOI] [PubMed] [Google Scholar]
- 5.Grandinetti K.B., Stevens T.A., Ha S., Salamone R.J., Walker J.R., Zhang J., Agarwalla S., Tenen D.G., Peters E.C., Reddy V.A. 2011. Overexpression of TRIB2 in human lung cancers contributes to tumorigenesis through downregulation of C/EBPα. Oncogene 30: 3328–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindholm-Perry A.K., Sexten A.K., Kuehn L.A., Smith T.P., King D.A., Shackelford S.D., Wheeler T.L., Ferrell C.L., Jenkins T.G., Snelling W.M., Freetly H.C. 2011. Association, effects and validation of polymorphisms within the NCAPG - LCORL locus located on BTA6 with feed intake, gain, meat and carcass traits in beef cattle. BMC Genet. 12: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loughran G., Huigsloot M., Kiely P.A., Smith L.M., Floyd S., Ayllon V., O’Connor R. 2005. Gene expression profiles in cells transformed by overexpression of the IGF-I receptor. Oncogene 24: 6185–6193. [DOI] [PubMed] [Google Scholar]
- 8.Makvandi-Nejad S., Hoffman G.E., Allen J.J., Chu E., Gu E., Chandler A.M., Loredo A.I., Bellone R.R., Mezey J.G., Brooks S.A., Sutter N.B. 2012. Four loci explain 83% of size variation in the horse. PLoS One 7: e39929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger J., Schrimpf R., Philipp U., Distl O. 2013. Expression levels of LCORL are associated with body size in horses. PLoS One 8: e56497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moritsu Y., Deki T.T., Miwa K., Ichikawa S. 1997. Estimates of heritability and environmental factors in age and body weight at first start among 3-year-old Thoroughbred horses. Anim. Sci. Agric. Hokkaido 39: 15–20(in Japanese). [Google Scholar]
- 11.Mosher D.S., Quignon P., Bustamante C.D., Sutter N.B., Mellersh C.S., Parker H.G., Ostrander E.A. 2007. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 3: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama K., Ogawa A., Miyashita H., Tabara Y., Igase M., Kohara K., Miki T., Kagawa Y., Yanagisawa Y., Katashima M., Onda T., Okada K., Fukushima S., Iwamoto S. 2013. Positive natural selection of TRIB2, a novel gene that influences visceral fat accumulation, in East Asia. Hum. Genet. 132: 201–217. [DOI] [PubMed] [Google Scholar]
- 13.Onoda T., Yamamoto R., Sawamura K., Inoue Y., Matsui A., Miyake T., Hirai N. 2011. Empirical growth curve estimation using sigmoid sub-functions that adjust seasonal compensatory growth for male body weight of Thoroughbred horses. J. Equine Sci. 22: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onoda T., Yamamoto R., Sawamura K., Inoue Y., Murase H., Nambo Y., Tozaki T., Matsui A., Miyake T., Hirai N. 2013. Empirical growth curve estimation considering multiple seasonal compensatory growths of body weights in Japanese Thoroughbred colts and fillies. J. Anim. Sci. 91: 5599–5604. [DOI] [PubMed] [Google Scholar]
- 15.Onoda T., Yamamoto R., Sawamura K., Murase H., Nambo Y., Inoue Y., Matsui A., Miyake T., Hirai N. 2013. Empirical percentile growth curves with z-scores considering seasonal compensatory growths for Japanese Thoroughbred horses. J. Equine Sci. 24: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onoda T., Yamamoto R., Sawamura K., Murase H., Nambo Y., Inoue Y., Matsui A., Miyake T., Hirai N. 2014. An approach of estimating individual growth curves for young thoroughbred horses based on their birthdays. J. Equine Sci. 25: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Rourke B.A., Greenwood P.L., Arthur P.F., Goddard M.E. 2013. Inferring the recent ancestry of myostatin alleles affecting muscle mass in cattle. Anim. Genet. 44: 86–90. [DOI] [PubMed] [Google Scholar]
- 18.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., Sham P.C. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signer-Hasler H., Flury C., Haase B., Burger D., Simianer H., Leeb T., Rieder S. 2012. A genome-wide association study reveals loci influencing height and other conformation traits in horses. PLoS One 7: e37282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soranzo N., Rivadeneira F., Chinappen-Horsley U., Malkina I., Richards J.B., Hammond N., Stolk L., Nica A., Inouye M., Hofman A., Stephens J., Wheeler E., Arp P., Gwilliam R., Jhamai P.M., Potter S., Chaney A., Ghori M.J., Ravindrarajah R., Ermakov S., Estrada K., Pols H.A., Williams F.M., McArdle W.L., van Meurs J.B., Loos R.J., Dermitzakis E.T., Ahmadi K.R., Hart D.J., Ouwehand W.H., Wareham N.J., Barroso I., Sandhu M.S., Strachan D.P., Livshits G., Spector T.D., Uitterlinden A.G., Deloukas P. 2009. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 5: e1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi F., Nabika T., Isono M., Katsuya T., Sugiyama T., Yamaguchi S., Kobayashi S., Yamori Y., Ogihara T., Kato N. 2009. Evaluation of genetic loci influencing adult height in the Japanese population. J. Hum. Genet. 54: 749–752. [DOI] [PubMed] [Google Scholar]
- 22.Tamioso P.R., Cosmo T.R., Pimentel C.M.M., Dias L.T., Teixeira R.A. 2012. Heritability estimates for body weight and height at withers in Brazilian army horses. Cienc. Rural. Santa Maria 42: 2246–2251. [Google Scholar]
- 23.Tozaki T., Hill E.W., Hirota K., Kakoi H., Gawahara H., Miyake T., Sugita S., Hasegawa T., Ishida N., Nakano Y., Kurosawa M. 2012. A cohort study of racing performance in Japanese Thoroughbred racehorses using genome information on ECA18. Anim. Genet. 43: 42–52. [DOI] [PubMed] [Google Scholar]
- 24.Tozaki T., Miyake T., Kakoi H., Gawahara H., Sugita S., Hasegawa T., Ishida N., Hirota K., Nakano Y. 2010. A genome-wide association study for racing performances in Thoroughbreds clarifies a candidate region near the MSTN gene. Anim. Genet. 41(Suppl 2): 28–35. [DOI] [PubMed] [Google Scholar]
- 25.Tozaki T., Sato F., Hill E.W., Miyake T., Endo Y., Kakoi H., Gawahara H., Hirota K., Nakano Y., Nambo Y., Kurosawa M. 2011. Sequence variants at the myostatin gene locus influence the body composition of Thoroughbred horses. J. Vet. Med. Sci. 73: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 26.Tozaki T., Sato F., Ishimaru M., Kikuchi M., Kakoi H., Hirota K.I., Nagata S.I. 2016. Sequence variants of BIEC2-808543 near LCORL are associated with body composition in Thoroughbreds under training. J. Equine Sci. 27: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsunoda T., Takashima Y., Tanaka Y., Fujimoto T., Doi K., Hirose Y., Koyanagi M., Yoshida Y., Okamura T., Kuroki M., Sasazuki T., Shirasawa S. 2010. Immune-related zinc finger gene ZFAT is an essential transcriptional regulator for hematopoietic differentiation in blood islands. Proc. Natl. Acad. Sci. U.S.A. 107: 14199–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner S.D. 2014. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. bioRxiv. [Google Scholar]
- 29.Wade C.M., Giulotto E., Sigurdsson S., Zoli M., Gnerre S., Imsland F., Lear T.L., Adelson D.L., Bailey E., Bellone R.R., Blöcker H., Distl O., Edgar R.C., Garber M., Leeb T., Mauceli E., MacLeod J.N., Penedo M.C., Raison J.M., Sharpe T., Vogel J., Andersson L., Antczak D.F., Biagi T., Binns M.M., Chowdhary B.P., Coleman S.J., Della Valle G., Fryc S., Guérin G., Hasegawa T., Hill E.W., Jurka J., Kiialainen A., Lindgren G., Liu J., Magnani E., Mickelson J.R., Murray J., Nergadze S.G., Onofrio R., Pedroni S., Piras M.F., Raudsepp T., Rocchi M., Røed K.H., Ryder O.A., Searle S., Skow L., Swinburne J.E., Syvänen A.C., Tozaki T., Valberg S.J., Vaudin M., White J.R., Zody M.C., Lander E.S., Lindblad-Toh K., Broad Institute Genome Sequencing PlatformBroad Institute Whole Genome Assembly Team 2009. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 326: 865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weedon M.N., Lettre G., Freathy R.M., Lindgren C.M., Voight B.F., Perry J.R., Elliott K.S., Hackett R., Guiducci C., Shields B., Zeggini E., Lango H., Lyssenko V., Timpson N.J., Burtt N.P., Rayner N.W., Saxena R., Ardlie K., Tobias J.H., Ness A.R., Ring S.M., Palmer C.N., Morris A.D., Peltonen L., Salomaa V., Davey Smith G., Groop L.C., Hattersley A.T., McCarthy M.I., Hirschhorn J.N., Frayling T.M., Diabetes Genetics InitiativeWellcome Trust Case Control Consortium 2007. A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat. Genet. 39: 1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willet P. 1991. A History of the General Stud-Book. Weatherbys, Northants. [Google Scholar]
- 32.Zhou X., Benson K.F., Ashar H.R., Chada K. 1995. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature 376: 771–774. [DOI] [PubMed] [Google Scholar]