Abstract
Background:
Relief of pain is very important goal intraoperatively and postoperatively. Neostigmine has been used successfully intrathecally with other agents such as clonidine and opioids for pain relief.
Aims:
This study aims to compare and evaluate the efficacy and safety of combining intrathecal (IT) neostigmine with IT clonidine and transdermal nitroglycerin (tNTG) patch for the relief of pain in patients after surgery.
Settings and Design:
This was a randomized, prospective, and comparative study
Materials and Methods:
In this study, recruited patients were randomly allocated into three groups. Groups I, II, and III received intrathecally 25 μg of neostigmine + 15 mg hyperbaric 0.5% bupivacaine, 25 μg of neostigmine + 25 μg clonidine + 15 mg hyperbaric 0.5% bupivacaine, and 25 μg of neostigmine + tNTG patch (3 cm × 5 cm, 5 mg/24 h) +15 mg hyperbaric 0.5% bupivacaine, respectively. Heart rate, mean arterial pressure, analgesic properties, and complications were assessed and compared among groups.
Statistical Analysis:
Mean and standard deviation were calculated. Test of analysis between two groups was done by t-test and among three groups by ANOVA, then P value was calculated.
Results:
Duration of analgesia was significantly longer in Group III in comparison to Group II (7.142 ± 1.81 vs. 4.408 ± 0.813 h) and was significantly longer in Group II in comparison to Group I (4.408 ± 0.813 vs. 2.583 ± 0.493 h). Analgesic requirement was significantly less in Group III in comparison to Group II (1.9 ± 0.76 vs. 2.5 ± 0.51) and was significantly less in Group II in comparison to Group I (2.5 ± 0.51 vs. 3.1 ± 0.48). Sedation score was found significantly high in Group II than other groups.
Conclusion:
Both IT clonidine and tNTG patch with bupivacaine + neostigmine spinal anesthesia were found effective in pain control. Results were found better with tNTG patch.
Keywords: Clonidine, neostigmine, Ramsay Sedation Scale, transdermal nitroglycerine, visual analog scale
INTRODUCTION
The relief of pain is one of the paramount goals of medical science. The surgical stress response peaks during the postoperative period and has major effects on almost all body systems. A pain- and stress-free postoperative period definitely reduces morbidity and mortality of any surgical operation. Neuraxial blockade is one of the effective modalities to control postoperative pain. Neostigmine is the universally used neuromuscular blocking reversal agent whose postoperative pain relief property was first described by Naguib and Yaksh et al.[1] in 1994. However, a higher dose of neostigmine shown to produce many untoward side effects such as nausea, vomiting, etc., and lower doses of neostigmine does not show much analgesic property.[2] Hence, as to reduce the dose of neostigmine and potentiate its analgesic property other adjuvants such as clonidine, opioids, and transdermal nitroglycerin (tNTG) patch have been added along with it. Clonidine, a selective partial α2 adrenergic agonist, is being extensively evaluated as an adjuvant to intrathecal (IT) local anesthetics and has proven to be a potent analgesic free of opioid-related side effects.[3] It is known to increase both sensory and motor blockade of local anesthetics.[4] IT clonidine has been used as an adjuvant to local anesthetics in various surgical procedures without any clinically significant side effects.[5,6] tNTG has been found to be useful for enhancing the postoperative analgesic effect of IT sufentanil[7] and neostigmine[8] by release of nitric oxide (NO). This NO increases the intracellular concentration of cyclic guanosine monophosphate, which produces pain modulation in the central and peripheral nervous system.[9]
The purpose of the present study was to compare and evaluate the efficacy and safety of combining IT neostigmine with IT clonidine and tNTG patch for the relief of pain in patients after surgery.
MATERIALS AND METHODS
After approval from the Ethical Committee and taking written consent from the patients, the study was conducted at Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, over a period of 1 year. This prospective study was conducted on 90 adult patients of the American Society of Anesthesiologists physical status Classes I and II, aged 18–60 years scheduled for surgery below the umbilicus. Patients were systematically randomized into three groups, consisting thirty patients in each group.
Group I: Patients received 15 mg of hyperbaric bupivacaine (0.5%) and 25 μg of neostigmine intrathecally
Group II: Patients received 15 mg of hyperbaric bupivacaine (0.5%) and 25 μg of neostigmine and 25 μg of clonidine intrathecally
Group III: Patients, in addition to 15 mg of hyperbaric bupivacaine (0.5%) and 25 μg of neostigmine intrathecally, received tNTG patch (3 cm × 5 cm, 5 mg/24 h) at chest wall in nonanesthetized area 20 min after IT administration of drug solution.
Exclusion criteria
Patient refusal, patients having cardiopulmonary illness, metabolic disorders, nervous system disorders, psychiatric disorders, history of hypersensitivity reaction to any of the study medications, patients having gastrointestinal disorders, patients with sinus bradycardia, bleeding disorder, infection at the site of lumbar puncture, patients on opioids, or chronic analgesic abuse.
Preanesthetic checkup was done 1 day earlier and nil per oral explained. All the patients were familiarized with 0–10 cm visual analog scale (VAS) for pain and nausea. Zero equal to “no pain or nausea” and ten equal to “worst possible pain or nausea”. All patients received tablet alprazolam 0.25 mg and tablet ranitidine 150 mg orally on the previous night of surgery. On arrival to the operation theater table, an intravenous (IV) infusion line was secured, and monitors were connected. Baseline recordings of pulse, systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate, (pulse oximetry) SpO2, and electrocardiography (ECG) tracings were taken.
Preloading of the patients with ringer lactate 10 ml/kg was done 15 min before procedure. Lumbar puncture was performed at L3–L4 level under aseptic conditions, with 26-gauge spinal needle, and the drug solution was injected intrathecally over 30 s as per the group allocation. The level of sensory loss was assessed by pinprick test. Heart rate, SBP, DBP, Respiratory rate, ECG, and SpO2 were monitored intraoperatively. Blood pressure and heart rate were monitored every 5 min throughout the surgery and a decrease >20% below the baseline value was treated by the incremental dose of injection ephedrine 4 mg IV. Any fall in the heart rate below 50 beats/min was treated with incremental doses of injection atropine 0.3 mg IV. Intraoperative nausea was treated initially with injection metoclopramide 10 mg IV followed by injection ondansetron 4 mg IV. Postoperatively, patients were assessed for pain by VAS rating scale at 15 min, 2 h, 4 h, 10 h, 18 h, and 24 h after the surgery was over.
Sedation score was evaluated at the above mentioned time intervals and was recorded using Ramsay scale:
1 = Anxious, agitated or restless or both
2 = Cooperative, oriented and tranquil
3 = Responding to command only
4 = Brisk response to light glabellar tap
5 = Sluggish response to light glabellar tap
6 = No response to light glabellar tap.
Patients were also assessed for the side effects such as nausea, vomiting, sedation, hypotension, bradycardia, sweating, and palpitation at the above-mentioned time intervals. Rescue analgesia was administered at VAS 4 or >4 (moderate pain). Patients were given injection tramadol (1 mg/kg) IV as a rescue analgesic. The total amount of tramadol consumed by the patients in each group was also recorded. Duration of effective analgesia was measured from the time of IT drug administration to the patient's first request for analgesic.
Statistical analysis
Data thus obtained were analyzed using Microsoft Excel 2010. Data obtained from the patients under study were recorded in a standard pro forma. The parametric data were expressed as mean ± standard deviation. Test for analysis among three groups was done by ANOVA for quantitative and Chi-square test for qualitative data. Comparison between two groups was done by Student's t-test. P < 0.05 was considered statistically significant and P > 0.05 was not considered statistically significant.
OBSERVATIONS AND RESULTS
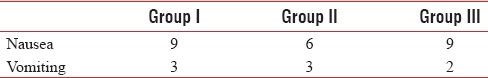
There was no significant difference found among the three study groups in respect of age, sex, weight, duration of surgery, and baseline laboratory investigations such as pulse rate, mean arterial pressure, and respiratory rate as shown in Table 1. There was no statistically significant difference in heart rate and mean arterial pressure among the groups (P > 0.05) as shown in Tables 2 and 3. Duration of analgesia was longest in Group III in comparison to other groups, and this difference was statistically significant (P = 0.00). As shown in Table 4, duration of analgesia was significantly longer in Group III in comparison to Group II (7.142 ± 1.81 vs. 4.408 ± 0.813 h, P < 0.05) and was significantly longer in Group II in comparison to Group I (4.408 ± 0.813 vs. 2.583 ± 0.493 h, P < 0.05). Table 4 shows that analgesic requirement was significantly less in Group III in comparison to Group II (1.9 ± 0.76 vs. 2.5 ± 0.51, P < 0.05) and was significantly less in Group II in comparison to Group I (2.5 ± 0.51 vs. 3.1 ± 0.48, P < 0.05). Sedation score was highest in Group II in comparison of other groups, and this difference was statistically significant (P = 0.006). There was no statistically significant difference in sedation score in Groups I and III (P > 0.05) [Table 4]. There was no significant difference found among the groups when compared nausea and vomiting [Table 5]. No incidence of respiratory depression seen in any group.
Table 1.
Demographic data
Table 2.
Comparison of heart rate (beats/min)

Table 3.
Comparison of mean arterial pressure (mm of Hg)

Table 4.
Comparison of duration of analgesia (h), number of analgesic requirement in the first 24 h and sedation score (n=30)
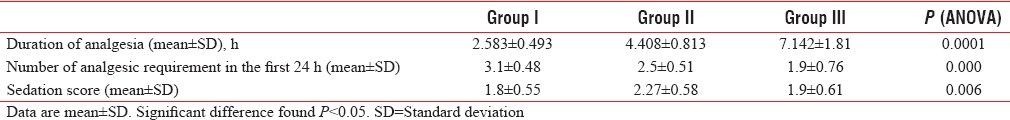
Table 5.
Comparison of adverse effects
DISCUSSION
Various drugs have been tried in the subarachnoid space along with local anesthetics with the aim of improving the duration of postoperative analgesia. The cholinesterase inhibitor neostigmine is one among such adjuvants. Even though neostigmine shown to produce an increase in duration of analgesia, it was also associated with many unwanted side effects particularly nausea and vomiting, especially in higher doses.[10] Hence, to reduce the adverse effects and to prolong postoperative analgesia, other adjuvants have been used along with neostigmine. The aim of this study was to systematically review current evidence of analgesic enhancement of IT neostigmine by the addition of IT clonidine and tNTG patch on bupivacaine spinal anesthesia. The rationale for combining IT neostigmine and clonidine was that IT alpha-2 agonists stimulate norepinephrine release from dorsal horn of spinal cord and cholinesterase inhibitors inhibit the breakdown of spinally released acetylcholine and increase the acetylcholine concentration in cerebrospinal fluid. These drugs exert their antinociceptive effect by mimicking the action of spinally released norepinephrine or acetylcholine, which acted on spinal α2 adrenoceptors or cholinergic receptors, respectively. An additive antinociceptive interaction was found between IT clonidine and cholinesterase inhibitor.[11] As acetylcholine-induced response have been shown to involve NO, the present study was designed to examine whether a combination of tNTG (source of exogenous NO) would enhance the analgesic efficacy of IT neostigmine (source of acetylcholine).
In our study, the duration of analgesia was analyzed as period between complete onset of sensory blockade to the time at which patient started complaining of pain or first rescue analgesic was given using VAS score. We found that the addition of either 25 μg of IT clonidine or a tNTG patch (5 mg/24 h) provides a good duration of postoperative analgesia and this correlates with the findings of Pan et al.,[12] Lauretti et al.,[8] and Kaur et al.[13] Pan et al.[12] conducted a study of enhancement of analgesic effect of IT neostigmine and clonidine on bupivacaine spinal anesthesia. Their study was designed to evaluate the efficacy and safety of the combining IT neostigmine and clonidine for the relief of pain in patients after cesarean delivery. They concluded that the combination of 150 μg clonidine intrathecally and 50 μg neostigmine provided longer postsurgical analgesia than with either drug used alone. Lauretti et al.[8] in 2000 conducted a study to determine whether the association of tNTG would enhance analgesia from a low dose of IT neostigmine in patients undergoing gynecologic surgery during spinal anesthesia. They concluded that neither IT 5 μg neostigmine alone nor tNTG alone (5 mg/day) delayed the time to administration of the first rescue analgesics, the combination of both provided an average of 14 h of effective postoperative analgesia after vaginoplasty, suggesting that transdermal nitroglycerin and the central cholinergic agent neostigmine may enhance each other's antinociceptive effects at the dose studied. Kaur et al. in 2007 conducted a study to assess the effect of transdermal nitroglycerin patch (5 mg/24 h) on the analgesia of IT neostigmine (5 μg) and incidence of untoward effects. They found a statistically significant longer duration of analgesia in patients who received both IT neostigmine and tNTG than in patients who received neostigmine alone.[8]
The present study did not show any significant changes on cardiovascular parameters. The mean heart rate and mean arterial pressures were comparable in all the groups in the intra and postoperative periods and was found to be statistically insignificant. This finding was consistent with the findings observed by Hood et al., Krukowski et al., Lauretti et al. (1999) and Lauretti et al. in 2000. Hood et al.[2] studied the effects of IT neostigmine in 28 healthy volunteers in doses ranging from 50 μg to 750 μg. They found that IT neostigmine of 50 μg did not affect any measured cardiovascular variable. IT neostigmine in doses of 750 μg was associated with increased blood pressure and heart rate probably due to excitatory action on preganglionic sympathetic neurons. Lauretti et al. in 2000 conducted a study to determine whether the association of tNTG would enhance analgesia from a low dose of IT neostigmine in patients undergoing gynecologic surgery during spinal anesthesia. Their study shown that there were no significant changes on cardiovascular parameters while using nitroglycerin transdermal patch along with IT neostigmine. Krukowski et al.[14] found that when neostigmine injection preceded epidural clonidine by 15 min, it reduces the incidence of hypotension. They also founded that IT neostigmine did not have any significant effect on mean arterial pressures.
In our study, we observed that Group II patients showed significantly higher sedation score than the other two groups. This difference can be attributed to the use of IT clonidine. Sethi et al.[6] conducted a study on the efficacy of analgesic effects of low-dose IT clonidine as adjuvant to bupivacaine. In their study, the incidence of sedation as assessed by sedation score was higher in the clonidine group than in the control group.
High incidence of nausea and vomiting was found in all the three groups. This is most probably occurred due to use of neostigmine. Cho et al.[15] conducted a study to analyze the effect of IT neostigmine on postcesarean section analgesia. This study was designed to evaluate the efficacy and safety of IT neostigmine for postcesarean section analgesia. The study was conducted using normal saline 0.2 ml, or neostigmine 12.5 μg, or neostigmine 25 μg intrathecally with 0.5% hyperbaric bupivacaine. There were significantly higher incidences of nausea and vomiting in neostigmine groups than in saline group.
CONCLUSION
We concluded in this study that the addition of IT clonidine and tNTG patch to bupivacaine + neostigmine spinal anesthesia produced significant increase in duration of analgesia and decrease in requirement of analgesics. The results were better with tNTG compared to IT clonidine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Naguib M, Yaksh TL. Antinociceptive effects of spinal cholinesterase inhibition and isobolographic analysis of the interaction with mu and alpha 2 receptor systems. Anesthesiology. 1994;80:1338–48. doi: 10.1097/00000542-199406000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–43. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Eisenach JC. Overview: First international symposium on alpha 2-adrenergic mechanisms of spinal anesthesia. Reg Anesth. 1993;18:207–12. [PubMed] [Google Scholar]
- 4.Chiari A, Eisenach JC. Spinal anesthesia: Mechanisms, agents, methods, and safety. Reg Anesth Pain Med. 1998;23:357–62. doi: 10.1016/s1098-7339(98)90006-2. [DOI] [PubMed] [Google Scholar]
- 5.van Tuijl I, van Klei WA, van der Werff DB, Kalkman CJ. The effect of addition of intrathecal clonidine to hyperbaric bupivacaine on postoperative pain and morphine requirements after Caesarean section: A randomized controlled trial. Br J Anaesth. 2006;97:365–70. doi: 10.1093/bja/ael182. [DOI] [PubMed] [Google Scholar]
- 6.Sethi BS, Samuel M, Sreevastava D. Efficacy of analgesic effects of low dose intrathecal clonidine as adjuvant to bupivacaine. Indian J Anaesth. 2007;51:415–9. [Google Scholar]
- 7.Lauretti GR, de Oliveira R, Reis MP, Mattos AL, Pereira NL. Transdermal nitroglycerine enhances spinal sufentanil postoperative analgesia following orthopedic surgery. Anesthesiology. 1999;90:734–9. doi: 10.1097/00000542-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Lauretti GR, Oliveira AP, Julião MC, Reis MP, Pereira NL. Transdermal nitroglycerine enhances spinal neostigmine postoperative analgesia following gynecological surgery. Anesthesiology. 2000;93:943–6. doi: 10.1097/00000542-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Duarte ID, Lorenzetti BB, Ferreira SH. Acetylcholine induces peripheral analgesia by the release of nitric oxide from l-arginine. In: Moncada S, Higgs EA, editors. A Bioregulatory system. Amsterdam: Elsevier; 1990. pp. 165–70. [Google Scholar]
- 10.Tan PH, Kuo JH, Liu K, Hung CC, Tsai TC, Deng TY. Efficacy of intrathecal neostigmine for the relief of postinguinal hemiorrhaphy pain. Acta Anaesthesiol Scand. 2000;44:1056–60. doi: 10.1034/j.1399-6576.2000.440904.x. [DOI] [PubMed] [Google Scholar]
- 11.Gordh T, Jr, Jansson I, Hartvig P, Gillberg PG, Post C. Interactions between noradrenergic and cholinergic mechanisms involved in spinal nociceptive processing. Acta Anaesthesiol Scand. 1989;33:39–47. doi: 10.1111/j.1399-6576.1989.tb02857.x. [DOI] [PubMed] [Google Scholar]
- 12.Pan PM, Huang CT, Wei TT, Mok MS. Enhancement of analgesic effect of intrathecal neostigmine and clonidine on bupivacaine spinal anesthesia. Reg Anesth Pain Med. 1998;23:49–56. doi: 10.1016/s1098-7339(98)90110-9. [DOI] [PubMed] [Google Scholar]
- 13.Kaur G, Osahan N, Afzal L. Effect of transdermal nitroglycerine patch on analgesia of low dose intrathecal neostigmine: An evaluation. J Anesth Clin Pharmacol. 2007;23:159–62. [Google Scholar]
- 14.Krukowski JA, Hood DD, Eisenach JC, Mallak KA, Parker RL. Intrathecal neostigmine for post-cesarean section analgesia: Dose response. Anesth Analg. 1997;84:1269–75. doi: 10.1097/00000539-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Cho SS, Kim JS, Chung CJ, Han IS, Jang SC. Effect of intrathecal neostigmine on post-cesarean section analgesia. Korean J Anesthesiol. 1998;35:545–52. [Google Scholar]


