Abstract
Context:
Brachial plexus block is effective with good postoperative analgesia in upper limb surgery has gained importance as it safe, low cost, and maintains stable hemodynamics intraoperatively. To decrease the onset time and prolong the duration of nerve block bicarbonate, opioids (morphine, fentanyl, etc.), sympathomimetic agents (epinephrine, phenylephrine, etc.), α-2 agonists (clonidine and dexmedetomidine), calcium channel blocker (verapamil), magnesium sulfate, etc., were studied with local anesthetics and their isomers. For their sedative, analgesic, perioperative sympatholytic, and cardiovascular stabilizing effects with reduced anesthetic requirements, α-2 adrenergic receptor agonists, such as more potent and highly selective dexmedetomidine, have been the focus of interest for regional anesthesia. Intravenous dexmedetomidine infusion resulted in significant opioid-sparing effects as well as a decrease in inhalational anesthetic requirements. Animal studies proved that dexmedetomidine enhances sensory and motor blockade along with increased duration of analgesia. In humans, dexmedetomidine has also shown to prolong the duration of block and postoperative analgesia when added to local anesthetic in various regional blocks. Bupivacaine, the widely used local anesthetic in regional anesthesia, is available in a commercial preparation as a racemic mixture (50:50) of its two enantiomers: levobupivacaine, S (−) isomer and dextrobupivacaine, R (+) isomer. Severe central nervous system and cardiovascular adverse reactions reported in the literature after inadvertent intravascular injection or intravenous regional anesthesia have been linked to the R (+) isomer of bupivacaine. The levorotatory isomers were shown to have a safer pharmacological profile with less cardiac and neurotoxic adverse effects. The decreased toxicity of levobupivacaine is attributed to its faster protein binding rate. The pure S (−) enantiomers of bupivacaine, i.e., ropivacaine and levobupivacaine were thus introduced into the clinical anesthesia practice. Such an increased usage mandates the documentation of evidence-based literature with regard to risk and safety concerns as well as clinical issues related to levobupivacaine. This study is designed to assess the efficacy of adding dexmedetomidine to levobupivacaine during placement of supraclavicular brachial plexus blockade.
Materials and Methods:
This prospective observational double-blinded study was conducted over a 1-year period among randomly selected seventy (n = 35) American Society of Anesthesiologists Classes I and II patients of ages between 18 and 60 years of both sexes scheduled to undergo upper limb surgery. With nerve locator, levobupivacaine (0.5%) 28 ml and 2 ml normal saline for Group L and levobupivacaine (0.5%) 28 ml and 0.75 μg/kg dexmedetomidine made up a solution of 2 ml, for Group D, a total 30 ml will be injected locally, in both the groups. Onset and duration of sensory and motor block will be assessed.
Results and Discussion:
One patient in Group L and two patients in Group D failed to achieve block within 30 min. Those three patients were then excluded from the analysis. Hence, the analysis was done by taking 34 patients in Group L and 33 patients in Group D. Onset of sensory and motor block was earlier in Group D (12.03 ± 0.85 and 13.58 ± 0.97) than Group L (14.32 ± 1.15 and 15 ± 0.98), and the difference is statistically significant (P < 0.0001). Duration of sensory and motor block was longer in Group D (563.94 ± 15.60 and 495.15 ± 10.34) than Group L (368.53 ± 9.89 and 321.47 ± 7.84), and the difference is also statistically significant (P < 0.0001). Duration of analgesia was longer in Group D (672.12 ± 11.39) than Group L (506.47 ± 9.497), and the difference is statistically significant (P < 0.0001). Heart rate and mean arterial pressure were well maintained within the presumed range of significant variation, i.e., 20% from baseline, though at some point of time, intergroup comparison was statistically significant. Visual analog scale score compared at the time for administration of rescue analgesic between the groups come out to be statistically significant.
Conclusion:
Addition of 0.75 μg/kg dexmedetomidine to 0.5% levobupivacaine for supraclavicular plexus block shortens sensory and motor block onset time and extends sensory block, motor block, and analgesia duration.
Keywords: Brachial plexus block, dexmedetomidine, levobupivacaine
INTRODUCTION
Supraclavicular brachial plexus blockade (SCBPB) is the common approach to provide surgical anesthesia of upper limb, was first described by Kulenkampff in 1911.[1] Nowadays, SCBPB has gained importance as a regional anesthetic technique of choice for surgical, diagnostic, and therapeutic purposes in interventional pain management. As here (cervical plexus) nerves are most compactly arranged, less amount of anesthetic solution required to block.[2] It provides ideal condition for surgery, maintains stable intraoperative hemodynamic, and decreases vasospasm, edema, and postoperative pain. Due to bupivacaine's long duration of action, it is used most frequently among the local anesthetic drugs for brachial plexus block though provides postoperative analgesia of varying duration when bupivacaine is used alone.[3] Bupivacaine is available in a commercial preparation as a racemic mixture (50:50) of its two enantiomers: levobupivacaine, S (−) isomer and dextrobupivacaine, R (+) isomer. The pure S (−) enantiomers of bupivacaine, i.e., ropivacaine and levobupivacaine were introduced into the clinical anesthesia practice due to less central nervous system and cardiovascular adverse reactions reported in the literature after inadvertent intravascular injection or intravenous regional anesthesia than R (+) isomer of bupivacaine.[4]
Various adjuvants have been clinically used so far, including clonidine, midazolam, neostigmine, hyaluronidase, bicarbonate, and dexamethasone along with local anesthetic (LA) for brachial plexus block.[5,6,7,8,9,10] Addition of any of the above agents is supposed to prolong the analgesic effect without any untoward systemic effects. For their sedative, analgesic, perioperative sympatholytic, and cardiovascular stabilizing effects with reduced anesthetic requirements, α-2 adrenergic receptor agonists have been the focus of interest and are used as epidural, intrathecal, and parenteral injections, either alone or in combination with another drug to prolong and intensify the anesthesia. Compared to clonidine, dexmedetomidine has higher affinity to α-2 receptors. Dexmedetomidine added to LAs shortens the onset, prolongs the duration of block, and enhances postoperative analgesia.[8,11,12,13,14] Addition of dexmedetomidine in clinically relevant doses to levobupivacaine results in a dose-dependent increase in the duration of sensory and motor block. Hence, this study was conducted to evaluate the onset time and analgesic efficacy of dexmedetomidine-levobupivacaine combination in comparison with levobupivacaine (0.5%) for brachial plexus block.
The aim of our study was to compare the onset and duration of sensory and motor blockade, to compare the duration of analgesia, and to find any adverse effect between the two study groups.
MATERIALS AND METHODS
Our prospective randomized double-blind controlled study was conducted after obtaining clearance from the Institutional Ethics Committee and written informed consent from the seventy adult patients of either sex, belonging to American Society of Anesthesiologists Classes I or II, aged between 18 and 60 years, admitted in the Department of General Surgery or Orthopedic of this hospital for forearm surgery at out tertiary care hospital. Patient with opioid or sedative medications in the week before surgery, a history of alcohol or drug abuse, known allergy to any of the test drugs, contraindication brachial plexus block (e.g., coagulation defects, infection at puncture site, and preexisting neurological deficits in the extremities), cardiovascular, respiratory, neurological, psychological, hepatic or renal disease, history of seizures, pneumothorax, and pregnancy were excluded from our study. Participants were allocated into two groups of an equal number of patients (n = 35 participants per group). Sample was designed as per computerized randomized table.
Heart rate (HR), mean arterial pressure (MAP), onset time for complete sensory blockade, duration of sensory block, duration of analgesia, onset time for complete motor blockade, duration of motor block, and visual analog scale (VAS) score at the time of rescue analgesic are studied.
Study technique
All patients were undergone through history taking, physical examination, and all routine investigations. Before performing the procedure, venous cannula 18G was secured in opposite hand, and routine monitors such as pulse oximetry, noninvasive blood pressure, and electrocardiogram were attached. Then, baseline parameter was recorded. Study medication was prepared in identical 30 ml by a person not involved in the study syringes to ensure blinding of anesthetist. Investigators, who have collected postoperative data, also be blinded to study drug administered.
Neural localization was achieved using a nerve locator (Fisher and Paykel, New Zealand) connected to a 22G, 50 mm long stimulating needle (Stimuplex, Braun, Germany). The location end point was a distal motor response with an output lower than 0.5 mA in the median nerve region. Following negative aspiration, levobupivacaine (0.5%) 28 ml and 2 ml normal saline for Group L and levobupivacaine (0.5%) 28 ml and 0.75 μg/kg dexmedetomidine made 2 ml, total 30 ml, for Group D was injected for brachial plexus block.
Sensory block was tested using alcohol swabs and motor blockade was done using the Bromage three point score (2 = normal motor function with full flexion and extension of elbow, wrist, and fingers, 1 = decrease motor strength with the ability to move fingers and/or wrist only, and 0 = complete motor blockade with the inability to move fingers). Patients were sedated with injection midazolam 2 mg intravenously, and oxygen is given by face mask. Vital parameters (pulse, respiration, and blood pressure) were recorded every 5 min for first 30 min and thereafter every 10 min till 120 min, followed by 4, 8, 12, 16, 20, and 24 h from the time of administration of the study drugs.
The duration of analgesia was taken from the time of onset of block to the first complaint of pain (i.e., first rescue analgesic or when VAS score >4). Injection diclofenac sodium intragluteally in a dose of 1.5 mg/kg was administered as a rescue analgesic.
Pain scale = VAS (0–10):
0 - No pain
5 - Moderate pain
10 - Maximum pain.
Episodes of perioperative hypotension (systolic blood pressure <20% of baseline), bradycardia (HR <50 beats/min), and desaturation (SpO2 <90%) were recorded.
Plan for analysis of data
Data were entered in Microsoft Office Excel database and analyzed by standard statistical software. Numerical variables would be compared between group-wise unpaired Student's t-test. If normally distributed categorical variables were compared between groups by Chi-square test or Fischer's exact test as appropriate. Analysis was two tailed and t < 0.05 considered statistically significant.
RESULTS AND ANALYSIS
A total of seventy patients were enrolled in our study. One patient in Group L and two patients in Group D failed to achieve block within 30 min. Those three patients were then excluded from the analysis. Hence, the analysis was done by taking 34 patients in Group L and 33 patients in Group D. Patients between the two groups are demographically comparable as per Table 1, and duration of surgery was not statistically significant [Table 2 and Figure 1].
Table 1.
Demographic parameters
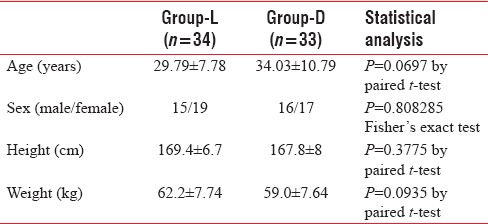
Table 2.
Duration of surgery with onset and duration of sensory ad motor block
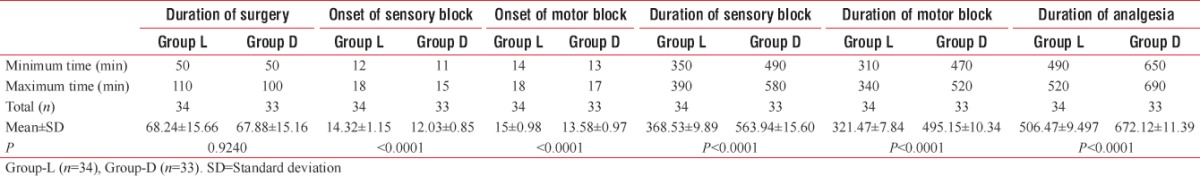
Figure 1.
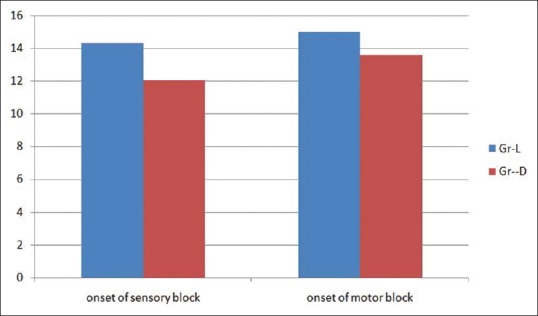
Comparison of onset of sensory and motor block
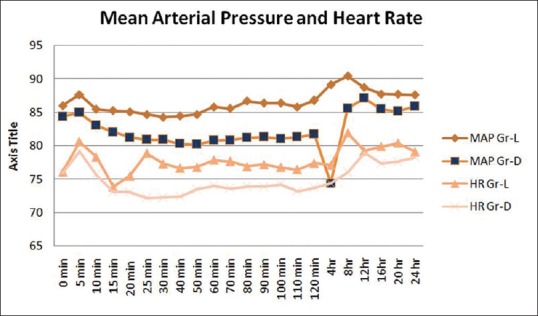
Onset of sensory and motor block was earlier in Group D (12.03 ± 0.85 and 13.58 ± 0.97) than Group L (14.32 ± 1.15 and 15 ± 0.98) and the difference is statistically significant (P < 0.0001). Duration of sensory and motor block was longer in Group D (563.94 ± 15.60 and 495.15 ± 10.34) than Group L (368.53 ± 9.89 and 321.47 ± 7.84), and the difference is also statistically significant (P < 0.0001) [Figure 2]. Duration of analgesia was longer in Group D (672.12 ± 11.39) than Group L (506.47 ± 9.497), and the difference is statistically significant (P < 0.0001) [Table 2 and Figure 2]. From the Table 3 and Figure 3, we can see HR and MAP were well maintained within the presumed range of significant variation, i.e., 20% from baseline though at some point of time, intergroup comparison was statistically significant.
Figure 2.
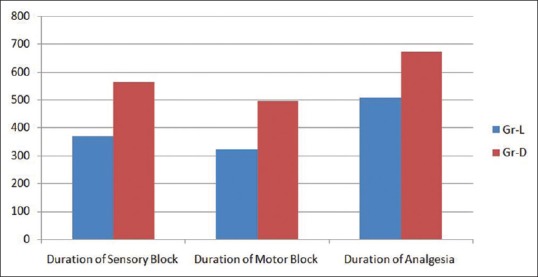
Comparison of duration of sensory block, motor block, and analgesia
Table 3.
Comparison of hemodynamics in different time intervals
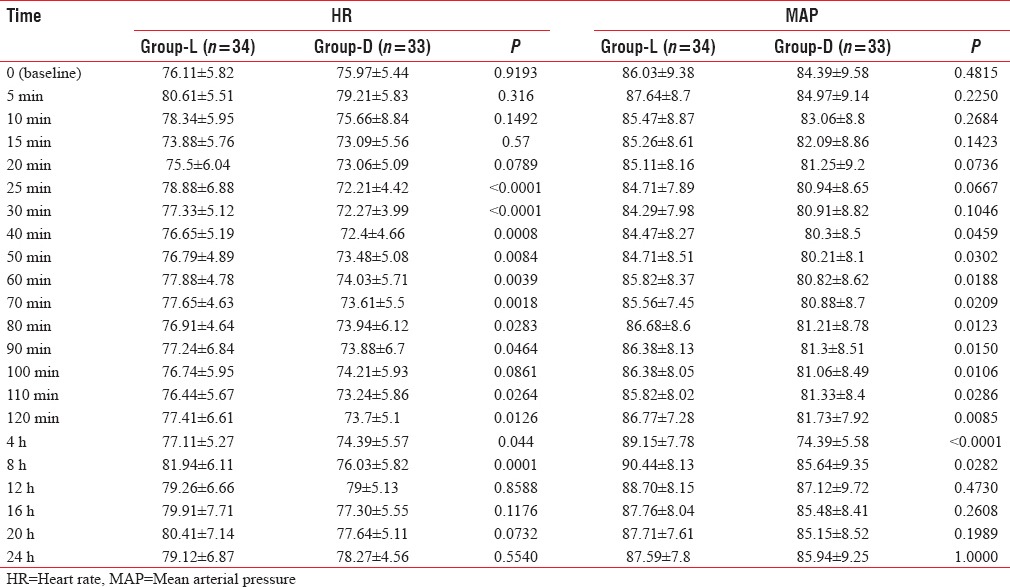
Figure 3.
Comparison of hemodynamics at different time intervals
There was a significant difference in VAS score at the time of rescue analgesia (P < 0.0001 by paired t-test) among the control group (Group L) and study group (Group D) as per Table 4.
Table 4.
Visual analog scale score at the time of rescue analgesic

DISCUSSION
In our study, we demonstrated that the addition of dexmedetomidine to levobupivacaine can significantly shorten the sensory and motor block onset time, reduce the offset time for motor block, and prolong the duration of postoperative analgesia.
Local anesthetic agent selection, dose, concentration, volume, and physical modifications can affect onset, spread, quality, and duration of anesthesia. Levobupivacaine, the S-enantiomer of bupivacaine, which has less cardiac and neural toxicity than bupivacaine, is currently the closest to the ideal agent for neural blockade; however, a large volume of drug is required for adequate block.[15] As dexmedetomidine has peripheral analgesic action,[16] so it was hypothesized that it may be helpful in reducing the onset of sensory and motor block on the other hand increasing duration of sensory and motor block as well as analgesia.
In our study, we found the following outcome as in Table 2
Onset of sensory block was earlier in Group D (12.03 ± 0.85) than Group L (14.32 ± 1.15), and the difference is statistically significant (P < 0.0001)
Onset of motor block was earlier in Group D (13.58 ± 0.97) than Group L (15 ± 0.98), and the difference is statistically significant (P < 0.0001)
Duration of sensory block was longer in Group D (563.94 ± 15.60) than Group L (368.53 ± 9.89), and the difference is statistically significant (P < 0.0001)
Duration of motor block was longer in Group D (495.15 ± 10.34) than Group L (321.47 ± 7.84), and the difference is statistically significant (P < 0.0001)
Duration of analgesia was longer in Group D (672.12 ± 11.39) than Group L (506.47 ± 9.497), and the difference is statistically significant (P < 0.0001).
In a randomized, double-blind trial performed by Esmaoglu et al.,[17] Biswas et al.,[18] and Kaur et al.[19] and a meta-analysis by Abdallah and Brull,[20] dexmedetomidine added to local anesthetic for brachial plexus blockade shortened the block onset time, prolonged the duration of motor and sensory effects, and extended postoperative analgesia that corroborate with our study.
Recently, Kaygusuz et al.[21] evaluated the addition of dexmedetomidine 1 μg/kg–0.5% levobupivacaine in axillary brachial plexus block and observed significantly decreased sensory block onset time, an increase in the sensory and motor block duration, and time to first analgesic use. In our study using dexmedetomidine 0.75 μg/kg with 0.5% levobupivacaine, we have found the same result.
Ammar and Mahmoud and Agarwal et al.[22,23] used dexmedetomidine with bupivacaine and compared it with plain bupivacaine and demonstrated enhancement of onset of sensory and motor blockade, prolonged duration of analgesia, increased duration of sensory and motor block, lower VAS pain scores, and reduction in supplemental opioid requirements.
Dexmedetomidine may lead to side effects such as hypotension and bradycardia with increased dosage, along with its effects such as sedation and anxiolysis.[22,23,24] As we have used dexmedetomidine 0.75 μg/kg for that reason, we may have not got any hemodynamic side effect.
As per Table 3 at 25 min, 30 min, 40 min, 50 min, 60 min, 70 min, 80 min, 90 min, 110 min, 120 min, 4 h, and 8 h after completion of block, HR in both the group was statistically significant where MAP was statistically significant; 60 min, 70 min, 80 min, 90 min, 100 min, 110 min, 120 min, 4 h, and 8 h after completion of block but that rise is not clinically significant. Furthermore, the entire change was well maintained within the presumed range of significant variation, i.e., 20% from baseline.
VAS score is generally used as a tool to determine appropriate time for administration of rescue analgesia, considering the subjective element in this type of assessment we anticipated that the effect of adjuvant to the local anesthetic will have some influence on the outcome of the score. VAS score compared at the time for administration of rescue analgesic between the groups come out to be statistically significant. Our study, therefore, points to a different aspect that dexmedetomidine as an adjuvant may also affect a subjective parameter of analgesia during the perioperative period. Further study toward this aspect utilizing tools such as patient satisfaction score may provide better insight to this finding. As per our finding, patient and surgeon's satisfaction is good.
Major limitations of our study were that we could not biochemically analyze the blood concentration of levobupivacaine and dexmedetomidine due to nonavailability of facilities at our institution, which would have further supported our conclusions. Use of ultrasound-guided nerve blocks may further help to reduce effective levobupivacaine concentration with advantage of injecting the LA mixtures in near proximity of nerve bundles. Further randomized trials need to be conducted to validate the findings of our study.
CONCLUSION
The results of the present study conclude that the addition of 0.75 μg/kg dexmedetomidine to 0.5% levobupivacaine for supraclavicular plexus block helps in decreased onset time for sensory and motor block on the other hand increased the duration of motor block, sensory block, and duration of analgesia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank our family and colleagues.
REFERENCES
- 1.Kulenkampff D. Anesthesia of the brachial plexus. Zentralbl Chir. 1911;38:1337–40. [Google Scholar]
- 2.Brown DL. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998. The upper extremity: The somatic block. Neural Blockade in Clinical Anaesthesia and Management of Pain; pp. 345–71. [Google Scholar]
- 3.Lund PC, Cwik JC, Vallesteros F. Bupivacaine – A new long-acting local anesthetic agent. A preliminary clinical and laboratory report. Anesth Analg. 1970;49:103–14. [PubMed] [Google Scholar]
- 4.Bajwa SJ, Kaur J. Clinical profile of levobupivacaine in regional anesthesia: A systematic review. J Anaesthesiol Clin Pharmacol. 2013;29:530–9. doi: 10.4103/0970-9185.119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picard PR, Tramèr MR, McQuay HJ, Moore RA. Analgesic efficacy of peripheral opioids (all except intra-articular): A qualitative systematic review of randomised controlled trials. Pain. 1997;72:309–18. doi: 10.1016/s0304-3959(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 6.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: A systematic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Kohane DS, Lu NT, Cairns BE, Berde CB. Effects of adrenergic agonists and antagonists on tetrodotoxin-induced nerve block. Reg Anesth Pain Med. 2001;26:239–45. doi: 10.1053/rapm.2001.23215. [DOI] [PubMed] [Google Scholar]
- 8.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (a2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth. 2012;56:243–9. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabaeizavareh MH, Omranifard M, Moalemi A. The effect of verapamil as an adjuvant agent with local anesthetic on sensory block level, hemodynamic and postoperative pain. Pak J Med Sci. 2012;28:259–62. [Google Scholar]
- 10.Gunduz A, Bilir A, Gulec S. Magnesium added to prilocaine prolongs the duration of axillary plexus block. Reg Anesth Pain Med. 2006;31:233–6. doi: 10.1016/j.rapm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi R, Shah A, Patel I. Use of dexmedetomidine along with bupivacaine for brachial plexus block. Natl J Med Res. 2012;2:67–9. [Google Scholar]
- 12.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R. Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med. 2010;35:427–31. doi: 10.1097/AAP.0b013e3181ef4cf0. [DOI] [PubMed] [Google Scholar]
- 14.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baskan S, Taspinar V, Ozdogan L, Gulsoy KY, Erk G, Dikmen B, et al. Comparison of 0.25% levobupivacaine and 0.25% bupivacaine for posterior approach interscalene brachial plexus block. J Anesth. 2010;24:38–42. doi: 10.1007/s00540-009-0846-0. [DOI] [PubMed] [Google Scholar]
- 16.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 17.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 18.Biswas S, Das RK, Mukherjee G, Ghose T. Dexmedetomidine an adjuvant to levobupivacaine in supraclavicular brachial plexus block: A randomized double blind prospective study. Ethiop J Health Sci. 2014;24:203–8. doi: 10.4314/ejhs.v24i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur H, Singh G, Rani S, Gupta KK, Kumar M, Rajpal AS, et al. Effect of dexmedetomidine as an adjuvant to levobupivacaine in supraclavicular brachial plexus block: A randomized double-blind prospective study. J Anaesthesiol Clin Pharmacol. 2015;31:333–8. doi: 10.4103/0970-9185.161668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: A systematic review and meta-analysis. Br J Anaesth. 2013;110:915–25. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 21.Kaygusuz K, Kol IO, Duger C, Gursoy S, Ozturk H, Kayacan U, et al. Effects of adding dexmedetomidine to levobupivacaine in axillary brachial plexus block. Curr Ther Res Clin Exp. 2012;73:103–11. doi: 10.1016/j.curtheres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammar AS, Mahmoud KM. Ultrasound-guided single injection infraclavicular brachial plexus block using bupivacaine alone or combined with dexmedetomidine for pain control in upper limb surgery: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6:109–14. doi: 10.4103/1658-354X.97021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal S, Aggarwal R, Gupta P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2014;30:36–40. doi: 10.4103/0970-9185.125701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross SA. Pathophysiology of pain. Mayo Clin Proc. 1994;69:375–83. doi: 10.1016/s0025-6196(12)62225-3. [DOI] [PubMed] [Google Scholar]


