Abstract
Background: Respiratory viral illnesses account for many hospitalizations and inappropriate antibiotic use. Respiratory viral panels by polymerase chain reaction (RVP-PCR) provide a reliable means of diagnosis. In 2015, the RVP-PCR assay at our institution was switched from respiratory viral panel (RVP) to rapid respiratory panel (rapid RP), which has a faster turnaround time (24 hours vs 12 hours, respectively). The purpose of this study was to evaluate the effect of RVP-PCR tests on duration of antibiotic use and length of stay (LOS) in hospitalized patients. Methods: We performed a retrospective chart review of patients who had a RVP-PCR ordered within a 1-year time period before and after the assay switch. Patients who were pregnant, had received antibiotics within 30 days prior to admission, were not discharged, or had not completed antibiotics by end of study period were excluded. Results: Data were obtained from a total of 140 patients (70 in each group). Of these, 25 (35.7%) in the RVP group and 28 (40.0%) in the rapid RP group had a positive result. The median LOS was 4.5 days (IQR, 3-9 days) in the RVP group and 5 days (IQR, 3-9 days) in the rapid RP group (P = .78). The median duration of antibiotic use was 4 days (IQR, 2-7 days) in the RVP group and 5 days (IQR, 1-7 days) in the rapid RP group (P = .8). Conclusion: Despite faster turnaround time, there was no significant difference in duration of antibiotic use, or LOS between the RVP and rapid RP groups.
Keywords: respiratory viral illnesses, respiratory viral panel, polymerase chain reaction, antibiotic use
Introduction
Early and accurate diagnosis of respiratory viral illnesses is important because these are among the most common reasons for hospitalization and account for a large proportion of inappropriate antibiotic use. Previous conventional methods of diagnosing viral etiologies by cultures have shown to be time-consuming and labor intensive, with poor sensitivity for detection.1 The availability of respiratory viral panels by polymerase chain reaction (RVP-PCR) has provided a reliable means of diagnosis and are rapid and sensitive compared with conventional methods.1
A small number of studies analyzed the impact of RVP-PCR testing on clinical outcomes in pediatric populations.2-4 In these studies, limited data showed a decrease in antibiotic usage and in length of stay (LOS) when compared with conventional methods of testing. While Schulert et al showed that positive results of RVP testing were associated with decreased duration of antibiotics and decreased LOS in pediatric patients,2 other studies demonstrated that RVP may not be associated with these benefits in pediatric pneumonia and cancer patients.3,4 In these studies, investigators considered a 24-hour turnaround time to be rapid for RVP-PCR testing.
Although studies that compared sensitivity and turnaround times of different RVP-PCR tests have been conducted, data comparing the clinical outcomes associated with these assays on antibiotic use and duration of hospitalization are scant.1,5 Earlier diagnosis of a viral infection with a RVP-PCR test may help in de-escalating or stopping empiric therapy to narrow-spectrum agents specific to the microorganism. This may potentially decrease costs, overall antibiotic use, antibiotic resistance, and drug-related adverse events.1,2,5,6
The purpose of this study was to examine the clinical outcomes of 2 different RVP-PCR tests: whether one is associated with a decrease in duration of antibiotic therapy and LOS.
Methods
Setting
This retrospective chart review was conducted at an 806-bed quaternary teaching hospital in Manhasset, NY, USA. The study protocol was approved by the institutional review board.
RVP-PCR Tests
The RVP-PCR molecular assays that were used for the purpose of this study are the rapid respiratory panel (rapid RP) by Biofire FilmArray and the respiratory viral panel (RVP) by Luminex xTAG.
The rapid RP is a multiplexed amplified nucleic acid test that uses a polymerase chain reaction (PCR) assay for the qualitative detection and identification of multiple respiratory viral and bacterial pathogens.7 The assay incorporates a reverse transcription-PCR amplification followed by suspension array detection, covering 20 different pathogens: influenza A (H1, H1-2009, H3) and B viruses, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), parainfluenza virus (types 1, 2, 3, 4), enterovirus/rhinovirus, coronavirus (229E, HKU1, NL63, OC43), adenovirus, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae.
The RVP is also an amplified nucleic acid test that detects 19 pathogens: influenza A (A matrix, H1, H1-2009, H3) and B viruses, RSV, hMPV, parainfluenza virus (types 1, 2, 3, 4), enterovirus/rhinovirus, coronavirus (229E, HKU1, NL63, OC43), adenovirus, and bocavirus. The 2 tests are very similar; however, there are some differences in the pathogens that are detected, the level of sensitivity of detection, and the time in which results are available.5 Babady et al showed that the rapid RP was more sensitive than the RVP and had a turnaround time much less than that of RVP.1 In our institution, the RVP typically produced results in about 24 hours while the rapid RP results are available after about 12 hours.
Patient Selection
The RVP was updated to the rapid RP at North Shore University Hospital in January 12, 2015. Patients who were 18 years of age or older and had an RVP-PCR done within a year before this period were included in the RVP group. Patients who were 18 years of age or older and were evaluated with an RVP-PCR within a year after this period were included in the rapid RP group. Patients were included regardless of receiving empiric antibiotic therapy for a respiratory infection (ie, community acquired pneumonia, health care–associated pneumonia, etc). Patients who were pregnant, had received antibiotics within 30 days prior to admission, were not discharged by end of study period, or had not completed antibiotics by end of study period were excluded.
Outcomes
Primary outcomes included LOS in days and duration of antibiotic use in days. LOS was defined as the time between RVP-PCR testing and discharge in this study. The duration of antibiotic use included any inpatient antibiotics ordered regardless of the number or class of antibiotics that were given, as well as outpatient antibiotics that were prescribed to complete a course of therapy after discharge. Secondary outcomes included administration of antibiotics prior to resulting of RVP-PCR tests.
Statistical Analysis
Data were obtained using a review of the electronic medical record. Information collected included patient demographics (age, gender), comorbidities, antibiotic(s) administered, duration of antibiotic use in days, and LOS in days. The results of the RVP-PCR tests, vital signs, and other laboratory findings (ie, white blood cells [WBC], serum creatinine, systemic inflammatory response syndrome [SIRS] criteria, blood/sputum/urine cultures, x-ray/computed tomographic [CT] scan findings, etc) were also collected.
The Mann-Whitney U test was used for continuous factors such as LOS and duration of antibiotic use. The chi-square test was used for the secondary outcome while the Fisher exact test was used for other categorical factors. Results were considered statistically significant at a P value < .05. The sample size for this study is based on feasibility and availability of resources and not a formal power calculation. We did not perform any multivariable analyses in this study. All analyses were carried out in SAS Version 9.4 (Cary, North Carolina).
Results
Data were obtained from a total of 140 patients who were included from January 2014 to January 2016, with 70 patients in each of the 2 groups. The demographic and baseline characteristics of the patients in the RVP and rapid RP groups were comparable (Table 1), except there were significantly more immunocompromised patients in the RVP group (35.7% vs 18.6%, P = .036) and a higher mean WBC count in the rapid RP group (10.3 ± 13.5 K/µL vs 12 ± 9.4 K/µL). While there was a trend toward more diabetic patients in the rapid RP group, this difference was not statistically significant (21.4% vs 34.3%, P = .13).
Table 1.
Baseline Characteristics.
| Variable | RVP (n = 70) | Rapid RP (n = 70) | P value |
|---|---|---|---|
| Male | 31 (44.3%) | 31 (44.3%) | NS |
| Age, median (IQR) | 70 (54-81) | 74 (64-84) | NS |
| Comorbidities | |||
| Immunocompromised | 25 (35.7%) | 13 (18.6%) | .036 |
| Type 2 diabetes mellitus | 15 (21.4%) | 24 (34.3%) | NS |
| Chronic kidney disease | 13 (18.6%) | 7 (12%) | NS |
| Chronic pulmonary diseasea | 25 (35.7%) | 26 (37.1%) | NS |
| WBC (K/µL) | 10.3 ± 13.5 | 12 ± 9.4 | .012 |
| T Max (°F) | 99.6 ± 1.7 | 99.2 ± 1.6 | NS |
| Heart rate | 92.9 ± 17.5 | 92.5 ± 17.6 | NS |
| Respiratory rate | 19.2 ± 2.6 | 18.6 ± 2 | NS |
| SIRSb | 33 (47.1%) | 22 (31.4%) | .08 NS |
| Antibiotics given | |||
| Penicillins | 18 (25.7%) | 17 (24.3%) | |
| Cephalosporins | 21 (30%) | 22 (31.4%) | |
| Carbapenems | 4 (5.7%) | 5 (7.1%) | |
| Aztreonam | 1 (1.4%) | 4 (5.7%) | |
| Macrolides | 17 (24.3%) | 12 (17.1%) | |
| Fluoroquinolones | 12 (17.1%) | 17 (24.3%) | |
| Vancomycin | 21 (30%) | 21 (30%) | |
| Other | 3 (4.3%) | 5 (7.1%) | |
Note. RVP = respiratory viral panel; rapid RP = rapid respiratory panel; WBC = white blood cells; T Max = maximum temperature; SIRS = systemic inflammatory response syndrome.
Chronic pulmonary diseases include patients with chronic obstructive pulmonary disease, asthma, and/or bronchiectasis.
Each factor in SIRS was recorded within 24 hours of respiratory viral panel polymerase chain reaction result.
Of the 140 patients, 25 out of 70 patients (35.7%) in the RVP group and 28 out of 70 patients (40.0%) in the rapid RP group had a positive result. The most frequently detected viral pathogen was enterovirus/rhinovirus followed by influenza A, RSV, and parainfluenza virus (Table 2). Both tests are unable to differentiate between enterovirus and rhinovirus due to the cross-reactivity between the 2 viruses. M pneumoniae was detected in 1 patient in the rapid RP group who was given antibiotics prior to RVP-PCR result and was continued on an appropriate regimen.
Table 2.
Pathogens Detected Among Patients With a Positive Respiratory Viral Panel Polymerase Chain Reaction Test.
| Pathogena | RVP (n = 25) | Rapid RP (n = 28) |
|---|---|---|
| Entero/rhinovirusb | 10 (40%) | 12 (42.9%) |
| Influenza A | 8 (32%) | 4 (14.3%) |
| Respiratory syncytial virus | 1 (4%) | 5 (17.9%) |
| Parainfluenza | 1 (4%) | 4 (14.3%) |
| Influenza B | 3 (12% | 0 (0%) |
| Other viral pathogensc | 1 (4%) | 2 (7.1%) |
| Mycoplasma pneumoniae | 0 (0%) | 1 (3.6%) |
Note. RVP = respiratory viral panel; rapid RP = rapid respiratory panel.
Each comparison was not statistically significant.
Both tests were not able to differentiate between enterovirus and rhinovirus due to the cross-reactivity between the 2 viruses.
Other viral pathogens included adenovirus, human metapneumovirus, and coronavirus OC43.
Diagnostic tests such as radiologic findings and cultures are shown in Table 3. Out of the 140 patients included in the study, about 86% and 46% of the patients had chest x-ray and CT results available, respectively. While some of these tests were questionable for pneumonia, only 6% of the available x-rays and 31% of the CT scans were positive for pneumonia (defined as an infiltrate or consolidation suggesting pneumonia as reported by the radiologist). Blood culture results were only available in 77% of all the patients, with about 3% of these results being positive. Overall, there were no differences in these diagnostic tests.
Table 3.
Available Radiologic Findings and Cultures (Cx).
| Diagnostic testa | RVP (n = 70) | Rapid RP (n = 70) |
|---|---|---|
| Chest x-rayb | 62 (88.6%) | 58 (82.9%) |
| Positive | 4 (6.5%) | 3 (5.2%) |
| Questionable | 7 (11.3%) | 13 (22.4%) |
| Chest CTb | 30 (42.9%) | 34 (28.6%) |
| Positive | 13 (18.6%) | 7 (12%) |
| Questionable | 25 (35.7%) | 26 (37.1%) |
| Blood Cx | 58 (82.6%) | 50 (71.4%) |
| Positive | 1 (1.7%) | 2 (4%) |
| Urine Cx | 39 (55.7%) | 27 (38.6) |
| Positive | 4 (10.3%) | 3 (11.1%) |
| Sputum Cx | 6 (8.6%) | 6 (8.6%) |
| Positive | 0 (0%) | 1 (16.7%) |
| BAL | 1 (1.4%) | 1 (1.4%) |
| Positive | 0 (0%) | 1 (100%) |
| Legionella antigen | 3 (4.3%) | 12 (17.1%) |
| Positive | 0 (0%) | 0 (0%) |
Note. RVP = respiratory viral panel; rapid RP = rapid respiratory panel; CT = computed tomography; BAL = bronchoalveolar lavage.
Each comparison was not statistically significant.
Testing for pneumonia.
Outcomes
The median LOS was 4.5 days (IQR, 3-9 days) in the RVP group and 5 days (IQR, 3-9 days) in the rapid RP group (P = .78). The median duration of antibiotic use was 4 days (IQR, 2-7 days) in the RVP group and 5 days (IQR, 1-7 days) in the rapid RP group with no significant difference (P = .8). In terms of the secondary outcome, 45.7% of patients in the RVP group received antibiotics before RVP-PCR test resulted, whereas it was 50% in the rapid RP group (P = .61). Twenty-three percent of patients in the RVP group and 27.1% in the rapid RP did not receive any antibiotics throughout the hospitalization.
A subanalysis was performed on duration of antibiotic use, specifically among patients who had a positive RVP-PCR result (Figure 1). The median duration of antibiotic use was 5 days in the RVP group and 2 days in the rapid RP group (P = .13).
Figure 1.
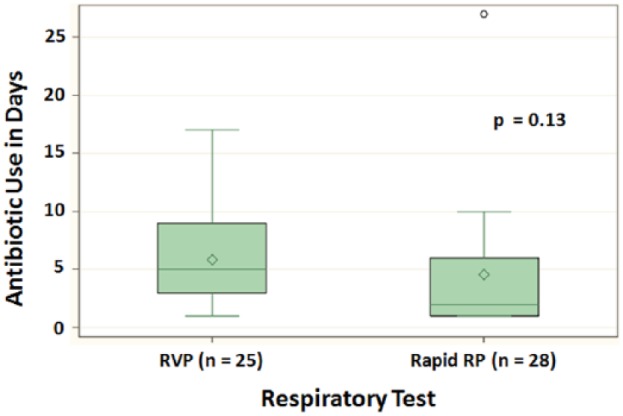
Duration of antibiotic use among patients with positive respiratory viral panel polymerase chain reaction results.
Note. RVP = respiratory viral panel; rapid RP = rapid respiratory panel.
Discussion
Previous studies have shown that rapid viral testing had a clinical impact in decreasing antibiotic use and hospital LOS in certain subgroups of patients.1,2,5,6 Although we were not able to detect a significant difference in duration of antibiotic use and LOS, it is likely that this outcome is due to differences seen in the current study compared with previous studies.
Our group looked at 2 different adult populations in 2 different time frames, with a unique RVP-PCR test used in each group. Babady et al looked at 1 set of pediatric patients with 2 RVP-PCR tests in 1 time frame, while Schulert et al focused on various subgroups within 1 pediatric population and 1 RVP-PCR test.1,3 In the latter study, the investigators found an association between positive results of RVP-PCR and LOS was dependent on patient’s admission service (particularly hematology/oncology).2,3
Also, some differences between our adult population and the previously studied pediatric populations may be contributing factors to our results. When compared with adults, children may generally be more susceptible to respiratory viral illnesses due to reduced innate and adaptive immune responses.8 Also, while up to 80% of respiratory infections may be due to viral pathogens in children, viruses may be implicated in 13% to 31% in elderly patients.8,9 In terms of RVP-PCR sensitivity, Popowitch et al studied both RVP and rapid RP in different age groups and found that sensitivities of these tests were significantly different in the <18-year and ≥18-year age groups.5 In our study, because the majority of patients were aged 54 to 84 years, it is likely that the individuals in our population may have responded differently than pediatric patients in other studies and were also less likely to have an infection of viral origin.
In our subanalysis of patients who tested positive with RVP (n = 25, 35.7%) and rapid RP (n = 28, 40%), we found a trend toward shorter duration of antibiotic use in patients who tested positive with the rapid RP; however, this difference was not considered significant when compared with that of the RVP group. Overall, we had a smaller subgroup of patients who had a positive RVP-PCR result compared with previous studies which had >50% of patients with positive RVP-PCR results in larger patient populations.1-3 Although Schulert et al lacked sufficient evidence to claim association between positive RVP and LOS, they found that positive RVP-PCR was associated with shorter duration of intravenous antibiotic use, in certain groups of patients and those with some common respiratory diagnoses.2
In our secondary outcome, we found that only about 48% of patients received antibiotics before the RVP-PCR tests resulted. Interestingly, we also found that about 25% of patients did not receive any antibiotic agents throughout their hospitalization despite testing with an RVP-PCR. Because severity of diagnoses was not captured in our study, it is possible that treatment of less severe respiratory conditions influenced these values. In a study that focused on children with severe pneumonia, the percentage of children receiving parenteral antibiotics were 96.1% and 89.2% in 2 groups.3
In this study, it is likely that we did not have an adequate sample size to find a difference, because we also did not formally conduct a power calculation. A larger sample size associated with a power calculation may be appropriate for future studies. Also, it is possible that a difference of 12 hours between the RVP and rapid RP may be too short to demonstrate a clinical significance.
In conclusion, despite faster turnaround time, there was no significant difference in duration of antibiotic use, or LOS between the RVP and rapid RP groups. Small sample size, positivity of RVP-PCR tests, a small difference of 12 hours between turnaround times, and confounding variables may have contributed to this result.
Acknowledgments
We would like to thank Cristina P. Sison, PhD, and Joanna Stein Fishbein, MPH, from the department of Biostatistics at the Feinstein Institute for Medical Research, and Roberta Shiavone, RN, BS, CIC, from the Department of Infection Control at North Shore University Hospital, for their participation in data collection and analysis.
Footnotes
Authors’ Note: All authors demonstrated adherence to ethics and reporting requirements. Sebastian Choi is currently affiliated with LECOM Senior Living Center, Erie, PA, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Babady NE, Mead P, Stiles J, et al. Comparison of the Luminex xTAG RVP fast assay and the Idaho Technology FilmArray RP assay for detection of respiratory viruses in pediatric patients at a cancer hospital. J Clin Microbiol. 2012;50:2282-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schulert GS, Lu Z, Wingo T, et al. Role of a respiratory viral panel in the clinical management of pediatric inpatients. Pediatr Infect Dis J. 2013;32(5):467-472. [DOI] [PubMed] [Google Scholar]
- 3. Schulert GS, Hain PD, Williams DJ. Utilization of viral molecular diagnostics among children hospitalized with community acquired pneumonia. Hosp Pediatr. 2014;4(6):372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen N, Palomino P, Gedrimaite Z, et al. Can the respiratory viral panel reduce antibiotic use in hospitalized pediatric patients with cancer? Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) September 2014;Washington, DC. Abstract G-304. [Google Scholar]
- 5. Popowitch EB, O’Neill SS, Miller MB. Comparison of the Biofire FilmArray RP, Genmark eSensor RVP, Luminex xTAG RVPv1, and Luminex xTAG RVP fast multiplex assays for detection of respiratory viruses. J Clin Microbiol. 2013;51(5):1528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woo P, Chiu SS, Seto WH, et al. Cost-effectiveness of rapid diagnosis of viral respiratory tract infections in pediatric patients. J Clin Microbiol. 1997;35(6):1579-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. North Shore LIJ Laboratories. Test: respiratory virus panel has been updated to the rapid respiratory panel by PCR assay by Biofire FilmArray. New Hyde Park, NY: 2015. [Google Scholar]
- 8. Tregoning JS, Jurgen S. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. J Clin Microbiol. 2010;23:74-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 2010;50(5):747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]


