Abstract
Introduction:
Ropivacaine has been studied previously and holds promise as an agent that offers a safe, efficacious, and better recovery profile than other conventional agents such as bupivacaine. The aim of the present study was to compare the safety and efficacy of equal volume of different concentration of ropivacaine for epidural analgesia in patients undergoing major lower limb orthopedic surgery.
Subjects and Methods:
One hundred and fifty adult patients were randomized into three groups to receive single dose of equal volume of ropivacaine through epidural route in concentrations of 0.2%, 0.5%, and 0.75%, respectively. All the groups received equal dose of ropivacaine of same concentration for subarachnoid block using combined spinal-epidural technique.
Results:
Modified Bromage Scale and Numeric rating scale was used to assess motor block and analgesia. Data analysis was done using WINDOW SPSS Student Version 17 ANOVA test. Student's t-test was performed for comparison between two groups, and qualitative data were analyzed by applying Chi-square test.
Conclusion:
0.5% and 0.75% ropivacaine were sufficient and effective for intrathecal subarachnoid block as well as for postoperative analgesia with epidural use. Shorter duration of motor blockade and analgesia was seen with ropivacaine 0.2%.
Keywords: Analgesia, epidural, orthopedic, postoperative, ropivacaine
INTRODUCTION
In this highly evolving and fast-paced world, regional anesthesia has emerged with wide-spread acceptability among anesthesiologists worldwide.[1] Epidural anesthesia has been well established as a safe and effective technique, not for only perioperative anesthesia but also for postoperative analgesia. Hence, the search is always on for a drug which is safer, efficacious, and less toxic with an early recovery profile, which provides an early ambulation.
Bupivacaine, introduced in 1965 was followed by the reports of central nervous system and cardiovascular system toxicity leading to restrictions of its use. This led to the development of newer pure agents with less toxic potential such as ropivacaine and levobupivacaine. Ropivacaine, a long-acting amide local anesthetic is an optically active pure S-enantiomer of propivacaine.[1,2] Ropivacaine has good hemodynamic stability, and there is a less chance of cardiotoxicity and neurotoxicity with ropivacaine and probably has the greatest margin of safety of all long-acting local anesthetics available at present.[2] It is nearly identical to bupivacaine in onset, quality, and duration of sensory block.[3] It has got lower lipid solubility; hence, less likely to penetrate large, myelinated, motor nerve fibers; hence, lesser propensity for motor blockade.[4,5]
This study was designed to compare the safety and efficacy of differing concentrations of isobaric ropivacaine for epidural analgesia in patients undergoing orthopedic surgery.
SUBJECTS AND METHODS
After obtaining the approval of the Ethical Committee of the institution, and written informed consent, a randomized, prospective, double-blinded study was conducted on patients undergoing major orthopedic lower limb surgery under spinal anesthesia. Inclusion criteria were patients of age 18–70 years, of either sex, and the American Society of Anesthesiologists (ASA) I and II undergoing major lower limb orthopedic surgeries. Exclusion Criteria included unwilling patients, emergency surgeries, known case of hypersensitive reactions to local anesthetics, coagulation disorder, and local site infection, history of drug or alcohol abuse, ASA III and above.
Group allocation and intervention plan
Patients were randomly divided into three groups (n = 50 in each group) using a computer-generated number table. Study medications were prepared by the site anesthesiologist, who was not involved in any other part of the study. The investigator was kept blind for the group of patient and also for the contents of syringes. The test drugs were given through the epidural catheter after motor recovery of neuraxial block.
Group A: 14 ml, single dose of ropivacaine (0.5%)
Group B: 14 ml, single dose of ropivacaine (0.75%)
Group C: 14 ml, single dose of ropivacaine (0.2%).
Preanesthetic management
A detailed preanesthetic checkup was done in all patients, which included a detailed history and thorough physical examination. The necessary routine investigations were carried out in all the patients. All the patients received tablet diazepam 10 mg at night and 5 mg in the morning of surgery and also tablet ranitidine 150 mg on the night of the surgery. Patients were kept nil per oral for at least 6 h.
On arrival to the operating room, 18-gauge intravenous (i.v.) line was secured and ringer's lactate solution was started. Total amount of fluid required intraoperatively was calculated on per kg basis in all patients. Noninvasive monitors connected and baseline values of heart rate, blood pressure, and oxygen saturation were noted before the procedure. Before the commencement of anesthesia, patients were instructed on the methods of sensory or motor assessments. After patient positioning, L3–L4 lumbar epidural space was identified by loss of resistance technique, under strict asepsis using a combined spinal-epidural set. Subarachnoid block was given with 4 ml of isobaric bupivacaine (7.5 mg/ml) using the same concentration which is used for epidural analgesia. Epidural catheter was inserted and advanced at 5 cm into the epidural space. The catheter was aspirated to exclude intrathecal or i.v. placement and then secured. The induction of anesthesia was considered when at least the T10 dermatome was anesthetized.
An anesthesiologist, who was blinded regarding which local anesthetic was used, assessed sensory and motor block after the intrathecal injection at 1 and 2 min and then, subsequently at 2 min intervals until surgical anesthesia was achieved. Sensory block was assessed by pinprick sensation using hypodermic needle. Motor blockade was tested by Modified Bromage Scale (0 = no block, 1 = inability to raise extended leg, 2 = inability to flex knee, and 3 = inability to flex ankle and foot). The duration of sensory or motor blockade was defined as the interval from intrathecal administration to the point of complete resolution of the sensory block or to the point in which the Bromage score was back to zero, respectively. Heart rate, blood pressure, and arterial oxygen saturation were recorded 5 min after the intrathecal drug administration and thereafter every 10 min till the end of the operation.
Epidural administration
Epidural top up in each group was given after 45 min of administration of subarachnoid block. 14 ml of ropivacaine of respective concentration of each group was given as test drug for epidural top up. Vitals monitored in postanesthesia care unit and inward before and after epidural top up for 12 h. Postoperative pain assessment was done by Numerical rating scale at 0, 0.5, 1, 2, 4, 6, 8, and at 12 h after surgery. Rescue analgesia given at Numerical rating scale level between 3 and 4. Injection diclofenac 75 mg i.v. infusion in 100 ml of 0.9% saline was used as rescue analgesic. The occurrence of adverse events including bradycardia, hypotension, hypoxia, shivering as well as nausea and vomiting was also recorded.
In Group C where 0.2% ropivacaine was given, patient complained of pain and surgeon found insufficient muscle relaxation in almost all cases. Hence, to continue the surgery in 12 patients’ general anesthesia with endotracheal intubation was administered. In six patients, general anesthesia with laryngeal mask airway and two patients’ moderate sedation was required to continue the surgery. This ineffectiveness of Group C drug concentration was informed to the ethical committee. After informing the ethical committee and taking its approval and consulting our institute statistician, the 0.2% ropivacaine group was removed from the study and thereon study was carried on only two groups of 0.5% and 0.75% concentration of ropivacaine. Our result was similar to Sia et al.[6] who compared effectiveness of 0.2% and 0.125% ropivacaine in patient-controlled epidural analgesia and reported that sufficient analgesia had been obtained in both concentrations, but motor block had been less in low concentration of ropivacaine.
Statistical analysis
Qualitative data were analyzed by applying Chi-square test. Paired/unpaired t-test and Chi-square test were used for comparison between two groups. P < 0.05 was considered statistically significant.
Both groups were comparable with regard to age, weight, height, ASA, and gender [Table 1].
Table 1.
Patient characteristics
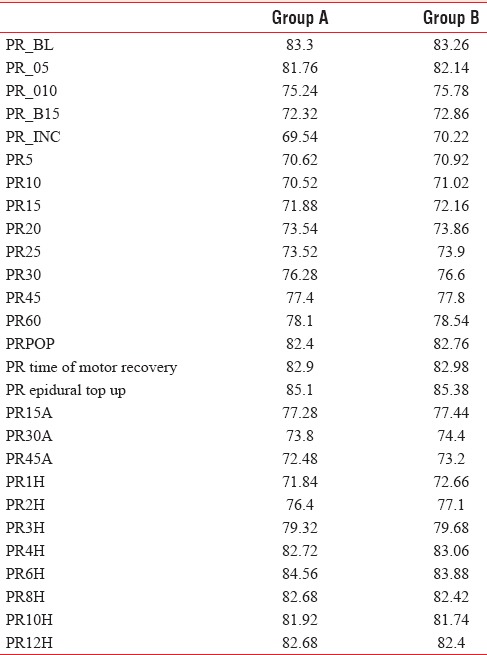
Changes in mean arterial pressure in Group A and B were almost similar and were not statistically significant [Table 2].
Table 2.
Comparison of mean arterial blood pressure

Heart rates in both groups were comparable with no statistical significant differences [Table 3].
Table 3.
Comparison of mean heart rate
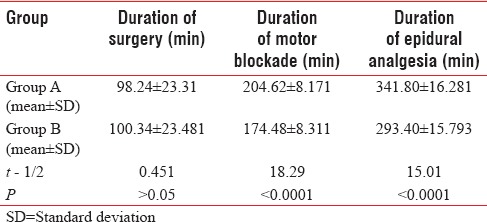
T-value between Group A and B is 0.451 and P > 0.05 means there is no significant difference in duration of surgery between Group A and B [Table 4].
Table 4.
Comparison of mean duration of surgery, meantime duration to achieve individual motor blockade, and epidural analgesia
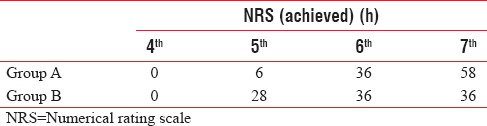
T-value between Group A and C is 18.29 and P < 0.0001 between both group means there is significant difference in duration of motor blockade in both concentrations of ropivacaine.
T-value between Group A and C is 15.01 but P < 0.0001 in between both groups means there is significant difference in duration of epidural analgesia. The comparisons of quality of postoperative epidural analgesia between the three groups are given in Table 5. Patients in Group A had pain relief for 6.52 h, patients in Group B had pain relief for 6.08 h.
Table 5.
Distribution of cases according to numerical rating scale pain achievement
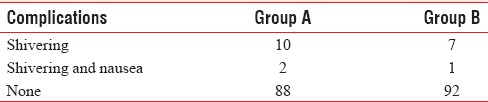
The incidence of complications between the study groups is listed in Table 6.
Table 6.
Perioperative side effects
DISCUSSION
The speed of neural block is proportional to the concentration of the local anesthetic solution.[7] We aimed to compare the duration of blockade and analgesia in epidural as well as the incidence of complications between different concentration of ropivacaine in patients who underwent lower limb orthopedic surgeries.
The degree of motor blockade, assessed by Modified Bromage score were comparable to previous studies by McNamee et al.[8] and Kallio et al.[9] The time required to achieve (individual Bromage score) motor blockade was also similar in both groups with no statistically significant difference which is supported by a study conducted by Gudul et al. by comparing isobaric solutions of ropivacaine 7.5 mg/ml and bupivacaine 5 mg/ml.[10]
We did not note any significant difference between the groups regarding hemodynamic variables, heart rate, and oxygen saturation. Only 4–5 patients had significant fall in blood pressure who required ephedrine. In his study, Danelli et al.[11] noticed no difference in clinical hypotension in sixty women undergoing elective cesarean delivery under spinal anesthesia with either ropivacaine or bupivacaine, which further supports our study findings.
Similar observations were made by Eryilmaz and Gunaydin.[10] They noticed although mean blood pressure significantly decreased with ropivacaine rather than with bupivacaine at 10 min and 20 min after the spinal block, they were within clinically acceptable limits and did not find any significant difference in the amount of ephedrine used between the groups. We also not found any significant fall with any concentration of ropivacaine.
In both Group A and B patients have good analgesia and muscle relaxation without any significant hemodynamic disturbances intraoperatively and postoperatively. There was complaint of shivering only in few patients that too was not significant. Hence, both 0.5% and 0.75% concentration of ropivacaine were sufficient and effective for intrathecal intraoperative along with epidural use for postoperative analgesia.
Both concentrations of ropivacaine were devoid of serious side effects, and hemodynamic changes were not significant in any group, even in older age group.
CONCLUSION
Ropivacaine is safe and effective for lower limb surgeries in intrathecal block as well as for postoperative pain relief in concentration of 0.5% and. 75%. Shorter duration of motor blockade and analgesia was seen with ropivacaine 0.2%.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McConachie I, McGeachie J, Barrie J. Regional anaesthetic techniques. In: Thomas EJ, Knight PR, editors. Wylie and Churchill Davidson's: A Practice of Anesthesia. Vol. 37. London: Arnold; 2003. pp. 599–612. [Google Scholar]
- 2.Zink W, Graf BM. The toxicity of local anesthetics: The place of ropivacaine and levobupivacaine. Curr Opin Anaesthesiol. 2008;21:645–50. doi: 10.1097/ACO.0b013e32830c214c. [DOI] [PubMed] [Google Scholar]
- 3.Owen MD, Dean LS. Ropivacaine. Expert Opin Pharmacother. 2000;1:325–36. doi: 10.1517/14656566.1.2.325. [DOI] [PubMed] [Google Scholar]
- 4.Simpson D, Curran MP, Oldfield V, Keating GM. Ropivacaine: A review of its use in regional anaesthesia and acute pain management. Drugs. 2005;65:2675–717. doi: 10.2165/00003495-200565180-00013. [DOI] [PubMed] [Google Scholar]
- 5.McConachie I, McGeachie J, Barrie J. Regional anaesthetic techniques. In: Healy TE, Knight PR, editors. Wylie and Churchill Davidson's A Practice of Anesthesia. Vol. 37. London: Arnold; 2003. pp. 599–612. [Google Scholar]
- 6.Sia AT, Chong JL. Epidural 0.2% ropivacaine for labour analgesia: Parturient-controlled or continuous infusion? Anaesth Intensive Care. 1999;27:154–8. doi: 10.1177/0310057X9902700204. [DOI] [PubMed] [Google Scholar]
- 7.Denson DD, Mazoit JX. Physiology, pharmacology, and toxicity of local anesthetic: Adult and pediatric concentrations. In: Raj PP, editor. Clinical Practice of Regional Anesthesia. New York: Churchill Livingstone; 1991. [Google Scholar]
- 8.McNamee DA, McClelland AM, Scott S, Milligan KR, Westman L, Gustafsson U. Comparison of plain ropivacaine 5 mg/ml with bupivacaine 5 mg/ml for orthopaedic surgery. Br J Anaesth. 2002;89:702–6. [PubMed] [Google Scholar]
- 9.Kallio H, Snäll EV, Kero MP, Rosenberg PH. A comparison of intrathecal plain solutions containing ropivacaine 20 or 15 mg versus bupivacaine 10 mg. Anesth Analg. 2004;99:713–7. doi: 10.1213/01.ANE.0000129976.26455.32. [DOI] [PubMed] [Google Scholar]
- 10.Gudul Z, Yumru C, Tokuc EC. A comparison of the effect of isobaric ropivacaine 7.5 mg/ml with isobaric bupivacaine 5 mg/ml for spinal anaesthesia for elective surgery. Reg Anesth Pain Med. 2004;29:221–6. [Google Scholar]
- 11.Danelli G, Fanelli G, Berti M, Cornini A, Lacava L, Nuzzi M, et al. Spinal ropivacaine or bupivacaine for cesarean delivery: A prospective, randomized, double-blind comparison. Reg Anesth Pain Med. 2004;29:221–6. doi: 10.1016/j.rapm.2004.02.003. [DOI] [PubMed] [Google Scholar]


