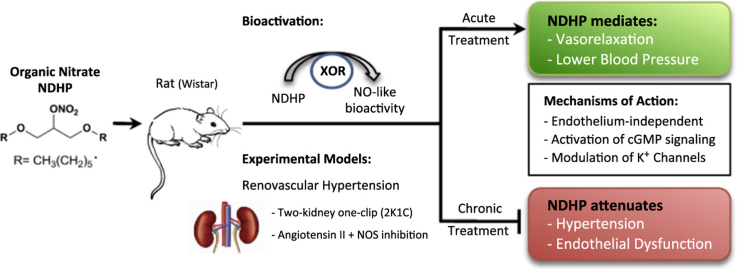
Abstract
Rationale
Development and progression of cardiovascular diseases, including hypertension, are often associated with impaired nitric oxide synthase (NOS) function and nitric oxide (NO) deficiency. Current treatment strategies to restore NO bioavailability with organic nitrates are hampered by undesirable side effects and development of tolerance. In this study, we evaluated NO release capability and cardiovascular effects of the newly synthesized organic nitrate 1, 3-bis (hexyloxy) propan-2-yl nitrate (NDHP).
Methods
A combination of in vitro and in vivo approaches was utilized to assess acute effects of NDHP on NO release, vascular reactivity and blood pressure. The therapeutic value of chronic NDHP treatment was assessed in an experimental model of angiotensin II-induced hypertension in combination with NOS inhibition.
Results
NDHP mediates NO formation in both cell-free system and small resistance arteries, a process which is catalyzed by xanthine oxidoreductase. NDHP-induced vasorelaxation is endothelium independent and mediated by NO release and modulation of potassium channels. Reduction of blood pressure following acute intravenous infusion of NDHP was more pronounced in hypertensive rats (two-kidney-one-clip model) than in normotensive sham-operated rats. Toxicological tests did not reveal any harmful effects following treatment with high doses of NDHP. Finally, chronic treatment with NDHP significantly attenuated the development of hypertension and endothelial dysfunction in rats with chronic NOS inhibition and angiotensin II infusion.
Conclusion
Acute treatment with the novel organic nitrate NDHP increases NO formation, which is associated with vasorelaxation and a significant reduction of blood pressure in hypertensive animals. Chronic NDHP treatment attenuates the progression of hypertension and endothelial dysfunction, suggesting a potential for therapeutic applications in cardiovascular disease.
Keywords: Nitric oxide, Cardiovascular disease, Hypertension, Organic nitrates, Nitrite
Graphical abstract
Highlights
-
•
The organic nitrate NDHP mediates NO formation in cell-free system and blood vessels.
-
•
NDHP-mediated NO release is dependent on functional XOR.
-
•
NDHP induces endothelium-independent vasorelaxation and significant reduction of blood pressure.
-
•
NDHP-mediated vasorelaxation involves activation of NO/cGMP/PKG pathway and K+ channels (Kv and BKCa).
-
•
Chronic treatment with NDHP attenuates the development of hypertension and endothelial dysfunction.
1. Introduction
Arterial hypertension is a chronic degenerative disease of multifactorial etiology that involves functional alterations of the vascular, renal and central nervous systems [1], [2], [3]. Of note, hypertension is closely related to endothelial dysfunction mainly attributed to reduced nitric oxide (NO) bioavailability in the vascular wall [4], [5] and increased oxidative stress, resulting in chronic and abnormal increase in vascular resistance [6].
NO is considered as the main endothelium-derived relaxing factor regulating arterial blood pressure in several ways, including relaxation of vascular smooth muscle cells, inhibition of platelet aggregation, smooth muscle cell replication and neuronal communication [7], [8], [9], [10]. This lipid soluble gas is synthesized from L-arginine by the action of NO synthases (NOS) [11], [12]. NO exerts its biological action on the vascular smooth muscle by activating soluble guanylate cyclase (sGC), leading to increased intracellular cyclic guanosine monophosphate (cGMP) levels, which in turn activates protein kinase G (PKG), thus mediating vasodilation [13]. In addition to modulating the sGC-cGMP-PKG pathway, NO may also directly activate K+ channels; including calcium-dependent K+ channels, whose action promotes vasorelaxation [5], [14], [15].
Thus, the search for new therapies which increase NO bioavailability in cardiovascular diseases (CVDs) has been the target of numerous studies. Of note, organic nitrates such as sodium nitroprusside and glyceryl trinitrate, which mimic the role of endogenous NO in biological systems, have been used in the treatment of several cardiovascular diseases; such as arterial hypertension and coronary artery disease [16], [17], [18], [19], [20], [21], [22]. Despite their beneficial effects in patients with cardiovascular disease, these drugs have several clinical limitations. These include short duration (half-life), high reactivity, low tissue selectivity and development of tolerance (reducing efficacy) with frequent dosing [23], [24], [25], [26]. Moreover, side-effects including headaches (cerebral vasodilation), flushing, hypotension and tachycardia, which augments angina via increasing oxygen demand [26].
In order to obtain a novel NO donor with reduced detrimental effects, 1,3-bis (hexyloxy) propan-2-yl nitrate (NDHP) obtained from glycerin was recently synthesized [27]. The aims of the present study were to characterize this novel organic nitrate as an NO donor and vasodilator, and to investigate both the acute and chronic cardiovascular effects of NDHP treatment in experimental models of hypertension.
2. Materials and methods
2.1. Ethics
All experimental procedures were approved by the ethics committee in Stockholm, Sweden (Protocol ID: N139/15) for animal experiments, and by Federal University of Paraiba Animal Care and Use Committee (CEUA/UFPB, Protocol ID: 142/2015) in João Pessoa, Brazil. The experiments were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2. Animals and surgical procedures
Male (250–300 g) and female (200–250 g) Wistar rats were kept in ordinary cages in a temperature-controlled room, 12:12 h light/dark cycle with standard rat chow (Labina®, Purina, Paulinea, SP, Brazil) and water ad libitum. Renovascular hypertension was induced by clamping the right renal artery (2K1C model) in rats as previously described [28]. Briefly, under combined ketamine and xylazine anesthesia (75 and 10 mg/kg, i.p., respectively), a midline abdominal incision was made. The right renal artery was exposed and carefully separated from the right renal vein and surrounding tissues. A U-shaped silver clip (0.2 mm, internal diameter) was placed around the vessel, proximal to the abdominal aorta, and the wound was closed. Control sham operations, were performed in exactly the same way, with the exception of clipping the renal artery. After surgery, the rats were kept in their cages and remained untouched for six weeks in order to develop arterial hypertension.
2.3. Nitric oxide measurements by fluorescence microscopy
Indirect NO measurements were performed using fluorescence microscopy with the cell permeable 4,5-diaminofluorescein diacetate (DAF-2 DA) probe. Normotensive rats were euthanized and superior mesenteric artery rings (2–3 mm) were isolated and maintained in physiological Tyrode's solution (composition in mM: NaCl 158.3; KCl 4.0; CaCl2 2.0; MgCl2 1.05; NaH2PO4 0.42; NaHCO3 10.0 and glucose 5.6), at 37 °C. Vascular rings were pre-incubated with DAF-2A (10 μM; Calbiochem, San Diego, CA, USA), in darkness for 30 mins. Vascular rings were then fixed in Tissue-Tek® O.C.T.™ and frozen at −20 °C. Sections were cut (8 µm) and collected using a CM 1900 cryostat (Leica Inc., Deerfield, IL, USA) and mounted on slides with coverslips. The sections were washed (PBS 0.1 M) and stimulated with: PBS 0.1 M; NDHP (1 mM); NDHP (1 mM) + L-NG-nitroarginine (L-NNA, 100 μM), an eNOS inhibitor; linsidomine (SIN-1, 10 μM), an NO donor, SIN-1 (10 μM) + L-NNA (100 μM) for 30 min. To test whether NDHP signaling was mediated by xanthine oxidoreductase (XOR); another set of DAF-2 DA experiments was performed. After loading and washing (as described above), the sections were incubated with the xanithine oxidase inhibitor febuxostat (50 μM, Sigma-Aldrich), or the combination of febuxostat (50 μM) + NDHP (1 mM) for 30 min. After stimulation the sections were washed and processed as described above. 4′,6-diamidino-2-phenylindole stain (DAPI) was used to visualize cell nuclei. The cytosolic NO levels were assessed by exciting DAF-2 DA at 480 nm using a xenon lamp and measuring the fluorescence at an excitation of 515–565 nm using a fluorescence microscope (Zeiss Observer Z1, Germany). The relative fluorescence intensity was calculated from images obtained using a digital camera (Axio Cam MRm, Zeiss, Germany) and ImageJ software 1.42q (Wayne Rasband, National Institutes of Health, USA). The NO content (A.U), represents the change in the fluorescence intensity, which is calculated as the difference between the final and basal florescence.
2.4. Assessment of NDHP-derived nitric oxide in purified XOR
NDHP-induced NO production was evaluated by using purified XOR enzyme. In brief, 2 mL of PBS containing XOR (0.05 U/mL, Roche, Cat. no: 10110434001), NADH (1 mM, Sigma, Cat. no: 10107735001) and increasing concentrations of NDHP (0.1, 1, and 1 mM) was added into a chamber of an Oxygraph-2k system (Oroboros instruments) under nitrogen atmosphere (oxygen ≤ 0.02%) at 37 °C. The oxygraph chamber was connected in line to a NO analyzer device (ECO Physics analyzer, CLD 77 A.M., Swiss), using nitrogen gas as carrier (400 mL/min). After a period of equilibration (approx. 5 min), NDHP was injected into the chamber and real-time NO production was recorded throughout the experiments, using a data acquisition system (AcqKnowledge v3.9, Biopac MP150), as described previously [27], [29]. While no changes in NO formation were detected by NADH, XOR or NDHP alone, the combination of these three components rapidly induced NO production. NO production was recorded within 1 h post NDHP injection and, in a subset of experiments, the XOR inhibitor oxypurinol (100 µM) was added 15 min before NDHP. The average NO signal (ppb) was calculated during 5 min periods at baseline and at maximum response to NDHP. NDHP-mediated NO release was calculated by subtracting the average NO values, after the addition of NDHP, from the NO values at baseline.
2.5. Vasorelaxant effect elicited by NDHP
After euthanasia, the superior mesenteric artery was isolated, placed in Tyrode's solution and adherent tissue was dissected. Vessels were cut into arterial rings (1–2 mm) which were continuously gassed with a carbogenic mixture (95% O2 and 5% CO2) and maintained at 37 °C, pH 7.4. Rings were mounted vertically on two Δ-shaped stainless steel wires in a 10 mL tissue chamber connected to a tension transducer (PowerLab™, ADInstruments, MA, EUA). All rings were stabilized under 0.75 g resting tension for 1 h. The presence of functional endothelium was assessed by responses of rings to acetylcholine (ACh, 10 µM), following phenylephrine stimulation (PHE, 10 μM). Experiments were conducted in rings with and without endothelium. After preconstriction of mesenteric artery rings with PHE (1 µM), cumulative doses of NDHP were added to the organ bath (10–12–10−3 M) in order to study the concentration-response. Data were expressed as maximum effect and potency (pD2). In some preparations, the rings were pre-incubated for 30 min with the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 100 µM), the NO scavengers 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO, 300 µM) and hydroxocobalamin (HDX, 30 µM), the soluble guanylyl cyclase inhibitor 1H-[1], [2], [4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 mM). Additional experiments investigated the effects of tetraethylammonium (TEA, 3 mM); a non-selective K+ channel blocker, TEA (1 mM); a large conductance Ca2+-sensitive K+ channel blocker, glibenclamide (GLIB, 1 mM); a KATP blocker, 4-aminopyridine (4-AP, 1 mM); a voltage-operated K+ channel blocker and barium chloride (BaCl2, 30 µM); an inward-rectifier potassium channels (KIR) blocker. Inhibition was calculated by comparing the response elicited by NDHP in the absence and presence of inhibitors in the preparations. All drugs were obtained from Sigma-Aldrich (São Paulo-SP, Brazil). ODQ and GLIB were solubilized in DMSO and the remaining compounds were dissolved in distilled water. For functional studies using ODQ and GLIB, the final concentration of DMSO never exceeded 0.01% in the bath. This had no effect when tested in control preparations (data not shown).
2.6. Acute preclinical toxicity test for NDHP
Toxicity test was performed according to the "Guidelines for Testing of Chemicals" from The Organization for Economic Co-operation and Development [30]. Female rats (n = 3/group) were treated with single doses of 300 or 2000 mg/kg by oral administration of NDHP, while the control group received vehicle only [5% (v/v) Cremophor in saline]. For the behavioral changes and signs of toxicity, characteristic signs of central nervous system (CNS) or autonomic nervous system (ANS) activity were carefully observed every 15 min (0, 15, 30 and 60 min), after 4 h, and on daily basis for the following 14 days [31]. On day 15, the animals were euthanized and the organs (liver, heart, spleen, kidneys and lung) were harvested, weighed and examined macroscopically. The dose responsible for the death of 50% of the experimental animals (LD50) was estimated accordingly [30].
2.7. Acute cardiovascular effects of NDHP in normotensive and hypertensive rats
After six weeks, sham and 2K1C operated rats were anesthetized with ketamine and xylazine (75 and 10 mg/kg, i.p., respectively) and polyethylene cannulae were inserted into the inferior vena cava and abdominal aorta through vein and femoral artery for drug injections and arterial pressure recordings, respectively. Animals were placed in individual cages for 24 h with food and water ad libitum. Blood pressure measurements were recorded in conscious rats using a pressure transducer connected to an acquisition system (PowerLab; ADInstruments, Castle Hill, NSW, Australia) and LabChart 5.0 software (ADInstruments). NDHP was dissolved in saline and administered through venous catheter following baseline recordings (20 min). Blood pressure was evaluated before and after the administration of NDHP (1; 5; 10 and 20 mg/kg, i.v., randomly). The interval between the doses was 15 min, and the results were expressed as changes in mean arterial pressure (ΔMAP).
2.8. Cardiovascular effects of repeated NDHP treatment in a rat model of angiotensin II-induced hypertension
Male Wistar rats (250–300 g, n = 16) from Janviers Labs (Saint Berthevin Cedex, France) were kept in ordinary cages in a temperature-controlled room, 12:12 h light/dark cycle with standard rat chow (Lantmännen, Kimstad, Sweden) and water ad libitum.
2.8.1. Cardiovascular function
Blood pressure and heart rate were monitored by noninvasive tail-cuff system (Kent Scientific Corporation Coda, Torrington, CT, USA), described previously [32], [33] accordingly to the manufacturer's protocol. After training, baseline measurements were taken and subsequently the rats were anesthetized by spontaneous inhalation of isoflurane (Forene®, Abbott Scandinavia AB, Solna, Sweden) in air (~ 2.2%). Osmotic minipumps (Alzet®, Durect™, CA, USA) were implanted subcutaneously to deliver ANG II (120 ng/kg/min) (Sigma-Aldrich, Stockholm, Sweden) for 14 days, as described previously [34]. After implantation all rats were given L-NAME (0.5 g/L; drinking water) and treated with either NDHP (10 mg/kg i.p.) or vehicle (cremophor in saline) twice per day (8 a.m. and 8 p.m.). Blood pressure and heart rate were monitored again on Day 10–14 following implantation.
2.8.2. Endothelial function
On Day 15 the rats were euthanized; tissues samples were collected and snap frozen (−80 °C) for later in vitro analyses. Freshly isolated mesenteric arteries were mounted in multiwire myograph system for vessel reactivity studies [27], [32].
2.8.3. Plasma nitrate and nitrite
Plasma samples were analyzed for their levels of nitrate and nitrite, using a dedicated high performance liquid chromatography (HPLC) system (ENO-20; Eicom) [27].
2.9. Statistical analysis
Results are expressed as the mean ± SEM. Statistical analysis was performed by Student's t-test or analysis of variance (ANOVA; one or two two-way analysis as appropriate) followed by the recommended post-hoc analysis in the Graph Pad Prism 5.0 program. Statistical significance was considered when p < 0.05.
3. Results
3.1. NDHP-mediated nitric oxide generation in cell-free system – role of xanthine oxidoreductase
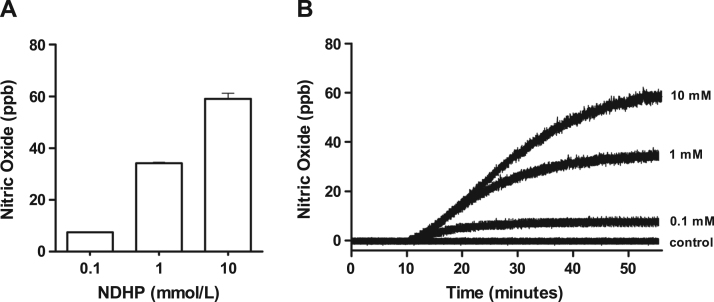
In the presence of NADPH and XOR, administration of NDHP (0.1, 1 and 10 mM) increased NO production in a dose-dependent manner compared with control. NO production was sustained for more than 50 min (Fig. 1), and this effect was solely observed after the addition of XOR, thus suggesting that NDHP-mediated NO release requires enzymatic activity.
Fig. 1.
Nitric oxide generation by NDHP. Effect of NDHP in different concentrations (0.1, 1 and 10 mM) on NO production (ppb) in a cell-free system. Data are shown as mean ± SEM. n = 3 per group.
3.2. NDHP-mediated nitric oxide fluorescence in mesenteric arteries is mediated by xanthine oxidoreductase
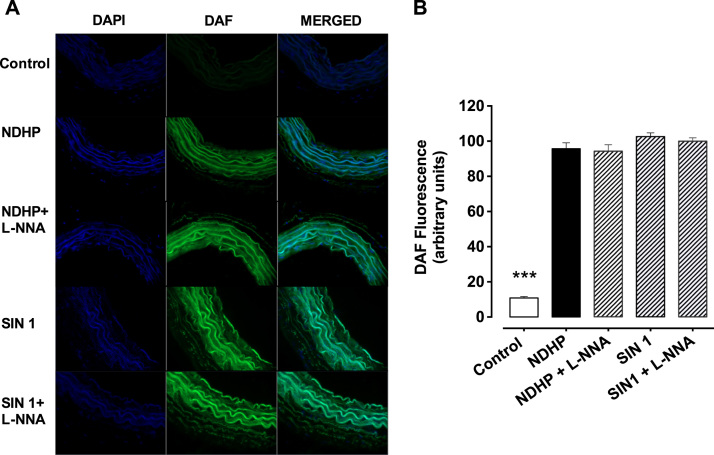
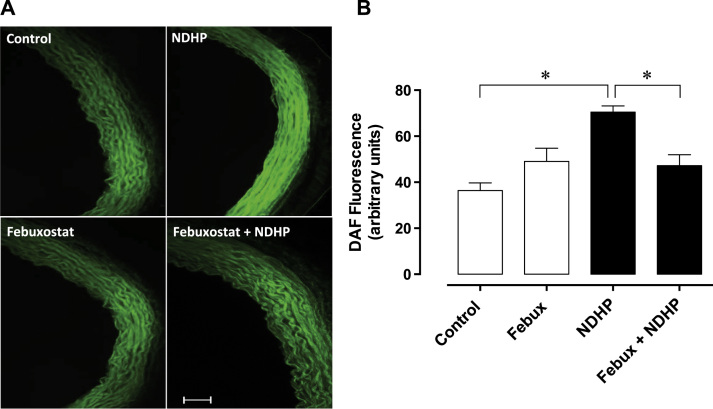
Fluorescence microscopy images (Fig. 2A) show the changes in the fluorescence emitted by DAF-2 DA, an index of NO production, in response to different treatments. Incubation with DAF-2 DA alone was set as baseline and served as control (Fig. 2B). Incubation with NDHP significantly increased fluorescence compared with control (95.7 ± 3.4 vs. 10.9 ± 0.8 a.u., respectively, p < 0.05). The positive control, SIN-1, also increased DAF-2 DA fluorescence, suggesting increased NO production (102.5 ± 2.1 vs. 10.9 ± 0.8 a.u., respectively, p < 0.05). In addition, L-NNA did not prevent the increase in fluorescence promoted by NDHP (94.2 ± 3.6 vs. 95.7 ± 3.4 a.u., respectively, p < 0.05) or SIN 1 (99.8 ± 1.9 vs. 102.5 ± 2.1 a.u., respectively, p < 0.05). Fluorescence microscopy images (Fig. 3A) show the changes in the fluorescence emitted by DAF-2 DA during inhibition of XOR with febuxostat. Simultaneous incubation with febuxostat tended to increase the fluorescence in mesenteric arteries. Increased fluorescence following treatment with NDHP was blocked by febuxostat (Fig. 3B).
Fig. 2.
Imaging of nitric oxide species in small arteries. (A) Representative images of fluorescence in superior mesenteric artery sections incubated with DAF-FM DA (10 μM), a fluorescent cell permeable probe for NO. (B) Bars graph showing the NO production after incubation with only DAF-2DA (Baseline) and treatments groups: NDHP; NDHP + L-NNA; Linsidomine (SIN-1); SIN-1 + L-NNA. Fluorescence was quantified by optic densitometry (arbitrary units, a.u.). Data are shown as mean ± SEM. n = 13–16 per group. *p < 0.05 DAF versus other groups.
Fig. 3.
NDHP-mediated generation of nitric oxide species in small arteries involves xanthine oxidoreductase. (A) Representative images of superior mesenteric artery sections incubated with the fluorescent cell permeable NO probe DAF-2 DA in the absence or presence of simultaneous treatment with febuxostat and/or NDHP. (B) Bar graph showing the NO production after incubation with only DAF-2 DA (Control), DAF-2 DA+Febuxostat, DAF-2 DA+NDHP and DAF-2 DA+NDHP+Febuxostat. Fluorescence was quantified by optic densitometry (arbitrary units). Data are shown as mean ± SEM. n = 13–16 per group. *p < 0.05 between indicated groups.
3.3. NDHP-mediated vasorelaxation of mesenteric artery – modulation of nitric oxide release and signaling
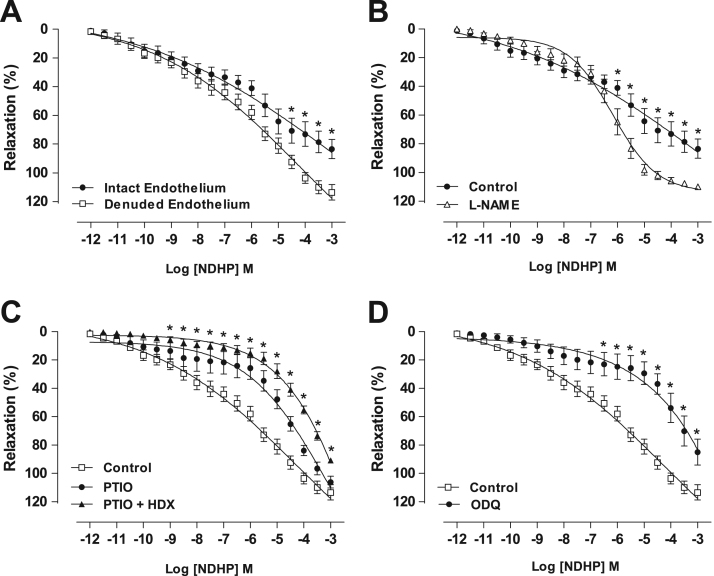
NDHP (10–12–10−3 µM) promoted concentration-dependent vasorelaxation in preconstricted mesenteric artery rings isolated from normotensive rats in the presence of functional endothelium (83.3 ± 6.7%; n = 6). After removal of the endothelium, vasorelaxation was potentiated (113.4 ± 5.3%, n = 7) (Fig. 4A). There was no significant difference between pD2 for rings with or without endothelium. However, pre-incubation with the NOS inhibitor L-NAME, increased NDHP-induced relaxation (109.7 ± 1.5%; n = 6) compared with control (83.3 ± 6.7%; n = 6) (Fig. 4B). Thus suggesting that NDHP-induced relaxation is attenuated by eNOS participation. Therefore, in all subsequent experiments the investigation of NDHP-mediated signaling was performed after removal of the vascular endothelium.
Fig. 4.
NDHP-mediated vasorelaxation and the role of nitric oxide. Concentration-response curves were induced by cumulative addition of NDHP (10–12–10−3 M) in PHE-precontracted (10 μM) superior mesenteric artery rings. Panel A shows NDHP inducing concentration-dependent vasorelaxation in both, intact and denuded-endothelium vessel segments (*p < 0.05 versus intact endothelium. Panel B shows the effect of NOS inhibition by L-NAME in intact-endothelium vessel segments (*p < 0.05 comparing to intact-endothelium control segments). Panel C shows the effect of NO scavenger PTIO alone or in combination with HDX in NDHP inducing vasorelaxation (*p < 0.05 for denuded-endothelium pretreated with PTIO + HDX versus denuded-endothelium control). Padel D shows the effect of sGC inhibitor ODQ in the vasorelaxation elicit NDHP (*p < 0.05 versus denuded-endothelium). Data are shown as mean ± SEM. n = 6–8 per group.
In preconstricted mesenteric artery rings and pre-incubated with the NO scavenger PTIO, the concentration-response curve was shifted to the right, decreasing the NDHP potency when compared to the control (pD2 = 5.1 ± 0.1 vs. 6.5 ± 0.1, n = 6, p < 0.05) (Fig. 4C). However, when the rings were preincubated with PTIO together with hydroxocobalamin (HDX), the right shift of the concentration-response curve was increased with reduced maximum effect compared to the control curve (90.8 ± 0.8 vs. 113.4 ± 5.3%, n = 6, p < 0.05). Moreover, the sGC inhibitor ODQ decreased NDHP-mediated vasorelaxation compared to the control (84.9 ± 9.5 vs 113.4 ± 5.3%, n = 6, p < 0.05) (Fig. 4D). Taken together, the data suggest that NDHP-induced vasodilation can be mediated by NO release and activation of cGMP signaling.
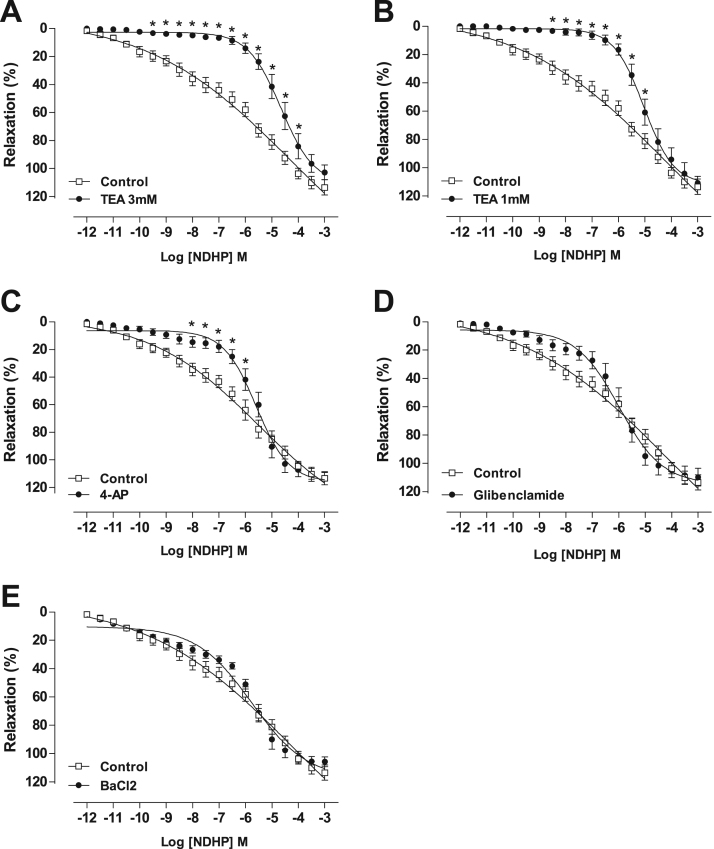
3.4. NDHP-mediated vasorelaxation of mesenteric artery – modulation of K+channels
Pre-incubation of mesenteric artery rings with TEA, in a concentration that predominantly acts as a non-selective K+ channels blocker, shifted the concentration-response curve to the right, decreasing the NDHP potency (Fig. 5A), shown by pD2 value when compared with the control (pD2 = 4.8 ± 0.1 vs. 6.5 ± 0.1, n = 6, p < 0.05). The curve displacement by NDHP was also present when K+ channel subtypes were evaluated using a threefold lower concentration of TEA to selectively block the voltage-gated and Ca2+-activated potassium channels (BKCa) (pD2 = 5.1 ± 0.1 vs. 6.5 ± 0.1, n = 6, p < 0.05) (Fig. 5B), or with inhibition of voltage-gated potassium channels (Kv) with 4-AP (pD2 = 5.7 ± 0.1 vs. 6.5 ± 0.1, n = 7, p < 0.05). On the other hand, vasorelaxation elicited by NDHP was not altered by inhibition of ATP-sensitive potassium channels (KATP) using GLIB (Fig. 5D) or by inhibition of inward-rectifier potassium channels (KIR) with BaCl2 (Fig. 5E).
Fig. 5.
NDHP-mediated vasorelaxation and the role of potassium channels. Vasorelaxation induced by cumulative addition of NDHP (10–12–10−3 M) in PHE-precontracted (10 μM) superior mesenteric artery rings. (A) Denuded Endothelium vs. Denuded Endothelium + TEA (3 mM). (B) Denuded Endothelium vs. Denuded Endothelium + TEA (1 mM). (C) Denuded Endothelium vs. Denuded Endothelium + 4-AP. (D) Denuded Endothelium vs. Denuded Endothelium + GLIB. (E) Denuded Endothelium vs. Denuded Endothelium + BaCl2. Data are shown as mean ± SEM. n = 6–8 per group. *p < 0.05 versus Control.
3.5. Acute preclinical toxicity of NDHP
Oral administration of NDHP (300 mg/kg) did not evoke any behavioral changes compared to the control group. However, animals treated with the much higher dose (2000 mg/kg) presented analgesia and decreased ambulation, when compared to the control. Regarding the LD50 assessment, no death was observed in animals treated with 300 and 2000 mg/kg of NDHP. Thus, LD50 for NDHP is around 5000 mg/kg (category 5). Treatment with NDHP in these two doses did not promote any changes of body weight and organs weights, as well as, on consumption of water and food compared with the control group (Table 1).
Table 1.
Acute preclinical toxicity test for NDHP in rats.
| Parameter |
Treatment |
||
|---|---|---|---|
| Control | NDHP (300 mg/kg) | NDHP (2000 mg/kg) | |
| Body weight(g) | |||
| Initial | 151 ± 4.50 | 154 ± 5.11 | 162 ± 3.50 |
| Final | 183 ± 4.48 | 182 ± 5.00 | 185 ± 6.46 |
| Organ to body weights(mg/g bw) | |||
| Liver | 38.6 ± 1.19 | 41.7 ± 1.42 | 38.4 ± 1.15 |
| Heart | 4.38 ± 0.18 | 4.16 ± 0.09 | 4.17 ± 0.19 |
| Kidneys | 8.23 ± 0.29 | 8.47 ± 0.22 | 8.24 ± 0.18 |
| Spleen | 2.71 ± 0.23 | 2.62 ± 0.12 | 2.59 ± 0.16 |
| Water intake(mL/rat/day) | 29.1 ± 0.56 | 30.6 ± 0.87 | 29.2 ± 0.90 |
| Food intake(g/rat/day) | 17.0 ± 0.30 | 17.6 ± 0.19 | 17.1 ± 0.46 |
Data are shown as mean ± SEM. n = 6 per group. *p < 0.05 compared to Control (vehicle; Cremophor 5%). For organ evaluation, values were expressed as organs index corresponding to division of organ weight (mg) by the body weight of the animal (g).
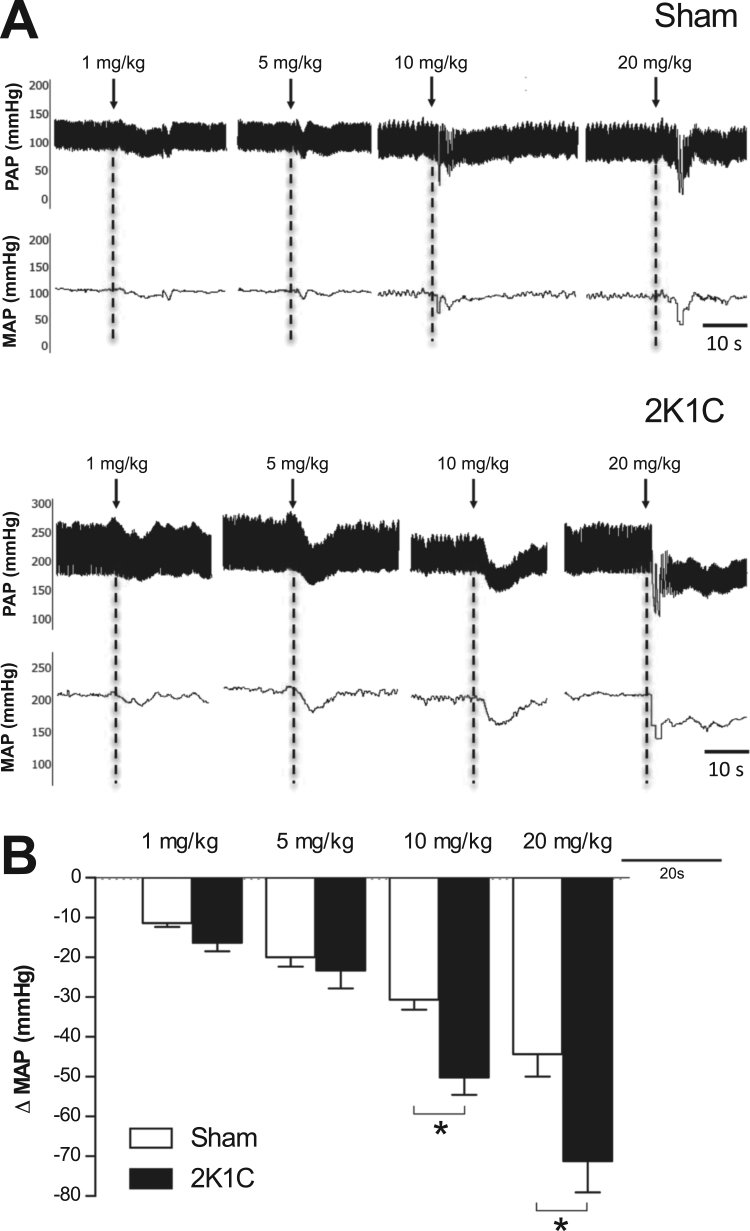
3.6. Acute treatment with NDHP reduces blood pressure in 2K1C hypertensive rats
Six weeks after 2K1C surgery, baseline MAP was increased compared with sham-operated rats (172 ± 10 vs. 117 ± 2 mmHg, n = 8, p < 0.05) as shown by representative tracings from sham-operated and 2K1C-hypertensive rats (Fig. 6A). In normotensive rats, acute intravenous administration of NDHP (1; 5; 10; 20 mg/kg) decreased blood pressure (−11 ± 1; −19 ± 2; −28 ± 2; −44 ± 5 mmHg, respectively) (Fig. 6B). NDHP (1; 5; 10; 20 mg/kg) reduced blood pressure dose-dependently in conscious hypertensive rats (−16 ± 3; −23 ± 4; −50 ± 1; and −71 ± 8 mmHg, respectively) (Fig. 6B).
Fig. 6.
Acute cardiovascular effects of NDHP in normotensive and hypertensive rats. Representative tracings (A) and grouped data on changes in mean arterial pressure (Δ MAP) in normotensive sham-operated and hypertensive-2K1C rats (B) following intravenous administration of NDHP (1, 5, 10 and 20 mg/kg). Data are shown as mean ± SEM. n = 8 per group. *p < 0.05 between indicated groups.
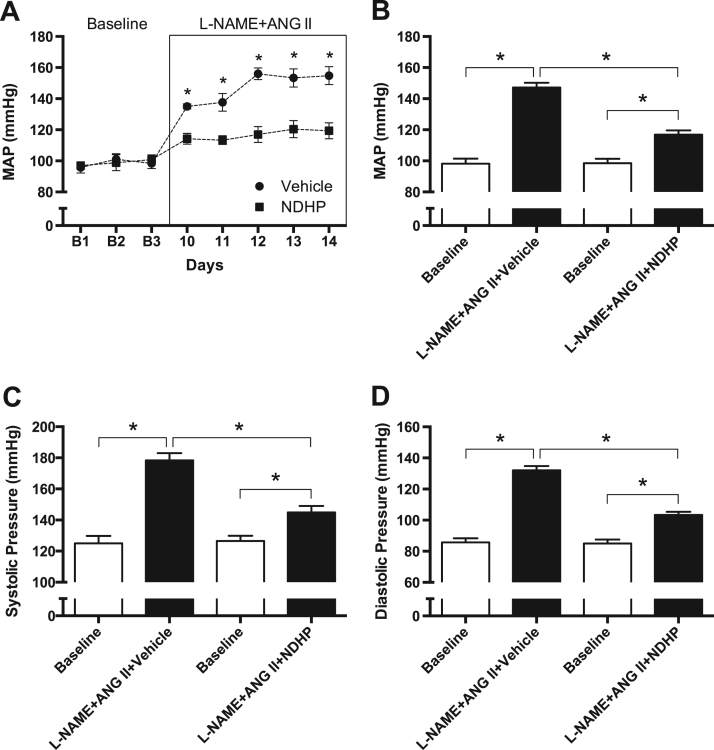
3.7. Chronic treatment with NDHP attenuates the progression of L-NAME and ANG II-induced hypertension
Before any treatment was started (i.e., Baseline), there were no differences in body weight (Supplemental Fig. 1A), blood pressure or heart rate between the vehicle group (MAP: 98 ± 2 mmHg, HR: 366 ± 14 bpm) and the NDHP group (MAP: 99 ± 3 mmHg, HR: 349 ± 16 bpm). Chronic treatment with L-NAME and ANG II significantly increased blood pressure in both groups compared with baseline (∆MAPvehicle: 49 mmHg; ∆MAPNDHP: 18 mmHg). However, simultaneous treatment with NDHP significantly attenuated the development of hypertension (Fig. 7A-D). A significant reduction of heart rate was observed in the vehicle group, and a clear trend was observed in the NDHP treated rats (Supplemental Fig. 1B). No differences in heart and kidney weights were observed between the vehicle and NDHP groups following chronic treatment with L-NAME and ANG II. Plasma nitrate was significantly higher in NDHP-treated rats with L-NAME+ANG II compared with placebo-treated rats (21.6 ± 2.9 vs 4.4 ± 1.1 µM, p < 0.05), whereas no significant difference was observed for nitrite (0.416 ± 0.156 vs 0.179 ± 0.051 µM) (Supplemental Fig. 2).
Fig. 7.
Cardiovascular effects of NDHP in a model of hypertension in rats. Effects of chronic treatment with NDHP in an in vivo model of L-NAME and ANG II-induced hypertension. Mean arterial pressure (A-B), systolic pressure (C) and diastolic pressure (D) during baseline (B1-B3) and during treatment with NOS inhibition (L-NAME, 0.5 g/L in drinking water) and infusion with angiotensin II (ANG II; 120 ng/kg/min) on Days 10–14. Data are shown as mean ± SEM (n = 8/group). In Panel A, *p < 0.05 between vehicle and NDHP group. In Panels C-D, *p < 0.05 between the indicated groups.
3.8. Chronic treatment with NDHP attenuates the development of endothelial dysfunction in rats with L-NAME + ANG II-induced hypertension
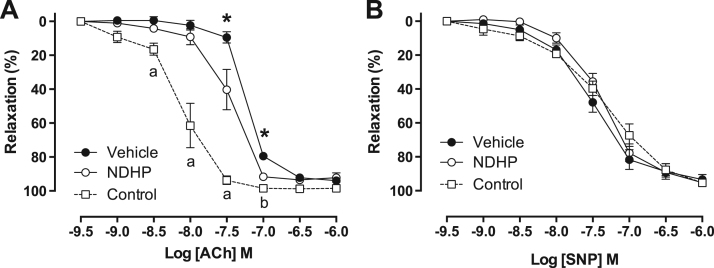
The contraction of mesenteric resistance arteries in response to PHE and ANG II were similar between NDHP- and vehicle-treated rats (Supplemental Fig. 3A-B). The sensitivity to ACh was significantly reduced in vehicle-treated rats compared with NDHP treatment (Fig. 8A), whereas similar vasorelaxation was observed to the NO donor SNP (Fig. 8B). Taken together, these findings suggest that NDHP, at least in part, can preserve endothelial function in this experimental model of hypertension.
Fig. 8.
Vascular responses in rats with L-NAME and ANG II-induced hypertension. Concentration-response curves in small mesenteric artery rings from healthy rats (Control) and rats treated with L-NAME and ANG II together with either vehicle or NDHP for two weeks. Acetylcholine-mediated vasorelaxation was stronger in rings from NDHP treated rats compared with vehicle (A), whereas no differences were observed in response to sodium nitroprusside (SNP) (D). Results are expressed as % of phenylephrine-mediated contraction, and shown as mean ± SEM. n = 7–8 per group. *p < 0.05 between NDHP versus vehicle. a p < 0.05 between Control and both vehicle or NDHP groups; b p < 0.05 between Control and vehicle group.
4. Discussion
Cardiovascular disease, including hypertension, is the main cause of death worldwide, and the therapeutic value of new strategies to increase NO signaling in the cardiovascular system have previously been discussed [35]. Several NO donors and organic nitrates have been studied in the past [16], [17], [18], [19], [20], [21], [22], but have been associated with adverse effects mainly due to short half-life and unspecific location for NO release, as well as the development of tolerance with repeated dosing [35], [36]. Due to the need for new NO donors with better pharmacological properties, we recently synthesized and characterized 1,3-bis(hexyloxy)propan-2-yl nitrate (NDHP) [27]. This novel organic mononitrate generates NO, which is catalyzed by XOR, and the effects of NDHP were not subjected to development of tolerance [27]. The present study extends our previous findings, demonstrating that acute treatment with NDHP mediated vasorelaxation and significant reduction of blood pressure in hypertensive animals. Moreover, we show that chronic treatment with NDHP can attenuate the progression of hypertension and endothelial dysfunction in a model of cardiovascular disease.
In this study we further evaluated the underlying mechanism(s) of NO formation and investigated the acute and chronic effects of NDHP in two animal models of hypertension (i.e. 2K1C and L-NAME+ANG II). Optical measurement techniques of NO using diaminofluorescenin (DAF) or other NO-sensitive dyes are useful in locating anatomically where NO is being produced [37]. Based on the assumption that the NDHP may generate NO in vitro, we quantified the intracellular NO production in vascular smooth muscle cells (VSMCs) of small mesenteric artery from normotensive rats using the probe DAF-2 DA. Mesenteric artery cross sections, stimulated with NDHP and incubated with DAF-2 DA, showed increased fluorescence emitted by the probe. Since the vascular endothelium is the main physiological source of NO, the eNOS blocker L-NNA was used to investigate the possible participation of this enzyme in NDHP-mediated NO production. Pre-treatment with L-NNA did not prevent the increase of fluorescence emission in both NDHP-treated tissues and tissues treated with another known NO donor, SIN-1.
Next we showed that NDHP promoted vasodilatation of small mesenteric arteries from normotensive rats. Our data showed that NDHP-mediated vasorelaxation was potentiated in vessels without endothelium or when eNOS was blocked with L-NAME. This interaction requires further investigation, but suggests that eNOS negatively modulates the effect of NDHP. This is in agreement with a previous study by Potje et al. [38], showing that endothelium removal or pharmacological inhibition of NOS increases the effects of the NO donor TERPY (metal complexes of ruthenium) in Wistar rat aortas, but not in spontaneous hypertensive rats (SHR). In addition, we speculate that by removing the endothelium, NADPH oxidase(s) expressed on endothelial cells are also removed, therefore basal superoxide generation is reduced and the bioavailability of NO donated by the compound is increased. In fact, it might not be only the presence of eNOS per se, but rather the lack of NADPH oxidase that influence the profile of the vasorelaxation curves elicited by NDHP. Taken together, our results show that NDHP-mediated NO release and vasorelaxation is endothelium-independent, which may be associated with favorable effects during cardiovascular disease when eNOS function is compromised.
Our previous and current data indicate that NDHP undergoes a metabolization process with consequent release of NO in VSMCs without the participation of eNOS. However, it is not possible from our ex vivo vessel studies with the DAF-FM probe to determine the absolute NO levels due to several technical limitations, such as irregular distribution of DAF in tissues or cells, interference from other chemical compounds, photoactivation and photobleaching [39], [40], [41]. Some NO-like compounds release NO spontaneously or non-enzymatically, while others require enzymatic catalysis to release NO [42]. Several enzymes play important biological roles in the release of NO from NO-donors, including, aldehyde oxidase and XOR [43], [44]. In agreement with our previous study [27] the current study demonstrates that NDHP-mediated NO production required functional XOR activity.
Next we aimed to further investigate the underlying mechanism for endothelium-independent vasorelaxation by NDHP. VSMCs express several plasma membrane K+ channels, which may be modulated by NO. Organic nitrates-mediate NO release in VSMCs and can activate the sGC-cGMP-PKG pathway leading to hyperpolarization and consequently decreased Ca2+ in the cell [45], [46]. Important targets of PKG which promote relaxation of VSMCs are the K+ channels, including voltage-dependent K+ channels (Kv), large-conductance Ca2+-activated K+ channels (BKCa), inwardly-rectifying K+ channels (KIR), ATP-sensitive K+ (KATP) channels [47], [48], [49], [50]. In order to investigate the involvement of K+ channels, preparations of mesenteric artery were incubated with above mentioned K+ channels inhibitors. Using TEA (3 mM), a decrease in the potency of NDHP was observed, suggesting that some of these channels may be involved in the compound-induced vasorelaxant effect. From these results we set out to investigate the different subtypes of K+ channels by using specific blockers. As manifested by the rightward shift of the concentration-response curve, TEA (1 mM) and 4-AP (1 mM) decreased the effect of NDHP. This suggested participation of Kv and BKCa channels, respectively. Conversely, GLIB or BaCl2 did not influence the vasorelaxant effect induced by NDHP, demonstrating no involvement of the KATP or the KIR channels.
These promising ex vivo findings with NDHP encouraged in vivo studies to assess the potential therapeutic effects in models of hypertension. Increasing doses of NDHP were used to evaluate the acute cardiovascular effects in normotensive animals and in rats with 2K1C-induced hypertension. Renovascular hypertension is considered to be the most frequent cause of secondary hypertension. The 2K1C renovascular hypertension model is associated with increased plasma renin activity and circulating ANG II concentration, increased sympathetic tone, uncoupling of eNOS and oxidative stress in specific organs [51], [52], [53]. Our findings showed that intravenous administration of NDHP reduced blood pressure in a dose-dependent manner in both groups. However, the observed blood pressure-lowering effect was more pronounced in hypertensive animals.
In order to assure safety for NDHP treatment an acute toxicity test was performed. Two high doses of NDHP were selected in this toxicity study (i.e. 300 and 2000 mg/kg body weight), to investigate the risk of lethality or the presence of any adverse effects. According to the behavioral parameters evaluated, it was observed that NDHP presented analgesia and decreased ambulation at the highest dose. Studies have shown that NO has important actions in the central nervous system. For example NO influences peripheral somatosensory actions antinociceptive by acting on specific channels in neurons [54], [55], which may explain the NDHP-mediated CNS depressant effect at higher doses. No death of the animals was observed for any of the tested doses, and NDHP had no significant effect on the body weight development, food or water intake, or on organ weights during the 14 days of evaluation compared with the placebo-treated control group.
Since our toxicity studies did not reveal any adverse effects of NDHP, we finally assessed the therapeutic value of chronic treatment with NDHP in an experimental model of endothelial dysfunction and hypertension. Supporting the acute blood pressure lowering effects of NDHP, we also observed that chronic treatment with NDHP attenuated L-NAME and ANG II induced hypertension. As expected, markers of NO homeostasis (i.e. nitrate and nitrite) were increased in rats treated with NDHP, which is consistent with the idea of NO generation from NDHP. Moreover, our findings from isolated vessels suggested that chronic treatment with NDHP, at least in part, prevented the development of endothelial dysfunction in this disease model.
Limitations of the current study include the use of different administration routes for the acute and chronic studies, the use of a single species (rat) and that additional doses of NDHP will be necessary to investigate its favorable cardiovascular effects. Future investigations are warranted to investigate the role of XOR in mediating the bioactivation of NDHP in vivo, and also to elucidate the role of different K+ channels modulating the vasorelaxant and antihypertensive effect of NDHP.
In conclusion, our data show that NDHP is a novel NO donor that induces vasorelaxation via activation of NO/cGMP/PKG pathway and specific K+ channels (Kv and BKca), and also exerts blood pressure lowering effects in hypertensive rats. The therapeutic value of this new organic mononitrate will be further evaluated in additional experimental studies and in planned clinical trials.
Acknowledgements
We thank Carina Nihlén, Annika Olsson and Margareta Stensdotter (Karolinska Institutet, Stockholm) for their technical assistance.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
Sources of funding
This work was supported by grants from the Swedish Heart and Lung Foundation (Dnr: 20140448 and 20170124 M.C.), the Swedish Research Council (Dnr: 2016-01381 M.C.), the William Harvey Research Institute Academy (Dnr: 608765 M.F.M.), Karolinska Institutet KID Grant (Dnr: 2‐3707/2013 M.C.), CAPES/STINT (Dnr: 1499/2014 V.A.B. & BR2013-5512 M.C.), the Brazilian National Council for Scientific and Technological Development (CNPq; Dnr: 304772/2014-3 V.A.B.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.12.004.
Appendix A. Supplementary material
Supplementary material
References
- 1.Harrison D.G., Marvar P.J., Titze J.M. Vascular inflammatory cells in hypertension. Front. Physiol. 2012;3 doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friso S., Carvajal C.A., Fardella C.E., Olivieri O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl. Res. 2015;165:154–165. doi: 10.1016/j.trsl.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Nascimento E.R., Maia A.C.O., Nardi A.E., Silva A.C. Sexual dysfunction in arterial hypertension women: the role of depression and anxiety. J. Affect. Disord. 2015;181:96–100. doi: 10.1016/j.jad.2015.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Griendling K.K., FitzGerald G.A. Oxidative Stress and Cardiovascular Injury Part I: basic Mechanisms and In Vivo Monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 5.Pereira A.C., Paulo M., Araujo A.V., Rodrigues G.J., Bendhack L.M. Nitric oxide synthesis and biological functions of nitric oxide released from ruthenium compounds. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Med. E Biol. 2011;44:947–957. doi: 10.1590/s0100-879x2011007500084. [DOI] [PubMed] [Google Scholar]
- 6.Kang N., Lee J.H., Lee W.W., Ko J.Y., Kim E.A., Kim J.S., Heu M.S., Kim G.H., Jeon Y.J. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ. Toxicol. Pharmacol. 2015;39:764–772. doi: 10.1016/j.etap.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S., Palmer R.M.J., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 8.Alzawahra W.F., Talukder M.A.H., Liu X., Samouilov A., Zweier J.L. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H499–H508. doi: 10.1152/ajpheart.00374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.L.J. Ignarro, Nitric Oxide: Biology and Pathobiology, 2009. 〈http://dx.doi.org/10.1017/CBO9781107415324.004〉.
- 10.Torregrossa A.C., Aranke M., Bryan N.S. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J. Geriatr. Cardiol. 2011;8:230–242. doi: 10.3724/SP.J.1263.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loscalzo J. The identification of nitric oxide as endothelium-derived relaxing factor. Circ. Res. 2013;113:100–103. doi: 10.1161/CIRCRESAHA.113.301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupp C., Baasner S., Ince C., Nocken F., Stover J.F., Westphal M. Differentiated control of deranged nitric oxide metabolism: a therapeutic option in sepsis? Crit. Care. 2013;17:311. doi: 10.1186/cc12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treuer A.V., Gonzalez D.R. Nitric oxide synthases, S-nitrosylation and cardiovascular health: from molecular mechanisms to therapeutic opportunities (Review) Mol. Med. Rep. 2015;11:1555–1565. doi: 10.3892/mmr.2014.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolotina V.M., Najibi S., Palacino J.J., Pagano P.J., Cohen R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 15.Félétou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br. J. Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feelisch M. The use of nitric oxide donors in pharmacological studies. Naunyn. Schmiede. Arch. Pharmacol. 1998;358:113–122. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- 17.Queiroz T.M., Mendes-Júnior L.G., Guimarães D.D., França-Silva M.S., Nalivaiko E., Braga V.A. Cardiorespiratory effects induced by 2-nitrate-1,3-dibuthoxypropan are reduced by nitric oxide scavenger in rats. Auton. Neurosci. Basic Clin. 2014;181:31–36. doi: 10.1016/j.autneu.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Naseem K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Thatcher G. Nitric oxide mimetic molecules as therapeutic agents in Alzheimer's disease. Curr. Alzheimer Res. 2005;2:1–11. doi: 10.2174/1567205053585945. [DOI] [PubMed] [Google Scholar]
- 20.França-Silva M.S., Balarini C.M., Cruz J.C., Khan B.A., Rampelotto P.H., Braga V.A. Organic nitrates: past, present and future. Molecules. 2014;19:15314–15323. doi: 10.3390/molecules190915314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshpande S.R., Satyanarayana K., Rao M.N.A., Pai K.V. Nitric oxide modulators: an emerging class of medicinal agents. Indian J. Pharm. Sci. 2012;74:487–497. doi: 10.4103/0250-474X.110572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porpino S.K.P., Zollbrecht C., Peleli M., Montenegro M.F., Brandão M.C.R., Athayde-Filho P.F., França-Silva M.S., Larsson E., Lundberg J.O., Weitzberg E., Persson E.G., Braga V.A., Carlström M. Nitric oxide generation by the organic nitrate NDBP attenuates oxidative stress and angiotensin II-mediated hypertension. Br. J. Pharmacol. 2016:2290–2302. doi: 10.1111/bph.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laursen J.B., Mulsch A., Boesgaard S., Mordvintcev P., Trautner S., Gruhn N., NielsenKudsk J.E., Busse R., Aldershvile J. In vivo nitrate tolerance is not associated with reduced bioconversion of nitroglycerin to nitric oxide. Circulation. 1996;94:2241–2247. doi: 10.1161/01.cir.94.9.2241. [DOI] [PubMed] [Google Scholar]
- 24.Kosmicki M.A. Long-term use of short- and long-acting nitrates in stable angina pectoris. Curr. Clin. Pharmacol. 2009;4:132–141. doi: 10.2174/157488409788185016. [DOI] [PubMed] [Google Scholar]
- 25.Ignarro L.J., Napoli C., Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ. Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 26.Omar S.A., Artime E., Webb A.J. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide – Biol. Chem. 2012;26:229–240. doi: 10.1016/j.niox.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Zhuge Z., Paulo L.L., Jahandideh A., Brandão M.C.R., Athayde-Filho P.F., Lundberg J.O., Braga V.A., Carlström M., Montenegro M.F. Synthesis and characterization of a novel organic nitrate NDHP: role of xanthine oxidoreductase-mediated nitric oxide formation. Redox Biol. 2017;13:163–169. doi: 10.1016/j.redox.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.das L., Mendes-Júnior G., Guimarães D.D., Gadelha D.D.A., Diniz T.F., Brandão M.C.R., Athayde-Filho P.F., Lemos V.S., do M., França-Silva S., Braga V.A. The new nitric oxide donor cyclohexane nitrate induces vasorelaxation, hypotension, and antihypertensive effects via NO/cGMP/PKG pathway. Front. Physiol. 2015;6 doi: 10.3389/fphys.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peleli M., Zollbrecht C., Montenegro M.F., Hezel M., Zhong J., Persson E.G., Holmdahl R., Weitzberg E., Lundberg J.O., Carlström M. Enhanced XOR activity in eNOS-deficient mice: effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radic. Biol. Med. 2016;99:472–484. doi: 10.1016/j.freeradbiomed.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 30.OECD OECD guidelines for the testing of chemicals, section 4, test No. 425: acute oral toxicity - up-and-down procedure. Guidel. Test. Chem. 2001:26. [Google Scholar]
- 31.A.C.C. Almeida R.N., Falcão A.C.G.M., Diniz R.S.T., Quintanas-Júnior L.J., Polari R.M., Barbosa-Filho J.M., Agra M.F., Duarte J.C., Ferreira C.D., Antoniolli A.R. Metodologia para avaliação de plantas com atividade no Sistema Nervoso central e alguns dados experimentais. Rev. Bras. Farm. 1999;80:72–76. [Google Scholar]
- 32.Hezel M., Peleli M., Liu M., Zollbrecht C., Jensen B.L., Checa A., Giulietti A., Wheelock C.E., Lundberg J.O., Weitzberg E., Carlström M. Dietary nitrate improves age-related hypertension and metabolic abnormalities in rats via modulation of angiotensin II receptor signaling and inhibition of superoxide generation. Free Radic. Biol. Med. 2016;99:87–98. doi: 10.1016/j.freeradbiomed.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Peleli M., Flacker P., Zhuge Z., Gomez C., Wheelock C.E., Persson A.E.G., Carlstrom M. Renal denervation attenuates hypertension and renal dysfunction in a model of cardiovascular and renal disease which is associated with reduced NADPH and xanthine oxidase activity. Redox Biol. 2017;13:522–527. doi: 10.1016/j.redox.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X., Yang T., Liu M., Peleli M., Zollbrecht C., Weitzberg E., Lundberg J.O., Persson A.E.G., Carlstrom M. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension. 2015;65:161–170. doi: 10.1161/HYPERTENSIONAHA.114.04222. [DOI] [PubMed] [Google Scholar]
- 35.Lundberg J.O., Gladwin M.T., Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 36.Münzel T., Daiber A., Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ. Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 37.Buerk D.G., Barbee K.A., Jaron D. Nitric oxide signaling in the microcirculation. Crit. Rev. Biomed. Eng. 2011;39:397–433. doi: 10.1615/critrevbiomedeng.v39.i5.40. http://www.ncbi.nlm.nih.gov/pubmed/22196161%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = PMC3608675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potje S.R., Munhoz F.C., Perassa L.A., Graton M.E., Pereira A.A.F., Nakamune A.C.M.S., Da Silva R.S., Bendhack L.M., Sumida D.H., Antoniali C. Mechanisms underlying the hypotensive and vasodilator effects of Ru(terpy)(bdq)NO]3+, a nitric oxide donor, differ between normotensive and spontaneously hypertensive rats. Eur. J. Pharmacol. 2014;741:222–229. doi: 10.1016/j.ejphar.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez J., Specian V., Maloney R., Jourd’Heuil D., Feelisch M. Performance of diamino fluorophores for the localization of sources and targets of nitric oxide. Free Radic. Biol. Med. 2005;38:356–368. doi: 10.1016/j.freeradbiomed.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Planchet E., Kaiser W.M. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: a comparison using abiotic and biotic NO sources. J. Exp. Bot. 2006;57:3043–3055. doi: 10.1093/jxb/erl070. [DOI] [PubMed] [Google Scholar]
- 41.Bryan N.S., Grisham M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achike F.I., Kwan C. Brief Review Nitric Oxide, Human Diseases and the Herbal Products That Affect the Nitric Oxide Signalling Pathway. Clin. Exp. Pharmacol. Physiol. 2003:605–615. doi: 10.1046/j.1440-1681.2003.03885.x. [DOI] [PubMed] [Google Scholar]
- 43.Maia L.B., Moura J.J.G. Nitrite reduction by xanthine oxidase family enzymes: a new class of nitrite reductases. J. Biol. Inorg. Chem. 2011;16:443–460. doi: 10.1007/s00775-010-0741-z. [DOI] [PubMed] [Google Scholar]
- 44.Kim-Shapiro D.B., Gladwin M.T. Mechanisms of nitrite bioactivation. Nitric Oxide – Biol. Chem. 2014;38:58–68. doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Follmann M., Griebenow N., Hahn M.G., Hartung I., Mais F.J., Mittendorf J., Schäfer M., Schirok H., Stasch J.P., Stoll F., Straub A. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew. Chem. – Int. Ed. 2013;52:9442–9462. doi: 10.1002/anie.201302588. [DOI] [PubMed] [Google Scholar]
- 46.Carlström M., Liu M., Yang T., Zollbrecht C., Huang L., Peleli M., Borniquel S., Kishikawa H., Hezel M., Persson A.E.G., Weitzberg E., Lundberg J.O. Cross-talk between nitrate-nitrite-NO and NO synthase pathways in control of vascular NO homeostasis. Antioxid. Redox Signal. 2014;0:1–12. doi: 10.1089/ars.2013.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quayle J.M., Standen N.B. KATP channels in vascular smooth muscle. Cardiovasc. Res. 1994;28:797–804. doi: 10.1093/cvr/28.6.797. http://ezproxy.lib.le.ac.uk/login?url = http://ovidsp.ovid.com/ovidweb.cgi?T = JS&CSC = Y&NEWS = N&PAGE = fulltext&D = med3&AN = 7923282%5Cnhttp://openurl.ac.uk/ukfed:le.ac.uk/?sid = OVID:medline&id = pmid:7923282&id = doi:&issn = 0008-6363&isbn = &volume = 28&issue = 6&spage = 797&pa [DOI] [PubMed] [Google Scholar]
- 48.Jackson W.F. Potassium channels and regulation of the microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- 49.Chrissobolis S., Sobey C.G. Inwardly rectifying potassium channels in the regulation of vascular tone. Curr. Drug Targets. 2003;4:281–289. doi: 10.2174/1389450033491046. [DOI] [PubMed] [Google Scholar]
- 50.Goonetilleke L., Quayle J. TREK-1 K+ channels in the cardiovascular system: their significance and potential as a therapeutic target. Cardiovasc. Ther. 2012;30:e23–e29. doi: 10.1111/j.1755-5922.2010.00227.x. http://ovidsp.ovid.com/ovidweb.cgi?T = JS&CSC = Y&NEWS = N&PAGE = fulltext&D = emed14&AN = 364156012 [DOI] [PubMed] [Google Scholar]
- 51.Nishi E.E., Ribeiro Campos R., Toledo Bergamaschi C., Rossi de Almeida V., Araki Ribeiro D. Vitamin C prevents DNA damage induced by renovascular hypertension in multiple organs of Wistar rats. Hum. Exp. Toxicol. 2010;29:593–599. doi: 10.1177/0960327109358267. [DOI] [PubMed] [Google Scholar]
- 52.Maia R.C.A., Sousa L.E., Santos R.A.S., Silva M.E., Lima W.G., Campagnole-Santos M.J., Alzamora A.C. Time-course effects of aerobic exercise training on cardiovascular and renal parameters in 2K1C renovascular hypertensive rats. Braz. J. Med. Biol. Res. 2015;48:1010–1022. doi: 10.1590/1414-431X20154499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveira-Sales E.B., Colombari E., Abdala A.P., Campos R.R., Paton J.F.R. Sympathetic overactivity occurs before hypertension in the two-kidney, one-clip model. Exp. Physiol. 2016;101:67–80. doi: 10.1113/EP085390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cury Y., Picolo G., Gutierrez V.P., Ferreira S.H. Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide - Biol. Chem. 2011;25:243–254. doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Gamper N., Ooi L. Redox and nitric oxide-mediated regulation of sensory neuron ion channel function. Antioxid. Redox Signal. 2015;22:486–504. doi: 10.1089/ars.2014.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material