Abstract
Background: Focus on antimicrobial use and infection prevention from accrediting or regulatory bodies such as the Joint Commission, as well as regulatory agencies such as the Centers for Medicare and Medicaid Services and the Centers for Disease Control, has highlighted the need for continuing development of antimicrobial stewardship programs at healthcare facilities across the country. Methods: Our institution utilized the 2007 Infectious Diseases Society of America and the Society for Healthcare Epidemiology guidelines to direct the evaluation of its antimicrobial use and develop a successful antimicrobial stewardship program. Three baseline evaluations were conducted. Retrospective chart reviews evaluating formulary restrictions for fluroquinolones and carbepenems, a dosing optimization program for meropenem, and the intravenous to oral conversion program for fluconazole and voriconazole were completed. Results: Approximately 40% of orders for levofloxacin were not supported with a clinical justification for nonformulary use in the patient chart. Forty-nine percent of orders written for meropenem did not follow the dose optimization program. Opportunity for fluconazole and voriconazole to be converted to oral therapy when appropriate was suggested. Conclusion: The baseline evaluations revealed the need for an antimicrobial stewardship program. This article outlines the process used to assess need, plan, implement, and evaluate the impact of an antimicrobial stewardship program.
Keywords: antimicrobial stewardship, antimicrobial resistance, antimicrobial use, antibiotics
Introduction
Antimicrobial resistance has become a threat to human health and is considered by many to be a crisis.1-7 Antimicrobial stewardship is an important strategy to address this challenge. In 2014, the Centers for Disease Control and Prevention (CDC) developed a campaign to prevent antimicrobial resistance in health care settings titled “Get Smart for Healthcare” focusing on the implementation of antimicrobial stewardship programs (ASPs) in hospitals.8 President Obama enacted an Executive Order, also in 2014, focused on appropriate antimicrobial use resulting in the National Action Plan for Combating Antibiotic Resistance published in 2015. Regulatory agencies and government payers have conveyed the urgency of antimicrobial stewardship through implementation of requirements for ASPs as a condition of accreditation and payment for services. In June 2016, the Centers for Medicare and Medicaid Services (CMS) proposed new Conditions for Participation for hospitals and critical access hospitals which included the requirement for hospital-wide ASPs for the surveillance, prevention and control of hospital-acquired infections, and the appropriate use of antibiotics.9 The CMS inpatient prospective payment system provides an additional incentive to implement stewardship programs by reducing payments for services in the setting of a hospital-acquired infection such as catheter-associated urinary tract infections, incentivizing health care facilities to focus on both infection prevention and control and antimicrobial stewardship.10 In addition, effective January 1, 2017, The Joint Commission has added a new standard (Standard MM.09.01.01) requiring hospitals, critical access hospitals, and nursing care centers to have an ASP “based on current scientific literature.”11
Prior to these new mandates, guidelines had been published in 2007 by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology (IDSA/SHEA) to guide health care facilities in the implementation of ASPs. The guidelines provided recommendations for the formation of successful stewardship teams along with a description of both core and supplemental activities for evidence-based implementation.12 Of note, in the 2016 iteration of these guidelines, the core team recommendation was reduced to the physician, pharmacist, and infection control practitioner and guidance is provided for implementation and measurement of stewardship initiatives in both hospitals and long-term care facilities.13
After assessment of our needs and opportunities, we utilized the original IDSA/SHEA guidelines as a foundation for the structure and operation of our ASP. The following is a step-by-step review of the assessment and implementation process, and a summary of the associated outcomes of the program. Descriptions of the programmatic changes that have been implemented over the past 7 years since the inception of the ASP are also discussed. It is our hope that sharing our approach will be of use to other health systems contemplating the development and initiation of a stewardship program.
Overview of the Health System
Our health system is a 785-bed academic medical center affiliated with 6 colleges including Medicine and Pharmacy. The average daily census of the medical center is approximately 700 patients, with approximately 47,000 admissions per year. It comprises the Children’s Hospital, Institute of Psychiatry, the Cancer Center, 2 adult hospital entities, and the Eye Institute. The Medical Center includes centers for specialized care including the Heart Center, Transplantation Center, Cancer Center, and Digestive Diseases Center, as well as numerous physician-based outpatient facilities such as the Family Medicine Center, University Diagnostic Center, and other affiliated faculty practice ambulatory care centers.
Step 1: Baseline Evaluation to Support Implementation of an Antimicrobial Stewardship Program
Figure 1 outlines the steps utilized to plan, implement, and adapt the ASP at our institution. According to the original IDSA/SHEA Guidelines for Developing an Institutional Program,12 there are both core and supplemental strategies that should be deployed to develop a program and these were carefully weighed in contemplating our needs and priorities. In 2007, baseline data reflecting current efforts related to stewardship were evaluated to assess needs and then garner administrative support for funding of the program. The Anti-infective Subcommittee of the Pharmacy and Therapeutics (P&T) Committee already oversaw many initiatives around antimicrobial use such as trending utilization and resistance patterns, reviewing formulary requests, and developing clinical pathways and orders sets. This committee remains intact today and is led by an infectious diseases physician who is also the medical director of the antimicrobial stewardship team (AST) resulting in an integral relationship between the committee and the AST. The committee has always served as a sounding board for AST proposals and receives and reacts to reports of AST accomplishments. The AST often contributes agenda items by presenting results from the AST or ideas for program implementation. The secretary of the committee, a pharmacist, assists the chair in preparing the agenda, takes and distributes minutes, and is responsible for scheduling a meeting time and location. The meeting is held once per month, in person, and a quorum is always achieved, as there is great commitment from the membership. Membership of the committee is comprised of representatives from surgery, infectious diseases, clinical microbiology, infection control and prevention, the hospitalist service, specialties such as hematology/oncology, solid organ transplant, pediatric infectious diseases, and critical care which are all services that heavily utilize antimicrobials. Both AST physicians and both AST pharmacists are on this committee. The secretary is also a member of the Infection Control Committee. This overlap in committee appointment assures ongoing and effective communication between major parties involved with our stewardship program. The facility antibiogram is updated annually by the clinical microbiology department and presented to the committee for review and approval. Consensus among the committee members is achieved utilizing majority vote. When initiatives or agenda items impact specialties not represented by the committee membership, they are invited to attend the meetings, and/or the chair is responsible for communicating with the individuals. The subcommittee reports to the P&T Committee which includes representatives from all major clinical specialties. Any pertinent agenda items reviewed by the subcommittee are vetted by the larger P&T Committee.
Figure 1.
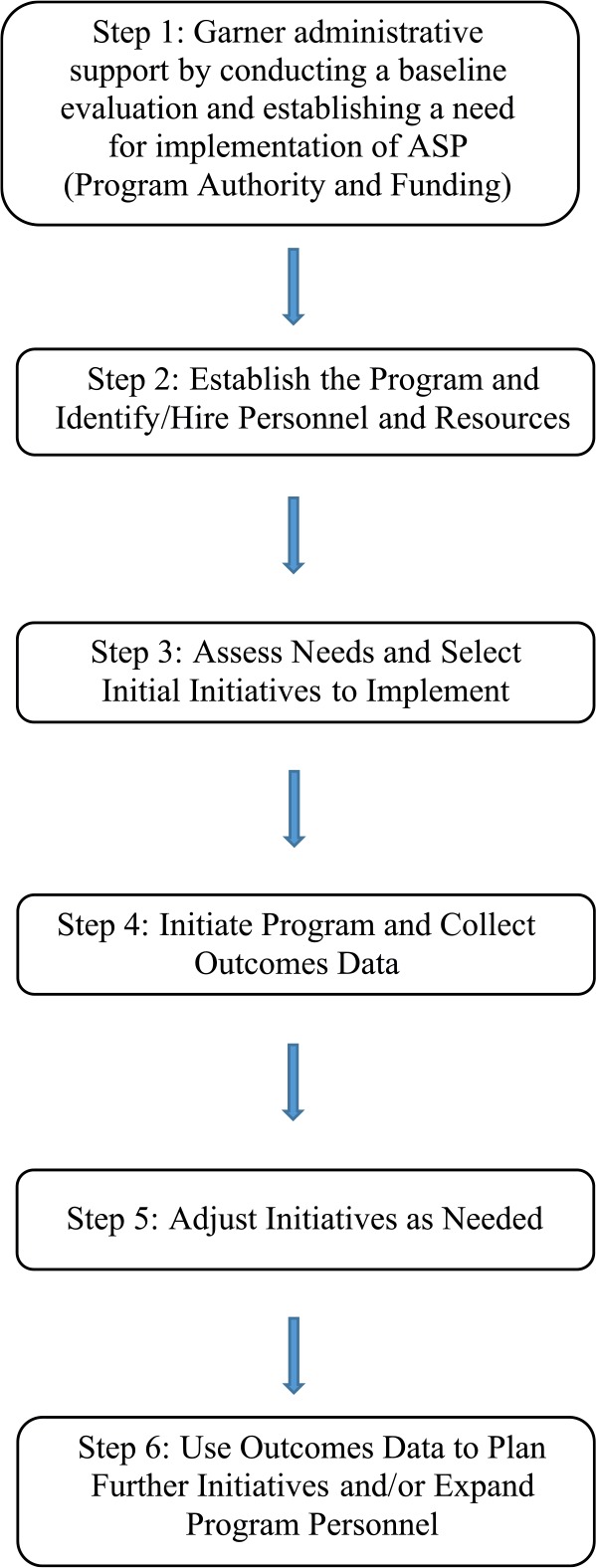
Steps for implementation of an ASP.
Note. ASP = antimicrobial stewardship program.
A well-staffed Medication Safety Use and Policy group has been in place for many years and actively supports the information needs of both the Subcommittee and the P&T Committee. Importantly, an active Infectious Diseases Division, full-service Clinical Microbiology Laboratory, and progressive Infection Control and Prevention Department were all in place, all with a strong history of cooperation among themselves as well as with the Department of Pharmacy Services. In addition, surveillance software for infection and antimicrobial monitoring was under joint consideration by Pharmacy Services and Infection Prevention and Control but had not yet been purchased.
Stewardship-specific initiatives that were evaluated for baseline data were the automatic therapeutic substitution (ATS) programs for levofloxacin and imipenem/cilastatin to alternative formulary agents; and a P&T-approved, pharmacist-driven dosing program for appropriate dosing of meropenem; and an intravenous (IV) to oral (PO) program for select antimicrobials including fluconazole and voriconazole. The following is a summary of some of the baseline analyses that were completed to justify the need for expanded ASP personnel and services.
Formulary Restriction Program Evaluation
ATS programs for both fluoroquinolones and carbapenems were implemented to support compliance with the list of approved formulary antimicrobial agents. The ATS for fluoroquinolones provided pharmacists a P&T-approved protocol to change orders written for levofloxacin (nonformulary agent at the time) to one of 2 formulary fluoroquinolones, ciprofloxacin or moxifloxacin, depending on indication. A chart review conducted in 2007 indicated that approximately 40% of orders verified for levofloxacin were not supported with a clinical justification for nonformulary use in the patient chart. The conclusion drawn from these data was that the formulary restriction program was not yielding the desired effect of formulary compliance, and further support from an ASP program was needed.
Meropenem Dosing Program Evaluation
A dose optimization program for meropenem was implemented to allow pharmacists to evaluate orders for clinical appropriateness and appropriate dosing. Any order written for meropenem 1 g IV every 8 hours (q8h) could be evaluated by the pharmacist for indication and renal function–appropriate dosing to ensure proper use. If the indication for use was anything other than cystic fibrosis, febrile neutropenia, or meningitis, the dose should be converted to 500 mg IV q6h or another appropriate renal dose. In preparation for a stewardship program, an evaluation of the efficacy of dosing program was completed.
A retrospective chart review was conducted. All meropenem orders processed for 1 g IV q8h during the month of March 2008 were included in the initial assessment. Orders for pediatric patients, or 1-time or STAT orders, were excluded from the evaluation. In total, 39 patient charts were reviewed. Forty-nine percent of patients received a dose of 1 g q8h, and should have been prescribed 500 mg IV q6h based on recommended dosing guidelines. In addition, if a modified glomerular filtration rate calculation were performed upon order entry, one patient should have had his or her dose adjusted because of renal insufficiency. Twenty patients received meropenem for febrile neutropenia, and were appropriately dosed based on renal function and indication. A secondary evaluation was completed to determine how many patients who received 500 mg IV q6h were prescribed this dose because of a pharmacy-initiated conversion from 1 g IV q8h. Of 30 patients who received meropenem 500 mg IV q6h during the evaluation period, only 2 received this dose due to a pharmacist intervention. It is unknown how many interventions to adjust the dose were attempted by a pharmacist and rejected by a prescriber, as this information was not documented for any of the reviewed orders. These data suggested the need for more prospective and routine monitoring of appropriate dosing of antimicrobials through alternate mechanisms. Daily monitoring from a stewardship team utilizing surveillance software was one such recommendation.
IV to PO Program Evaluation
Adult and pediatric IV and PO fluconazole and voriconazole utilization data from the beginning of 2003 to the third quarter of 2007 were analyzed to determine the trend in IV:PO use of these agents at our institution as influenced by an active, pharmacy-initiated IV to PO conversion program. Adult and pediatric data were considered separately. Usage was expressed as both grams and doses administered per quarter. The data were normalized by census and expressed as use per 1000 patient days. Institution-specific drug prices from 2003 to 2007 (adjusted to 2007 dollars) by the Medical Component of the Consumer Price Index were used to determine the impact of IV to PO conversion on drug expense. Prices from 2003 to 2005 were assumed to be the same as 2005, because data prior to 2005 were unavailable. The drug expense and IV:PO ratio for each quarter were compared with the baseline (first quarter of 2003) expense and IV:PO ratio to calculate variations in expenditure. Compared with baseline, the IV:PO ratios for fluconazole declined in the adult population but hovered around 1:1 in the pediatric population for both doses and grams dispensed. Voriconazole IV:PO ratios also hovered around 1:1 in adults. Usage of this drug in pediatrics was too infrequent to allow a similar analysis. Although the trends do not indicate dramatic shifting to more oral antifungal therapy, the ratio remained about 1:1 and indicated that patients were generally converted to oral therapy if indicated. It was also shown that when compared with baseline, these conversions led to a savings of $3710.33 per 1000 patient days over the duration of the study ($1.8 million adjusted to 2007 dollars). This was deemed to be an area/effort that should be sustained and could possibly be improved upon once a formal stewardship program was in place.
Summary of Baseline Evaluation
Areas of opportunity that were identified based on our assessments described above included a need for a more structured formulary restriction/review program, IV to PO conversion program improvements, and the need for more prospective monitoring of some therapeutic situations and/or drug use. In addition, other evaluations of our antimicrobial utilization that are not described in this manuscript revealed the following needs for optimization: updating of clinical pathways/order sets and creation of additional ones, de-escalation of therapy, detection and actions on pathogen-drug mismatches, and evaluation of possible overutilization of some empiric antimicrobial agents such as piperacillin/tazobactam, and ciprofloxacin for presumed gram-negative infections. Additionally, development of a formal ASP was identified as a major area of opportunity as a programmatic approach to evaluate and implement these identified initiatives. In addition, software programs were considered for automation of various aspects of surveillance and reporting functionality to the ASP. It was also recognized that these preexisting initiatives focused on appropriate drug use/dosing and cost savings rather than quality of care, which is the main focus and purpose of antimicrobial stewardship. Thus, a formal stewardship program, focused on assessment and improvement of patient outcomes through optimization of antimicrobial use, represented an unmet need.
Step 2: Establishing the Program and Acquiring Personnel and Resources
The plans for program implementation and the associated personnel needs were incorporated into a business plan proposal to the hospital’s medical director requesting line-item funding for creation of an AST. The proposal included various initiatives to improve infection- and antimicrobial use–related patient outcomes as well as projections for potential cost savings. In this plan, we proposed that based on published literature referenced in the IDSA/SHEA Guidelines,12 we could reduce antibiotic expenditures at our institution by about 20% which at the time was estimated to yield about $900 000 in initial annual savings. Additional impacts were expected in a reduction in length of stay, and hopefully a reduction in microbial resistance which could yield improved patient outcomes and decreased costs of care for the institution. A list of mechanisms for reduction of costs was also included in the proposal. Examples of efforts presented were restriction of microbial use, reduction of treatment duration, dose optimization, development and use of clinical guidelines and pathways, evaluation of current usage and susceptibility patterns to direct future interventions, and, of course, development of a stewardship team along with its structure and function.
The original request was for 1.0 full-time equivalent (FTE) Infectious Diseases trained pharmacy clinical specialist, 1.0 FTE PGY2 Pharmacy Antimicrobial Stewardship pharmacy resident (a PGY2 Infectious Diseases residency was already in place), and a pharmacist supervisor (0.1 FTE), along with 0.2 FTE medical director, 0.1 FTE data analyst, and 0.25 FTE data manager. Not all positions were approved, but in 2009, the ASP was formally launched with the following funded positions: 1.0 FTE ID trained pharmacist, 0.25 FTE Medical Director, and 0.25 co-medical director. Although integrally involved, clinical microbiology, infection control, and data management participation was not funded.
Steps 3 and 4: Assess Needs, Select Initiatives to Implement, and Collect Outcome Data
At the time of program initiation, a stewardship steering committee was formed, and was comprised of the Head of Infection Prevention and Control, the Division Chief of Infectious Diseases, the Director and Medical Director of the Clinical Microbiology Lab, and a Clinical Pharmacy Specialist trained in Infectious Diseases. This committee advised the core stewardship team about desirable initiatives and data to collect and present to administration for approval of both concept and resources. The steering committee has since dissolved and is represented in the Anti-infective Subcommittee of the P&T Committee previously described. The program also maintained the strong working relationship with the Anti-infective Subcommittee. Among the first initiatives implemented in 2009 was assessment and acquisition of a clinical decision support system to assist the AST in evaluating real-time antimicrobial use. This system was originally implemented by the Infection Control department, and was later adopted by the Department of Pharmacy Services including the stewardship pharmacist and the PGY2 Infectious Diseases (ID) resident. It was used for purposes of stewardship initiatives such as evaluating the daily use of vancomycin and aminoglycoside dosing, bug-drug mismatches, organ dysfunction for possible dose adjustments, and utilization of nonformulary antimicrobials. These reports were monitored daily, and prescribers were contacted (typically via pager) by the pharmacist to address concerns with backup from the ID physician on the AST. Today, the 2 pharmacy clinical specialists who work with the AST attend daily Technical Rounds in the Clinical Microbiology Lab where culture results are reported, and review of susceptibilities is evaluated real-time to determine a course of therapy for a patient in a more timely manner.
In 2010, treatment guidelines for the empiric treatment of hospital acquired pneumonia, ventilator associated pneumonia, healthcare associated pneumonia and febrile neutropenia were updated to reflect a change in preferred broad-spectrum antimicrobial from piperacillin/tazobactam and meropenem, respectively, to cefepime based on our then current antibiogram. In addition, antimicrobial dosing recommendations/guidelines were created to assist practitioners in selecting the most appropriate empiric dosing of all antibiotics as well as appropriate dose adjustments for renal dysfunction, and the various types of dialysis utilized at our facilities.
In 2011, formulary management and restriction of the use of certain agents were utilized to both preserve susceptibility of some antimicrobial agents and conserve financial resources. For example, ciprofloxacin was restricted to treatment of infections that had culture-confirmed susceptibility (with select exceptions) reports due to dramatically rising resistance of gram-negative pathogens, especially Pseudomonas aeruginosa. In addition, the preferred formulary echinocandin antifungal was changed to a lesser priced product to reduce the cost of therapy.
Over the past several years, additional initiatives surrounding dose optimization and clinical pathways have been executed. For example, extended infusion of piperacillin/tazobactam is now standard of care, and the bacteremia clinical pathway now includes multiplex polymerase chain reaction diagnostic testing. Since initiation of the ASP, members of the AST have been integrated into the Antimicrobial Subcommittee of the P&T Committee which also has representation from Pharmacy Services, Infection Control, Clinical Microbiology, and a number of pertinent medical specialties. This allows the subcommittee to act as a “think tank” that ultimately proposes new initiatives, clinical treatment pathways, and formulary changes (including restrictions) for consideration by the P&T Committee. Fodder for subcommittee consideration is supplied by AST members (eg, antimicrobial use and resistance trends; clinical treatment pathways/order forms) and the Medication Safety Use and Policy group within the Department of Pharmacy Services (eg, preparation of drug monographs).
Other initiatives underway are also focusing on infection prevention protocols, such as antibiotic locks for the prevention of central line–associated blood stream infections, and also educational initiatives targeting appropriate dosing and monitoring of aminoglycosides and vancomycin use. In addition, initiatives to ensure appropriate dose adjustments for other anti-infectives for organ dysfunction and de-escalation of empiric therapies were put in place.
Steps 5 and 6: Post ASP Implementation Evaluation
Since the inception of the ASP, 2 publications from our program have reported examples of the efficacy of various ASP efforts. Data from these publications were used to provide feedback and ideas for adjustment of current initiatives, and to support expansion of future initiatives with the addition of a second pharmacist.
O’Brien et al14 evaluated the impact of a stewardship-initiated restriction on empirical use of ciprofloxacin on the nonsusceptibility of Escherichia coli urinary isolates to ciprofloxacin over time. Due to concerns for gram-negative resistance to ciprofloxacin, the ASP implemented an Anti-infective Subcommittee–approved formulary restriction for ciprofloxacin. The empiric use of ciprofloxacin was banned with few exceptions. Therapeutic use was restricted to patients with documented infections caused by ciprofloxacin-susceptible pathogens. If the prescribed use did not meet the restriction, consultation with the AST or the on-call infectious diseases physician was required for approval but often resulted in recommendation for an alternative agent. An evaluation of impact of this initiative revealed that it dramatically decreased the ciprofloxacin use by greater than 70%, and the percentage of E coli urinary isolates nonsusceptible to ciprofloxacin decreased from 41.5% to 32.8% between the first quarter of 2011 when the ciprofloxacin restriction was implemented and December 2012 when the analysis period ended.
Timbrook et al15 conducted an investigation to evaluate the impact of our ASP on the utilization, susceptibilities, and financial expenditures of our antibiotics post implementation of our ASP. The findings indicate that our ASP has positively impacted antibiotic utilization, financial expenditures, and susceptibilities. Utilizing strategies such as formulary restriction, prospective audit and feedback, dose optimization, and IV to PO conversions resulted in reduction in utilization of targeted agents by 16%. In addition, total systemic antibiotic expenditures were reduced by $1.8 million when expenditures from fiscal year 2010 were compared with those in fiscal year 2013. Susceptibility changes were also noted between the pre-ASP implementation and post-ASP implementation periods for Staphylococcus aureus, P aeruginosa, E coli, Serratia marcescens, and Acinetobacter baumannii.
Future Directions
The core AST is now comprised of two 1.0 FTE stewardship pharmacists and two 0.25 FTE stewardship physician medical directors. Additional support is provided by 1 clinical pharmacist in the HIV clinic, 1 part-time pharmacist in the ID consult service, and members from infection control and microbiology, as well as a PGY2 Infectious Diseases pharmacy resident. In addition, surveillance software has been implemented and is used on a daily basis to monitor antimicrobial usage through reports such as bug-drug mismatches, duration of therapy, the need for escalation/de-escalation of therapy, and therapy initiation with medications requiring monitoring or dose adjustments based on organ dysfunction. To date, an antimicrobial stewardship pharmacy resident has not been instituted, but the PGY2 ID resident is integrally involved in the ASP when on applicable rotations.
As the ASP continues to develop, we look to identify potential opportunities for future stewardship initiatives. One such example is the data from Timbrook et al16 who investigated clinician prescribing behavior of antimicrobial therapy upon receipt of viral respiratory panels (RP) and/or procalcitonin (PCT) results that were suggestive of viral respiratory infection. A retrospective chart review, evaluating 4869 patients with PCT and/or RP results (2031 patients ultimately included in the analysis), was conducted. Of the patients included, 503 had PCT results, and 1823 patients had RP results. Of those patients, 295 patients had results for both PCT and RP tests. Test results indicated that 789 patients were potential candidates for discontinuation of therapy or antimicrobial avoidance. However, only 10% of patients who had a negative RP or PCT or both had their antimicrobial therapy discontinued. These findings suggest a need for AST intervention and programmatic implementation of interventions to reduce the use of antimicrobials in the setting of viral pneumonia.
Closing Statement
Implementation of our ASP positively impacted empiric use of antimicrobials and susceptibility of organisms to selected agents, and reduced expenses through formulary management and IV to PO conversion, and other systematic approaches. With the recent emphasis from national initiatives through CMS, The Joint Commission, and the White House, continued development and expansion of stewardship initiatives will continue to gain momentum. By sharing our experience in development and implementation of an ASP, we hope that this information can guide programs beginning their programs or who are looking to advance their practice.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Division of Healthcare Quality Promotion, National Center for Infectious Diseases, Centers for Disease Control and Prevention. Antimicrobial Resistance: A Growing Threat to Public Health. Atlanta, GA: Division of Healthcare Quality Promotion, National Center for Infectious Diseases, Centers for Disease Control and Prevention; 2002. [Google Scholar]
- 2. Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293:1786-1790. [DOI] [PubMed] [Google Scholar]
- 3. Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36:697-705. [DOI] [PubMed] [Google Scholar]
- 4. Infectious Diseases Society of America. Bad Bugs, No Drugs: As Antibiotic Discovery Stagnates, a Public Health Crisis Brews. Alexandria, VA: Infectious Diseases Society of America; 2004. [Google Scholar]
- 5. Centers for Disease Control and Prevention, Office of Infectious Diseases. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013. Published April 2013. Accessed November 16, 2016.
- 6. Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155-164. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Antimicrobial resistance: global report on surveillance 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/. Published April 2014. Accessed November 16, 2016.
- 8. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. http://www.cdc.gov/drugresistance/index.html. Updated August 17, 2016. Accessed November 16, 2016.
- 9. Centers for Medicare and Medicaid Services Press Release. CMS issues proposed rule that prohibits discrimination, reduces hospital-acquired conditions, and promotes antibiotic stewardship in hospitals. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2016-Fact-sheets-items/2016-06-13.html. Published June 13, 2016. Accessed November 16, 2016.
- 10. Centers for Medicare and Medicaid Services. Hospital value-based purchasing. Date unknown. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html. Accessed August 4, 2016.
- 11. Joint Commission. Approved: new antimicrobial stewardship standard. https://www.jointcommission.org/assets/1/6/New_Antimicrobial_Stewardship_Standard.pdf. Accessed July 12, 2017. [PubMed]
- 12. Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177. [DOI] [PubMed] [Google Scholar]
- 13. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2017;62:e51-e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Brien K, Shang J, Mauldin P, et al. Impact of a stewardship-initiated restriction on empirical use of ciprofloxacin on nonsusceptibility of Escherichia coli urinary isolates to ciprofloxacin. Pharmacotherapy. 2015;35(5):464-469. [DOI] [PubMed] [Google Scholar]
- 15. Timbrook TT, Hurst JM, Bosso JA. Impact of an antimicrobial stewardship program on antimicrobial utilization, bacterial susceptibilities, and financial expenditures at an academic medical center. Hosp Pharm. 2016;51:703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Timbrook T, Maxam M, Bosso J. Antibiotic discontinuation rates associated with positive respiratory viral panel and low procalcitonin results in proven or suspected respiratory infections. Infect Dis Ther. 2015;4:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]


