Abstract
Background:
Awake fiberoptic intubation (AFOI) is a recommended technique for anticipated difficult airway. An ideal regime should provide patient comfort, cooperation, amnesia, hemodynamic stability, and blunt airway reflexes and maintain a patent airway with spontaneous ventilation. The aim of our study was to compare intubation conditions between dexmedetomidine and fentanyl–midazolam combination during AFOI.
Methods:
This prospective, randomized study was conducted on a total of sixty patients of the American Society of Anesthesiologists physical status I and II of either sex, in the age group of 18–60 years having predicted difficult intubation undergoing elective surgeries and the patients were allocated to two groups of thirty patients each. After premedication and topicalization of airways, dexmedetomidine group (Group I, n = 30) received dexmedetomidine 1 μg/kg over 10 min and midazolam–fentanyl group (Group II, n = 30) received fentanyl 2 μg/kg plus midazolam 0.02 mg/kg over 10 min. Adequacy of intubation condition was evaluated by cough score and postintubation score. Incidence of desaturation, hemodynamic changes, and sedation using Ramsay sedation scale were noted and compared between two groups.
Results:
The demographic characteristics were comparable in the two groups (P > 0.05). The mean Ramsay sedation score in Group I was 3.13 ± 0.937 and Group II was 3.16 ± 0.949, and the comparison between two groups was statistically insignificant (P = 0.891). Cough scores and postintubation scores were favorable in dexmedetomidine group than midazolam–fentanyl group and were statistically significant with P < 0.001 and 0.0001, respectively. Group I also showed better hemodynamics and less episodes of desaturation than Group II.
Conclusions:
Dexmedetomidine is more effective than midazolam–fentanyl during AFOI, as it provides better intubation condition, hemodynamic stability, and preservation of airway and spontaneous ventilation.
Keywords: Awake fiberoptic, dexmedetomidine, fentanyl, intubation, midazolam
INTRODUCTION
Predicted difficult airway has always been challenging for the anesthesiologist, and awake fiberoptic intubation (AFOI) has been accepted as one of the best techniques for its management.[1] Difficult airway scenarios arise in various clinical syndromes, head and neck malignancies, trauma, and previous failed intubation attempts, and hence, adequate preparation is needed to prevent any airway compromise. AFOI is practiced for these recognized difficult airway conditions, and it needs expertise on the part of the anesthesiologist to master the technique and allay the anxiety and discomfort of the patient. AFOI is also known to cause the sympathetic response in the patients.[2,3] Hence, a sedative agent which allays anxiety and discomfort of the patient and decreases the sympathetic response without causing respiratory depression is ideal for such situations. Various drugs have been used previously such as benzodiazepines, opioids, and propofol. These drugs cause sedation, amnesia, and attenuate the hemodynamic response. However, they are known to cause respiratory depression and hence hypoxemia.[4,5] Dexmedetomidine, a selective α2 agonist, has been recently tried for AFOI and provides both sedation and analgesia without causing respiratory depression or airway compromise.[6,7,8] Hence, this study was undertaken to compare the effectiveness of dexmedetomidine and fentanyl plus midazolam combination for evaluating intubating condition during AFOI.
METHODS
This prospective, randomized study was conducted in a tertiary care hospital for a period of 2 years from July 2015 to June 2017. A total of sixty patients of the American Society of Anesthesiologists (ASA) physical status (PS) I and II of either sex, in the age group 18–60 years having predicted difficult intubation undergoing elective surgeries were allocated using computer-generated randomization list into two groups of thirty patients each after taking written informed consent and approval from the institutional review board. Uncooperative patients, pregnant patients, patients with age below 18 years and allergy to drugs used, patients requiring emergency surgery, and patients with coagulopathies were excluded from the study. Tablet ranitidine 150 mg and tablet ondansetron 4 mg were given as premedication 2 h before surgery. A patient was shifted to operating room on trolley, and baseline monitors such as electrocardiogram, noninvasive blood pressure, and oxygen saturation (SpO2) were connected after securing wide bore (18 G) cannula. Puffs of 10% lidocaine were sprayed over tongue and hypopharynx after nebulization with 2% lidocaine 4 ml (80 mg) given for topicalization. Lidocaine jelly and xylometazoline nasal drops were applied to both the nostrils. After that, dexmedetomidine (1 μg/kg over 10 min) in Group I and fentanyl (2 μg/kg over 10 min) plus midazolam 0.02 mg/kg in Group II were infused according to the subjects inclusion number. Bronchoscope was loaded with appropriate size cuffed polyvinyl chloride endotracheal tube after proper lubrication. Ramsay sedation score (RSS)[9] was used to assess the sedation at the end of study drug infusion as follows:
Anxious, agitated, or restless
Cooperative, oriented, and tranquil
Sedated but responds to command
Asleep, brisk glabellar reflex responds to loud noise,
Asleep, sluggish glabellar reflex, or responds to loud noise
Asleep with no response to a painful stimulus.
Bronchoscopy was performed only when RSS ≥2. General anesthesia was induced after proper placement of the tube and after that surgery was allowed to proceed. Cough score[10] during bronchoscopy was used to evaluate the intubation conditions as follows:
No cough
Slight cough (no more than two cough in sequence)
Moderate cough (3–5 cough in sequence)
Severe cough (>5 cough in sequence).
Tolerance to intubation was evaluated by postintubation score[11] after placement of tube in the trachea as follows:
Cooperative
Minimal resistance
Severe resistance.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and SpO2 were noted at baseline, postintubation, 30 min, 1 h, 1.5 h, 2 h, 2.5 h, and 3 h. Hypotension, bradycardia, and desaturation were treated with intravenous fluids, drugs, and oxygen supplementation, respectively.
Statistical analyses were carried out using the statistical package for the social sciences 16.0 statistical software packages. Numerical data were compared between two groups using independent t-test and within the same group using paired t-test. Categorical data were compared between two groups using Chi-square test. All analyses were two tailed and the results were discussed on 5% level of significance, i.e., P < 0.05 was considered statistically significant.
RESULTS
The demographic characteristics (age, weight, male:female ratio, and ASA-PS) were comparable between two groups [Table 1]. The baseline HR, SBP, DBP, and SpO2 were comparable in two groups [Table 2]. There was rise of HR, SBP, and DBP compared to baseline values in both the groups; however, the rise was more in group II than group I and was statistically significant. These parameters were comparable in two groups at all intervals of time except the postintubation period [Figures 1–3]. The mean HR of Group I at postintubation was 87.33 ± 9.14 and of Group II was 98.40 ± 4.91 with P < 0.0001 and was statistically significant; the mean SBP of Group I at postintubation was 127.37 ± 7.568 and of Group II was 133.2 ± 6.96 with P = 0.003 and was statistically significant; and the mean DBP of Group I at postintubation was 84.00 ± 5.705 and of Group II was 90.60 ± 5.870 with P < 0.0001 and was statistically significant [Table 2]. In the groups when compared with reference to overall mean HR, mean SBP, and mean DBP at various intervals postoperatively, the difference was found to be statistically insignificant with P > 0.05 [Table 2]. No patients in both groups developed bradycardia (HR <60/min); however, 6 patients in group I and 7 patients in group II developed hypotension in perioperative period and were treated with intravenous (i.v.) fluids and/or phenylephrine 50 μg i.v. bolus according to study protocol to normalize arterial pressure. A nonsignificant difference (P > 0.05) was observed with respect to baseline and intraoperative SpO2 in between the two groups at all intervals of time during study [Table 2 and Figure 4]. However, during intubation period, five patients in Group I and 13 patients in Group II had desaturation (SpO2 <95%) with P = 0.024 and was statistically significant [Table 3].
Table 1.
Comparison of patient characteristics in two groups
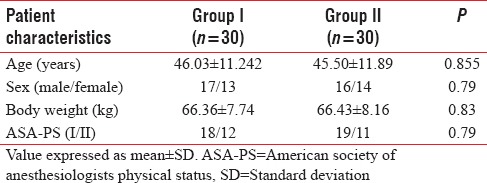
Table 2.
Comparison of mean heart rate, systolic blood pressure, diastolic blood pressure, and oxygen saturation
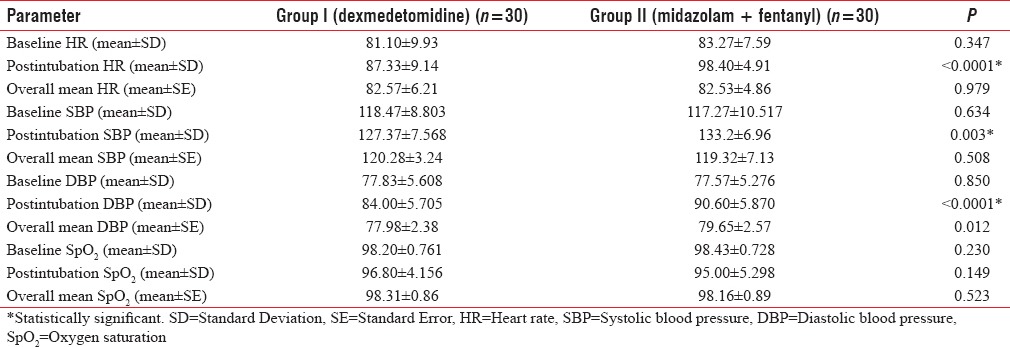
Figure 1.
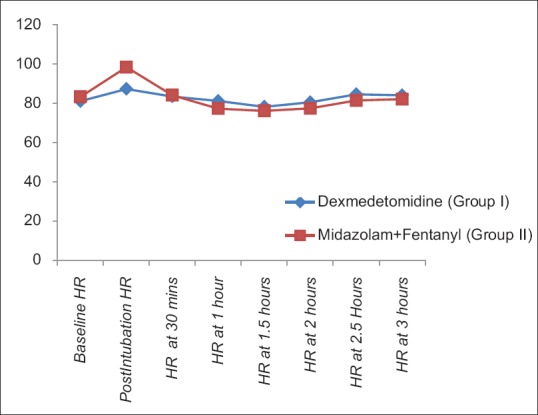
Comparison of mean heart rate in two groups at different intervals of time
Figure 3.
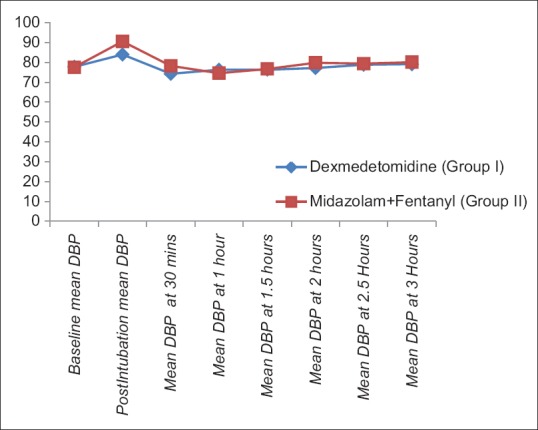
Comparison of mean diastolic blood pressure in two groups at different intervals of time
Figure 4.
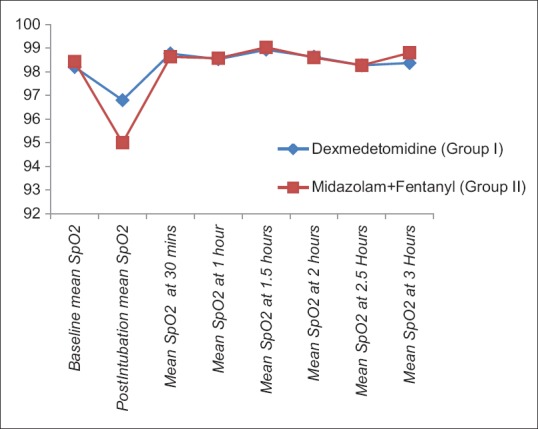
Comparison of mean oxygen saturation (spO2) in two groups at different intervals of time
Table 3.
Comparison of Ramsay sedation score, cough score, postintubation score, and oxygen saturation
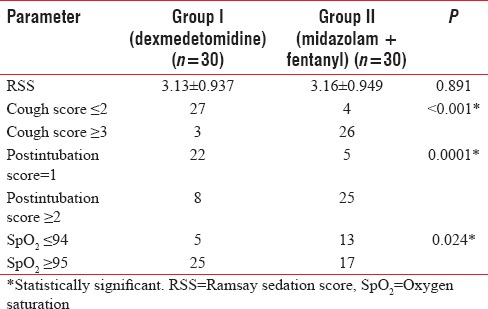
Figure 2.
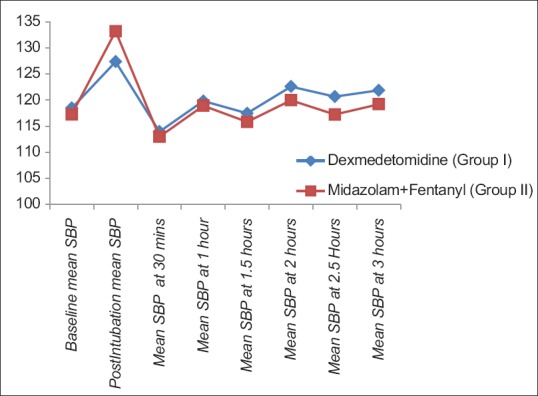
Comparison of mean systolic blood pressure in two groups at different intervals of time
The mean RSS in Group I was 3.13 ± 0.937 and of Group II was 3.16 ± 0.949 and the comparison between two groups was statistically insignificant with P = 0.891 [Table 3]. Cough score of ≤2 was considered favorable and was found in 27 patients in Group I and four patients from group II while three patients from Group I and 26 patients from Group II had cough scores of ≥3 and hence considered unfavorable. The comparison between two groups was statistically significant with P < 0.001 with a favorable result with dexmedetomidine (Group I) group [Table 3]. Postintubation Score of 1 was considered favorable and was found in 22 patients in Group I and five patients from Group II while eight patients from Group I and 25 patients from Group II had postintubation scores of ≥2 and hence considered unfavorable. The comparison between two groups was statistically significant (P = 0.0001) with a favorable result with dexmedetomidine (Group I) group [Table 3].
DISCUSSION
AFOI is a recommended method for securing airway in the predicted difficult airway scenarios. It is a troublesome procedure for the patients as well as the anesthetist. Hence, the goals are to maintain the comfort level of the patient and ease for the anesthetist. The goals for the drug administration are patient comfort, cooperation, amnesia, hemodynamic stability, and blunt airway reflexes and maintain a patent airway with spontaneous ventilation. Various drugs have been tried to achieve conscious sedation for AFOI.
Chu et al.[12] in their study compared effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fiberoptic nasal intubation and observed better tolerance to intubation without respiratory depression and upper airway obstruction in dexmedetomidine group compared with fentanyl group. Reduced hemodynamic response, better intubation score, and postintubation score were seen in dexmedetomidine group. Bergese et al.[13] in their study compared dexmedetomidine plus midazolam and midazolam alone for sedation doing elective AFOI and observed that dexmedetomidine–midazolam patients were significantly calmer and more cooperative during AFOI and had fewer adverse reaction to AFOI than did the midazolam patients. They also were more satisfied with AFOI than that the midazolam only patients. Sayeed et al.[14] in their study compared the safety and effectiveness of dexmedetomidine with a combination of midazolam and fentanyl for sedation during awake fiberoptic nasotracheal intubation and concluded that dexmedetomidine provides better intubating conditions and patient satisfaction without adversely affecting the airway or hemodynamic stability during AFOI.
Literature shows that studies done earlier were mostly comparative studies between individual drugs as benzodiazepines, opioids, propofol, and dexmedetomidine and little studies done for combination of these drugs. Hence, we conducted a study on comparison between dexmedetomidine and midazolam–fentanyl combination for conscious sedation in AFOI. We compared two groups by giving dexmedetomidine 1 μg/kg over 10 min in one group and other group received fentanyl 2 μg/kg plus midazolam 0.02 mg/kg over 10 min.
Favorable cough score of ≤2 was found in 27 patients in Group I and four patients from Group II while three patients from Group I and 26 patients from Group II had cough scores of ≥3 and hence considered unfavorable (P < 0.001). Favorable postintubation score of 1 was found in 22 patients in Group I and five patients from Group II while eight patients from Group I and 25 patients from Group II had postintubation scores of ≥2 and hence considered unfavorable (P = 0.0001). In concordance with our study, Chu et al.[12] also observed better tolerance to intubation without respiratory depression and upper airway obstruction in dexmedetomidine group (1 μg/kg) compared with fentanyl group (1 μg/kg). In our study, dexmedetomidine produced better intubating conditions than midazolam–fentanyl. Bergese et al.[13] noted that dexmedetomidine at 1 μg/kg bolus was safe and beneficial for patients undergoing AFOI even without airway nerve block or topical anesthesia. Bergese et al.[13] also found that dexmedetomidine in combination with low-dose midazolam is more effective than midazolam alone for sedation in AFOI. This also correlates with our study with favorable results with dexmedetomidine. Our results are differed from the study carried by Sayeed et al.[14] who observed that comfort scores were comparable in both the groups. Agrawal et al.[15] also observed similar comfort levels in the two groups.
In our study, both the groups achieved RSS >2 with the mean RSS in Group I as 3.13 ± 0.937 and Group II as 3.16 ± 0.949, and the comparison between two groups was statistically insignificant (P = 0.891). That indicates that the sedation levels after the infusion of the drug were comparable in both the groups. Our study was in concordance with Ryu et al.[16] who compared remifentanil with dexmedetomidine for conscious sedation during bronchoscopy. They found that there were no significant differences of sedation level, mean arterial pressure (MAP), HR, and patient satisfaction score (P > 0.05), but cough score and incidence of desaturation were significantly lower (P < 0.01) in dexmedetomidine group than remifentanil group. In contrast with our study, Mondal et al.[17] observed higher sedation scores (3 ± 0.371) with dexmedetomidine group than fentanyl group (2.07 ± 0.254).(P < 0.0001). This difference could be because of infusion of fentanyl only in comparison to dexmedetomidine; however, our study has a combination of fentanyl and midazolam which could enhance the sedative effect and were comparable.
HR, SBP, DBP, and SpO2 by pulse oximetry were recorded at various intervals in perioperative period starting from baseline, postintubation period, 30 min, 1 h, 1.5 h, 2 h, 2.5 h, and 3 h [Figures 1–4]. In the groups when compared with reference to overall mean HR, mean SBP, and mean DBP at various intervals postoperatively, the difference was found to be statistically insignificant (P > 0.05). However, when hemodynamic parameters such as HR, SBP, and DBP were compared at different intervals of time, they were comparable and statistically insignificant at all intervals of time except the postintubation period. There was a significant increase in HR, SBP, and DBP in both the groups in postintubation period, but the increase was more in Group II than Group I (P < 0.05). This shows that dexmedetomidine group has more favorable hemodynamics than midazolam–fentanyl group. In concordance with our study, Mondal et al.[17] also observed similar changes in hemodynamics in their study. They observed that patients of dexmedetomidine group showed better hemodynamic stability. Initial HR and MAP were similar in both groups. There was a significant change of HR in the postintubation period in comparison with the baseline value in Group B (fentanyl), which was statistically significant (P < 0.0001). However, there were no significant changes of HR in the postintubation period in comparison with baseline value in Group A (dexmedetomidine). Yavascaoglu et al.[18] reported that dexmedetomidine prevented the hemodynamic response to tracheal intubation more effectively than esmolol.
Sayeed et al.[14] also observed that within the group, statistically significant increase in HR, mean blood pressure, and SBP at intubation compared to baseline value in midazolam–fentanyl group. However, DBP change (fall from baseline) was statistically significant in dexmedetomidine group during fiberoscopy. Our study showed a significant difference in hemodynamics between the two groups at intubation with minimal increase in dexmedetomidine group as compared to midazolam–fentanyl group. In contrast to our study, Bergese et al.[13] observed that when dexmedetomidine was used alone or with midazolam, SBP and DBP responses were not different for both patient groups. However, dexmedetomidine–midazolam patients had a lower baseline mean HR than the midazolam patients. This difference could be possibly due to variable patient profile.
There was drop in saturation of <95% in 5 out of thirty patients from Group I (dexmedetomidine) and 13 out of thirty patients from Group II (midazolam + fentanyl) during intubation period, and the comparison was statistically significant with P = 0.024. Better results were in dexmedetomidine group where less patients experienced desaturation (SpO2 <95%). Our study was in concordance with Agrawal et al.[15] who also observed that respiratory rate and SpO2 were significantly low in group FM before and during intubation. Bailey et al.[4] observed that fentanyl alone produced hypoxemia in half of the subjects and significant depression of the ventilatory response to carbon dioxide but did not produce apnea. Mondal et al.[17] also observed that the incidence of desaturation was less in dexmedetomidine group (four patients) than fentanyl group B (25 patients) (P < 0.0001). Ryu et al.[16] also showed less incidence of desaturation with dexmedetomidine than remifentanil.
Limitations of the study
The study has not been blinded and hence there is a possibility of an observational bias. Furthermore, RSS, cough score, and postintubation score are to be assessed by the researcher on the subjective response of the study individuals, there may be variability of responses elicited, and it is difficult to standardize the variables. Some patients may tolerate intubation better than others at same levels of sedation and may add to bias in the study.
CONCLUSIONS
We concluded that dexmedetomidine provides better and safe intubating conditions than midazolam–fentanyl combination for patients undergoing AFOI. It also provides better hemodynamic stability and preservation of patent airway and spontaneous ventilation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Popat M. Practical Fiberoptic Intubation. 1st ed. Oxford: Butterworth-Heinemann Publishers; 2001. pp. 75–95. [Google Scholar]
- 2.Sutherland AD, Williams RT. Cardiovascular responses and lidocaine absorption in fiberoptic-assisted awake intubation. Anesth Analg. 1986;65:389–91. [PubMed] [Google Scholar]
- 3.Malcharek MJ, Bartz M, Rogos B, Günther L, Sablotzki A, Gille J, et al. Comparison of Enk Fibreoptic Atomizer with translaryngeal injection for topical anaesthesia for awake fibreoptic intubation in patients at risk of secondary cervical injury: A randomised controlled trial. Eur J Anaesthesiol. 2015;32:615–23. doi: 10.1097/EJA.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 4.Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826–30. doi: 10.1097/00000542-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Rai MR, Parry TM, Dombrovskis A, Warner OJ. Remifentanil target-controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: A double-blinded randomized controlled trial. Br J Anaesth. 2008;100:125–30. doi: 10.1093/bja/aem279. [DOI] [PubMed] [Google Scholar]
- 6.Abdelmalak B, Makary L, Hoban J, Doyle DJ. Dexmedetomidine as sole sedative for awake intubation in management of the critical airway. J Clin Anesth. 2007;19:370–3. doi: 10.1016/j.jclinane.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fiberoptic intubation: A report of three cases. J Clin Anesth. 2004;16:124–6. doi: 10.1016/j.jclinane.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Bergese SD, Khabiri B, Roberts WD, Howie MB, McSweeney TD, Gerhardt MA. Dexmedetomidine for conscious sedation in difficult awake fiberoptic intubation cases. J Clin Anesth. 2007;19:141–4. doi: 10.1016/j.jclinane.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue FS, He N, Liao X, Xu XZ, Xu YC, Yang QY, et al. Clinical assessment of awake endotracheal intubation using the lightwand technique alone in patients with difficult airways. Chin Med J (Engl) 2009;122:408–15. [PubMed] [Google Scholar]
- 11.Tsai CJ, Chu KS, Chen TI, Lu DV, Wang HM, Lu IC. A comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation. Anaesthesia. 2010;65:254–9. doi: 10.1111/j.1365-2044.2009.06226.x. [DOI] [PubMed] [Google Scholar]
- 12.Chu KS, Wang FY, Hsu HT, Lu IC, Wang HM, Tsai CJ. The effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fibreoptic nasal intubation. Eur J Anaesthesiol. 2010;27:36–40. doi: 10.1097/EJA.0b013e32832e0d2b. [DOI] [PubMed] [Google Scholar]
- 13.Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22:35–40. doi: 10.1016/j.jclinane.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Sayeed T, Shenoy A, Umesh G. Comparison of the safety and effectiveness of dexmedetomidine with a combination of midazolam and fentanyl for sedation during awake fiberoptic nasotracheal intubation. Indian J Respir Care. 2013;2:320–7. [Google Scholar]
- 15.Agrawal A, Jadon A, Parida SS, Chakraborty S, Sinha N, Chandra O. Comparative evaluation of dexmedetomidine and fentanyl – Midazolam combination as sedative adjunct to fibreoptic intubation under topical anaesthesia. Am J Adv Med Sci. 2014;2:29–37. [Google Scholar]
- 16.Ryu JH, Lee SW, Lee JH, Lee EH, Do SH, Kim CS. Randomized double-blind study of remifentanil and dexmedetomidine for flexible bronchoscopy. Br J Anaesth. 2012;108:503–11. doi: 10.1093/bja/aer400. [DOI] [PubMed] [Google Scholar]
- 17.Mondal S, Ghosh S, Bhattacharya S, Choudhury B, Mallick S, Prasad A. Comparison between dexmedetomidine and fentanyl on intubation conditions during awake fiberoptic bronchoscopy: A randomized double-blind prospective study. J Anaesthesiol Clin Pharmacol. 2015;31:212–6. doi: 10.4103/0970-9185.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yavascaoglu B, Kaya FN, Baykara M, Bozkurt M, Korkmaz S. A comparison of esmolol and dexmedetomidine for attenuation of intraocular pressure and haemodynamic responses to laryngoscopy and tracheal intubation. Eur J Anaesthesiol. 2008;25:517–9. doi: 10.1017/S0265021508003529. [DOI] [PubMed] [Google Scholar]


