Abstract
Background
Cholera is a diarrheal disease that produces rapid dehydration. The infection is a significant cause of mortality and morbidity. Oral cholera vaccine (OCV) has been propagated for the prevention of cholera. Evidence on OCV delivery cost is insufficient in the African context. This study aims to analyze Shanchol vaccine delivery costs, focusing on the vaccination campaign in response of a cholera outbreak in Lake Chilwa, Malawi.
Methods
The vaccination campaign was implemented in two rounds in February and March 2016. Structured questionnaires were used to collect costs incurred for each vaccination related activity, including vaccine procurement and shipment, training, microplanning, sensitization, social mobilization and vaccination rounds. Costs collected, including financial and economic costs were analyzed using Choltool, a standardized cholera cost calculator.
Results
In total, 67,240 persons received two complete doses of the vaccine. Vaccine coverage was higher in the first round than in the second. The two-dose coverage measured with the immunization card was estimated at 58%. The total financial cost incurred in implementing the campaign was US$480275 while the economic cost was US$588637. The total financial and economic costs per fully vaccinated person were US$7.14 and US$8.75, respectively, with delivery costs amounting to US$1.94 and US$3.55, respectively. Vaccine procurement and shipment accounted respectively for 73% and 59% of total financial and economic costs of the total vaccination campaign costs while the incurred personnel cost accounted for 13% and 29% of total financial and economic costs. Cost for delivering a single dose of Shanchol was estimated at US$0.97.
Conclusion
This study provides new evidence on economic and financial costs of a reactive campaign implemented by international partners in collaboration with MoH. It shows that involvement of international partners’ personnel may represent a substantial share of campaign’s costs, affecting unit and vaccine delivery costs.
Electronic supplementary material
The online version of this article (10.1186/s12879-017-2885-8) contains supplementary material, which is available to authorized users.
Keywords: Cholera, Shanchol, Delivery costs, Choltool, Malawi
Introduction
Cholera is a diarrheal disease that produces rapid dehydration. Caused by ingestion of toxigenic Vibrio cholerae [1], the infection represents a serious public health problem in the developing world. The disease is a significant cause of mortality and morbidity, particularly for children and vulnerable people. The provision of safe water coupled with adequate hygiene and sanitation have been acknowledged as measures for the prevention of cholera [2, 3] and agreed upon by the international community through their adoption in the Sustainable Development Goals [4]. In spite of this, 2.9 million cases of cholera continue to occur worldwide every year with an estimated annual mortality of 95,000 [5]. A larger part of these deaths occur in sub-Saharan Africa where access to an improved drinking-water source remain problematic for thousands of people [6].
It has been largely accepted that improvement in water and hygiene conditions is unlikely to occur in the near future due to suboptimal funding and infrastructure. In recognition of this, OCVs have been recommended for cholera prevention by the World Health Organization (WHO) [1]. Consequently, Dukoral, and more recently Shanchol, a low-cost cholera vaccine, were prequalified for the prevention of cholera [7]. There is an abundance of literature demonstrating the protective efficacy of Shanchol vaccine [8–12]. As a consequence, there has been an increased enthusiasm in its use for the prevention of cholera. A review from the WHO reported that up to 35 OCV campaigns were conducted between July 2013 to July 2016 in response to emergency and endemic contexts in various settings [13].
Although Shanchol has been largely propagated as inexpensive, ongoing questions regarding its delivery costs exist. High vaccine delivery costs may discourage its use in resource-limited settings. However, evidence on Shanchol delivery costs is questionable due to inconsistent collection and reporting of cost data [14]. This study aims to provide a detailed analysis of Shanchol delivery cost focusing on the case study of a reactive vaccination campaign in Lake Chilwa, Malawi.
Methods
Study setting
The campaign was implemented in three administrative districts, including Machinga, Phalombe, and Zomba. In 2016, all three districts had an estimated total population of 1,895,625 inhabitants [15]. The districts’ economies are driven by agriculture and fishing. Health services are mainly delivered to these communities through health posts, clinics, health centers, and hospital facilities. People living on and around the Lake use it as a source of drinking water, for bathing and as a toilet. As a consequence, levels of fecal contamination are high, particularly in parts of the lake with stagnant water [16]. This exposure to unsafe water, in turn, leads to a high mortality rate, at 26.1 per 100,000 inhabitants in 2012 [17]. Of the total Malawian population, 59% has no sustainable access to improved sanitation, and 10% do not have safe drinking water sources [17].
Vaccine procurement
A total of 200,025 doses of Shanchol were shipped from the International Coordinating Group (ICG) emergency stockpile. Vaccine costs were covered by GAVI, the Vaccine Alliance and transported from Kuala Lumpur to Blantyre Expanded Program on Immunization (EPI) storage cold room via Lilongwe.
Cold chain management
Upon arrival, the vaccines were stored temporarily at the central cold room managed by the Ministry of Health (MoH) in Lilongwe at 2–8 °C. From central level they were transported to the regional cold room in Blantyre. Both cold rooms had sufficient capacity available to absorb the cholera vaccine doses. The cold room in Blantyre is at two hours distance from Machinga, one hour from Zomba and one hour and half from Phalombe. Vaccines were kept at central stores and send at last time possible for distribution. Each vaccination site used one cold box RCW25 and one vaccine carrier for vaccine transportation, with most items borrowed from the EPI program.
Social mobilization
Before and throughout the vaccination campaign, advocacy and social mobilization activities were conducted to 1) inform the public about the availability of the vaccine against cholera during the campaign; 2) encourage people to get vaccinated against cholera and 3) reinforce good hygiene and sanitation practices. The target areas for social mobilization included gathering places (trading centers, schools, markets, community existing health support groups and fisheries’ organizations). Communication activities were implemented using health education sessions, posters and banners.
Personnel and training
Training sessions organized all at district level were conducted to train supervisors, vaccinators, and volunteers on vaccine delivery. Sessions were initiated by a two day orientation meeting with the District Executive Committee to gain support from the district administration and partners in the implementation of the campaign. Supervisors were trained simultaneously to ensure consistency between districts. They were subsequently responsible of training vaccination staff and volunteers. Vaccinators were all community based health workers known as health surveillance assistants. Approximately 250 people were trained in the week preceding the vaccination campaign.
Transportation
A combination of car and boats were used for vaccine delivery to recipients. The transport of vaccine and teams to villages around the Lake Chilwa was done by car and transportation to villages located on the Lake (islands) by boats. For populations in areas not accessible by car or boat, some of the teams moved on foot, bicycle and motorbike for vaccine delivery.
Data collection
The Global Task Force on Cholera Control (GTFCC) recommended the use of standardized methods for costing assessments of vaccination campaigns. In line with these recommendations, Choltool, a standardized Excel-based cholera cost calculator developed by the International Vaccine Institute, WHO, and DOVE was used for the analysis [18]. The tool quantifies resources required to introduce OCV vaccination campaigns to existing immunization programs. The tool provides estimates of two main cost measures, including the total costs of adding the OCV to specific areas and the cost per fully immunized person. It differentiates financial and economic costs incurred for implementing the OCV vaccination campaign by vaccination related activity (see Table 1).
Table 1.
Study objectives and type of cost data collected
| Financial costs | Economic costs | |
|---|---|---|
| Specific objective | To assess the incurred financial costs of implementing the vaccination campaign | To assess the incurred economic costs of the vaccination campaign, including opportunity costs of resources’ utilization |
| Vaccine purchase | Vaccine costs | Vaccine costs |
| Vaccine shipment | International transport (freight), clearance insurance, transport and storage | International transport (freight), clearance insurance, transport and storage |
| Microplanning | Per diems, venue rental, transportation, stationery, printing, fuel and lubricant, catering and communication | Salaried labor, per diems, venue rental, transportation, stationery, printing, fuel and lubricant, catering and communication |
| Training | Per diems, venue rental, transportation, stationery, printing, fuel and lubricant, catering and communication material, equipment | Salaried labor, per diems, venue rental, transportation, stationery, printing, fuel and lubricant, catering and communication, material, equipment |
| Sensitization/social mobilization | Per diems, transportation, material, stationery, printing, fuel and lubricant, catering and communication, rental equipment | Salaried labor, volunteer labor, per diems, transportation, stationery, printing, fuel and lubricant, catering and communication, material, equipment |
| Vaccination rounds | Per diems, transportation, material, stationery, printing, fuel and lubricant, catering and communication, rental equipment, maintenance, operating costs | Salaried labor, volunteer labor, per diems, transportation, material, stationery, printing, fuel and lubricant, catering communication, rental, equipment maintenance, operating costs |
Data collection was conducted from February 2016 to March 2017 using various structured questionnaires based on Choltool to estimate the value of resources used to implement the campaign. The data were extracted by reviewing programmatic documents, microplanning, budget, and financial reports basing on actual expenditures and economic costs borne by institutions that supported the implementation of the campaign, including Agence de Médecine Préventive (AMP), Médecins Sans Frontrières (MSF), United Nations Children’s Fund (UNICEF) and WHO.
Measurement of the vaccination campaign
The vaccination campaign was implemented in two rounds from February 16–22, 2016 and from March 8–15, 2016. The target consisted of 90,000 individuals aged more than one-year-old, including pregnant women. To reach the target, a total of 98 vaccination posts were set up in the selected three districts. Each post, opened daily for a minimum of eight hours, was led by a health surveillance assistant. In total, 53 teams were constituted for the first vaccination round and 56 for the second. Each team consisted of five people, including a vaccinator, a register, a tally person, a logistician, and a social mobiliser. In addition, 23 senior health surveillance assistants were hired to supervise the campaign. Prior to and throughout each round, social mobilization activities were implemented to foster vaccine uptake.
Four structured questionnaires were used to collect costs incurred for conducting each vaccination related activity, including vaccine procurement and shipment, training, microplanning, sensitization, social mobilization and vaccination rounds. Questionnaires, which were derived from Choltool structure, were adapted to data collection at the various levels of campaign implementation and to the Malawian context, including the MoH and various partners which supported the campaign. Financial and economic costs linked to the implementation of the campaign were collected. Data collected included vaccine procurement, shipment of vaccines, personnel and per diems, material and equipment, fuel, lubricant, maintenance, transportation, rental, catering, operating costs, and miscellaneous costs. Capital costs (buildings, cold rooms, vehicles) were excluded from the analysis. Private household costs to receive oral cholera vaccine and costs incurred for monitoring adverse events following immunization (AEFI) were also excluded from this analysis. Details on costs included are described in Table 1. Because the campaign was conducted concurrently with research and treatment activities, efforts were made to disentangle costs of these activities from that of the vaccination.
Data analysis
The data was entered and analyzed in Choltool [18]. The calculation of the campaign costs followed an ingredient approach to estimate the financial and economic costs of implementing the campaign from the government perspective. The total financial cost was estimated as the sum total of costs incurred for all vaccination related activities described in Table 1. The total cost for each vaccination related activity was obtained by adding up the total costs of all inputs used for that given activity. Each input cost was calculated by multiplying quantities used by the corresponding unit price for recurrent inputs, and annualized before being accounted for some equipment (vaccine carriers, cold boxes). The estimated time costs of each human resource were estimated by multiplying the daily wage by the corresponding time involved. The economic costs captured all resources used, combining financial and opportunity costs for non-marketable resources. Opportunity costs covered some equipment and civil servants’ time. Civil servants’ time costs were accounted for by multiplying the corresponding daily wage of each of these human resources by the corresponding time involved.
The total financial and economic costs of the campaign were reported by vaccination related activities, and further by line input including an estimation of involved international staff costs. This international staff comprised epidemiologists, nurses, logisticians and support staff who contributed to the campaign organization, implementation, supervision as well as vaccine delivery to recipients. The total delivery economic cost was divided by the total number of people receiving the complete vaccine doses to generate the total delivery cost per fully vaccinated person.
Cost data were reported in 2016 US dollars based on OANDA data [19]. To allow comparisons, costs were further converted to international dollars (I$) using the appropriate conversion factor from the International Monetary Fund [20], and estimates reported in supplementary files.
Results
Characteristics of the vaccination campaign
Table 2 describes key characteristics of the vaccination campaign. In total, 177,523 doses of vaccine were used, with 108,483 receiving the first dose, and 67,240 persons receiving the complete two doses. About 27% of the first dose was delivered during the second vaccination round. The vaccine coverage with two doses measured with the immunization card and oral reporting was estimated at 58%. Seven mild adverse events following immunization, including vomiting, abdominal cramps, and headache were reported. The proportion of vaccines wasted was very low at, approximately, 1%. The main reasons for wastage included broken vials, empty vials and spillage.
Table 2.
Descriptive characteristics of the mass oral vaccination campaign
| Oral vaccination campaign characteristics | Total |
|---|---|
| Target population | 90,000 |
| Receiving OCV 1st and 2nd dose | 67,240 |
| Receiving OCV 1st dose only | 108,483 |
| Total number of vaccine doses’ distributed | 175,723 |
| Number of vaccine doses’ wasted | 1,800 |
| Total number of doses used (distributed plus wasted) | 177,523 |
| Two-dose vaccine coverage (%) | 58 |
Cholera vaccination campaign costs by vaccination related activities and input type
Table 3 reports total financial and economic costs of the vaccination campaign by vaccination related activities. The total economic costs of the vaccination campaign amounted to US$588637. International dollar figures are given in [see Additional file 1]. Economic costs exceeded financial costs by US$108362, with financial costs amounting to US$480275. The total financial and economic costs of vaccination without vaccine totaled US$130319 and US$238681, respectively.
Table 3.
Distribution of total vaccination costs by activity in 2016 US dollars
| Financial costs | Economic costs | |||
|---|---|---|---|---|
| 2016 US$ | Percentage | 2016 US$ | Percentage | |
| Vaccine procurement and shipmenta | 349,956 | 72.87 | 349,956 | 59.45 |
| Vaccine purchase | 331,748 | 69.08 | 331,748 | 56.36 |
| Vaccine shipment, clearance and custom fees | 18,208 | 3.79 | 18,208 | 3.09 |
| Vaccine delivery | 130,319 | 27.13 | 238,681 | 40.55 |
| Microplanning | 11,648 | 2.42 | 78,649 | 13.36 |
| Sensitization | 2,865 | 0.60 | 9,512 | 1.62 |
| Training | 10,191 | 2.12 | 11,097 | 1.89 |
| Social mobilization | 29,377 | 6.12 | 29,377 | 4.99 |
| Vaccination Round 1 | 34,221 | 7.12 | 48,796 | 8.29 |
| Vaccination Round 2 | 42,017 | 8.75 | 61,250 | 10.40 |
| Total | 480,275 | 100.00 | 588,637 | 100.00 |
aIncluding wastage
Looking at costs’ distribution by vaccination activities, vaccine procurement and shipment accounted for almost 73% and 59% of total financial and economic costs, respectively, while vaccine delivery costs represented broadly 27% and 41%.
Of the total vaccine delivery economic costs, microplanning was the largest delivery cost component (13%) followed by the vaccination activity itself (8% for round 1 and 10% for round 2). Sensitization activities appeared to be a minor cost component of the delivery cost.
Table 4 reports the breakdown of the total financial and economic costs of vaccination campaign costs by input type in 2016 US$. International dollar figures are given in [see Additional file 2]. The findings show that financial and economic costs for all categories of personnel amounted to US$62386 and US$169313, respectively.
Table 4.
Distribution of total vaccination costs by input type in 2016 US dollars
| Financial costs | Economic costs | |||
|---|---|---|---|---|
| 2016 US$ | Percentage | 2016 US$ | Percentage | |
| Vaccine procurement and shipmenta | 349,956 | 72.87 | 349,956 | 59.45 |
| Vaccine purchase | 328,418 | 68.38 | 328,418 | 55.79 |
| Vaccine shipment, clearance and custom fees | 21,538 | 4.49 | 21,538 | 3.66 |
| Vaccine delivery | 130,319 | 27.13 | 238,681 | 40.55 |
| Vehicle, fuel, lubricant, and maintenance | 32,342 | 6.73 | 32,661 | 5.55 |
| Fuel and ground transportation | 25,278 | 5.26 | 25,278 | 4.30 |
| Lubricant and maintenance | 124 | 0.03 | 124 | 0.02 |
| Rental (car, boat, etc.) | 6,940 | 1.44 | 7,259 | 1.23 |
| Personnel from international partners | 0 | 0.00 | 90,353 | 15.35 |
| Salary | 0 | 0.00 | 62,420 | 10.61 |
| Per diems | 0 | 0.00 | 24,326 | 4.13 |
| International transport and visas | 0 | 0.00 | 3607 | 0.61 |
| Personnel, local | 62,386 | 12.99 | 78,960 | 13.41 |
| Salary (opportunity cost MoH staff) | 0 | 0.00 | 16,574 | 2.81 |
| Per diems (mobilizers, volunteers, local staff, etc.) | 62,386 | 12.99 | 62,386 | 10.60 |
| Material | 14,483 | 3.02 | 15,599 | 2.65 |
| Banners, T-shirts | 12,943 | 2.70 | 12,943 | 2.20 |
| Supplies (printings, plastic bags, etc) | 117 | 0.02 | 117 | 0.02 |
| Equipment | 1,423 | 0.30 | 2,539 | 0.43 |
| Operating costs | 19,753 | 4.11 | 19,753 | 3.36 |
| Operating costs (on-site expenses) | 10,299 | 2.14 | 10,299 | 1.75 |
| Communication | 9,454 | 1.97 | 9,454 | 1.61 |
| Catering & other expenses | 1,355 | 0.28 | 1,355 | 0.23 |
| Beverages, drinks, water, etc | 1,092 | 0.23 | 1,092 | 0.19 |
| Miscalleneous | 263 | 0.05 | 263 | 0.04 |
| Total costs | 480,275 | 100.00 | 588,637 | 100.00 |
aIncluding wastage
Moreover, the distribution of financial and economic costs shows that, apart from vaccines, incurred total personnel costs were the largest cost component of the total vaccination costs, representing approximately 13% and 29% of total financial and economic costs, respectively. Incurred costs for vehicle, fuel, lubricant, and maintenance came in second place, representing approximately 7% and 6%, respectively, of the total financial and economic costs.
Cholera vaccine delivery costs by input type
Table 5 shows the breakdown of total vaccine delivery costs by input type. Of the total financial costs for vaccine delivery of US$130319, the incurred total personnel costs represented almost 48%. Considering the economic costs, the incurred total personnel costs accounted for approximately 71% of the total economic costs for vaccine delivery of US$238681. International dollar estimates by input type are shown in [see Additional file 3].
Table 5.
Distribution of total vaccine delivery costs by input type in 2016 US dollars
| Financial costs | Economic costs | |||
|---|---|---|---|---|
| 2016 US$ | Percentage | 2016 US$ | Percentage | |
| Vehicle, fuel, lubricant, and maintenance | 32,342 | 24.82 | 32,661 | 13.68 |
| Fuel and ground transportation | 25,278 | 19.40 | 25,278 | 10.59 |
| Lubricant and maintenance | 124 | 0.10 | 124 | 0.05 |
| Rental (car, boat, etc.) | 6,940 | 5.32 | 7,259 | 3.04 |
| Personnel from international partners | 0 | 0.00 | 90,353 | 37.85 |
| Salary | 0 | 0.00 | 62,420 | 26.15 |
| Per diems | 0 | 0.00 | 24,326 | 10.19 |
| International transport and visas | 0 | 0.00 | 3,607 | 1.51 |
| Personnel, local | 62,386 | 47.87 | 78,960 | 33.08 |
| Salary (opportunity cost MoH staff) | 0 | 0.00 | 16,574 | 6.94 |
| Per diems (mobilizers, volunteers, local staff, etc.) | 62,386 | 47.87 | 62,386 | 26.14 |
| Material | 14,483 | 11.11 | 15,599 | 6.54 |
| Banners, T-shirts | 12,943 | 9.93 | 12,943 | 5.43 |
| Supplies (printings, plastic bags, etc) | 117 | 0.09 | 117 | 0.05 |
| Equipment | 1,423 | 1.09 | 2,539 | 1.06 |
| Operating costs | 19,753 | 15.16 | 19,753 | 8.28 |
| Operating costs (on-site expenses) | 10,299 | 7.90 | 10,299 | 4.32 |
| Communication | 9,454 | 7.26 | 9,454 | 3.96 |
| Catering & other expenses | 1,355 | 1.04 | 1,355 | 0.57 |
| Beverages, drinks, water, etc | 1,092 | 0.84 | 1,092 | 0.46 |
| Miscalleneous | 263 | 0.20 | 263 | 0.11 |
| Total costs | 130,319 | 100.00 | 238,681 | 100.00 |
Of the latter total, costs incurred for personnel from international partners was the largest vaccine delivery cost item, amounting to US$90353, and accounting for approximately 38% of the total incurred economic costs for vaccine delivery. Costs incurred for local personnel represented 33% of the total economic costs of vaccine delivery. In addition, the economic costs incurred for vehicle, fuel, lubricants, and maintenance represented approximately 14% of the total incurred economic costs for vaccine delivery.
Unit delivery costs per dose administered and fully vaccinated person
Figure 1 shows the distribution of OCV campaign financial costs per dose administered by vaccination related activities. The financial cost per dose administered amounted to US$0.97. The distribution of the unit cost per dose administered by vaccination activities showed that vaccination rounds 1 and 2 were the most important contributors of the unit cost per dose administered.
Fig. 1.
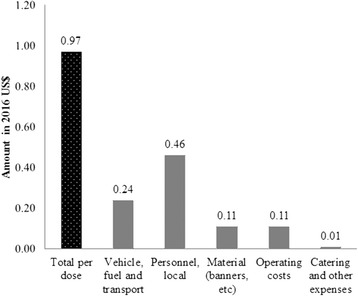
OCV financial delivery cost per dose administered, by activity in 2016 US dollars
The breakdown by input type shows that the incurred local personnel costs constituted the largest contributors of the financial cost per dose administered (Fig. 2).
Fig. 2.
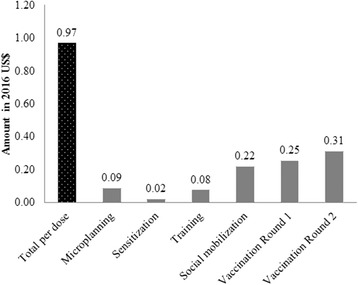
OCV financial delivery cost per dose administered, by input type in 2016 US dollars
Table 6 further shows financial and economic unit costs of vaccine delivery per fully vaccinated person. The financial and economic unit costs incurred for delivering the vaccine amounted to US$1.94 and US$3.55, respectively. International dollar estimates by input type are shown in [see Additional file 4]. In total, US$0.93 was spent directly out-of-pocket in personnel costs. In comparison, the unit costs incurred for personnel to deliver the vaccine to recipients amounted to US$2.52 when including the economic costs, with the incurred personnel cost from international partners representing approximately 38% of the incurred unit economic cost per fully vaccinated person.
Table 6.
Distribution of unit delivery costs per fully vaccinated person by input type in 2016 US dollars
| Financial costs | Economic costs | |||
|---|---|---|---|---|
| 2016 US$ | Percentage | 2016 US$ | Percentage | |
| Vehicle, fuel, lubricant, and maintenance | 0.48 | 24.82 | 0.49 | 13.68 |
| Fuel and ground transportation | 0.38 | 19.40 | 0.38 | 10.59 |
| Lubricant and maintenance | 0.00 | 0.10 | 0.00 | 0.05 |
| Rental (car, boat, etc.) | 0.10 | 5.32 | 0.11 | 3.04 |
| Personnel from international partners | 0.00 | 0.00 | 1.34 | 37.85 |
| Salary | 0.00 | 0.00 | 0.93 | 26.15 |
| Per diems | 0.00 | 0.00 | 0.36 | 10.19 |
| International transport and visas | 0.00 | 0.00 | 0.05 | 1.51 |
| Personnel, local | 0.93 | 47.87 | 1.18 | 33.08 |
| Salary (opportunity cost MoH staff) | 0.00 | 0.00 | 0.25 | 6.94 |
| Per diems (mobilizers, volunteers, local staff, etc.) | 0.93 | 47.87 | 0.93 | 26.14 |
| Material | 0.22 | 11.11 | 0.23 | 6.54 |
| Banners, T-shirts | 0.19 | 9.93 | 0.19 | 5.43 |
| Supplies (printings, plastic bags, etc) | 0.00 | 0.09 | 0.00 | 0.05 |
| Equipment | 0.03 | 1.09 | 0.04 | 1.06 |
| Operating costs | 0.29 | 15.16 | 0.29 | 8.28 |
| Operating costs (on-site expenses) | 0.15 | 7.90 | 0.15 | 4.32 |
| Communication | 0.14 | 7.26 | 0.14 | 3.96 |
| Catering & other expenses | 0.02 | 1.04 | 0.02 | 0.57 |
| Beverages, drinks, water, etc | 0.02 | 0.84 | 0.02 | 0.46 |
| Miscalleneous | 0.00 | 0.20 | 0.00 | 0.11 |
| Total costs | 1.94 | 100.00 | 3.55 | 100.00 |
Total cholera vaccination costs
With the total financial and economic costs for vaccine procurement and shipment amounting to US$349956 (Table 1), the estimated vaccine procurement and shipment expenditure per fully vaccinated person for this campaign was US$5.20 (349,956/67240). Adding up unit costs of cholera vaccine delivery, total financial and economic costs per fully vaccinated person were US$7.14 and US$8.75, respectively. Total financial and economic costs of vaccination for partially immunized person were US$4.43 and US$5.43, respectively.
Discussion
This study represents one of the most detailed and comprehensive vaccine delivery costs analysis. With increasing interest in controlling cholera through vaccination, literature estimating the cost of Shanchol™, a low-cost oral cholera vaccine, is growing [21–24]. A limited number of review papers has also been published, reporting cholera vaccination costs in various settings [10, 14]. Some of these studies reported cost estimates disaggregated by inputs [22, 24], while others reported costs by activities [21, 23]. However, none of these studies has detailed enough the costs collected, making it difficult to make comparisons across settings. The comprehensive analysis we propose in this study may therefore orient future studies and contribute to enhancing quality and comparability of costing assessments. Moreover, a number of previous studies only estimated financial costs of vaccination campaigns [21, 23], while this study reported both financial and economic costs. By only reporting financial costs, implications from these previous studies are limited to information on financial needs and do not inform the opportunity costs for the health system (e.g., the costs of MoH staff involvement). This, in turn, limits the potential of studies that only reported financial costs of vaccine delivery in informing cost-effectiveness studies.
The findings showed that total personnel costs accounted to almost 71% of the total economic costs for vaccine delivery and slightly more than 47% of incurred financial costs. The latter result is consistent with the result of a previous review paper, which found that staff salaries accounted for 45% of non-vaccine expenditures [25]. However, this estimate was lower than the 55% estimate given by a previous paper in Bangladesh [24]. The difference in study findings may be due to inflation given that the two studies were from different periods. Moreover, our findings showed that the total incurred economic costs for international partners constituted slightly more than 37% of delivery costs. In their study in Zanzibar, Tanzania, Schaetti et al. reported that international personnel costs accounted for 45% of Dukoral vaccine delivery costs [26]. These two findings suggest that international staff involvement in cholera vaccination campaigns have an important impact on vaccine delivery costs.
The results also showed that cholera vaccine delivery costs accounted for approximately 41% of the total economic costs of the vaccination campaign. Our estimate appeared similar to that of a study which estimated cholera-vaccine delivery costs to account for 42% of total economic costs of vaccination in Bangladesh [24]. It was higher than the study conducted in Zanzibar by Schaetti and al, which found that cholera-vaccine delivery costs represented 32% of total costs of vaccination with Dukoral [26]. Cholera vaccine delivery costs have been estimated at 12% in Odisha, India [23] and 36% of the total vaccination costs in Guinea [21]. The above mentioned three studies may have underestimated the true share of cholera vaccine delivery costs in total vaccination given that economic costs were not accounted for.
The incurred financial cost for vaccine delivery per fully vaccinated person has been estimated at US$1.94. This finding appeared consistent with a previous review paper, which found that incurred financial cost for Shanchol vaccine delivery per fully vaccinated person varied between US$1.14 in India to US$3.05 in South Sudan [14]. However, our estimates may have been pulled up by the fact that external partners were involved in campaign activities implementation on top of local staff and that costs related to international staff salaries and travels may have increased the campaign costs.
Finally, the financial cost incurred per dose administered was US$0.97 of which the incurred personnel cost was US$0.46. This incurred delivery cost per dose administered of US$0.97 was above GAVI’s vaccine operational support for campaigns of US$0.65 per targeted person per vaccination round, intended to cover 80% of MoH-led campaigns’ operational costs [27]. However, our estimate of US$0.97 per dose administered included the incurred international personnel cost which is not covered by GAVI’s support policy. When removing this cost item, GAVI’s support of US$0.65 represented 127% of the financial delivery cost per dose of US$0.51.
This study has limitations. Implementation of vaccination campaigns in many settings have occurred in response to outbreaks. As with previous research [26], some cost data were collected retrospectively. This may have affected our cost estimates. Moreover, salaried labor was estimated retrospectively based on the corresponding daily wage. This may have also contributed to distorting economic costs due to under/overestimation of the time. Capital assets (cold rooms and related equipment, vehicles), particularly from the MoH, were important contributing factors to the vaccination campaign. However, they were not incorporated in our analysis. This also may have contributed to underestimating the true cost of the vaccination campaign. Time and pecuniary costs incurred by household members to travel to vaccination sites and receive free OCV have been shown significant by previous studies [24, 28]. Our study did not analyze private costs incurred by households to receive OCV. Cost estimates may have been higher in comparison of what we presented in this study if the analysis was conducted from the societal perspective, with private costs to receive the vaccine accounted for. Finally, the cost figures we presented in this paper may have also been distorted by the fact that costs incurred for monitoring AEFI were not incorporated.
Conclusion
Unlike previous costing assessments of cholera vaccination campaign, this study comprehensively presented costs collected and analyzed while clearly distinguishing financial and economic costs as well as activities and inputs. Despite challenges in conducting a detailed analysis of vaccination campaign costs, this type of in-depth analysis should be encouraged for improving understanding and fostering cross-country comparison of costs. The clear description of costs included in this study may therefore be of contribution in this area. The study also showed that international staff expenses are one of the cost drivers that have a substantial impact on cholera vaccine delivery costs.
Additional files
Distribution of total vaccination costs by activity in 2016 US dollars and in international dollars (I$). (DOCX 19 kb)
Distribution of total vaccination costs by input type in 2016 US dollars and in international dollars (I$). (DOCX 23 kb)
Distribution of total vaccine delivery costs by input type in 2016 US dollars and in international dollars (I$). (DOCX 21 kb)
Distribution of unit delivery costs per fully vaccinated person by input type in 2016 US$ and international dollars (I$). (DOCX 22 kb)
Acknowledgements
The authors would like to acknowledge the following: MSF, particularly, Adriana Palomares who provided budget and financial documents from her organization; Maurice Mwesawina and Bagrey Ngwira for facilitating study implementation and access to MoH information, Vittal Mogasale for providing Choltool and Winthrop Morgan for continuous support and amendments to the tool throughout the data collection process; Federica Joele, Florentina Rafael, Philippe Cavailler and Martin Mengel for their support. Xiao Xian Huang for her contribution to the development of the study protocol and for initiating data collection.
Funding
This work was funded by the Bill and Melinda Gates foundation (Grant No. 1106078).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMP
Agence de Médecine Préventive
- Choltool
Cholera Costs Calculator
- EPI
Expanded Program on Immunization
- GAVI
Global Alliance for Vaccination
- GTFCC
Global Task Force on Cholera Control
- I$
Global Task Force on Cholera Control
- MoH
Ministry of Health
- MSF
Médecins Sans Frontières
- OCV
Oral Cholera Vaccination
- UNICEF
United Nations Children’s Fund
- US Dollars
United States Dollars
- WHO
World Health Organization
Authors’ contributions
JBL developed the costing protocol; PGC and JBL participated in collecting data; PGC and JBL participated in planning the data analysis and/or analyzing the data; PGC drafted the paper and revised the subsequent drafts with specific inputs from JBL; PGC and JBL contributed to the interpretation of findings and commented on manuscript drafts. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The overall study protocol received clearance from the National Health Sciences Research Committee (NHSRC) of the Ministry of Health in Malawi (approval number: NHSRC # 16/3/1559). The costing study was part of the cholera vaccination campaign monitoring and evaluation, which was approved by the MoH.
Consent for publication
Not applicable.
Competing interests
PGC and JBL work for AMP, which receives unrestricted funding from Sanofi Pasteur and grant-specific support from Crucell, Sanofi Pasteur, Pfizer, Merck, and GlaxoSmithKline.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-017-2885-8) contains supplementary material, which is available to authorized users.
Contributor Information
Patrick G. Ilboudo, Email: gilboudo@aamp.org, Email: ipatrickgc@gmail.com
Jean-Bernard Le Gargasson, Email: jblegargasson@aamp.org, Email: jblegargasson@gmail.com.
References
- 1.WHO: Cholera vaccines: WHO position paper. In: Weekly Epidemiol Rec. 2010: 117–128. [PubMed]
- 2.WHO: Prevention and control of cholera outbreaks: WHO policy and recommendations. Global Task Force on Cholera Control. In.; 2011.
- 3.Clemens J, Holmgren J. When, how, and where can oral cholera vaccines be used to interrupt cholera outbreaks? Curr Top Microbiol Immunol. 2014;379:231–258. doi: 10.1007/82_2013_353. [DOI] [PubMed] [Google Scholar]
- 4.Nations Unies: Projet de document final du Sommet des Nations Unies consacré à l’adoption du programme de développement pour l’après-2015. In., vol. A/69/L.85: Nations Unies, Assemblée générale; 2015.
- 5.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9(6):e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO/UNICEF: Progress on Drinking Water and Sanitation: 2012 Update In Edited by Sanitation WUJMPfWSa; 2012.
- 7.WHO Prequalified Vaccines [https://extranet.who.int/gavi/PQ_Web/].
- 8.Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis. 2011;5(10):e1289. doi: 10.1371/journal.pntd.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, Sah B, Niyogi SK, Park JK, Sarkar B, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2013;13(12):1050–1056. doi: 10.1016/S1473-3099(13)70273-1. [DOI] [PubMed] [Google Scholar]
- 10.Martin S, Lopez AL, Bellos A, Deen J, Ali M, Alberti K, Anh DD, Costa A, Grais RF, Legros D, et al. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ. 2014;92(12):881–893. doi: 10.2471/BLT.14.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabir S. Critical analysis of compositions and protective efficacies of oral killed cholera vaccines. Clinical and vaccine immunology : CVI. 2014;21(9):1195–1205. doi: 10.1128/CVI.00378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali M, You YA, Kanungo S, Manna B, Deen JL, Lopez AL, Wierzba TF, Bhattacharya SK, Sur D, Clemens JD. Assessing different measures of population-level vaccine protection using a case-control study. Vaccine. 2015;33(48):6878–6883. doi: 10.1016/j.vaccine.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 13.Desai SN, Pezzoli L, Alberti KP, Martin S, Costa A, Perea W, Legros D. Achievements and challenges for the use of killed oral cholera vaccines in the global stockpile era. Human vaccines & immunotherapeutics. 2017;13(3):579–587. doi: 10.1080/21645515.2016.1245250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogasale V, Ramani E, Wee H, Kim JH. Oral cholera vaccination delivery cost in low- and middle-income countries: an analysis based on systematic review. PLoS Negl Trop Dis. 2016;10(12):e0005124. doi: 10.1371/journal.pntd.0005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GeoHive - Malawi population statistics [http://www.nsomalawi.mw/index.php?option=com_content&view=article&id=134%3Apopulationprojections-for-malawi&catid=8&Itemid=3].
- 16.Khonje A, Metcalf CA, Diggle E, Mlozowa D, Jere C, Akesson A, Corbet T, Chimanga Z. Cholera outbreak in districts around Lake Chilwa, Malawi: lessons learned. Malawi Med J. 2012;24(2):29–33. [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . World health statistics 2016: monitoring health for the SDGs, sustainable development goals. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 18.IVI, WHO, DOVE: CHOLTOOL: Planning and costing. User's Guide, Seoul, South Korea. In. Seoul, South Korea; 2016.
- 19.19.-2016 OANDA [https://www.oanda.com/lang/fr/].
- 20.World Economic Outlook Database, October 2016 [http://www.imf.org/external/pubs/ft/weo/2016/02/weodata/download.aspx].
- 21.Ciglenecki I, Sakoba K, Luquero FJ, Heile M, Itama C, Mengel M, Grais RF, Verhoustraeten F, Legros D. Feasibility of mass vaccination campaign with oral cholera vaccines in response to an outbreak in Guinea. PLoS Med. 2013;10(9):e1001512. doi: 10.1371/journal.pmed.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan IA, Saha A, Chowdhury F, Khan AI, Uddin MJ, Begum YA, Riaz BK, Islam S, Ali M, Luby SP, et al. Coverage and cost of a large oral cholera vaccination program in a high-risk cholera endemic urban population in Dhaka, Bangladesh. Vaccine. 2013;31(51):6058–6064. doi: 10.1016/j.vaccine.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Kar SK, Sah B, Patnaik B, Kim YH, Kerketta AS, Shin S, Rath SB, Ali M, Mogasale V, Khuntia HK, et al. Mass vaccination with a new, less expensive oral cholera vaccine using public health infrastructure in India: the Odisha model. PLoS Negl Trop Dis. 2014;8(2):e2629. doi: 10.1371/journal.pntd.0002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarker AR, Islam Z, Khan IA, Saha A, Chowdhury F, Khan AI, Cravioto A, Clemens JD, Qadri F, Khan JA. Estimating the cost of cholera-vaccine delivery from the societal point of view: a case of introduction of cholera vaccine in Bangladesh. Vaccine. 2015;33(38):4916–4921. doi: 10.1016/j.vaccine.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Lydon P, Levine R, Makinen M, Brenzel L, Mitchell V, Milstien JB, Kamara L, Landry S. Introducing new vaccines in the poorest countries: what did we learn from the GAVI experience with financial sustainability? Vaccine. 2008;26(51):6706–6716. doi: 10.1016/j.vaccine.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Schaetti C, Weiss MG, Ali SM, Chaignat CL, Khatib AM, Reyburn R, Duintjer Tebbens RJ, Hutubessy R. Costs of illness due to cholera, costs of immunization and cost-effectiveness of an oral cholera mass vaccination campaign in Zanzibar. PLoS Negl Trop Dis. 2012;6(10):e1844. doi: 10.1371/journal.pntd.0001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen A, Furrer E: GAVI Alliance Board: Vaccine introduction grants and operational support for campaigns. In.; 2012.
- 28.Mogasale V, Kar SK, Kim JH, Mogasale VV, Kerketta AS, Patnaik B, Rath SB, Puri MK, You YA, Khuntia HK, et al. An estimation of private household costs to receive free oral cholera vaccine in Odisha, India. PLoS Negl Trop Dis. 2015;9(9):e0004072. doi: 10.1371/journal.pntd.0004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of total vaccination costs by activity in 2016 US dollars and in international dollars (I$). (DOCX 19 kb)
Distribution of total vaccination costs by input type in 2016 US dollars and in international dollars (I$). (DOCX 23 kb)
Distribution of total vaccine delivery costs by input type in 2016 US dollars and in international dollars (I$). (DOCX 21 kb)
Distribution of unit delivery costs per fully vaccinated person by input type in 2016 US$ and international dollars (I$). (DOCX 22 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


