Abstract
Snakebite envenomation is an important global health concern. The current standard treatment approach for snakebite envenomation relies on antibody-based antisera, which are expensive, not universally available, and can lead to adverse physiological effects. Phage display techniques offer a powerful tool for the selection of phage-expressed peptides, which can bind with high specificity and affinity towards venom components. In this research, the amino acid sequences of Phospholipase A2 (PLA2) from multiple cottonmouth species were analyzed, and a consensus peptide synthesized. Three phage display libraries were panned against this consensus peptide, crosslinked to capillary tubes, followed by a modified surface panning procedure. This high throughput selection method identified four phage clones with anti-PLA2 activity against Western cottonmouth venom, and the amino acid sequences of the displayed peptides were identified. This is the first report identifying short peptide sequences capable of inhibiting PLA2 activity of Western cottonmouth venom in vitro, using a phage display technique. Additionally, this report utilizes synthetic panning targets, designed using venom proteomic data, to mimic epitope regions. M13 phages displaying circular 7-mer or linear 12-mer peptides with antivenom activity may offer a novel alternative to traditional antibody-based therapy.
Keywords: Antivenom, phage display, M13 phage, phospholipase A2, Agkistrodon piscivorus, species, in silico design, Western cottonmouth
Introduction
Snakebite envenomation is a global health concern and classified as a neglected tropical disease by the World Health Organization (WHO, 2010; Chippaux, 2017). Approximately 5.4 million people are victims of snakebite worldwide each year, with 81,000-138,000 deaths and over 400,000 suffering from long-term disability, constant wound care, amputations and ongoing psychological morbidity. Nearly 9,000 cases of snakebite envenomation are reported in the United States and Canada every year (Smith et al, 2014; Mowry et al, 2013).
Venoms are extremely complex, consisting of hundreds of proteins, peptides, enzymes and non-enzymatic toxins (Hall et al, 2016). Through advancements in whole genome studies, venomics has evolved to assess toxin composition directly and indirectly via proteome- or transcriptome-based methodologies. This research has shown that venom composition varies between and within species due to ontogenetic and geographical variability, and this complexity of venom has led to inconsistent antivenom potency. For example, the same antivenom produced in different geographic regions of India exhibits different therapeutic potential, indicating that the current antivenom manufacturing techniques do not adequately account for the diverse venom composition (Warrell et al, 2013). Restricted availability of species-specific antivenoms can be circumvented by designing and producing high potency multipurpose antivenoms effective against multiple species of snakes (Calvete et al, 2009). Antibody-based antivenoms are routinely prepared from the plasma of host animals immunized with venoms from multiple snake species. While this is a successful therapeutic approach for some snake species, there remain limitations in safety, efficacy, and economic aspects of manufacturing. Immediate hypersensitivity reactions following administration of Crotalidae polyvalent immune Fab antivenom, including urticaria, dyspnea, hypotension, tachycardia, chest pain, and nausea may occur in 19% of patients, and the range of serum sickness incidence including fever, myalgia, epigastric pressure, and arthralgia is 6% - 23% (Schaeffer et al, 2012). In the United States the most typical antivenom therapy using Crotalidae polyvalent immune Fab could cost more than $18,000 and its usage can be a financial burden for patients and hospitals (Weant et al, 2010). Thus, there is a need for a novel approach to develop antivenom solutions that are capable of rapid production and maximal efficacy without adverse reactions. Phage display techniques offer a comprehensive tool for the selection of phage-expressed peptides which have affinity for unique targets normally inaccessible to antibody-based methods (Wu et al, 2016). Hence, a phage display technique using composite consensus targets based on venom proteomic data could lead to the development of a broad spectrum inhibitor of multiple venoms.
To demonstrate proof-of-concept of phage display for the development of venom component inhibitor, the venom of the Western cottonmouth, Agkistrodon piscivorus leucostoma, a venomous New World pit viper found in North and Central America (Lomonte et al, 2014), was selected. Western cottonmouths inhabit a large geographic area and are found along the coast of the Gulf of Mexico into southeastern and central Texas, presenting a concern to civilian populations, as well as military operational and training sites across this region. Major components of the Western cottonmouth venom are phospholipases A2 (PLA2, 43.2%), metalloproteases (26.1%) and serine proteases (10.1%) (Lomonte et al, 2014). The clinical effects of envenomation are the initiation of the coagulation cascade and blockage of neuromuscular transmission. Snake venom PLA2 enzymes interfere in the normal physiological processes of victim animals and induce a wide variety of pharmacological effects including neurotoxicity (Harris and Scott-Davey 2013), myotoxicity (Teixeira et al, 2003), and anticoagulant effects (Saikia et al, 2013).
In this study, conserved PLA2 sequences from several species of cottonmouth snakes were identified and mapped to the three-dimensional (3D) crystal structure of PLA2 to determine their solvent accessibility. Phage-displayed peptides with affinity towards the target consensus sequence were tested for the generation of potential venom inhibitors.
Materials and Methods
Designing consensus sequence
Queries against the UniProt database were used to gather amino acid sequences of target peptides. Sequences were uploaded to Basic Local Alignment Search Tool (BLAST) to determine homologous regions using default parameters. Consensus peptides were designed based on areas of homology, and areas corresponding to active sites were given priority during the design process. The original sequences were aligned to the consensus sequence to ensure homology and identity. Once the validity of the consensus sequence was confirmed, the sequence was overlaid onto a crystal structure of the target protein. Crystal structure information was unavailable for Western cottonmouth PLA2, thus Eastern cottonmouth PLA2 (PBD: 1PPA) was used to map consensus sequence. JSmol Viewer, an open-source Java viewer, from Research Collaboratory for Structural Bioinformatics Protein Data Bank (Berman et al, 2002) was used to visualize the 3D mapping of the solvent exposed regions of the target peptide.
Screening of peptides with affinity to the consensus sequence
A cleavable crosslinker, Sulfo-LC-SPDP (sulfosuccinimidyl 6-[3’-(2-pyridyldithio)propionamido]hexanoate) was used to bind the target peptide to the interior of an aminosilylated glass capillary tube. Briefly, a glass capillary tube was washed with acetone and treated with aminosilane reagent. The silylated glass capillary tube was then modified with 10 mM Sulfo-LC-SPDP. After one hour incubation, the crosslinked capillary tube was rinsed twice with Coupling Buffer (50mM Phosphate, 150mM NaCl, 10mM EDTA, pH 7.2). The Sulfo-LC-SPDP modified capillary tube was filled with 5 μl of the target PLA2 consensus peptide (10mg/ml) and incubated overnight at 4oC to complete the crosslinking reaction. Phages were panned against target peptide using a modified panning method (Fralick, Chadha-Mohanty, and Li 2008). Briefly, a Marprene 0.5mm bore 1.6mm wall tubing (Watson-Marlow, Inc., Wilmington, MA) circuit containing a 10μl capillary tube with crosslinked peptide was inserted into a peristaltic pump (Heidolph North America, Elk Grove Village, IL). The tubing circuit was blocked for one hour with Blocking Buffer [100mM NaHCO3, (pH 8.6), 5mg/ml bovine serum albumin (BSA)], then rinsed six times with 1x TBST [TBS + 0.1% (v/v) Tween-20]. Phage libraries (PhD -7, -12, -C7C Phage Display Peptide Library Kits, New England Biolabs, Ipswich, MA) were diluted to a working concentration of 1x1011 PFU/ml in 1x TBST, added to the circuit, and slowly circulated at a speed of <5 rpm for one hour at room temperature (RT). The tubing circuit was rinsed ten times with 1x TBST to facilitate removal of non-binding phages. After siphoning off non-binding phages, phages with high affinity to the target peptide were eluted using either Elution Buffer (25mM Dithiothreitol, pH 8.5) at 37oC for Ph.D.-7 and Ph.D.-12 libraries or Acidic Elution Buffer [200mM Glycine-HCl (pH 2.2), 1mg/ml BSA] at RT for Ph.D.-C7C phage library. Eluted high-affinity phages were collected in Eppendorf tubes and the Ph.D.-C7C eluent was neutralized using 1M Tris-HCl (pH 9.1). The eluate was diluted 1:1000 in phosphate buffered saline (PBS), then serially diluted and titered out for individual plaques on soft agar plates. An aliquot of the primary panning eluate was amplified for a new population of M13 phages up to 1x1012-13 PFU/ml for secondary panning. A second round of panning was carried out with a more stringent wash step (5x TBST). Individual phage clones from the secondary panning elute were selected on soft agar plates and amplified as before up to 1x1012-13 PFU/ml. These clones were picked separately and grown up individually for PLA2 inhibition assay. To monitor the accuracy of panning process, the polyclonal phage isolates from the primary panning were mixed with venom at varying concentrations (3.2x1012 PFU/ml and 8x 1011 PFU/ml) and their anti-PLA2 activity was measured.
Phage harvesting and quantification
Phages were harvested and quantified according to the manufacturer’s protocol. Briefly, phages were harvested using a polyethylene glycol precipitation method (Yu and Smith, 1996) and resuspended in PBS (pH 7.5). Collected phages were titered on isopropyl β-D-1-thiogalactopyranoside/5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (50mM IPTG/40mM X-Gal) Luria-Bertani medium. Phage clones were individually picked from soft agar titer plates and amplified based on the manufacturer’s protocol for further testing.
Venoms
Snake venoms [Western cottonmouth, Eastern diamondback rattlesnake (Crotalus adamanteus), Western diamondback rattlesnake (Crotalus atrox), Mojave rattlesnake (Crotalus scutulatus), and broad-banded copperhead (Agkistrodon contortrix laticinctus)], were obtained in lyophilized form from the National Natural Toxins Research Center (Kingsville, TX). Venoms were reconstituted at a concentration of 10mg/ml in PBS (pH 7.5) and diluted to working concentrations.
PLA2 Activity Assay
PLA2 activity was measured using the EnzChek Phospholipase A2 Assay kit (Invitrogen, Eugene, OR) with modification. Briefly, the concentration of Western cottonmouth venom was optimized to be 4.88 mg/ml for the linearity of fluorescence signal from the plate reader. The selected phage clones were diluted to 1x 1012 PFU/ml and incubated with Western cottonmouth venom for 30min at RT. A non-specific M13 phage clone was used as a negative control. After adding reaction substrate to phage and venom mixture, fluorescence (excitation at 485nm and emission at 528nm) was measured for 20min at one minute intervals to monitor the reaction. The overall PLA2 activity was defined by the fluorescence level at the 10min time point.
Cross-species Inhibition
The inhibitory effect of monoclonal phage isolates on venoms beyond Western cottonmouth was determined using the EnzChek Phospholipase A2 Assay kit with modification. Venom from the Western cottonmouth, Eastern diamondback rattlesnake, Western diamondback rattlesnake, Mojave rattlesnake, and broad-banded copperhead, was diluted to a working concentration of 4.88μg/ml. Diluted venom was incubated with 1x1012 PFU/ml phage clones for 30min at RT. A non-specific M13 phage clone was used as a negative control. After adding reaction substrate to phage and venom mixture, fluorescence (excitation at 485nm and emission at 528nm) was measured for 20min at one minute intervals to monitor the reaction. The overall PLA2 activity was defined by the fluorescence level at the 10min time point.
DNA Sequencing
The double-stranded DNA (dsDNA) replicative form (RF) isolates of monoclonal phage from single plaque-infected ER2738 cultures were extracted using the QIAprep Spin Miniprep Kit (Qiagen, Germantown, MD) following manufacturer’s directions. Extracted dsDNA RFs were sequenced upstream of the peptide insertion using the -96 gIII sequencing primer supplied with the Ph.D. Phage Display Peptide Library Kit. DNA sequences were translated to amino acid sequences using the ExPASy Translate tool from the Swiss Institute of Bioinformatics.
Statistical Analysis
All experiments were conducted in triplicate and each fluorescence reading from the PLA2 reaction mixture was measured from three separate wells. The means and standard deviations were calculated from relative fluorescence units. Student’s t-test was used to determine the level of significance (p <0.05).
Results
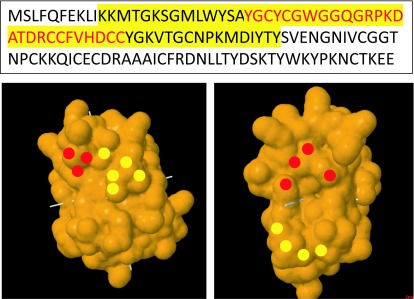
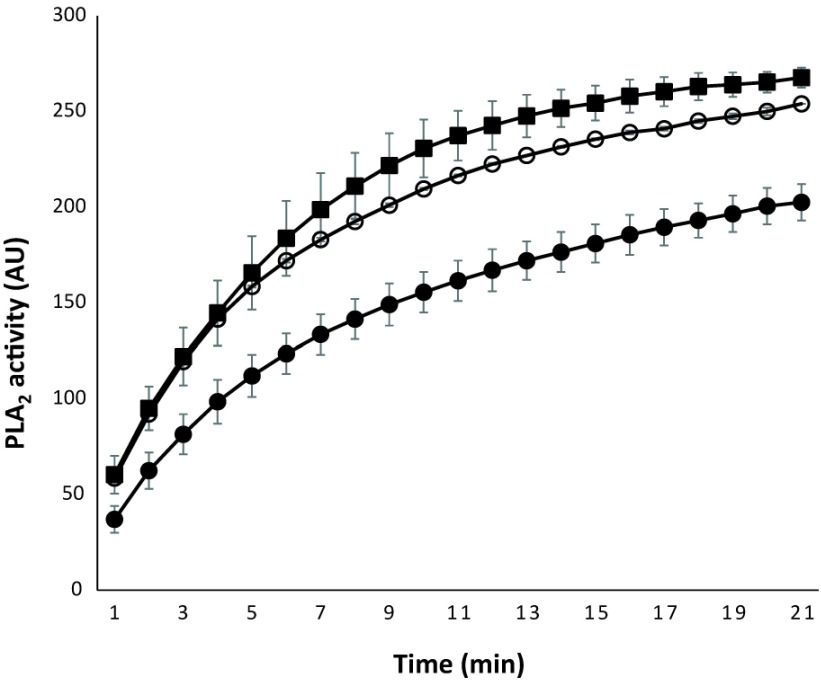
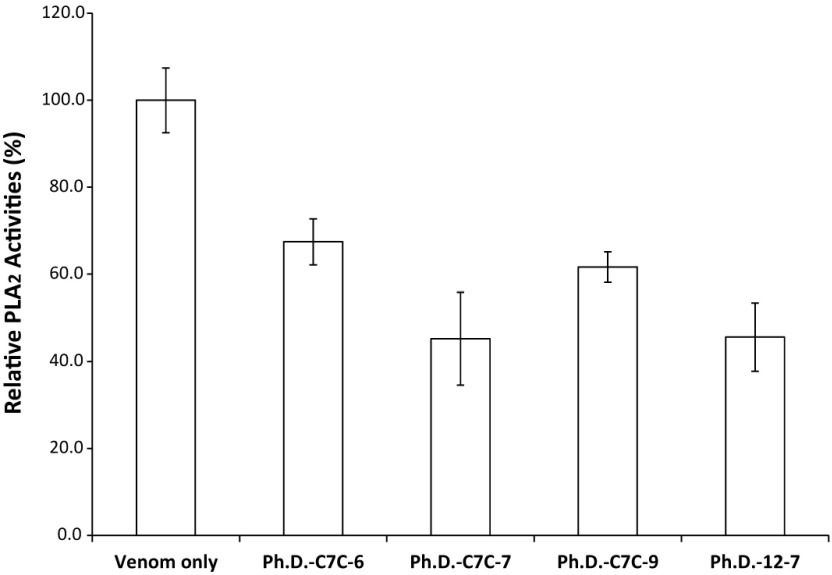
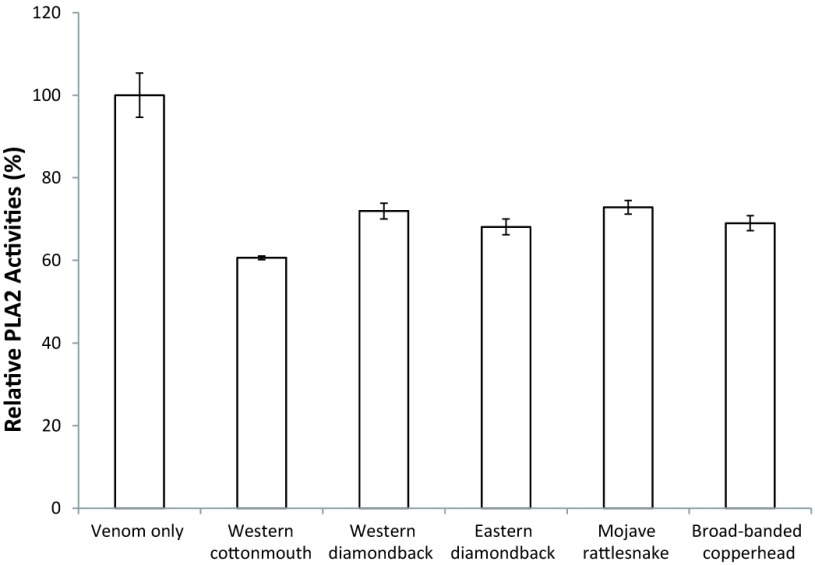
The BLAST report identified a region of 120 amino acids with high consensus. A section of 57 amino acids annotated as the active (yellow highlight) and metal binding site (red characters) for PLA2 (Figure 1a) is shown. The section corresponding to the active site was matched to the predicted crystal structure of PLA2 (PDB: 1PPA) from Eastern cottonmouth venom, and the surface-exposed residues were annotated (Figure 1b). The consensus peptide used as the panning target shares ≥95% homology with the major North American Crotalid (rattlesnakes, copperheads and cottonmouths) PLA2 proteins. During the panning process, polyclonal phage isolates from the primary panning process showed PLA2 inhibition activity in a concentration-dependent manner (Figure 2). Two different concentrations of polyclonal anti-PLA2 isolates (3.2x1012 PFU/ml and 8x1011 PFU/ml) were incubated with Western cottonmouth venom, inhibiting PLA2 activity by 55% and 15% of uninhibited venom. Since phages from the primary panning showed inhibition of PLA2 activity, the secondary panning was conducted with these eluted phages in more stringent conditions (5x TBST). After the second round of phage panning, the eluent was amplified and tested against Western cottonmouth venom again. Four individual phage clones were selected from polyclonal mixture based on their anti-PLA2 activity (Figure 3). These clones inhibited 30% to 60% of PLA2 activity from Western cottonmouth venom. Phage DNA sequencing from the selected monoclonal isolates identified four unique sequences and their corresponding peptide sequences (Table 1). Three of the sequenced isolates were from the Ph.D.-C7C library, showing three circular 7-mer motifs (Ph.D.-C7C-6, Ph.D.-C7C-7, and Ph.D.-C7C-9). The fourth was from the Ph.D.-12 library with a linear 12-mer peptide (Ph.D.-12-7). Cross-species anti-PLA2 activity was tested against five major snake venoms in North America (Western cottonmouth, Eastern diamondback rattlesnake, Western diamondback rattlesnake, Mojave rattlesnake, and broad-banded copperhead) using one of selected anti-PLA2 clones (Ph.D.-12-7). Results showed approximately 40% inhibition in Western cottonmouth venom and 30% inhibition in the other crotalid venoms (Figure 4).
Figure 1.
In silico design of consensus peptide from Cottonmouth PLA2. A. Homology sequence of PLA2 from cottonmouth species. This sequence corresponds to the homologous sequence between three PLA2 proteins from cottonmouth species. The yellow section corresponds to the active site and was synthesized as the target molecule for panning. Characters in red are the metal binding site, based on BLAST annotations. B. Crystal Structure of A. p. leucostoma PLA2. The front and 90° counterclockwise rotated views of PLA2 are shown. Colored circles annotate shared solvent-exposed residues with the synthesized homologous sequence (red: metal binding site, yellow: active site).
Figure 2.
activity of polyclonal M13 phages after the primary panning. Two different concentration of polyclonal anti-PLA2 M13 phages after the primary panning inhibited 55% and 15% of PLA2 activity of Western cottonmouth venom after 30min incubation. [◾: venom only (4.88μg/ml); ○: venom with 8x1011 PFU/ml of anti-PLA2 phages; •: venom with 3.2x1012 PFU/ml of anti-PLA2 phages.]
Figure 3.
Anti-PLA2 activities of selected M13 phage clones. Incubation with selected phage clones isolated from phage display libraries inhibited the PLA2 activity of A. p. leucostoma venom by 30-60% during the first 10min. Phage clones were incubated with 4.88μg/ml of venom for 30min.
Table 1.
Nucleotide sequences and corresponding peptide sequences of selected monoclonal phage binding motifs.
| Monoclonal Isolate | Nucleotide Sequence | Peptide Sequence |
|---|---|---|
| Ph.D.-C7C-6 | TCGCCGTTGCATAAGACTATG | SPLHKTM |
| Ph.D.-C7C-7 | TCGGGGATGAAGAAGACGAAG | SGMKKTK |
| Ph.D.-C7C-9 | AAGACGACGAAGATGGGGTTG | KTTKMGL |
| Ph.D.-12-7 | AAGCTTATTCATGGTAATGGTGTTATGGATGAGGGG | KLIHGNGVMDEG |
Figure 4.
Cross-species anti-PLA2 activity of Ph.D.-12-7 phages against five major snake venoms in North America. Cross-species anti-PLA2 activity was tested against five major snake venoms in North America (Western cottonmouth, Eastern diamondback rattlesnake, Western diamondback rattlesnake, Mojave rattlesnake, and broad-banded copperhead) using one of selected anti-PLA2 clones (Ph.D.-12-7). Approximately 40% inhibition in Western cottonmouth venom and 30% inhibition in the other crotalid venoms were observed.
Discussion
A consensus peptide, based on sequence homologous of PLA2 in cottonmouth species venom, proved to be a viable target for the selection of anti-PLA2 peptides displayed on M13 phages. Utilizing venom proteomic data, epitope design could be optimized in silico to produce a consensus antigenic determinant. This in silico approach could enhance the effective selection from a phage display library by targeting areas closely related to the venom activity, in contrast to serum-based methods that produce 5% - 36% therapeutically relevant antibodies (Laustsen et al, 2017). Successful selection of anti-PLA2 display phages which can inhibit a major component of Western cottonmouth venom, PLA2, could open the door to targeting other venom components, including metalloprotease or serine protease, utilizing this approach. Selection of antivenom phages was completed within four months, including design and synthesis of a novel consensus peptide, panning several Ph.D. libraries, and characterizing inhibitory effects.
While the observed highest inhibitory rate was 60%, a synergistic inhibitory effect could be expected after combining all the selected anti-PLA2 phages. Because four unique sequences of selected peptides displayed on M13 phages would target different regions of the PLA2 active sites, a more comprehensive inhibitory effect could be observed. Expansion of target venom components is currently underway and the design of consensus peptides for both metalloprotease and serine protease is being formulated. Once inhibitory phages for the three major venom components have been identified, a phage cocktail will be assembled and its synergetic effect will be tested against whole venom in an animal model to confirm the efficacy in practical application. In addition to screening individual venom components in Western cottonmouth venom, the universal consensus sequences of six other venoms from the Crotalidae subfamily have been identified for screening, incorporating features of all seven Crotalidae snake venoms.
Antivenom development and production towards the venom of small animals (eg. scorpion and spider) is hindered by the limited amounts of venom available for collection (Laustsen et al, 2016). In this study, only 5 μl of consensus venom peptide (10 mg/ml) was needed for the panning process. In silico design and synthesis of consensus peptides could provide an alternative solution to the limitation of venom availability. Design of in silico peptides could utilize transcriptome annotations to identify potential epitopes of unknown or punitive toxins. In addition, the design of consensus peptides could be based on shared homology across several genera or species. A future therapeutic product developed from multiple composite consensus peptides could show general effectiveness across venom components from several snake species in the geographic region. This design methodology could be expanded to include target molecules beyond proteins like carbohydrates and metals that are integral to venom components.
Conclusions
To the best of our knowledge, this is the first report identifying short peptide sequences expressed on M13 phages that can inhibit PLA2 activity of Western cottonmouth venom in vitro. The phage display methods conducted in this study could be implemented for the development of novel antivenom. Using BLAST search tool and subsequent categorization of venom sequences, the consensus target peptides of multiple peptide families could be systematically selected and used for the development of a universal antivenom covering snakes, reptiles, arachnids, sea jellies, or other venomous species. Future work is needed to investigate the synergetic effects of selected phages on target venom components.
Disclaimer
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. This work was supported and funded by the Naval Medical Research Center's In-house Laboratory Independent Research program using work unit number G1606. The authors are military service members or contract employees of the United States Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties. Approved for public release; distribution unlimited.
List of Abbreviations
- 3D:
Three-dimensional
- BLAST:
Basic Local Alignment Search Tool
- IPTG:
Isopropyl β-D-1-thiogalactopyranoside
- PBS:
Phosphate buffered saline
- PFU:
Plaque-forming unit
- PLA2:
Phospholipase A2
- RF:
Replicative form
- RT:
Room temperature
- Sulfo-LC-SPDP:
Sulfosuccinimidyl 6-[3’-(2-pyridyldithio)propionamido]hexanoate
- TBS:
Tris-buffered saline
- TBST:
TBS with 0.1% (v/v) Tween-20
- X-Gal:
5-bromo-4-chloro-3-indolyl β-D-galactopyranoside
Competing Interests
One patent is pending on the selected phages with antivenom activity and their displayed peptides. No additional competing interests were declared by the authors.
References
- Berman HM, Battistuz T, Bhat TN, et al. The Protein Data Bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Sanz L, Angulo Y, Lomonte B, Gutierrez JM. Venoms, venomics, antivenomics. FEBS Lett. 2009;583:1736–1743. doi: 10.1016/j.febslet.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Chippaux J-P. Snakebite envenomation turns again into a neglected tropical disease. J Venomous Animals Toxins Incld Trop Dis. 2017;23:38. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralick J, Chadha-Mohanty P, Li G. Phage Display and Its Application for the Detection and Therapeutic Intervention of Biological Threat Agents. In: Kendall R, Presley S, Austing G, Smith P, editors. Advances in Biological and Chemical Terrorism Countermeasures. Boca Raton: CRC Press; FL, USA: 2008. pp. 179–202. (Ed) [Google Scholar]
- Hall A, Papacostas N, Gauthier G, et al. Bites, Stings, and Envenomation - Guide providers in the evaluation and treatment of patients with bites and stings from animal life with focus on envenomation. Joint Trauma System Clinical Practice Guideline. 2016:1–12. [Google Scholar]
- Harris JB, Scott-Davey T. Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins (Basel) 2013;5:2533–2571. doi: 10.3390/toxins5122533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen AH, Johansen KH, Engmark M, Andersen MR. Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical disease. PLoS Negl Trop Dis. 2017;11:e0005361. doi: 10.1371/journal.pntd.0005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen AH, Sola M, Jappe EC, Oscoz S, Lauridsen LP, Engmark M. Biotechnological Trends in Spider and Scorpion Antivenom Development. Toxins (Basel) 2016;8:226. doi: 10.3390/toxins8080226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte B, Tsai WC, Urena-Diaz JM, et al. Venomics of New World pit vipers: genus-wide comparisons of venom proteomes across Agkistrodon. J Proteomics. 2014;96:103–116. doi: 10.1016/j.jprot.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry JB, Spyker DA, Cantilena LR, Jr, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila) 2013;51:949–1229. doi: 10.3109/15563650.2013.863906. [DOI] [PubMed] [Google Scholar]
- Saikia D, Majumdar S, Mukherjee AK. Mechanism of in vivo anticoagulant and haemolytic activity by a neutral phospholipase A(2) purified from Daboia russelii russelii venom: correlation with clinical manifestations in Russell's Viper envenomed patients. Toxicon. 2013;76:291–300. doi: 10.1016/j.toxicon.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Schaeffer TH, Khatri V, Reifler LM, Lavonas EJ. Incidence of immediate hypersensitivity reaction and serum sickness following administration of Crotalidae polyvalent immune Fab antivenom: a meta-analysis. Acad Emerg Med. 2012;19:121–131. doi: 10.1111/j.1553-2712.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- Smith S, Sammons SS, Carr J, King TR, Ambrose HS, Zimmet L, Repasky TM. Bedside management considerations in the treatment of pit viper envenomation. J Emerg Nurs. 2014;40:537–545. doi: 10.1016/j.jen.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Teixeira CF, Landucci EC, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42:947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Warrell DA, Gutierrez JM, Calvete JJ, Williams D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J Med Res. 2013;138:38–59. [PMC free article] [PubMed] [Google Scholar]
- Weant KA, Johnson PN, Bowers RC, Armitstead JA. Evidence-based, multidisciplinary approach to the development of a crotalidae polyvalent antivenin (CroFab) protocol at a university hospital. Ann Pharmacother. 2010;44:447–455. doi: 10.1345/aph.1M527. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) "WHO guidelines for the production, control and regulation of snake antivenom immunoglobulins.". WHO Press; Geneva, Switzerland: 2010. pp. 8–9. [Google Scholar]
- Wu CH, Liu IJ, Lu RM, Wu HC. Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci. 2016;23:8. doi: 10.1186/s12929-016-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Smith GP. Affinity maturation of phage-displayed peptide ligands. Methods Enzymol. 1996;267:3–27. doi: 10.1016/s0076-6879(96)67003-7. [DOI] [PubMed] [Google Scholar]