Abstract
Single-agent sunitinib, an oral small molecule inhibitor of multiple tyrosine kinase receptors, was evaluated for treatment of patients with recurrent glioblastoma (GB) and anaplastic astrocytoma (AA). Fourteen AA and 16 GB patients, all previously treated with surgery, radiotherapy, and temozolomide, were enrolled in a prospective phase II study at either first or second relapse. Patients were treated with daily sunitinib for 4 consecutive weeks, followed by a 2-week break. For AA patients, the most common side effects were fatigue (86 %), diarrhea (43 %), hand-foot syndrome (36 %), neutropenia (36 %), thrombocytopenia (36 %), and nausea (29 %). In the GB cohort, the most common side effects were fatigue (56 %), diarrhea (44 %), neutropenia (31 %), and thrombocytopenia (25 %). Six of 14 (43 %) AA and 5 of 16 (31 %) GB patients experienced grade 3 or greater toxicities. Five patients discontinued study due to drug toxicities. There were no partial or complete responses in either cohort; 8/14 (57 %) AA and 5/16 (31 %) GB patients had stable disease at the first planned assessment. Progression-free survival at 6 months was 21.5 % (AA) and 16.7 % (GB). Median overall survival was 12.1 months (AA) and 12.6 months (GB). These results are comparable to those reported in the literature in patients treated with standard cytotoxic therapies. This is the largest reported trial of sunitinib in recurrent malignant astrocytic gliomas to date, as well as contains the largest AA cohort. Nonetheless, sunitinib did not demonstrate significant anti-glioma activity in patients with recurrent malignant astrocytic gliomas.
Keywords: Sunitinib, Anaplastic astrocytoma, Glioblastoma, Recurrent malignant glioma, Prospective trial
Introduction
Patients with malignant astrocytic gliomas (MAG) generally have poor survival. Median overall survival (OS) for patients with glioblastoma (GB) is ~14.6 months and ~2.5 years for patients with anaplastic astrocytomas (AA) [1, 2]. The recurrence rate for MAG is nearly 100 %. Despite multimodal treatment with maximal safe resection, external beam radiation therapy, and chemotherapy, outcomes after recurrence are poor, with median OS of 25 weeks for recurrent GB and 47 weeks for recurrent AA [3]. There is a compelling and unmet need to identify new therapeutic agents that improve survival and quality of life for these patients. Unfortunately, standard cytotoxic chemotherapy agents have proven to be only modestly effective [4, 5].
Due to the highly angiogenic nature of MAG, particularly GB, treatment strategies have shifted toward anti-angiogenic targeted agents. GB and AA highly express potent angiogenic factors such as vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1-α [6, 7]. Bevacizumab, a humanized monoclonal antibody against the VEGF ligand, has demonstrated 3- to 5-fold higher radiographic response than standard cytotoxic chemotherapies and is approved as a single agent by the U.S. Food and Drug Administration (FDA) for patients with recurrent GB [8–10]. In addition, evidence regarding bevacizumab efficacy for recurrent AA, a non-approved indication, is limited, suggesting activity similar to that seen with recurrent GB [8, 11, 12].
In addition to targeting the VEGF ligand, therapies targeting multiple angiogenic receptors are also being investigated. Sunitinib (Sutent; SU11248; Pfizer, Inc., New York, NY) is an oral multi-targeted small molecule inhibitor of the receptor tyrosine kinases involved in tumor proliferation and angiogenesis, including VEGF receptors-1, -2, and -3, platelet-derived growth factor receptor-alpha and -beta (PDGFR-α and -β), stem cell receptor factor (c-KIT), the receptor tyrosine kinase receptor encoded by the (rearranged during transfection) ret proto-oncogene (RET), and fms-like tyrosine kinase 3 (Flt3). Sunitinib is currently approved by the FDA for gastrointestinal stromal cell tumors and renal cell carcinoma [13] and has been shown to inhibit angiogenesis in orthotopic GB mouse models both in vivo and in vitro [14]. These factors provided the rationale to evaluate the safety and efficacy of sunitinib in recurrent MAG patients.
Materials and methods
Protocol objectives
Our primary objective was to determine progression-free survival at 6 months (PFS6), which is the proportion of patients who remained alive and free of disease progression at 6 months from the date of first treatment. Secondary objectives were to estimate best response rates (proportion of patients who ever had a radiographic response equal to or better than stable disease during course assessment), PFS (time from the first treatment with sunitinib until the first evidence of disease progression or death from any cause), and OS (time from first treatment with sunitinib to date of death from any cause) and to evaluate toxicities associated with sunitinib treatment (grade 3 and greater toxicities). Note that PFS was censored for patients who were given any non-protocol antitumor treatment before progression or who were alive and who did not have objective evidence of tumor progression as of their last follow-up. All patients who received at least one treatment of sunitinib were included.
Patient eligibility
The protocol was IRB approved, and all study patients signed an informed consent before trial entry. Patients must have had pathologically or neuroradiographically recurrent AA or GB and prior pathologic confirmation of primary tumor histology. Patients with prior low-grade glioma were eligible if histological transformation to MAG was confirmed before enrollment. Inclusion criteria included age ≥18 years, Karnofsky performance score ≥60, and adequate organ function, as defined by serum aspartate transaminase (serum glutamic oxaloacetic transaminase) and serum alanine transaminase (serum glutamic pyruvic transaminase) ≤3× the institutional upper limit of normal (ULN), total serum bilirubin ≤1.5 × ULN, absolute neutrophil count ≥1500/µL, platelets ≥100,000/µL, hemoglobin ≥9.0 g/dL, serum calcium ≤12.0 mg/dL, and serum creatinine ≤1.5 × ULN. In addition, patients had to have recovered from all expected toxicities related to previous therapies and had measurable disease on contrast-enhanced brain MRI. Study patients must have completed external beam radiation therapy, may have received up to two prior chemotherapy regimens, and were allowed to have received Gliadel wafers at initial resection only.
Exclusion criteria included major surgery or radiation therapy within 4 weeks of starting study treatment, National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grade 3 hemorrhage within 4 weeks of starting study treatment, and history of or known spinal cord compression or carcinomatous meningitis or evidence of leptomeningeal disease on screening CT or MRI scan. Other exclusion criteria included any of the following within 6 months before study treatment: myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, symptomatic congestive heart failure, cerebrovascular accident or transient ischemic attack or pulmonary embolism, ongoing cardiac dysrhythmias of NCI CTCAE grade ≥2, prolonged QTc interval on baseline ECG, hypertension not controlled by medications (>150/100 mmHg despite optimal medical therapy), preexisting thyroid abnormality with thyroid function that cannot be maintained at normal range with medication, known HIV or acquired immunodeficiency syndrome-related illness or other active infection, concurrent treatment on another clinical trial (supportive care trials, e.g., quality of life, were allowed), concomitant use of ketoconazole and other agents known to inhibit CYP3A4, concomitant use of theophylline and phenobarbital and/or other agents metabolized by the cytochrome P450 system, ongoing treatment with therapeutic doses of warfarin, pregnancy or breastfeeding patients, and prior stereotactic radiosurgery treatment. Prior use of bevacizumab or other anti-angiogenic agents was not an exclusion criterion.
Female subjects were required to be surgically sterile, postmenopausal, or agreed to use effective contraception during treatment, and all with reproductive potential required a negative pregnancy test (serum or urine) before enrollment. Male subjects were surgically sterile or agreed to use effective contraception during treatment.
Trial design
This study was a prospective phase II trial of sunitinib in patients with either first or second recurrence of histologically confirmed AA or GB (ClinicalTrials.gov registry NCT00606008). Study patients were stratified by tumor histology (AA or GB). A total of 55 patients were planned for enrollment (31 GB, 24 AA). A planned interim analysis was performed after 16 GB and 14 AA patients were enrolled.
Sunitinib was provided free of charge by Pfizer, Inc. for this study. Trial patients received sunitinib 50 mg daily for 4 weeks without regard to meals, followed by a 2-week rest period. This 6-week regimen constituted 1 cycle. Patients were treated for up to 9 cycles (~1 year) or until disease progression or death or if persistent toxicities occurred. Complete blood count with differential, complete metabolic profile, neurologic exam, and brain MRI with contrast were obtained after each cycle. Toxicity assessments were obtained after each cycle.
Toxicity was graded according to NCI CTCAE, version 3.0. Response evaluations were performed using modified Macdonald criteria [15]; the response assessment in neuro-oncology (RANO) criteria was not defined before completion of this study [16].
Initially, patients were started on sunitinib at a dose of 50 mg daily. If 50 mg daily resulted in unacceptable toxicity, 2 dose modifications were allowed (to 37.5 and to 25 mg daily, if necessary). Study patients who could not tolerate 25 mg daily of sunitinib were taken off study. Unacceptable toxicity was defined as any grade 3 non-hematologic toxicity not reversible to grade 1 or less within 96 h, any grade 4 non-hematologic toxicity or any grade 4 hematologic toxicity not resolving to grade 1 or less within 5 days, despite best supportive care, grade 4 neutropenia associated with fever, or grade 4 thrombocytopenia.
The first 14 study patients (7 AA, 7 GB) were started on sunitinib 50 mg daily. Of those, 7 progressed after one cycle of sunitinib (3 AA, 4 GB). Another GB patient did not complete 1 cycle and withdrew due to excessive toxicity. Of the remaining 6 patients who completed >1 cycle of sunitinib, 3 were dose reduced to 37.5 mg daily due to toxicity. Thus, the study was amended to start subsequent patients at 37.5 mg daily. Twelve of the next 16 subjects enrolled at 37.5 mg tolerated this dose well, while 4 required a dose reduction to 25 mg daily.
Statistical analyses
A minimax two-stage design [17] was employed. The type I error rate was set at 10 % (i.e., α = 0.10) and the type II error rate at 20 % (i.e., β = 0.20 and power = 80 %).
For the AA cohort, an interim analysis occurred after 14 patients were treated and assessable for PFS6 at the end of stage 1. If 2 (14.3 %) or fewer of these first 14 patients were alive and had not progressed at 6 months, then this study cohort would be terminated early and further study of sunitinib would not be recommended in patients with recurrent AA. If 3 (21.4 %) or more patients were alive and progression free 6 months after start of sunitinib, then the study would continue (provided that toxicities were acceptable) until 24 patients had been treated and were evaluable for PFS6 at the end of stage 2. If 7 or fewer patients of these 24 patients were alive and progression free at 6 months, then we would conclude that this schedule of sunitinib was not active enough to warrant further study. With the proposed design, if the PFS6 rate was 20 % or less, then there was a 9 % chance that we would recommend this schedule of sunitinib for further study in these patients; if the true response rate was 40 % or more, then there was only a 20 % chance that we would observe too few patients with benefit (i.e., alive and progression-free at 6 months) at final target accrual and therefore not recommend this regimen for further study.
For the GB cohort, an interim analysis occurred after 16 patients were treated and assessable for PFS6 at the end of stage 1. If none or only 1/16 patients were alive and progression-free at 6 months, then this study cohort would be terminated early. If 2 or more patients experienced benefit (i.e., PFS6), then the study would be potentially continued (providing that the toxicities were acceptable) until 31 patients were treated and evaluable for PFS6 at the end of stage 2. If 5 or fewer patients of these 31 patients were alive and progression free at 6 months, then we would conclude that this schedule of sunitinib was not active enough to warrant further study. With the proposed design, if the PFS6 rate was ≤10 %, then there was an 8 % chance that we would recommend this schedule of sunitinib for further study; if the true response rate was ≥25 %, then there was only a 19 % chance that we would observe too few patients with benefit at final target accrual, and therefore we would not recommend this regimen for further study.
For the interim analysis, PFS6 was calculated based on the binary counts and the binomial distribution. For the final reporting, however, PFS, PFS6, and their 95 % confidence intervals (CIs) were constructed using the Kaplan–Meier product-limit method for both AA and GB groups. The Kaplan–Meier product-limit method was preferred because it takes censoring into account in data analysis while the binary approach is conservative, as all censored observations are treated as failures. Similarly, Kaplan–Meier curves of OS, median OS, and its 95 % CI were also calculated for both cohorts. Best overall response rates and their exact 95 % CIs were reported based on the binomial distribution for both cohorts. Grade 3 and greater toxicities possibly, probably, or definitely attributable to sunitinib were reported by toxicity category, type, and grade for each cohort. All patients who received at least one sunitinib treatment were accounted for in both the efficacy and toxicity analyses.
Results
Demographics and treatment characteristics
We enrolled 30 patients (16 GB, 14 AA) from March 2007 to December 2010 at the Moffitt Cancer Center (see Table 1). All but one patient received prior temozolomide. Sixteen patients enrolled at first recurrence and 14 enrolled at second recurrence. Nine of thirty (30 %) patients had an additional resection for recurrent MAG before study entry. Prior chemotherapy regimens included enzastaurin, hydroxyurea with imatinib, cyclophosphamide, PCV, bevacizumab-irinotecan, and carboplatin. The 2/14 AA patients and 3/16 GB patients who had received bevacizumab prior to study enrollment progressed after only 1 cycle of sunitinib. One GB patient was taken off study due to excessive toxicity (grade 3 elevated transaminases and grade 3 thrombocytopenia), and 1 AA patient withdrew <1 month after starting treatment due to grade 1 nausea and vomiting. In addition, 3 other AA patients withdrew before disease progression due to toxicities that included grade 1 diarrhea, rash, arthralgias, grade 2 fatigue, and grade 3 hand-foot syndrome.
Table 1.
Demographics and treatment duration (n = 30)
| Variable | AA (%) (n = 14) |
GB (%) (n = 16) |
Total (n = 30) |
|---|---|---|---|
| Sex, n (%) | |||
| Women | 2 (14.3) | 6 (37.5) | 8 (26.7) |
| Men | 12 (85.7) | 10 (62.5) | 22 (73.3) |
| Race, n (%) | |||
| Asian | 0 (0.0) | 1 (6.3) | 1 (3.3) |
| African American | 2 (14.3) | 0 (0.0) | 2 (6.7) |
| Unknown | 0 (0.0) | 1 (6.3) | 1 (3.3) |
| White | 12 (85.7) | 14 (87.5) | 26 (86.7) |
| Age, years | |||
| Mean (SD) | 46.9 (10.7) | 57.8 (10.0) | 52.7 (11.6) |
| Median (range) | 48.5 (30.0–64.0) | 57.5 (44.0–77.0) | 52.0 (30.0–77.0) |
| Duration of treatment, months | |||
| Mean (SD) | 3.1 (3.0) | 2.7 (3.2) | 2.9 (3.1) |
| Median (range) | 2.7 (0.6–12.3) | 1.4 (0.5–13.6) | 1.4 (0.5–13.6) |
Outcomes
Table 2 summarizes the number of completed cycles. The median duration (range) on treatment was 2.7 (0.6–12.3) and 1.4 (0.5–13.6) months for the AA and GB cohorts, respectively. The median follow-up for all study participants was 12 (range: 1.2–43.9) months. All patients died except for three AA patients who remain alive on alternative therapy. Best overall response rates (stable disease or better) were 57.1 % (8 of 14, 95 % CI: 28.9–82.3 %) and 31.3 % (5 of 16, 95 % CI: 11.0–58.7 %) for the AA and GB cohorts, respectively. Specifically, all of the best overall responses were stable disease; there were no partial or complete responses in any of the study patients.
Table 2.
Number of sunitinib cycles completed
| Cohort | Cycle | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| 1 | 2 | 3 | 5 | 9 | Total | |
| AA, n (%) | 6 (42.86) | 2 (14.29) | 5 (35.71) | 0 (0.00) | 1 (7.14) | 14 |
| GB, n (%) | 12 (75.00) | 1 (6.25) | 1 (6.25) | 1 (6.25) | 1 (6.25) | 16 |
| Total, n | 18 | 3 | 6 | 1 | 2 | 30 |
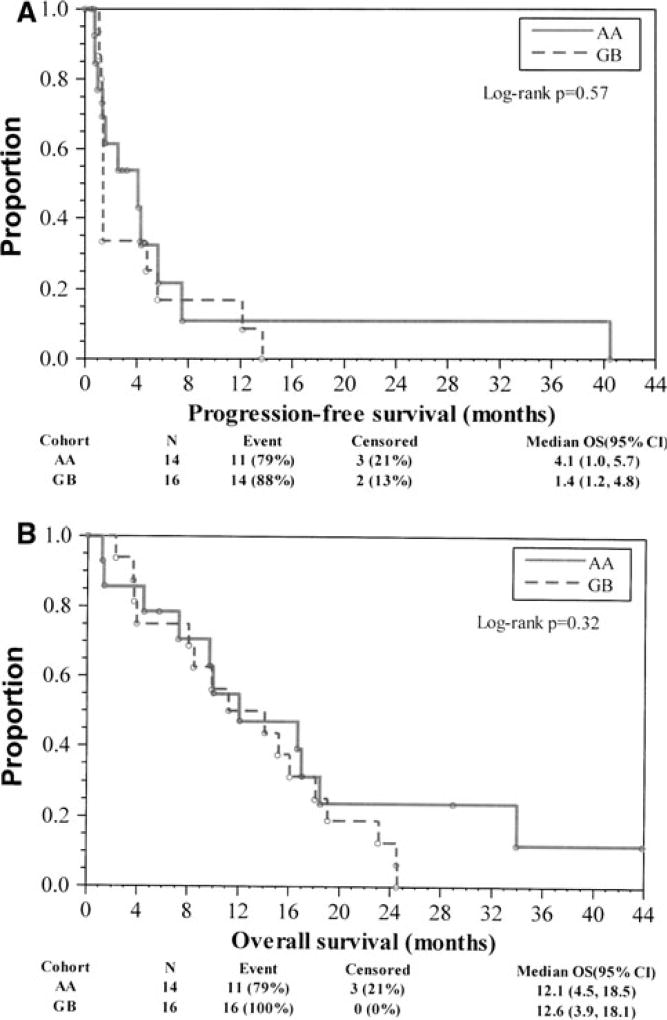
Based on the binomial approach for the interim analysis, 2/14 AA patients (14.3 %) and 2/16 GB patients (12.5 %) reached PFS6. Regarding final estimates based on the Kaplan–Meier product-limit approach, which takes censored observations on PFS into account, PFS6 was 21.5 % (95 % CI: 3.6–49.2 %) and 16.7 % (95 % CI: 2.9–40.2 %) for the AA and GB cohorts, respectively. All of the censored observations were not known at the interim analysis; thus, the decision to not expand the trial was based on the number of patients that did not reach the predetermined PFS6 targets. Median PFS was 4.1 (95 % CI: 1.0–5.7) and 1.4 (95 % CI: 1.2–4.8) months for AA and GB cohorts, respectively (Fig. 1a). Median OS was 12.1 (95 % CI: 4.5–18.5) and 12.6 (95 % CI: 3.9–18.1) months for AA and GB cohorts, respectively (Fig. 1b).
Fig. 1.
a Progression-free survival by cohort. b Overall survival by cohort
Toxicities
In the AA cohort, 4 patients (28.6 %) had grade 3 toxicities and 1 patient (7.1 %) had grade 4 neutropenia. The most common toxicities attributed to sunitinib (any grade) that occurred in at least 25 % of AA patients included fatigue (85.7 %), diarrhea (42.9 %), hand-foot syndrome (35.7 %), thrombocytopenia (35.7 %), leucopenia (35.7 %), neutropenia (28.6 %), rash (28.6 %), and nausea (28.6 %). In the GB cohort, 4 patients (25 %) had grade 3 toxicities, whereas 1 patient (6.3 %) had grade 4 neutropenia and thrombocytopenia. The most common toxicities attributed to sunitinib (any grade) that occurred in at least 25 % of GB patients included fatigue (56.3 %), diarrhea (43.8 %), neutropenia (31.3 %), and thrombocytopenia (25 %).
Discussion
Sunitinib was chosen as a potential anti-glioma agent due to its ability to inhibit multiple VEGF and PDGF receptors as well as c-KIT, all of which are overexpressed in malignant gliomas [18–20]. Preclinical studies have also demonstrated sunitinib activity against malignant gliomas [14, 21]. However, our results do not suggest sufficient activity to proceed with further studies of this agent in recurrent MAG. Only 2/14 (14.3 %) AA patients were alive and progression-free 6 months after starting treatment and only 2/16 (12.5 %) GB patients were progression-free at 6 months. Thus, the AA cohort did not reach its goal of at least 3 or more patients reaching PFS6. Although the GB cohort did have at least 2 patients reach PFS6, given the lack of partial or complete MRI responses and that 12/16 GB patients tolerated or progressed after only 1 cycle of sunitinib, the decision was made to not proceed with the second-stage expansion cohort. There were not enough patients who received bevacizumab prior to study enrollment (2 AA, 3 GB) to significantly affect the study outcomes. Thus, no definitive conclusions can be made regarding outcome differences between the bevacizumab versus the bevacizumab-naïve study patients.
The response outcomes in our study were similar to another phase II trial of single-agent sunitinib for recurrent malignant gliomas. In the study by Neyns et al. [22], 21 recurrent MAG patients who received sunitinib 37.5 mg daily had no objective response. The median OS in their study was 3.8 months, compared to 12.6 months for the GB cohort and 12.1 months for the AA cohort in our study. Given that 75 % of the GB patients completed only 1 cycle of sunitinib, it is likely that the difference in median OS in our study versus theirs may be attributed to subsequent treatments after sunitinib rather than the effect of sunitinib. In our study, 10 of 16 (63 %) GB patients progressing on sunitinib proceeded onto bevacizumab. In another study for recurrent malignant gliomas, Reardon et al. evaluated 25 recurrent MAG patients who received sunitinib and irinotecan. PFS6 in that study was 24 %, median PFS was 1.7 months, median OS was 13.3 months, and 1 patient had a partial radiographic response [23]. This study also utilized the 4 weeks on/2 weeks off sunitinib regimen at 50 mg daily. The authors also concluded that sunitinib with irinotecan did not demonstrate significant anti-glioma activity.
Tables 3 and 4 list toxicities >grade 3 for the AA and GB cohorts, respectively. After our protocol was amended to a dose of 37.5 mg daily, only four patients in each cohort (28.6 % of AA patients; 25 % of GB patients) had grade 3 or higher toxicities with only 1 patient in each cohort (7.1 % in AA; 6.3 % in GB) with grade 4 neutropenia. The most common side effects (fatigue, diarrhea, neutropenia, and hand-foot syndrome) were also among the most common toxicities reported in the other sunitinib studies [22, 23]. Overall, sunitinib at 37.5 mg daily was reasonably tolerated. Only 1 patient withdrew from the study, specifically because of toxicity at the 50 mg dose.
Table 3.
Grade ≥ 3 toxicities associated with sunitinib: AA cohort (n = 14)
| Toxicity category | Description (CDUS toxicity type code) |
Grade 3 n (%) |
Grade 4 n (%) |
Grade 5 n (%) |
Total grade 3–5 |
|---|---|---|---|---|---|
| Blood/bone marrow | Leukocytes (total WBC) | 2 (14.3) | 1 (7.1) | 3 (21.4) | |
| Lymphopenia | 1 (7.1) | 1 (7.1) | |||
| Platelets | 2 (14.3) | 2 (14.3) | |||
| Constitutional Symptoms | Fatigue (asthenia, lethargy, malaise) | 1 (7.1) | 1 (7.1) | ||
| Death | Death not associated with CTCAE term—disease progression NOS | 1 (7.1) | 1 (7.1) | ||
| Dermatology/skin | Dermatology/skin—other | 1 (7.1) | 1 (7.1) | ||
| Rash: hand-foot skin reaction | 2 (14.3) | 2 (14.3) | |||
| Hemorrhage/bleeding | Hemorrhage/bleeding—other | 1 (7.1) | 1 (7.1) | ||
| Overall | Maximum toxicity | 4 (28.6) | 1 (7.1) | 1 (7.1) | 6 (42.9) |
Table 4.
Grade ≥ 3 Toxicities associated with sunitinib: GB cohort (n = 16)
| Toxicity category | Description (CDUS toxicity type code) |
Grade 3 n (%) |
Grade 4 n (%) |
Grade 5 n (%) |
Total grade 3–5 |
|---|---|---|---|---|---|
| Blood/bone marrow | Neutrophils/granulocytes (ANC/AGC) | 1 (6.3) | 1 (6.3) | ||
| Platelets | 1 (6.3) | 1 (6.3) | 2 (12.5) | ||
| Constitutional symptoms | Fatigue (asthenia, lethargy, malaise) | 2 (12.5) | 2 (12.5) | ||
| Gastrointestinal | Mucositis/stomatitis (functional/symptomatic)—anus | 1 (6.3) | 1 (6.3) | ||
| Metabolic/laboratory | ALT, SGPT | 1 (6.3) | 1 (6.3) | ||
| AST, SGOT | 1 (6.3) | 1 (6.3) | |||
| Overall | Maximum toxicity | 4 (25.0) | 1 (6.3) | 5 (31.3) |
This is the largest reported trial of sunitinib in recurrent malignant gliomas (30 patients), as well as the largest cohort of recurrent AAs treated with sunitinib (14 patients). However, the outcomes of patients in the recurrent AA cohort were comparable to those in prior studies using cytotoxic chemotherapy (median PFS of 17.8 weeks compared to 13 weeks historically). The PFS6 of this cohort was 21.5 %, which is worse than the historical PFS6 benchmark of 31 % [3]. Based on the present study, it appears that patients with recurrent AA sustained a similar and meager benefit from sunitinib when compared with patients in the recurrent GB cohort, recognizing the small study cohort sizes. The results of the GB cohort were comparable to those reported by Wong et al. [3] PFS6 of 16.7 %, median PFS of 6.1 weeks, and median OS of 54.6 weeks, compared to historical rates of 15 %, 10 weeks, and 30 weeks, respectively.
Although sunitinib was deemed to be a potentially effective treatment for recurrent MAG, the current study is concordant with prior studies confirming lack of significant anti-glioma activity [22, 23]. This may be due to the possibility that the tolerated dose of sunitinib may be insufficient to significantly affect tumor growth or induce tumor responses. Alternatively, the 4-week on/2-week off schedule initially employed at 50 mg daily proved to be too toxic, thus preventing assessment of response. Because the 37.5 mg dose proved tolerable in patients with recurrent glioma and contemporary studies of renal cancer employ a continuous daily dose of 37.5 mg (with no drug interruptions except for toxicity), the study drug schedule may have been incorrect. In their study, Neyns et al. [22] noted modest reductions of gadolinium enhancement, cerebral blood flow, and cerebral blood volume in 4 of 14 (29 %) patients, but notwithstanding these anti-angiogenic effects were insufficient to induce true tumor regression. There is limited evidence that sunitinib penetrates the blood–brain barrier from published reports of brain metastases from renal cell carcinoma responding to sunitinib treatment [24] and reports of sunitinib treatment reducing the incidences of brain metastases from renal cell carcinoma [25]. However, no animal studies have shown conclusively that sunitinib achieves therapeutic concentrations in brain, suggesting another mechanism (failure to access the target) of sunitinib that would limit anti-glioma effectiveness.
Although there is limited evidence to suggest that sunitinib as a primary treatment for recurrent MAG is effective, it is possible that combining sunitinib with up-front chemoradiotherapy may be more efficacious. For example, when sunitinib is administered at various doses concurrently with temozolomide, sunitinib had a differential effect on the distribution of temozolomide in glioma mouse models. Optimal tumor exposure to temozolomide was achieved with lower sunitinib doses [26]. Despite sunitinib not demonstrating activity as a single agent in recurrent MAG, future studies evaluating other experimental anti-angiogenic agents, possibly in combination with cytotoxic agents, may demonstrate more efficacy in recurrent MAG.
Acknowledgments
The authors thank Catrina Montgomery for her work with data management and Rasa Hamilton for manuscript editing. Pfizer, Inc. provided funding for research support but did not contribute to the study design or interpretation of the study results.
Footnotes
Conflict of interest None.
Contributor Information
Edward Pan, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr., Tampa, FL 33612-9416, USA.
Daohai Yu, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr., Tampa, FL 33612-9416, USA.
Binglin Yue, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr., Tampa, FL 33612-9416, USA.
Lisa Potthast, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr., Tampa, FL 33612-9416, USA.
Sajeel Chowdhary, Department of Neuro-Oncology, Florida Hospital Cancer Institute, Orlando, FL, USA.
Pamela Smith, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr., Tampa, FL 33612-9416, USA.
Marc Chamberlain, Department of Neurology and Neurological Surgery, Seattle Cancer Care Alliance, Seattle, WA, USA.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann RD, Pietsch T, Wiestler OD, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 3.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 4.Groves MD, Puduvalli VK, Chang SM, Conrad CA, Gilbert MR, Tremont-Lukats IW, Liu TJ, Peterson P, Schiff D, Cloughesy TF, Wen PY, Greenberg H, Abrey LE, DeAngelis LM, Hess KR, Lamborn KR, Prados MD, Yung WK. A North American brain tumor consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81:271–277. doi: 10.1007/s11060-006-9225-y. [DOI] [PubMed] [Google Scholar]
- 5.Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S, Musib L, Liepa AM, Thornton DE, Fine HA. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korkolopoulou P, Patsouris E, Konstantinidou AE, Pavlopoulos PM, Kavantzas N, Boviatsis E, Thymara I, Perdiki M, Thomas-Tsagli E, Angelidakis D, Rologis D, Sakkas D. Hypoxia-inducible factor 1alpha/vascular endothelial growth factor axis in astrocytomas. Associations with microvessel morphometry, proliferation and prognosis. Neuropathol Appl Neurobiol. 2004;30:267–278. doi: 10.1111/j.1365-2990.2003.00535.x. [DOI] [PubMed] [Google Scholar]
- 7.Lamszus K, Ulbricht U, Matschke J, Brockmann MA, Fillbrandt R, Westphal M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9:1399–1405. [PubMed] [Google Scholar]
- 8.Vredenburgh JJ, Desjardins A, Herndon JE, II, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 9.Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9:403–407. doi: 10.6004/jnccn.2011.0037. [DOI] [PubMed] [Google Scholar]
- 10.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain MC, Johnston S. Salvage chemotherapy with bevacizumab for recurrent alkylator-refractory anaplastic astrocytoma. J Neurooncol. 2009;91:359–367. doi: 10.1007/s11060-008-9722-2. [DOI] [PubMed] [Google Scholar]
- 12.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 13.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, Booth BP, Verbois SL, Morse DE, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 14.de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, Guillamo JS. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9:412–423. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 16.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 17.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 18.Fleming TP, Saxena A, Clark WC, Robertson JT, Oldfield EH, Aaronson SA, Ali IU. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–4553. [PubMed] [Google Scholar]
- 19.Laird AD, Christensen JG, Li G, Carver J, Smith K, Xin X, Moss KG, Louie SG, Mendel DB, Cherrington JM. SU6668 inhibits Flk-1/KDR and PDGFRbeta in vivo, resulting in rapid apoptosis of tumor vasculature and tumor regression in mice. FASEB J. 2002;16:681–690. doi: 10.1096/fj.01-0700com. [DOI] [PubMed] [Google Scholar]
- 20.Puputti M, Tynninen O, Sihto H, Blom T, Maenpaa H, Isola J, Paetau A, Joensuu H, Nupponen NN. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res. 2006;4:927–934. doi: 10.1158/1541-7786.MCR-06-0085. [DOI] [PubMed] [Google Scholar]
- 21.Chahal M, Xu Y, Lesniak D, Graham K, Famulski K, Christensen JG, Aghi M, Jacques A, Murray D, Sabri S, Abdulkarim B. MGMT modulates glioblastoma angiogenesis and response to the tyrosine kinase inhibitor sunitinib. Neuro Oncol. 2010;12:822–833. doi: 10.1093/neuonc/noq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A, De Greve J. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103:491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 23.Reardon DA, Vredenburgh JJ, Coan A, Desjardins A, Peters KB, Gururangan S, Sathornsumetee S, Rich JN, Herndon JE, Friedman HS. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neurooncol. 2011;105:621–627. doi: 10.1007/s11060-011-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutras AK, Krikelis D, Alexandrou N, Starakis I, Kalofonos HP. Brain metastasis in renal cell cancer responding to sunitinib. Anticancer Res. 2007;27:4255–4257. [PubMed] [Google Scholar]
- 25.Verma J, Jonasch E, Allen P, Tannir N, Mahajan A. Impact of tyrosine kinase inhibitors on the incidence of brain metastasis in metastatic renal cell carcinoma. Cancer. 2011;117:4958–4965. doi: 10.1002/cncr.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q, Gallo JM. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro Oncol. 2009;11:301–310. doi: 10.1215/15228517-2008-088. [DOI] [PMC free article] [PubMed] [Google Scholar]