Abstract
Background
The main aim of this study was to use proton Magnetic Resonance Spectroscopy (MRS) to identify brain biomarkers for emotional dysregulation in youth as measured by subscales of the Child Behavior Checklist (CBCL).
Methods
We measured glutamate (Glu) concentrations in the anterior cingulated cortex (ACC) of 37 pediatric subjects (aged 6-17 years) using high field (4.0 Tesla) proton Magnetic Resonance Spectroscopy (MRS). Subjects were grouped based on combined T scores on three subscales (Anxiety/Depression, Aggression and Attention) of the CBCL previously associated with deficits in the regulation of emotion. Subjects were stratified into those with high (>180) (N=10) and low (<180) (N=27) scores.
Limitations
Limitations include small sample size, wide age range studied, focus on Anterior Cingulate Cortex (ACC) only, and that some subjects received psychopharmacological treatments.
Results
We found a statistically significant correlation between Glu levels in the ACC and CBCL dysregulation profile scores among subjects with high dysregulation profile scores.
Conclusions
These results suggest that glutamatergic dysregulation in the ACC may represent a useful biomarker of emotional dysregulation in youth. Further investigation into the causality, time line and utility as a predictive metric is warranted.
Introduction
Despite ongoing controversy on how to best categorize emotional volatility in the young, there is no debate that a sizeable minority of youth is affected with various forms of emotional regulation deficits which are associated with high levels of morbidity and disability (1-8). Recent efforts at operationalizing emotional regulation deficits have relied on the Child Behavior Checklist (CBCL), a paper and pencil empirically derived scale with excellent psychometric properties (9-18).
Recent work by our group and others have documented that a profile consisting of marked (>2SD) elevations of three of the CBCL clinical scales (Anxiety/Depression, Aggression and Attention [A-A-A profile]) was associated with very severe morbidity and dysfunction including suicidality and need for hospitalization, regardless of diagnosis, hence termed by some the “dysregulation profile” (6, 19-28). The same profile has also been associated with increased likelihood to satisfy diagnostic criteria on structured diagnostic interview for bipolar disorder (29, 30) and hence termed the CBCL-Juvenile bipolar profile. More recent work has linked an intermediate profile characterized by moderate scores (>1 SD) on the same CBCL scales with deficient emotional self regulation (DESR). However, whether deficits in emotional regulation are associated with unique biomarkers remains unknown.
One approach non-invasively to identify brain bio-markers is magnetic resonance imaging neuroimaging methodology. Proton Magnetic Resonance Spectroscopy (1HMRS) examines brain biochemistry in various regions of the brain and allows in vivo quantification of metabolic changes including those related to glutamate (Glu), the most abundant neurotransmitter in the brain. However, Glu analysis via 1HMRS is challenging due to its J-coupled multiple resonance patterns and overlapping resonances from other metabolites primarily glutamine (Gln). Although separating glutamate from glutamine levels can help in understanding the pathophysiology of various psychopathological states, field strengths less than 2.0 Tesla do not allow to resolve the resonances of Glu and Gln. Thus, often times the composite peak (Glx) is reported rather than the individual Glu and Gln levels (31).
A number of prior studies have linked abnormalities in Glx to mood disorders. In bipolar disorder, almost all studies report elevated Glx independent of disease state (32-39). However, as the majority of these previous 1HMRS results come from 1.5 Tesla strength imagers, few studies have quantified glutamate and glutamine separately. Furthermore, children and adolescents have been relatively understudied in general as well as with high field strength magnets.
The main aim of this study was to use 1HMRS to identify biomarkers of emotional regulation deficits in youth using a high field scanner capable of differentiating Glu from other metabolites. To this end, we conducted a 4.0 T proton Magnetic Resonance Spectroscopy study focusing on the anterior cingulate cortex (ACC) in 37 youth with high (>1SD) and low (<1SD) score on the CBCL A-A-A profile. The ACC was chosen because of its importance in cognitive and emotional regulation and because previous studies have reported neurometabolite abnormalities in mood disordered youth in the ACC (40-42). We hypothesized that Glu may represent a useful biomarker of emotional dysregulation in youth and that higher Glu levels would predict more emotional regulation deficits as indicated by higher CBCL scores. To the best of our knowledge this is the first examination of biomarkers of emotional regulation deficits in youth using a high field 1HMRS scan.
Methods
Participants
The 37 participants were ages 6-17 years old and either probands (N=24) or controls (N=13) from a high risk offspring study of youth (6-24 years) recruited based on having a parent with bipolar disorder or in the case of controls, without a family history of mood disorder or personal history of mood disorder or major psychiatric disorder. Controls were recruited to match the age and sex of the high risk sample. For this high risk offspring study 91 potential participants were screened, 61 met the inclusion and exclusion criteria, and of this group 37 were aged 6-17 years and had a completed CBCL. Participants were recruited from advertisements to the public in the local media as well from the Massachusetts General Hospital Pediatric Psychopharmacology Clinic and Research Program. Exclusion criteria included clinically significant chronic medical conditions, organic brain disorders, documented mental retardation, phobia of small spaces, contraindication to MRI including presence of metal or surgical devices, and pregnancy. Female participants of child bearing potential received a urine pregnancy test prior to scanning.
Procedures
Prior to enrollment, participants were screened by phone to describe study procedures and evaluate study eligibility. Study procedures were approved by the MGH and McLean Hospital human subjects Internal Review Boards (IRBs). Consent was obtained from a parent and the child provided written assent. Participants were compensated for their participation. Only anonymous de-identified data are presented.
Prior to scanning, all subjects were assessed diagnostically using the Kiddie Schedule for Affective Disorders and Schizophrenia, Epidemiologic Version for DSM-IV (K-SADS-E) (43). In addition to a diagnostic interview, participants were assessed using clinician-administered measures of mania and depression: the Young Mania Rating Scale (YMRS) (44) and the Child Depression Rating Scale (CDRS) (45). These measures were administered by board-certified child and adult psychiatrists who had been trained to reliability. Socioeconomic Status (SES) was assessed using the Hollingshead Socioeconomic Status scale. IQ was assessed using the Wechsler Abbreviated Scale of Intelligence Scale (WASI) (46) Vocabulary and Matrix Reasoning subtests.
Parents (usually the mother) completed the Child Behavior Checklist (CBCL) (9). T scores from subscales of interest included the Anxiety/Depression, Aggression and Attention subscales (CBCL A-A-A). Due to small sample size, 37 subjects (N=13 healthy comparison participants and N=24 high-risk offspring) were grouped into two groups based on their T-scores on the CBCL A-A-A profile: high score group (>180) (N=10) and low score group (<180) (N=27). The 10 subjects in the high score group included only high risk offspring. The 27 subjects in the low score group comprised all 13 healthy controls and 14 high risk offspring. A T-score of 60 is one standard deviation from normal based on well established norms for the CBCL. The high score group (>180) reflects subjects whose scores on the three subscales on average are at least one standard deviation from normal. A T-score of 60 or greater is considered to be of clinical concern and thus the high score group comprises subjects with a clinical picture generally meeting standards for psychiatric intervention.
Imaging Procedures
Data acquisition was performed on a 4.0 T Varian Unity/Inova whole body MR scanner (Varian NMR Instruments, Palo Alto, CA) equipped with proton volumetric head coil. The MR protocol consisted of anatomical and spectral data acquisitions. Anatomical MR images were used for patient positioning, voxel localization and tissue segmentation. Spectral data were acquired from a 2cm×2cm×2cm voxel localized on the ACC using PRESS (point-resolved spectroscopic sequence) (TR=2000ms, TE=30ms, number of averages=128, acquisition time<5 minutes). Manual shimming within the voxel produced unsuppressed water signal linewidths of less than 11 Hz. A systematic approach to voxel positioning was used in all subjects. Voxels were placed on the ACC on midsagittal T1-weighted images, anterior to genu of the corpus callosum, and positioned on the midline on axial images.
MRS Data Processing
All MRS processing was conducted blinded to diagnosis and group assignment. The automated spectral-fitting package LC Model (version 6.2-1F) and the standard vendor-supplied simulated basis set were used for quantification of metabolite concentrations (Figure 1).
Figure 1.
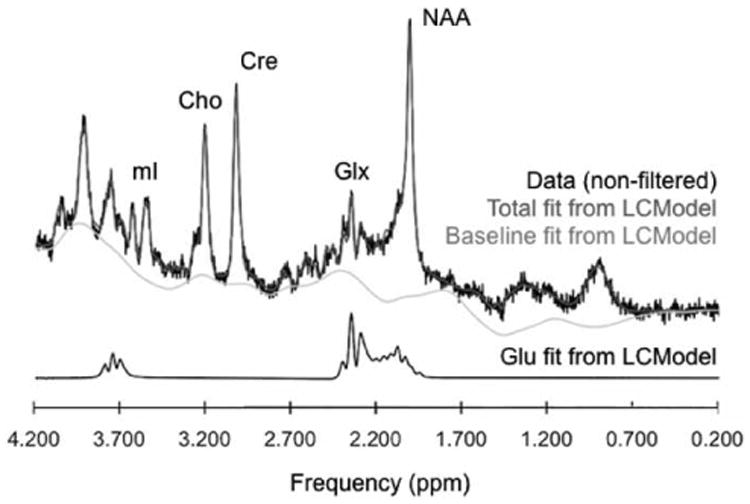
Representative proton magnetic resonance spectrum of the anterior cingulate cortex at 4 Tesla collected at TE=30ms with a point-resolved spectroscopy sequence along with spectra of Glu. The real part of the frequency-domain data (phased and referenced FFT of raw input data with no smoothing) is plotted as the black curve. The red curve is the LCModel fit to this data. Also plotted as the gray curve is the baseline. Below is the fiting line for Glu only. Cho = Choline; Cr = Creatine; Glx = Glutamine + Glutamate; Glu = Glutamate; NAA = N-Acetyl Aspartate; mI = myo-inositol; ppm = parts per million.
The basis set included alanine, aspartate, creatine, phosphocreatine, gamma-aminobutyric acid, glucose, glutamine, glutamate, glycerophosphocholine, phosphocholine, myo-inositol, lactate, n-acetylaspartate, n-acetylaspartylglutamate, syllo-inositol and taurine. Data and fitting quality were visually verified and further assessed by the percent standard deviation of the estimated concentration of each metabolite (CRLB), linewidth (FWHM) and signal-to-noise (SNR), all calculated by LCModel. The results were presented in institutional units (I.U.) and no attempt was made to convert IU to absolute concentrations due to the lack of knowledge about the Glu T1 and T2 relaxation times. Glu levels were corrected for the cerebrospinal fluid (CSF) and gray matter (GM) fraction. Tissue-segmentation of T1-weighted images into GM, white matter (WM), and CSF was automatically done using an open source software, “NVM” (freely available from Neuromorphometrics, Inc. at http://neuromorphometrics.org:8080/nvm/).
Statistical Analysis
Statistical analysis was performed using the IBM SPSS software (version 19.0.0.1 for Macintosh). Chi-Squared tests (for categorical variables) and t-tests (for continuous variables) were used to compare demographic and clinical characteristics across groups (low score and high score). Correlation between the clinical index and metabolite levels was carried out with Pearson bivariate correlation as well as partial correlation controlling for age, sex, and medication status (on/off). All tests were two-tailed, except for correlation analysis. Since a directional prior hypothesis had been made, the correlations were evaluated with one-tailed tests. A p-value of < 0.05 was considered statistically significant.
Results
Subject characteristics are summarized in Table 1. Groups were comparable with respect to age, gender, IQ and socioeconomic status. YMRS, CDRS and CBCL A-A-A scores were statistically significantly higher in the high CBCL score group than the low CBCL score group.
Table 1. Demographic and Clinical Characteristics of Study Participants in Low versus High CBCL Score Groups.
| Subgroups | Low Score Group mean ± SD | High Score Group mean ± SD | Comparison (Low Score-Combined vs High Score Group) | ||
|---|---|---|---|---|---|
|
| |||||
| Controls (n=13) | High Risk Offspring (n=14) | Combined (n=27) | High Risk Offspring (n=10) | ||
|
| |||||
| Age (years) | 11.50 ± 3.90 | 12.04 ± 3.11 | 11.78 ± 3.56 | 11.50 ± 3.50 | NS: t = 0.212, d.f. = 35, p = 0.833 |
|
| |||||
| Males (N; %) | 9; 69 | 8; 57 | 17; 63 | 8; 80 | NS: d.f. = 1, χ2 = 0.967, p = 0.326 |
|
| |||||
| iQ | 103.00 ± 17.08 | 104.43 ± 12.66 | 10374 ± 14.51 | 106.44 ± 12.80 | NS: t = 0.497, d.f. = 34, p = 0.622 |
|
| |||||
| SES | 1.83 ± 0.58 | 2.23 ± 0.941 | 2.04 ± 0.81 | 1.90 ± 0.74 | NS: d.f. = 3, χ2 = 0.437, p = 0.932 |
|
| |||||
| YMRS | 0.17 ± 0.39 | 5.50 ± 7.47 | 2.96 ± 5.98 | 14.01 ± 11.03 | t = 3.006, d.f. = 11.025, p = 0.012 |
|
| |||||
| CDRS | 1775 ± 1.36 | 22.63 ± 8.42 | 20.30 ± 6.73 | 38.41 ± 12.25 | t = 4.432, d.f. = 11.077, p = 0.001 |
|
| |||||
| CBCL (T score A-A-A) | 154.38 ± 7.37 | 161.29 ± 10.49 | 157.96 ± 9.61 | 207.40 ± 15.51 | t = 11.688, d.f. =35, p < 0.001 |
|
| |||||
| Diagnoses (N) | |||||
| Bipolar Disorder | 0 | 5 | 5 | 8 | |
| Major Depression | 0 | 3 | 3 | 7 | |
| Generalized Anxiety Disorder | 0 | 0 | 0 | 5 | |
| Oppositional Defiant Disorder | 0 | 2 | 2 | 7 | |
| Conduct Disorder | 0 | 1 | 1 | 3 | |
| Attention Deficit Hyperactivity Disorder | 0 | 2 | 2 | 6 | |
|
| |||||
| Medication Classes (N) | |||||
| A typical Antipsychotics | 0 | 3 | 3 | 3 | |
| Antidepressants | 0 | 4 | 4 | 4 | |
| Stimulants | 0 | 0 | 0 | 1 | |
| Mood Stabilizers | 0 | 0 | 0 | 2 | |
| other | 0 | 0 | 0 | 4 | |
Continuous variables expressed as mean ± SD. YMRS, Young Mania Rating Scale; CDRS, Children's Depression Ratio Scale; CBCL, Child Behavior Checklist; SES, Socio-economic status; iQ, intelligence Quotient; NS, non-significant.
Four subjects (15%) in the low CBCL score group and seven subjects (70%) in the high CBCL score group (all high risk offspring subjects) were taking one or more types of medication at the time of scans. The medication class rates are shown in Table 1.
Good quality MRS data were obtained with low CRLB, high SNR and low FWHM from both groups as shown in Table 2. There were no between group differences in any of these measures. Unobstructed clear Glu peak is demonstrated in Figure 1.
Table 2. Magnetic Resonance Spectroscopy Data of Study Participants in Low versus High CBCL score groups.
| Low Score Control Group (n=13) | Low Score At Risk Group (n=14) | Low Score Group Combined (n=27) | High Score Group (n=10) | Comparison | |
|---|---|---|---|---|---|
| SNR | 1237 ± 5.02 | 11.60 ± 5.70 | NS: t = 0.400, d.f. = 35, p = 0.692 | ||
| FWHM (ppm) | 0.05 ± 0.01 | 0.05 ± 0.02 | NS: t = 0.514, d.f. = 35, p = 0.610 | ||
| Glu CRLB (%) | 10.04 ± 3.11 | 10.11 ± 2.89 | NS: t = 0.063, d.f. = 35, p = 0.950 | ||
| Mean Glu (i.U.) | 4.88 ± 2.07 | 6.00 ± 1.95 | 5.47 ± 2.05 | 5.45 ± 1.77 | NS: t = 0.016, d.f. = 35, p = 0.987 |
All variables expressed as mean ± SD. SNR, Signal to Noise Ratio; FWHM, Full Width at Half Max; CRLB, Cramer-Rao Lower Bound; Glu, Glutamate; NS, non-significant.
Within the low dysregulation profile score group, Glu levels were increased in high-risk offspring subjects (n=14; mean CBCL score=161.29±10.49; mean Glu level=6.00±1.95) when compared with the healthy controls (n=13; mean CBCL score=154.38±7.37; mean Glu level=4.88±2.07) but did not reach statistical significance (two sample t-test; t = 1.963, d.f. = 25, p = 0.06 (2-tailed). Hence control and high-risk offspring subjects in the low dysregulation profile score group have been combined into a single group and compared with the high dysregulation profile group.
Despite absence of statistically significant differences in Glu levels between the low and high dysregulation profile groups (Table 2), there was a positive correlation between glutamate levels with the CBCL dysregulation profile scores in the high score group (Pearson correlation=0.659, p=0.019 (1-tailed)) (Figure 2). This finding held true when partial correlation controlling for age, sex, and medication status (on/off) was carried out (correlation=0.759, p=0.024 (1-tailed), df=5). The CBCL-Glu correlation was not significant in the low score group (p=0.111 (1-tailed)) or in the total (low+ high score groups) dataset (p=0.170 (1-tailed)).
Figure 2. Study Participant CBCL A-A-A Scores (Low and High) versus ACC Glutamate Levels.
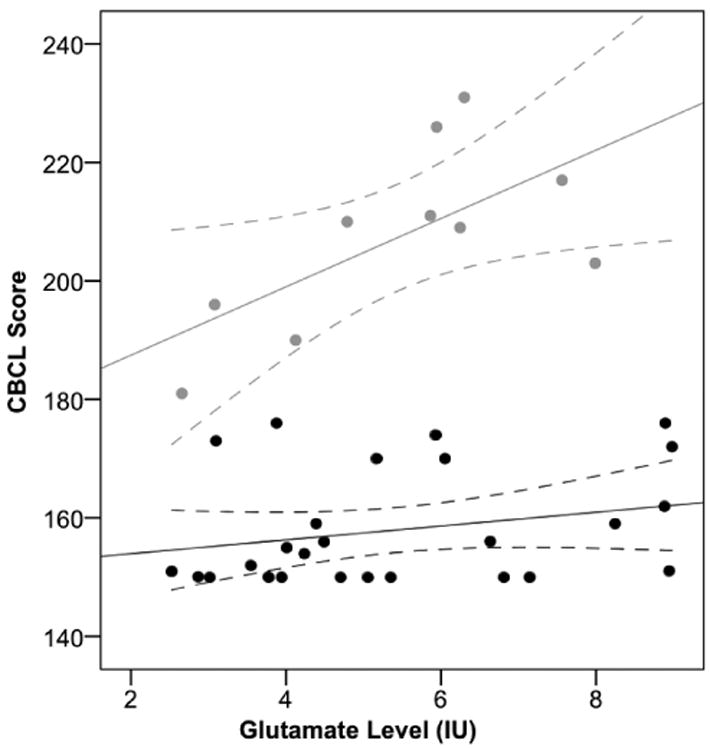
Solid lines represent the linear fits to the low score group data (black) and high score group data (grey). Dashed lines represent 95% confidence intervals. CBCL, Child Behavior Checklist; A-A-A, Anxiety/Depression, Aggression, Attention subscale; ACC, Anterior Cingulate Cortex.
Glu levels were increased in youth with high dysregulation profile scores (n=10; mean CBCL score=207.40±15.51; mean Glu level=5.45±1.77) when compared with just the healthy controls from the low dysregulation profile score group (n=13; mean CBCL score=154.38±7.37; mean Glu level=4.88±2.07) (two sample t-test; t = 10.88, d.f. = 21, p < 0.001 (2-tailed)).
Discussion
This study found a positive correlation between emotional dysregulation as measured by CBCL A-A-A scores (>180) and glutamate concentrations in the ACC in youth at high risk for bipolar disorder. Although in need of confirmation in larger studies, these findings suggest that glutaminergic dysregulation could represent a biomarker for emotional dysregulation in youth at risk for bipolar disorder.
Our finding of higher Glu levels in mood disordered youth with high CBCL dysregulation profile score is consistent with previously reported glutamatergic abnormalities in bipolar disorder and with a literature that suggests that glutamatergic abnormalities are a prominent feature of mood disorders (47). In major depressive disorder and bipolar disorder, serum, plasma and ACC levels of glutamate have been found to be altered (47-49). Glutamate level in the frontal cortex has been reported to be elevated in postmortem brains of patients with bipolar disorder and major depression (50). Glutamate is thought to be a marker of glial cell functioning and glial cell number and density reduction has been consistently demonstrated in mood disorders in the ACC in postmortem studies (51, 52). In bipolar disorder, almost all MRS studies report elevated Glx independent of disease state (31, 36, 38, 48, 53-57), making it a most consistent finding in the MRS literature.
Our 1HMRS ACC findings are also consistent with the literature that has previously found the ACC to be the site of neurometabolite abnormalities in mood-disordered youth. Davanzo et al. found significantly higher myo-inositol/creatine-phosphocreatine and mI levels in the ACC in bipolar youth versus healthy subjects or those with intermittent explosive disorder (40, 41). Cecil et al. (42) also found ACC abnormalities in mood disordered children, while Auer et al. reported ACC abnormalities in mood disordered adults (55).
On the other hand, our findings are discrepant with those of Singh et al. (58) who reported that high-risk offspring for bipolar disorder with subsyndromal symptoms of mania did not exhibit differences in Glu or Gln. They are also discrepant with findings by Moore and colleagues who reported that unmedicated youth with bipolar disorder had significantly lower Glx/Cr levels than healthy comparison subjects and medicated subjects with bipolar disorder (39, 59). More work is needed to reconcile these discrepant findings.
However, despite the positive correlation between Glu levels with the CBCL dysregulation profile score, there were no statistically significant Glu differences between the low versus high CBCL dysregulation profile groups. There could be several possible explanations for this finding: First, the low dysregulation profile group comprised a mixture of offspring of controls and bipolar disorder parents. It is possible that Glu is elevated only among youth at risk for bipolar disorder who also exhibit emotional dysregulation. This possibility is supported by our finding that there was a significant difference between high-risk offspring and healthy controls among the high emotional dysregulation group, but not the low dysregulation group. Thus, it would be important for subsequent studies to examine whether emotional dysregulation, as indexed by the CBCL represents a marker of risk or an endophenotype in these offspring at risk for bipolar disorder.
While no previous study has specifically correlated HMRS findings with the CBCL dysregulation profile, our 1HMRS results are also consistent with those from several studies that have connected the CBCL A-A-A dysregulation profile to genetic and other biomarkers. Althoff et al. have demonstrated in a very large sample that this CBCL profile is heritable, using latent class analysis (60). Doyle et al. in a genetic linkage study of 154 families estimated the heritability of this CBCL profile at 0.71 (61). Boomsma et al. (62) examined longitudinal data on Dutch mono- and dizygotic twin pairs (N = 8013 pairs) and found that 80% of the stability in childhood CBCL-Dysregulation profile was a result of additive genetic effects.
Zepf et al. (63) linked the profile to brain chemistry and reaction time. These authors used a placebo-controlled double-blind within-subject crossover design to compare the reaction times of high and low scorers on this CBCL-Dysregulation profile after a rapid tryptophan depletion test (RTD) (which lowers the central-nervous system 5-HT synthesis rate). Subjects with a high CBCL-Dysregulation score showed a slower reaction time under RTD compared to patients with low CBCL-Dysregulation profile. Another study found endocrino-logical correlates to the CBCL-Dysregulation profile. Basal serum TSH was measured in 114 children and adolescents with (N=53) and without (N=61) the CBCL-Dysregulation profile; TSH was elevated in those with the CBCL-Dysregulation profile compared to controls (64). Ducharme et al. reported on 193 healthy children aged 6-18 and found the Aggressive Behavior CBCL subscale alone to be correlated with bilateral striatal volumes and right ACC cortical thickness (65). Taken together, these studies all provide evidence that the CBCL A-A-A profile may be uniquely useful in the search for biomarkers of emotional dysregulation in the young.
This study has important strengths. Our definition of deficits in emotional regulation was anchored on a unique profile of the CBCL, an empirically derived scale with excellent psychometric properties, previously shown to discriminate youth with deficits in emotional regulation. By using a high field MRI scanner, the size of brain tissue volumes from which chemical information was obtained, was decreased which was an important consideration for acquiring MRS data from young children who have smaller brain volumes than adults. In addition, the improved signal to noise ratio at high field increased the metabolite signal enabling more accurate quantification including differentiation of Glu and Gln.
On the other hand, results of this study must be considered in light of some limitations. Our sample size was relatively small, resulting in very small cell sizes limiting the power of the study and increasing the possibility of spurious findings. Thus, our findings must be considered as preliminary until replicated with larger samples. To facilitate recruitment, this study included youth with a wide age range 6-17 years providing an additional confounding factor. Little is known about neurodevelopmental changes occurring during these years in the functioning of the ACC among typically developing youth. Such disparate ages would likely provide a confounding factor making a significant finding less likely. It is all the more remarkable that a correlation between CBCL scores and glutamate was noted. In addition, age was not statistically different between our two groups of interest, low and high scorers. Nonetheless, future confirmatory studies would benefit from examination of this brain region in youth of a narrower age range to remove any effects occurring during normal maturation. Although our focus on the ACC was well grounded on previous studies and theoretical considerations, future studies should examine other brain regions as well. Some of the subjects received pharmacologic treatment, which may have confounded the findings. In fact, that 70% of the high score group were taking one or more types of psychotropic medications at the time of the scan and that these medications were varied is a significant weakness of the study. Future studies would benefit from study of either treatment naïve or treatment free subjects.
Despite these considerations, our findings suggest that, among youth at risk for bipolar disorder, there is a relationship between emotional regulation deficits and neurometabolite glutamate in the ACC. Although additional work is needed to replicate these findings and further examine the implications of glutaminergic dysregulation in the ACC on the development of emotional dysregulation, our findings may have important scientific and clinical implications. Biomarkers of risk for emotional dysregulation may allow the identification of subjects at risk for this serious clinical problem as well as increase our understanding of the neural and biochemical bases of emotional dysregulation in youth. In addition, the construct of emotional dysregulation is consistent with the NIMH Research Domain initiative and may provide a fruitful area of scientific inquiry in the quest for biomarkers of psychopathological dysfunction.
Acknowledgments
This study was funded, in part, by National institutes of Health (NiH) grants K08MH001503 and R01MH066237 to Dr. Wozniak, the Susan G. Berk Endowed Fund for Juvenile Bipolar Disorder, the Heinz C. Prechter Bipolar Research Fund, and the support of members of the MGH Pediatric Psychopharmacology Council. In addition this work was supported by a NARSAD Young investigator Award in collaboration with a donation from the SHINE initiative (Henin), and a Massachusets General Hospital Claflin Distinguished Scholars Award to Dr. Henin. This study was also funded in part by MH073998 to Dr. Moore. We would like to acknowledge Dave Crowley, BA, Caroline Rycyna, BA, and Laura Rindlaub for their contributions to the study.
References
- 1.Staton D, Volness LJ, Beatty WW. Diagnosis and classification of pediatric bipolar disorder. J Affect Disord. 2008;105:205–212. doi: 10.1016/j.jad.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Wozniak J. Pediatric Bipolar disorder: The new perspective on severe mood dysfunction in children. J Child Adolesc Psychopharmacology. 2003;13:449–451. doi: 10.1089/104454603322724832. [DOI] [PubMed] [Google Scholar]
- 3.Towbin KE, Dykens EM, Pearson GS, Cohen DJ. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry. 1993;32:775–782. doi: 10.1097/00004583-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Carlson GA. Who are the children with severe mood dysregulation, a.k.a. “rages”? Am J Psychiatry. 2007;164:1140–1142. doi: 10.1176/appi.ajp.2007.07050830. [DOI] [PubMed] [Google Scholar]
- 5.Leibenluft E, Charney D, Towbin K, Bhangoo R, Pine D. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 6.Holtmann M, Goth K, Wockel L, Poustka F, Bolte S. CBCL-pediatric bipolar disorder phenotype: Severe ADHD or bipolar disorder? J Neural Transm. 2008;115:155–161. doi: 10.1007/s00702-007-0823-4. [DOI] [PubMed] [Google Scholar]
- 7.McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107–125. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 8.Barkley R. Attention-deficit/hyperactivity disorder, self-regulation, and time: Toward a more comprehensive theory. Dev Behav Pediatrics. 1997;18:271–279. [PubMed] [Google Scholar]
- 9.Achenbach T, Edelbrock C. Manual for the child behavior checklist and revised child behavior profile. Burlington, Vt: Department of Psychiatry, University of Vermont; 1983. [Google Scholar]
- 10.Achenbach TM. “Comorbidity” in child and adolescent psychiatry: Categorical and quantitative perpectives. J Child Adolesc Psychopharmacology. 1990;1(1991):271–278. [Google Scholar]
- 11.Achenbach TM. Manual for the child behavior checklist/4-18 and the 1991 profile. Burlington, Vt: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 12.Achenbach TM. Empirically based taxonomy: How to use syndromes and profile types derived from the CBCL/4-18 TRF, and YSR. Burlington, Vt: University of Vermont Department of Psychiatry; 1993. [Google Scholar]
- 13.Achenbach TM. Manual for the child behavior checklist/1-1/2-5. Burlington, Vt: ASEBA; 2000. [Google Scholar]
- 14.Achenbach TM, Dumenci L. Advances in empirically based assessment: revised cross-informant syndromes and new DSM-oriented scales for the CBCL, YSR, and TRF: Comment on Lengua, Sadowksi, Friedrich, and Fischer (2001) J Consult Clin Psychol. 2001;69:699–702. [PubMed] [Google Scholar]
- 15.Achenbach TM, Edelbrock C. The child behavior checklist. Burlington, Vt: University Associates in Psychiatry; 1983. [Google Scholar]
- 16.Achenbach TM, Howell CT, McConaughy ST, Stranger C. Six-year predictors of problems in a national sample of children and youth: I. Cross-informant syndromes. J Am Acad Child Adolesc Psychiatry. 1995;34 doi: 10.1097/00004583-199503000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach TM, McConaughy SH. In: Empirically based assessment of child and adolescent psychopathology: Practical applications. Kazdin AE, editor. Newbury Park, Cal: Sage; 1987. [Google Scholar]
- 18.Achenbach TM, Verhulst FC, Baron GD, Althaus M. A comparison of syndromes derived from the child behavior checklist for American and Dutch boys aged 6-11 and 12-16. J Child Psychol Psychiatry. 1987;28:437–453. doi: 10.1111/j.1469-7610.1987.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 19.Meyer SE, Carlson GA, Youngstrom E, Ronsaville DS, Martinez PE, Gold PW, et al. Long-term outcomes of youth who manifested the CBCL-Pediatric Bipolar Disorder phenotype during childhood and/or adolescence. J Afect Disord. 2009;113:227–235. doi: 10.1016/j.jad.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Holtmann M, Bolte S, Goth K, Dopfner M, Pluck J, Huss M, et al. Prevalence of the child behavior checklist-pediatric bipolar disorder phenotype in a German general population sample. Bipolar Disord. 2007;9:895–900. doi: 10.1111/j.1399-5618.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 21.Hudziak JJ, Althoff RR, Derks EM, Faraone SV, Boomsma DI. Prevalence and genetic architecture of Child Behavior Checklist-juvenile bipolar disorder. Biol Psychiatry. 2005;58:562–568. doi: 10.1016/j.biopsych.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Volk HE, Todd RD. Does the child behavior checklist juvenile bipolar disorder phenotype identify bipolar disorder? Biol Psychiatry. 2007;62:115–120. doi: 10.1016/j.biopsych.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Ayer L, Althoff R, Ivanova M, Rettew D, Waxler E, Sulman J, et al. Child behavior checklist juvenile bipolar disorder (CBCL-JBD) and CBCL posttraumatic stress problems (CBCL-PTSP) scales are measures of a single dysregulatory syndrome. J Child Psychol Psychiatry. 2009;50:1291–300. doi: 10.1111/j.1469-7610.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 24.Althoff RR, Rettew DC, Ayer LA, Sulman JS, Hudziak JJ. Requiem to the CBCL–Mania proxy. Pediatric Bipolar Conference; 2008, March 28-29; Boston, Mass. 2008. [Google Scholar]
- 25.Biederman J, Wozniak J, Kiely K, Ablon S, Faraone S, Mick E, et al. CBCL clinical scales discriminate prepubertal children with structured-interview derived diagnosis of mania from those with ADHD. J Am Acad Child Adolesc Psychiatry. 1995;34:464–471. [PubMed] [Google Scholar]
- 26.Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the child behavior checklist in pediatric bipolar disorder. Biol Psychiatry. 2003;53:1021–1027. doi: 10.1016/s0006-3223(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 27.McGough JJ, Loo SK, McCracken JT, Dang J, Clark S, Nelson SF, et al. CBCL pediatric bipolar disorder profile and ADHD: Comorbidity and quantitative trait loci analysis. J Am Acad Child Adolesc Psychiatry. 2008;47:1151–1157. doi: 10.1097/CHI.0b013e3181825a68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diler RS, Birmaher B, Axelson D, Goldstein B, Gill M, Strober M, et al. The child behavior checklist (CBCL) and the CBCL-bipolar phenotype are not useful in diagnosing pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2009;19:23–30. doi: 10.1089/cap.2008.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faraone SV, Althof RR, Hudziak JJ, Monuteaux MC, Biederman J. The CBCL predicts DSM bipolar disorder in children: A receiver operating characteristic curve analysis. Bipolar Disord. 2005;7:518–524. doi: 10.1111/j.1399-5618.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 30.Biederman J, Petty CR, Monuteaux MC, Evans M, Parcell T, Faraone SV, et al. The child behavior checklist-pediatric bipolar disorder profile predicts a subsequent diagnosis of bipolar disorder and associated impairments in ADHD youth growing up: A longitudinal analysis. J Clin Psychiatry. 2009;70:732–740. doi: 10.4088/JCP.08m04821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novotny EJ, Jr, Fulbright RK, Pearl PL, Gibson KM, Rothman DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54:S25–31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- 32.Benes FM. Altered glutamatergic and GABAergic mechanisms in the cingulate cortex of the schizophrenic brain. Arch Gen Psychiatry. 1995;52:1015–1018. doi: 10.1001/archpsyc.1995.03950240033007. discussion 9-24. [DOI] [PubMed] [Google Scholar]
- 33.Carrey N, MacMaster FP, Sparkes SJ, Khan SC, Kusumakar V. Glutamatergic changes with treatment in attention deficit hyperactivity disorder: A preliminary case series. J Child Adolesc Psychopharmacol. 2002;12:331–336. doi: 10.1089/104454602762599871. [DOI] [PubMed] [Google Scholar]
- 34.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 35.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 36.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7:S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 37.Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, et al. Reduced anterior cingulate cotrex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 39.Moore CM, Biederman J, Wozniak J, Mick E, Aleardi M, Wardrop M, et al. Mania, glutamate/glutamine and risperidone in pediatric bipolar disorder: A proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Affect Disord. 2007;99:19–25. doi: 10.1016/j.jad.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davanzo P, Yue K, Tomas MA, Belin T, Mintz J, Venkatraman TN, et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry. 2003;160:1442–1452. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- 41.Davanzo P, Tomas MA, Yue K, Oshiro T, Belin T, Strober M, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 42.Cecil K, DelBello M, Sellars MC, Strakowski SM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacology. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- 43.Orvaschel H. Schedule for affective disorder and schizophrenia for school-age children epidemiologic version. 5th. Ft. Lauderdale: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- 44.Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: Reliability, validity and sensitvity. Brit J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 45.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- 46.Wechsler D. Wechsler abbreviated scale of intelligence (WASI) 4th. San Antonio, Tx: The Psychological Corporation; 1999. [Google Scholar]
- 47.Palomino A, Gonzalez-Pinto A, Aldama A, Gonzalez-Gomez C, Mosquera F, Gonzalez-Garcia G, et al. Decreased levels of plasma glutamate in patients with first-episode schizophrenia and bipolar disorder. Schizophr Res. 2007;95:174–178. doi: 10.1016/j.schres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Frye MA, Watzl J, Banakar S, O'Neill J, Mintz J, Davanzo P, et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- 49.Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, et al. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 51.Drevets W, Ongur D, Prince J. Neuroimaging abnormalities in the subgenual prefrontal cortex: Implications for the pathophysiology of familial mood disorders. Molecular Psychiatry. 1998;3:220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 52.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanacora G, Rothman DL, Mason G, Krystal JH. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann NY Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 54.Eastwood SL, Harrison PJ. Markers of glutamate synaptic transmission and plasticity are increased in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry. 2010;67:1010–1016. doi: 10.1016/j.biopsych.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 56.Cecil KM, DelBello MP, Morey R, Strakowski SM. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord. 2002;4:357–365. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- 57.Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh M, Spielman D, Adleman N, Alegria D, Howe M, Reiss A, et al. Brain glutamatergic characteristics of pediatric offspring of parents with bipolar disorder. Psychiatry Res. 2010;182:165–171. doi: 10.1016/j.pscychresns.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore CM, Frazier JA, Glod CA, Breeze JL, Dieterich M, Finn CT, et al. Glutamine and glutamate levels in children ad adolescents with bipolar: A 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adoles Psychiatry. 2007;46:524–534. doi: 10.1097/chi.0b013e31802f5f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Althoff RR, Rettew DC, Boomsma DI, Hudziak JJ. Latent class analysis of the child behavior checklist obsessive-compulsive scale. Compr Psychiatry. 2009;50:584–592. doi: 10.1016/j.comppsych.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doyle AE, Biederman J, Ferreira MA, Wong P, Smoller JW, Faraone SV. Suggestive linkage of the child behavior checklist juvenile bipolar disorder phenotype to 1p21, 6p21, and 8q21. J Am Acad Child Adolesc Psychiatry. 49:378–387. [PMC free article] [PubMed] [Google Scholar]
- 62.Boomsma DI, Rebollo I, Derks EM, van Beijsterveldt TC, Althoff RR, Rettew DC, et al. Longitudinal stability of the CBCL-juvenile bipolar disorder phenotype: A study in Dutch twins. Biol Psychiatry. 2006;60:912–920. doi: 10.1016/j.biopsych.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 63.Zepf FD, Wockel L, Poustka F, Holtmann M. Diminished 5-HT functioning in CBCL pediatric bipolar disorder-profiled ADHD patients versus normal ADHD: Susceptibility to rapid tryptophan depletion influences reaction time performance. Hum Psychopharmacol. 2008;23:291–299. doi: 10.1002/hup.934. [DOI] [PubMed] [Google Scholar]
- 64.Holtmann M, Duketis E, Goth K, Poustka L, Boelte S. Severe affective and behavioral dysregulation in youth is associated with increased serum TSH. J Affect Disord. 2010;121:184–188. doi: 10.1016/j.jad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Ducharme S, Hudziak JJ, Botteron KN, Ganjavi H, Lepage C, Collins DL, et al. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biol Psychiatry. 2011;70:283–290. doi: 10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


