Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a life-threatening hypersensitivity reaction to medications. We report a case of a 75-year-old African American female who presented with generalized rash with desquamation and malodorous secretions. She was febrile and hypotensive, and required vasopressors for hemodynamic instability. Sepsis secondary to skin or soft tissue infection was considered initially. However, she recently was started on lenalidomide for treatment of her multiple myeloma, and her white blood cell count of 17 K/µL with 55% eosinophils along with peripheral smear showing eosinophilia suggested lenalidomide-induced rash. Lenalidomide was discontinued, and methylprednisolone was initiated. Four days after lenalidomide discontinuation, vasopressors were discontinued. Blood cultures did not exhibit any growth. The Niranjo Adverse Drug Reaction Probability Scale score of 9 suggests lenalidomide was a highly probable cause of DRESS syndrome. The temporal relation of lenalidomide administration and development of symptoms plus improvement of rash with the discontinuation of lenalidomide and reappearance on restarting lenalidomide in the follow-up clinic strengthens our suspicion of lenalidomide-induced DRESS syndrome. Cases of lenalidomide-induced DRESS syndrome are sparse; however, DRESS syndrome is fatal in approximately 10% of patients. Providers should be aware and keep a vigilant eye out for this adverse reaction with lenalidomide.
Keywords: lenalidomide, DRESS syndrome, drug reaction with eosinophilia and systematic symptoms, adverse drug reactions, rash
Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a life-threatening and idiosyncratic hypersensitivity reaction to a medication. Classic presentation is fever, rash, lymphadenopathy, eosinophilia, and variable systemic symptoms depending on the internal organs involved. DRESS syndrome has an incompletely understood pathogenesis, likely resulting from one or more of the following: genetic associations between human leukocyte antigens and moieties in a medication’s structure, virus-medication interaction leading to a viral reactivation, and genetic deficiency affecting detoxifying enzymes that leads to the accumulation of medication metabolites.1 The common medications known to precipitate DRESS syndrome are sulfonamides and anticonvulsants. However, recently other medications such as allopurinol, abacavir, and biological agents have been implicated as well.2-6 Prompt management is essential for better patient prognosis as the condition is reversible when the drug is discontinued early.7 Treatment includes immediate cessation of the culprit drug alongside supportive management. Steroid use has been proposed in a few case reports of DRESS syndrome involving systemic symptoms. However, because viruses like human herpesvirus–6 and human herpesvirus–7, Epstein-Barr virus, cytomegalovirus, and hepatitis C virus have been speculated to reactivate DRESS, corticosteroid use in DRESS syndrome has been controversial. Relapses after tapering of corticosteroids have been reported, suggesting that corticosteroids aid in DRESS syndrome when internal organ involvement is present; however, no randomized and controlled trial has been done proving the same.2, 8-10
Lenalidomide is a less toxic and more potent oral analogue of thalidomide. It modulates the immune system by activating T lymphocytes, increasing Th1-type cytokine release, inhibiting interleukin-6 growth factor, and stimulating pro-apoptotic signals, all of which can lead to antitumor activity.11-14 Its use has been proposed in multiple myeloma, chronic lymphocytic leukemia, mantle cell lymphoma, and myelodysplastic syndrome with the deletion of chromosome 5q.15,16 Severe dermatological reactions such as Stevens-Johnson syndrome, toxic epidermal necrolysis, and erythema multiforme secondary to lenalidomide have been reported,17,18 but evidence for lenalidomide causing DRESS syndrome is sparse.19-21 The precise mechanism for DRESS syndrome with lenalidomide is not clear; it is possible that the immunomodulatory activity of lenalidomide contributes in the hypersensitivity reaction presenting as DRESS syndrome. Lenalidomide is known to cause adverse medication reaction affecting the respiratory system,22 but nonpulmonary reactions have been rarely reported. We report a case of DRESS syndrome caused by lenalidomide used for the treatment of multiple myeloma.
Case Report
A 75-year-old African American female presented to our hospital with fatigue and weakness over the previous few days. On the morning of her presentation, she fell from a standing position and landed on her buttocks, but she denied syncope or presyncopal episode prior to the fall. She attributed the fall to feeling weak. On examination, she was noted to be hypotensive and responded only partially to a 1-L bolus of 0.9% sodium chloride. On physical examination, she had a diffuse erythematous skin rash covering 90% to 95% of her body surface area with evidence of skin breakdown and desquamation in addition to malodorous secretions in her skin folds (Figures 1 and 2).
Figure 1.
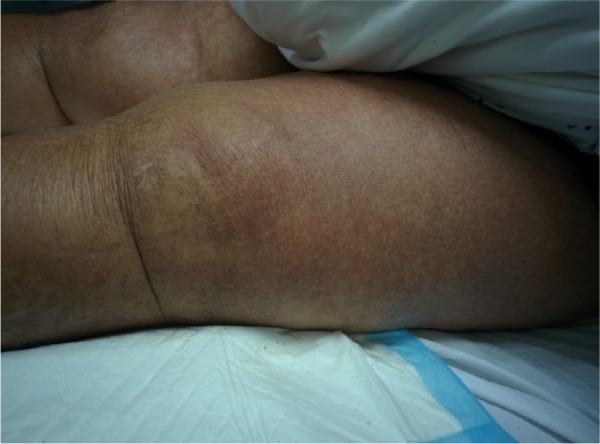
Extensive rash on patient’s legs.
Figure 2.
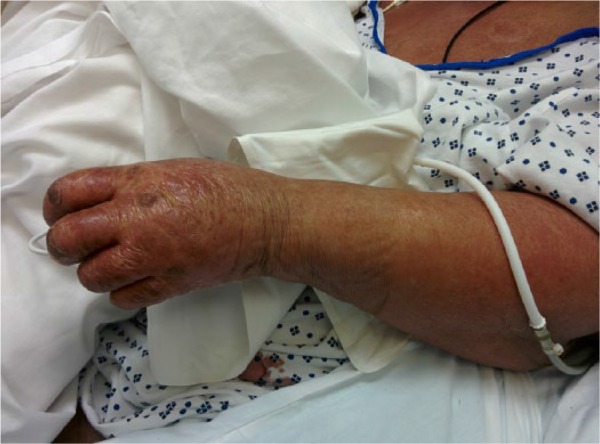
Extensive rash on patient’s left arm and chest.
One month prior to the patient presenting to the hospital, she was diagnosed with multiple myeloma based on serum protein electrophoresis that revealed serum M protein of 2.3 g/dL in addition to the presence of serum kappa-free light chains, 2.82 mg/dL (normal range: 0.22-1.94 mg/dL), and lambda-free light chains, 73.5 mg/dL (normal range: 0.57-2.63 mg/dL) and serum immunofixation that revealed the presence of IgG lambda plus free lambda M-proteins. Diagnosis was confirmed by bone marrow biopsy showing 30% plasma cells on aspiration and 25% on core biopsy. Concurrent flow cytometric analysis showed plasma cell population with an atypical immunophenotype, comprising about 8.94% of analyzed events. An immunohistochemical stain for CD138 and in situ hybridization studies for kappa and lambda light chains performed on AZF-fixed, paraffin-embedded sections of the bone marrow biopsy showed 25% plasma cells, predominantly lambda-expressing cells with the expansion of interfatty spaces by aggregates of monotypic plasma cells. She was started on lenalidomide 5 mg by mouth daily on days 1 to 21 of a 28-day cycle and dexamethasone 40 mg by mouth on days 1, 8, 15, and 22 every 28 days.
The patient’s other significant past medical history was type II diabetes mellitus, hypertension, heart failure with preserved ejection fraction of 60%, atrial myxoma with mitral valve prolapse for which the patient had received a bioprosthetic mitral valve replacement, sacral teratoma that had been resected, and stage III chronic kidney disease. Home medications included metoprolol succinate 25 mg by mouth daily, olmesartan 5 mg by mouth daily, aspirin 325 mg by mouth daily, furosemide 80 mg by mouth daily, spironolactone 25 mg by mouth daily, and a multivitamin taken once by mouth daily. She was known to develop an allergic rash and swelling with blue food coloring and amitriptyline but indicated that she had not taken either recently. She had no history of alcohol or tobacco use.
The review of systems was significant for the new-onset and worsening rash, malaise, and possible chills. Otherwise, the patient denied fever, abdominal pain, arthralgia, history of easy bruising, lymphadenopathy, and numbness or tingling in her extremities. On physical examination, initial vital signs were blood pressure 94/55 mmHg, pulse 73 beats per minute, temperature 99.3°F, and respiratory rate 17 breaths per minute. She was lying in bed with an ill and weak appearance but was well oriented to time, place, and person. Her cardiac and respiratory exams were unremarkable, and no organomegaly or tenderness to palpation was noted on abdominal exam. Majorly, she had an expanded rash extending from her face, chest, and back to the extremities but sparing her palms, soles, and nails. The rash was erythematous, scaly, and showed evidence of desquamation and malodorous secretions.
Labs on admission were remarkable for a white blood cell count of 17.01 K/µL with 55% eosinophils (lab standard: 1%-7%), hemoglobin 12.4 g/dL, hematocrit of 39.9%, blood urea nitrogen 27 mg/dL, serum creatinine 1.6 mg/dL, and lactate 3.7 mmol/L. Chest radiograph at admission did not show any pulmonary vascular congestion, consolidation, effusion, or hyperinflation. Urinalysis was unremarkable. Radiograph of the pelvis and upper extremities was performed to rule out the possibility of fractures due to her recent fall and was negative as well. Given her presentation with generalized rash, hypotension, and acute on chronic kidney disease, sepsis secondary to skin or soft tissue infection was high on the initial differential diagnosis. However, a peripheral smear showing eosinophilia combined with the white count differential revealing 55% eosinophils suggested the possibility of lenalidomide-induced rash; therefore, lenalidomide was held on admission and methylprednisolone 80 mg by intravenous injection 3 times daily was initiated. She was resuscitated with fluids and later admitted to the intensive care unit for hemodynamic instability requiring vasopressors. Following 2 blood cultures being drawn, she was initiated on vancomycin and cefepime. Four days after admission and lenalidomide discontinuation, vasopressors were discontinued. Blood cultures did not exhibit any growth and infection as a cause of her symptoms was later thought to be unlikely. Significant improvement in the rash and a decrease in eosinophilia were observed prior to discharge. In the follow-up clinic appointment 4 months after her discharge, the patient was restarted on lenalidomide at the same dose and frequency. However, the rash resurfaced within a day, and lenalidomide was discontinued immediately and indefinitely.
Discussion
Nonspecific rash has been noted in approximately 27% of patients treated with lenalidomide for an oncological disorder.15 Lenalidomide has been used more commonly in hematologic disorders only recently, and hence, there are only 3 cases of lenalidomide-associated DRESS syndrome in the literature.19-21 Shaaban et al reported a case of DRESS syndrome with manifestations of fever, pruritic rash, and cough with acute interstitial nephritis resulting from the first course of lenalidomide given for multiple myeloma, which improved following discontinuation of the medication.19 Similarly, Vlachopanos and colleagues provided care for a patient who was dependent on dialysis and developed DRESS syndrome after receiving lenalidomide at an appropriate dose for her renal function for 5 days prior to admission. The diffuse and infiltrating rash did not abate following discontinuation, and the patient succumbed from relapsed multiple myeloma 8 weeks after admission.20 Finally, another patient reported by Foti et al also experienced DRESS syndrome from lenalidomide, which improved with the termination of the drug. No recurrences of rash, facial edema, lymphadenopathy, or fever were noted on follow-up visits over 6 months.21
In our patient, the Naranjo Adverse Drug Reaction Probability Scale score was 9, suggesting lenalidomide was a highly probable cause of DRESS syndrome23 (Figure 3). Limitations of the case report include our inability to measure serum concentrations of lenalidomide and lack of biopsy results. However, the chronological relation of lenalidomide administration with the clinical symptoms including a classic DRESS syndrome rash, improvement of manifestations with discontinuation of lenalidomide, presence of significantly elevated eosinophils, and reappearance of the rash on a rechallenge of the agent strengthen the suspicion of lenalidomide-associated DRESS syndrome.
Figure 3.
Naranjo Adverse Drug Reaction Probability Scale.
A Naranjo Adverse Drug Reaction Probability Scale score of 9 suggests lenalidomide was a highly probable cause of this patient’s presentation.
Conclusion
DRESS syndrome is fatal in approximately 10% of patients and should be considered a rare but potential complication with lenalidomide.24 Practitioners could have easily missed lenalidomide as the highly probable culprit medication in our patient as sepsis secondary to skin or soft tissue infection was probable. Hence, with the increasing use of oral lenalidomide in hematological disorders, practitioners need to be aware of this adverse reaction, hold the medication if the diagnosis is being considered, and complete a thorough patient work-up while providing supportive care.
Footnotes
Authors’ Note: Presentation of this content was provided at the Society of Critical Care Medicine 46th Annual Congress in Honolulu, Hawaii.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Choudhary S, McLeod M, Torchia D, Romanelli P. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J Clin Aesthet Dermatol. 2013;6(6):31-37. [PMC free article] [PubMed] [Google Scholar]
- 2. Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206(4):353-356. [DOI] [PubMed] [Google Scholar]
- 3. Roujeau J-C. Treatment of severe drug eruptions. J Dermatol. 1999;26(11):718-722. [DOI] [PubMed] [Google Scholar]
- 4. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331(19):1272-1285. [DOI] [PubMed] [Google Scholar]
- 5. Callot V, Roujeau JC, Bagot M, et al. Drug-induced pseudolymphoma and hypersensitivity syndrome. Two different clinical entities. Arch Dermatol. 1996;132(11):1315-1321. [PubMed] [Google Scholar]
- 6. Sullivan JR, Shear NH. The drug hypersensitivity syndrome: what is the pathogenesis? Arch Dermatol. 2001;137(3):357-364. [PubMed] [Google Scholar]
- 7. Santiago F, Gonçalo M, Vieira R, Coelho S, Figueiredo A. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62(1):47-53. [DOI] [PubMed] [Google Scholar]
- 8. Chiou C-C, Yang L-C, Hung S-I, et al. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol. 2008;22(9):1044-1049. [DOI] [PubMed] [Google Scholar]
- 9. Kocaoglu C, Cilasun C, Solak ES, Kurtipek GS, Arslan S. Successful treatment of antiepileptic drug-induced DRESS syndrome with pulse methylprednisolone. Case Rep Pediatr. 2013;2013:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2(46):46ra62. [DOI] [PubMed] [Google Scholar]
- 11. Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380-386. [PubMed] [Google Scholar]
- 12. Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210-216. [DOI] [PubMed] [Google Scholar]
- 13. Dredge K, Marriott JB, Macdonald CD, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87(10):1166-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson KC. Lenalidomide and thalidomide: mechanisms of action–similarities and differences. Semin Hematol. 2005;42(4)(suppl 4):S3-8. [DOI] [PubMed] [Google Scholar]
- 15. Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. [DOI] [PubMed] [Google Scholar]
- 16. Ferrajoli A, Lee B-N, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. FDA Drug Safety Newsletter: Postmarketing reviews. Summer 2008;1(4):41-44. [Google Scholar]
- 18. Castaneda CP, Brandenburg NA, Bwire R, Burton GH, Zeldis JB. Erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis in lenalidomide-treated patients. J Clin Oncol. 2009;27(1):156-157. [DOI] [PubMed] [Google Scholar]
- 19. Shaaban H, Layne T, Guron G. A case of DRESS (drug reaction with eosinophilia and systemic symptoms) with acute interstitial nephritis secondary to lenalidomide. J Oncol Pharm Pract. 2013;20(4):302-304. [DOI] [PubMed] [Google Scholar]
- 20. Vlachopanos G, Kokkona A, Zerva A, et al. Atypical DRESS syndrome induced by lenalidomide in chronic hemodialysis. J Clin Exp Pathol. 2016;6(3):1-3. [Google Scholar]
- 21. Foti C, Antelmi A, Mazzocca A, et al. Drug reaction with eosinophilia and systemic symptoms caused by lenalidomide. Eur J Dermatol. 2012;22(6):799-800. [DOI] [PubMed] [Google Scholar]
- 22. Lerch E, Györik S, Feilchenfeldt J, Mazzucchelli L, Quadri F. A case of lenalidomide-induced hypersensitivity pneumonitis. Onkologie. 2010;33(5):249-252. [DOI] [PubMed] [Google Scholar]
- 23. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. [DOI] [PubMed] [Google Scholar]
- 24. Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36(1):6-11. [DOI] [PubMed] [Google Scholar]



