Abstract
National guidelines recommend screening for latent tuberculosis infection (LTBI) in all HIV-infected patients. Thus, the objective of this study was to measure protocol adherence to national guidelines regarding LTBI screening for HIV-infected patients entering care at an urban primary care clinic specializing in HIV care, identify clinical and other characteristics associated with adherence, and determine whether transitioning from the tuberculin skin test (TST) to the interferon-gamma release assay (IGRA) improved adherence. We conducted a retrospective study using protocol adherence to LTBI screening guidelines within twelve months of entering care at an HIV clinic as the primary outcome. Successful protocol adherence was defined as the placement and reading of a TST, performance of an IGRA, or a note in study clinic records documenting prior testing or treatment for tuberculosis in an outside setting. Multivariable modified Poisson regression models were used in analyses. Overall, 32% (n = 118/372) of patients received LTBI screening within twelve months of entering care. Protocol adherence to LTBI screening guidelines increased from 28% to 37% following the transition from TST to IGRA screening. IGRA screening [adjusted prevalence ratio: 1.45, 95% confidence limits: (1.07, 1.96)], male sex [1.47 (1.05, 2.07)], transfer patient status [1.51 (1.05, 2.18)], and greater than one year of clinic attendance [1.62 (1.06, 2.48)] were independently associated with protocol adherence. Among patients without prior LTBI screening or treatment, patients entering the clinic in 2013 under the IGRA screening protocol were more likely to be screened for LTBI compared to patients entering under the TST screening protocol (34.3% vs. 9.7%, p < 0.001). In conclusion, transitioning from TST to IGRA-based screening improved adherence to screening guidelines. However, further work on improving adherence to LTBI screening guidelines among HIV-infected patients is needed.
Keywords: HIV/AIDS, latent tuberculosis infection, interferon-gamma release assays, tuberculin test, epidemiology
Introduction
Globally, tuberculosis (TB) is the leading cause of death for persons living with HIV/AIDS (World Health Organization, 2015). While antiretroviral therapy has markedly decreased the incidence of concurrent HIV/TB infection in the United States, the risk of active TB among HIV-infected individuals remains twice that of the general population (Horsburgh, 2004). HIV-infected individuals are more likely to progress from latent to active TB and are at increased risk of developing multi-drug resistant TB, extrapulmonary manifestations, and disseminated disease compared to HIV-uninfected individuals (Corbett et al., 2003; Talati et al., 2011). Therefore, national guidelines strongly endorse screening and treatment of latent tuberculosis infection (LTBI) in HIV-infected patients (Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents, 2016).
In 2012, an HIV primary care clinic in Philadelphia, Pennsylvania transitioned from tuberculin skin test (TST) to interferon-gamma release assay (IGRA)-based screening. This transition provided an opportunity to compare protocol adherence to national guidelines based on the LTBI screening method. Thus, the objective of this retrospective study was to measure protocol adherence and identify clinical and other characteristics associated with adherence. We hypothesized that the transition to IGRA-based screening would improve adherence due to logistical advantages of the IGRA method (Cattamanchi et al., 2011; Foster-Chang, Manning, & Chandler, 2014; Linas, Wong, Freedberg, & Horsburgh, 2011).
Methods
Study population
Study participants were patients with laboratory-confirmed HIV entering care at an HIV primary care clinic in Philadelphia from January-December 2010 or January-December 2013. Patients were classified as transferring HIV care when a recorded CD4 or HIV viral load result from another outpatient setting preceded the initial visit to the study clinic. This observational, retrospective study was approved by the Institutional Review Board at Drexel University College of Medicine.
Data collection
Primary exposure
The primary exposure was entering care from January-December 2013 during IGRA-based screening versus entering care from January-December 2010 during TST-based screening.
Outcome
The outcome was protocol adherence to LTBI screening guidelines at the study clinic at or within the twelve months following the first clinic visit. Medical charts for enrolled patients were reviewed for a year following the initial visit by trained investigators. Successful protocol adherence was defined as the placement and reading of a TST, performance of an IGRA, or a note in study clinic records documenting prior testing or treatment for tuberculosis in an outside setting.
Secondary exposures and covariates
Secondary exposures and covariates included characteristics previously shown to be associated with LTBI screening (Backus et al., 2010; Lee, Lobato, Buskin, Morse, & Costa, 2006) including CD4 count, receipt of antiretroviral therapy, clinic attendance, and sociodemographic characteristics.
Statistical analysis
We performed χ2 analyses, t-tests, and Wilcoxon rank-sum tests to assess associations between sociodemographic and clinical characteristics with testing method and protocol adherence to screening guidelines. We then performed unadjusted and adjusted analyses using modified Poisson regression models to estimate associations with adherence to guidelines (McNutt, Wu, Xue, & Hafner, 2003; Zou, 2004). Statistical analyses were performed using SAS (Version 9.3, SAS Institute, Inc., Cary, NC).
Results
A total of 272 patients were identified as new to the clinic from December-January 2010 and 178 patients as new from December-January 2013. We excluded patients with a single visit (n = 41), patients missing baseline CD4 counts (n = 31), and transgender individuals (n = 5), resulting in a total study population of 372.
Demographic, behavioral, and clinical characteristics are presented in Table 1. Patients entering the study clinic were mostly male, non-Hispanic Black or White, transferring HIV care, and had an initial CD4 count >350 cells/uL. A significantly greater proportion of patients in 2013 were Black, non-smokers, living independently, on ART with significantly lower HIV RNA levels and higher CD4 cell counts by the end of follow-up.
Table 1.
Demographic, behavioral and clinical characteristics of HIV-infected patients entering an HIV primary care clinic in Philadelphia, Pennsylvania by year of entry into care, 2010 and 2013, N = 372.
| Characteristica | TST-based screening, 2010 n = 219, 58.9%b | IGRA-based screening, 2013 n = 153, 41.1%b | p-valuec |
|---|---|---|---|
| Sex | |||
| Male | 153 (69.9) | 100 (65.4) | 0.36 |
| Female | 66 (30.1) | 53 (34.6) | |
| Age | |||
| 16–24 | 29 (13.2) | 33 (21.6) | 0.22 |
| 25–34 | 53 (24.2) | 35 (22.9) | |
| 35–44 | 50 (22.8) | 34 (22.2) | |
| 45–54 | 60 (27.4) | 39 (25.5) | |
| 55–79 | 27 (12.3) | 12 (7.8) | |
| Race | |||
| Black, Non-Hispanic | 141 (64.4) | 120 (78.4) | 0.02 |
| White, Non-Hispanic | 38 (17.4) | 20 (13.1) | |
| Hispanic | 24 (10.9) | 7 (4.6) | |
| Multi-racial, other | 16 (7.3) | 6 (3.9) | |
| CD4 closest to initial visit | |||
| 0–200 cells/uL | 60 (27.4) | 30 (19.6) | 0.34 |
| 201–350 cells/uL | 37 (16.9) | 28 (18.3) | |
| 351–500 cells/uL | 51 (23.3) | 36 (23.5) | |
| 501–2558 cells/uL | 71 (32.4) | 59 (38.6) | |
| Transfer patient | |||
| Yes | 160 (73.1) | 107 (69.9) | 0.51 |
| No | 59 (26.9) | 46 (30.1) | |
| Current smoker | |||
| Yes | 123 (56.2) | 67 (43.8) | 0.02 |
| No | 96 (43.8) | 86 (56.2) | |
| Current illicit substance use | |||
| Yes | 46 (21.0) | 38 (24.8) | 0.38 |
| No | 173 (79.0) | 115 (75.2) | |
| Living situation | |||
| Independent | 145 (66.2) | 135 (88.2) | <0.01 |
| Transient | 25 (11.4) | 7 (4.6) | |
| In patient/long term care | 49 (22.4) | 11 (7.2) | |
| Start of follow-up | |||
| On ART (transfer only) | 90 (56.3) | 64 (59.8) | 0.56 |
| CD4 cells/uL median (quartiles) | 392 (177, 568) | 400 (262, 621) | 0.07 |
| HIV VL log10 copies/mL, mean(SD) | 3.32 (1.49) | 3.27 (1.56) | 0.57 |
| End of follow-up | |||
| On ART (all patients) | 187 (85.4) | 143 (93.5) | 0.02 |
| CD4 cells/uL, median (quartiles) | 428 (247, 612) | 479 (323, 746) | 0.01 |
| HIV VL log10 copies/mL, mean(SD) | 2.27 (1.30) | 1.79 (0.98) | <0.01 |
| Clinic attendance for more than one year | |||
| Yes | 159 (72.6) | 113 (73.9) | 0.79 |
| No | 60 (27.4) | 40 (26.1) |
Abbreviations: HIV, Human Immunodeficiency Virus; TST, tuberculin skin test, IGRA, interferon gamma release assay; ART, Antiretroviral Therapy; VL, Viral load; SD, standard deviation.
Characteristic at initial visit unless stated otherwise.
Count and percentages presented as n (%).Counts and percentages add down columns to obtain total sample size and 100%.
Chi-square test, t-test (CD4 cell count) or Wilcoxon rank sum test (HIV RNA) comparing the “TST-based Screening” and “IGRA-based Screening” study populations. Boldface font indicates a p < 0.05.
Overall, 31.7% of patients received care adhering to LTBI screening guidelines: 27.9% of patients entering care in 2010 and 37.3% of patients entering care in 2013 (Figure 1). Of the 67 screening tests performed at the study clinic during both years, one patient had a positive TST result. This patient self-reported a previous positive TST result; however, there was no record of previous LTBI screening within clinic records. A follow-up chest radiograph showed no acute lung disease. Individuals with no signs of active TB disease who test positive for LTBI typically receive treatment to prevent active TB disease and the associated risk of TB-related morbidity and mortality (Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents, 2016).
Figure 1.
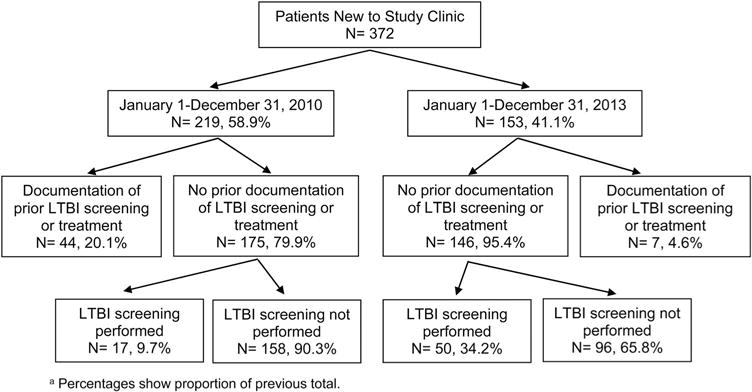
Flowchart of patient identification and LTBI screening outcomes among patients entering an HIV primary care clinic, Philadelphia, Pennsylvania, N = 372.
Note: Percentages show proportion of previous total.
In adjusted analyses (Table 2), patients entering the study clinic in 2013 under the IGRA protocol were 1.45 (95% confidence limits, 1.07, 1.96) times more likely to have received care adherent to guidelines. Patients who were male [1.47 (1.05, 2.07)], had transferred [1.51 (1.05, 2.18)], and had greater than one-year of clinic attendance [1.62 (1.06, 2.48)] were also significantly more likely to have received care adherent to guidelines.
Table 2.
Unadjusted and adjusted prevalence ratios for adherence to LTBI screening guidelines by latent tuberculosis screening method, sociodemographic, and clinical characteristics among new patients attending an HIV primary care clinic, Philadelphia, Pennsylvania, N = 372.
| Exposurea | Adhered to LTBI screening guidelines n = 118, 31.7%b | Failure to adhere to LTBI screening guidelines n = 254, 68.3%b | Unadjusted prevalence ratio (95% confidence limits) | Adjusted prevalence ratioc (95% confidence limits) |
|---|---|---|---|---|
| Year of entry into care | ||||
| 2010 TST screening | 61 (27.9) | 158 (72.2) | ref | ref |
| 2013 IGRA screening | 57 (37.3) | 96 (62.8) | 1.34 (0.99, 1.80) | 1.45 (1.07, 1.96) |
| Sex | ||||
| Male | 88 (34.8) | 165 (65.2) | 1.38 (0.97, 1.96) | 1.47 (1.05, 2.07) |
| Female | 30 (25.2) | 89 (74.8) | ref | ref |
| Age | ||||
| 16–24 | 18 (29.0) | 44 (71.0) | ref | ref |
| 25–34 | 25 (28.4) | 63 (71.6) | 0.98 (0.59, 1.63) | 1.06 (0.64, 1.76) |
| 35–44 | 31 (36.9) | 53 (63.1) | 1.27 (0.79, 2.05) | 1.24 (0.76, 2.01) |
| 45–54 | 31 (31.3) | 68 (68.7) | 1.08 (0.66, 1.75) | 0.98 (0.61, 1.60) |
| 55–79 | 13 (33.3) | 26 (66.7) | 1.15 (0.64, 2.07) | 1.27 (0.71, 2.29) |
| Race | ||||
| Black, Non-Hispanic | 92 (35.3) | 169 (64.8) | ref | ref |
| White, Non-Hispanic | 12 (20.7) | 46 (79.3) | 0.59 (0.35, 1.00) | 0.60 (0.35, 1.04) |
| Hispanic | 9 (29.0) | 22 (71.0) | 0.82 (0.46, 1.46) | 0.88 (0.51, 1.54) |
| Multi-racial, other | 5 (22.7) | 17 (77.3) | 0.64 (0.29, 1.42) | 0.61 (0.30, 1.27) |
| CD4 closest to initial visit | ||||
| 0–200 cells/uL | 28 (31.1) | 62 (68.9) | 0.99 (0.66, 1.47) | 94 (0.63, 1.41) |
| 201–350 cells/uL | 24 (36.9) | 41 (63.1) | 1.17 (0.78, 1.76) | 0.1.20 (0.81, 1.78) |
| 351–500 cells/uL | 25 (28.7) | 62 (71.3) | 0.91 (0.60, 1.38) | 1.06 (0.70, 1.59) |
| 500–2558 cells/uL | 41 (31.5) | 89 (68.5) | ref | ref |
| Current smoker | ||||
| Yes | 63 (33.2) | 127 (66.8) | 1.09 (0.81, 1.48) | 1.09 (0.81, 1.46) |
| No | 55 (30.2) | 127 (69.8) | ref | ref |
| Current illicit substance use | ||||
| Yes | 28 (33.3) | 56 (66.7) | 1.07 (0.75, 1.51) | 1.06 (0.77, 1.46) |
| No | 90 (31.3) | 198 (68.8) | ref | ref |
| Living situation | ||||
| Independent | 81 (28.9) | 199 (71.1) | ref | ref |
| Transient | 14 (43.8) | 18 (56.3) | 1.14 (0.69, 1.89) | 1.05 (0.64, 1.71) |
| In patient/long term care | 23 (38.3) | 37 (61.7) | 0.75 (0.52, 1.09) | 0.68 (0.45, 1.00) |
| Transfer patient | ||||
| Yes | 92 (34.5) | 175 (65.5) | 1.39 (0.96, 2.02) | 1.51 (1.05, 2.18) |
| No | 26 (24.8) | 79 (75.2) | ref | ref |
| Receipt of HAART by end of follow-up | ||||
| Yes | 108 (32.7) | 222 (67.3) | 1.37 (0.78, 2.41) | 1.26 (0.70, 2.29) |
| No | 10 (23.8) | 32 (76.2) | ref | ref |
| Clinic attendance for more than one year | ||||
| Yes | 96 (35.3) | 176 (64.7) | 1.60 (1.07, 2.39) | 1.62 (1.06, 2.48) |
| No | 22 (22.0) | 78 (78.0) | ref | ref |
Abbreviation: LTBI, Latent Tuberculosis Infection; HIV, Human Immunodeficiency Virus; TST, tuberculin skin test; IGRA, interferon gamma release assay; HAART, Highly Active Antiretroviral Therapy.
Characteristic at initial visit unless stated otherwise.
Count and row percentages presented as n (%). Counts and percentages add across rows to obtain total sample size and 100%.
All variables in table are included in the adjusted model. Boldface font indicates that p < 0.05.
Discussion
Screening for latent tuberculosis infection is an essential component of preventive care for HV-infected patients (Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents, 2016). We found that 68% of HIV-infected new patients at an HIV primary clinic in Philadelphia did not receive care adhering to national guidelines on LTBI screening. Our study is the first to show that a transition from TST to IGRA-based screening within an HIV primary care clinic significantly improved protocol adherence; however, adherence remained low. Protocol adherence was also significantly positively associated with male gender, transfer patient status, and clinic attendance for more than a year. These findings suggest that further research is needed on how to most effectively improve adherence to LTBI screening guidelines in HIV primary care settings.
Progress over the past two decades toward TB elimination has decreased TB incidence from 10.5 cases per 100,000 in 1992 to 3.0 per 100,000 in 2015 (Centers for Disease & Prevention, 1993; Salinas et al., 2016). While the incidence of active tuberculosis has decreased in recent years, Philadelphia reported 71 cases of active TB in 2015 and 12% were co-infected with HIV (Philadelphia Department of Public Health, 2015). The co-infection rate is more than double the national average and highlights the continued need for screening in this clinic setting (Philadelphia Department of Public Health, 2015). LTBI screening remains a vital and low-cost component of HIV preventive care.
Previous studies with healthcare workers have demonstrated the advantages of IGRA-based over TST-based screening (Shah et al., 2012; Wrighton-Smith, Sneed, Humphrey, Tao, & Bernacki, 2012). A 2014 study found that adoption of IGRA-based screening increased compliance to employee screening guidelines at a medical center from 77% to 97% and reduced screening-associated costs (Foster-Chang et al., 2014). Within our study, screening tests performed at the study clinic increased from 7% to 33% following the transition to IGRA-based screening. Logistical advantages associated with IGRA such as testing within a single visit may help explain this finding.
Within our study population, women were significantly less likely to be screened compared to men. Unfortunately, gender disparities in HIV-care outcomes are common (Aziz & Smith, 2011; Shapiro et al., 1999). Women-focused HIV care that addresses these barriers may help decrease disparities (Aziz & Smith, 2011). Patients transferring HIV care and patients who remained at the study clinic for over a year were significantly more likely to have received care adhering to guidelines. However, transfer patient status and clinic attendance for more than one year no longer significantly impacted adherence when analysis was limited to patients without prior evidence of LTBI screening or treatment (results not shown).
Limitations of this study include the use of medical records and reliance on the completeness and validity of these records. We did not include health insurance coverage as an exposure, and type of coverage may have influenced screening. Not accounting for other factors including travel history, birthplace, or immigration status in analyses may have impacted results. We were unable to disentangle the impact of screening method and period effects. A change in clinic workflow or in healthcare providers across periods may have impacted screening. LTBI screenings received under the TST screening protocol could have been IGRA as outside clinics may have adopted IGRA earlier than the study clinic. Finally, the study sample was selected from one HIV clinic in Philadelphia and results may not be generalizable to other HIV-infected populations, particularly those living outside the United States. However, sociodemographic attributes of our study population are very similar to those of HIV-infected populations living in other economically disadvantaged urban areas in the United States including Baltimore, Newark, and New Orleans (Laffoon et al., 2015). Therefore, our findings may generalize to other urban HIV-infected populations in the United States. Despite these limitations, this is the first study to examine the impact of transitioning from TST to IGRA-based screening on protocol adherence for HIV-infected patients.
In summary, this study found poor protocol adherence to LTBI screening guidelines for HIV-infected patients at an urban HIV clinic. Furthermore, protocol adherence was associated with IGRA-based screening, male sex, transfer patient status, and remaining a patient at the clinic for over a year. The observed poor protocol adherence suggests a change in clinic policy regarding LTBI screening may be needed.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Presentations
This study was presented in part at the World Congress of Epidemiology 2014, ASM Microbe 2016, and IDWeek 2016.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
J.W. Adams http://orcid.org/0000-0002-7703-5944
References
- Aziz M, Smith KY. Challenges and successes in linking HIV-infected women to care in the United States. Clinical Infectious Diseases. 2011;52(Suppl 2):S231–S237. doi: 10.1093/cid/ciq047. [DOI] [PubMed] [Google Scholar]
- Backus LI, Boothroyd DB, Phillips BR, Belperio PS, Halloran JP, Valdiserri RO, Mole LA. National quality forum performance measures for HIV/AIDS care: The department of veterans affairs’ experience. Archives of Internal Medicine. 2010;170(14):1239–1246. doi: 10.1001/archinternmed.2010.234. [DOI] [PubMed] [Google Scholar]
- Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, Pai M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: A systematic review and meta-analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;56(3):230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease & Prevention. Tuberculosis morbidity–United States, 1992. MMWR Morbidity and Mortality Weekly Report. 1993;42(36):696–697. 703–704. [PubMed] [Google Scholar]
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis. Archives of Internal Medicine. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- Foster-Chang SA, Manning ML, Chandler L. Tuberculosis screening of new hospital employees: Compliance, clearance to work time, and cost using tuberculin skin test and interferon-gamma release assays. Workplace Health & Safety. 2014;62(11):460–467. doi: 10.3928/21650799-20140902-02. [DOI] [PubMed] [Google Scholar]
- Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. New England Journal of Medicine. 2004;350(20):2060–2067. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- Laffoon BT, Hall HI, Surendera Babu A, Benbow N, Hsu LC, Hu YW, Urban Areas HIVSW. HIV infection and linkage to HIV-related medical care in large Urban Areas in the United States, 2009. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;69(4):487–492. doi: 10.1097/QAI.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Lobato MN, Buskin SE, Morse A, Costa OS. Low adherence to guidelines for preventing TB among persons with newly diagnosed HIV infection, United States. International Journal of Tuberculosis and Lung Disease. 2006;10(2):209–214. [PubMed] [Google Scholar]
- Linas BP, Wong AY, Freedberg KA, Horsburgh CR., Jr Priorities for screening and treatment of latent tuberculosis infection in the United States. American Journal of Respiratory and Critical Care Medicine. 2011;184(5):590–601. doi: 10.1164/rccm.201101-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from the centers for disease control and prevention the national institutes of health, and the HIV medicine association of the infectious diseases society of America. 2016 Retrieved from http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- Philadelphia Department of Public Health, T. C. P. Summary surveillance data: 2015. 2015 Retrieved from https://hip.phila.gov/Portals/_default/HIP/DataReports/Tuberculosis/2015/TB_SummarySurveillanceData_2015.pdf.
- Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence– United States, 2013–2015. MMWR Morbidity and Mortality Weekly Report. 2016;65(11):273–278. doi: 10.15585/mmwr.mm6511a2. [DOI] [PubMed] [Google Scholar]
- Shah M, Miele K, Choi H, DiPietro D, Martins-Evora M, Marsiglia V, Dorman S. QuantiFERON-TB gold in-tube implementation for latent tuberculosis diagnosis in a public health clinic: A cost-effectiveness analysis. BMC Infectious Diseases. 2012;12:360. doi: 10.1186/1471-2334-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, Bozzette SA. Variations in the care of HIV-infected adults in the United States: Results from the HIV Cost and Services Utilization Study. JAMA. 1999;281(24):2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- Talati NJ, Gonzalez-Diaz E, Mutemba C, Wendt J, Kilembe W, Mwananyanda L, Blumberg HM. Diagnosis of latent tuberculosis infection among HIV discordant partners using interferon gamma release assays. BMC Infectious Diseases. 2011;11(1) doi: 10.1186/1471-2334-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. HIV-associated tuberculosis. 2015 Retrieved from http://www.who.int/tb/challenges/hiv/tbhiv_factsheet_2015.pdf?ua=1.
- Wrighton-Smith P, Sneed L, Humphrey F, Tao X, Bernacki E. Screening health care workers with interferon-gamma release assay versus tuberculin skin test: Impact on costs and adherence to testing (the SWITCH study) Journal of Occupational and Environmental Medicine. 2012;54(7):806–815. doi: 10.1097/JOM.0b013e318254620f. [DOI] [PubMed] [Google Scholar]
- Zou G. A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]


