Abstract
Background
The burden of mast cell (MC) infiltration and their phenotypes, MC-tryptase (MCT) and MC-tryptase/chymase (MCTC), after lung transplantation (LT) has not been evaluated in human studies.
Methods
We reviewed 20 transbronchial lung biopsy (TBLB) specimen from patients with early normal allograft (<6 months post-LT, n=5), late normal allograft (>6 months, n=5), A2 or worse acute cellular rejection (ACR, n=5), and chronic lung allograft dysfunction (CLAD, n=5). Slides were immunostained for tryptase and chymase. Total MC, MCT, MCTC and MCTC to-MCT ratio were compared between the four groups using a generalized linear mixed model.
Results
Irrespective of clinicopathologic diagnosis, MC burden tends to increase with time (r2=.56, P=.009). MCTC phenotype was significantly increased in the CLAD group (8.2±4.9 cells per HPF) in comparison with the other three groups (early normal: 1.6±1.7, P=.0026; late normal: 2.5±2.3, P=.048; ACR: 2.7±3.5, P=.021). Further, the ratio of MCTC to MCT was significantly increased in CLAD group as compared to the other three groups (P<.001 for all comparisons).
Conclusions
The burden of MC may increase in the allograft as function of time. Patients with CLAD have an increased relative and absolute burden of MCTC phenotype MC. Future studies are needed to confirm these findings and evaluate the potential pathologic role of MCTC in allograft dysfunction.
Keywords: acute cellular rejection, chronic lung allograft dysfunction, innate immunity, transbronchial lung biopsy
1 | INTRODUCTION
An increasing number of lung transplantations (LTs) are being performed every year.1 Despite better perioperative outcomes, long-term survival of patients with LT remains inferior to those after other forms of solid organ transplantation. This is largely driven by early development of chronic rejection, which is the most common cause of mortality after first year.1 The pathologic hallmark of chronic rejection after LT is obliterative bronchiolitis (OB)2,3 with bronchiolitis obliterans syndrome (BOS) as its clinical correlate.4 However, it has been recognized that diverse clinical entities (or phenotypes) may lead to a decline in allograft function and the use of an umbrella term, chronic lung allograft dysfunction (CLAD), has been recommended by the International Society for Heart and Lung Transplantation (ISHLT).5
There is growing evidence implicating the innate immune system in causing allograft injury. In this context, mast cells (MCs) modulate the adaptive immune response through release of cytokines such as tumor necrosis factor-α, facilitate the recruitment T cells to sites of inflammation, and enhance interactions with antigen presenting cells.6,7 Several investigators have confirmed an increase in the number of MC in the allograft after heart,8 kidney,9 and lung10 transplantation with further increases in the presence of acute rejection. Although this was initially believed to suggest a proinflammatory role for MCs, subsequent analyses pointed toward an immunomodulatory effect.11,12 Notwithstanding the short-term potentially favorable effects, prolonged presence of activated MC appears to be detrimental to the allograft.10,13–17 These data support a dual role for MCs where the protective effect against acute rejection coexists with profibrotic potential that may be mediated via different MC phenotypes. It is noteworthy that profibrotic potential of MCs is well recognized across diverse disease processes and organ systems including lung (idiopathic pulmonary fibrosis, airway remodeling in asthma), skin (chronic atopic dermatitis), and kidneys (renal fibrosis).18
Mast cells can be histologically classified based upon their protease content, with those expressing tryptase only designated as MCT and those positive for tryptase and chymase designated MCTC.19,20 MC phenotype has been reported to change with time after kidney transplantation with increase in MCTC correlating with severity of interstitial fibrosis.21 It has also been demonstrated that MCT are the predominant phenotype in normally functioning kidney allograft, and MCTC become the dominant phenotype at 100 days with their numbers correlating with the extent of allograft fibrosis.22
The role of MC phenotypes among patients with LT has not been studied. It is not known if a “phenotypic switch” similar to renal allograft also occurs after LT. In this study, we explore the extent of MC infiltration and their phenotypes among patients with LT during different clinicopathologic stages.
2 | METHODS
This was a retrospective chart review study with immunofluorescence staining of transbronchial lung biopsy (TBLB) samples. The study was conducted with institutional review board approval with waiver of informed consent.
From the lung transplant database, we identified five patients where TBLB specimens were available corresponding to the following four clinical stages: early post-transplant (<6 months) with no clinical, spirometry or histological evidence of allograft dysfunction (early normal group); late post-transplant (>6 months) with no clinical, spirometry or histological evidence of allograft dysfunction (late normal group); acute cellular rejection (ACR) of A2 or higher severity on TBLB (ACR group); and clinical diagnosis of CLAD (CLAD group). The diagnosis and severity of ACR had been made previously by the lung pathologist per standard guidelines.23 Diagnosis of CLAD was based upon persistent (at least 3 months), unexplained allograft dysfunction defined by FEV1 and/or FVC <90% from baseline.5 Baseline lung function was defined as the average of two best post-transplant values for FEV1 and FVC obtained at least 3 weeks apart.
2.1 | Clinical and demographic data
Variables recorded from the patient’s chart included donor and recipient demographics, panel-reactive antibody (PRA), date, type, and indication for transplant, cross-match results, cytomegalovirus and Epstein-Barr virus matching status, type of surgery, post-transplant course including primary graft dysfunction, number of ACR episodes, and severity before CLAD, presence of any donor-specific antibodies preceding the diagnosis of CLAD, time to development of CLAD, and its phenotype. Stage of obstructive CLAD was also recorded.5 Per the institutional protocol, all patients had been started on azithromycin after transplantation and were already on it when CLAD was diagnosed. After the workup for alternate causes of allograft dysfunction including bronchoscopy, patients were treated with antithymocyte globulin with stabilization of lung functions in one of the patients with obstructive CLAD.
2.2 | Immunofluorescence staining
Five micron tissue sections from TBLB samples were deparaffinized and rehydrated. Antigen retrieval was performed using proteinase K treatment and heating in citrate buffer pH.6.0. Slides were immunostained using the mouse antitryptase antibody (1:500 dilution; Promega, G3361, Madison, WI, USA) and the goat antichymase (1:150 dilution; Abcam, clone CC1, Cambridge, MA, USA) antibodies and detected with donkey anti-mouse FITC (1:1000 dilution) and donkey anti-goat alexa 594 (1:500 dilution; Life Technologies, Carlsbad, CA, USA). Sections were counterstained with DAPI for nuclear staining and analyzed using the Image-Pro software (MediaCybernetics, Rockville, MD, USA) with cell counts reported per high-power fields (HPF). Ten HPF focused on the alveolar tissue in the TBLB were counted for each specimen. MC phenotypes were differentiated in to MCT and MCTC based on immunofluorescence staining. The number of MCT, MCTC and the ratio of MCT to MCTC were compared between the four groups. Samples were independently analyzed by two investigators (AB and YH) who were blinded to the clinical data.
2.3 | Statistical analysis
Data were described using mean with standard deviations and proportions as appropriate. Spearman correlation analysis was utilized to study the association between the days since transplantation and mast cell counts on TBLB. Generalized linear mixed modeling (GLM) was used for comparing each outcome variable (total MC, MCT, MCTC and ratio) among the four groups. Data on the total MC, MCT and MCTC counts were considered continuous, and a negative binomial distribution was used. The ratio of MCT to MCTC was considered continuous data and analyzed as a normal distribution. Pairwise comparisons among the least square means of each group were performed with adjustment for multiple comparisons using the Tukey-Kramer method. All analyses are two-tailed and were performed at a significance level of .05. SAS 9.3 software (Cary, NC, USA) was used for all analyses.
3 | RESULTS
Baseline demographic and clinical characteristics of the patients are presented in Table 1. Idiopathic pulmonary fibrosis was the most common indication for transplantation. Mean number of days post-transplant at which the biopsies were performed for each groups was 79 ±23 days (range 38–103 days) for early normal group, 309±38 days (range 268–381 days) for late normal group, 170±117 days (range 30–332 days) for ACR group, and 715±219 days (365–912 days) for CLAD group.
TABLE 1.
Demographic and clinical profile of the study group
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age | 63 | 56 | 60 | 68 | 48 |
| Gender | Male | Male | Male | Female | Male |
| Race | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| Underlying diagnosis | IPF | IPF | IPF | COPD | IPF |
| Lung allocation score | 50.45 | 53.55 | 85.49 | 31.47 | 90.54 |
| CMV mismatch | No | Yes | No | No | Yes |
| Donor gender | Female | Female | Female | Male | Female |
| Donor race | Hispanic | African American | Caucasian | African American | Caucasian |
| PRA Class 1/Class 2 | 5%/4% | 4%/4% | 7%/3% | 3%/3% | 5%/8% |
| Type of transplant | Right Single | Left Single | Bilateral | Right Single | Bilateral |
| Cardiopulmonary bypass | Yes | Yes | Yes | No | Yes |
| Primary graft dysfunction at 72 hours | No | No | Yes | No | No |
| Number of ACR episodes during first year | 1 | 2 | 4 | 2 | 1 |
| Severity of worst A grade rejection | A2 | A3 | A2 | A2 | A2 |
| Severity of worst A grade rejection | B0 | B1 | B0 | Non-diagnostic | B0 |
| Time to CLAD development (days) | 849 | 912 | 550 | 897 | 365 |
| CLAD Phenotype | Obstructive | Obstructive | Obstructive | Obstructive | Restrictive |
| Severity of obstructive CLAD | Stage III | Stage II | Stage II | Stage III | |
| Response to antithymocyte globulin | No | Yes | No | No | No |
C, Caucasian; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease; CMV, cytomegalovirus; PRA, panel-reactive antigens; ACR, acute cellular rejection; CLAD, chronic lung allograft dysfunction.
Mean total MC, MCT, MCTC and the ratio of MCTC to MCT in each biopsy specimen for all the groups are presented in Table 2. Irrespective of the group, there appeared to be a trend toward progressive increase in the number of MC with time after transplantation. There was a positive correlation between the number of days post-transplantation and the number of MC. This association was statistically significant for total MC (Spearman correlation coefficient, r2=.56, P=.009, Fig. 1A), MCTC cells (r2=.65, P=.002, Fig. 1C) as well as for the ratio of MCTC to MCT cells (r2=.58, P=.008, Fig. 1D) but not for MCT cells (r2=.42, P=.07, Fig. 1B).
TABLE 2.
Total, MCT cells, MCTC cells, and ratio of MCTC to MCT cells for all the groups*
| Early normal group | Late normal group | ACR group | CLAD group | |
|---|---|---|---|---|
| Days since transplant | 79.6±22.7 | 309.4±38.4 | 169.6±117 | 714.6±219 |
| Total MC (per HPF) | 11.4±6.7 | 21.5±3.9 | 17.5±7.2 | 27±10.8 |
| MCT cells(per HPF) | 9.8±6.4 | 19±6.1 | 14.8±7.3 | 18.9±10.5 |
| MCTC cells (per HPF) | 1.6±1.7 | 2.5±2.3 | 2.7±3.5 | 8.2±4.9 |
| Ratio (%) | 14.4±11.8 | 12.4±10.4 | 15.8±18 | 43.5±12.5 |
ACR, acute cellular rejection; CLAD, chronic lung allograft dysfunction.
Mean with standard deviation calculated using results of individual high-power field reads for each biopsy (n=10 per biopsy for 5 patients) resulting in total of 50 variables per group
FIGURE 1.
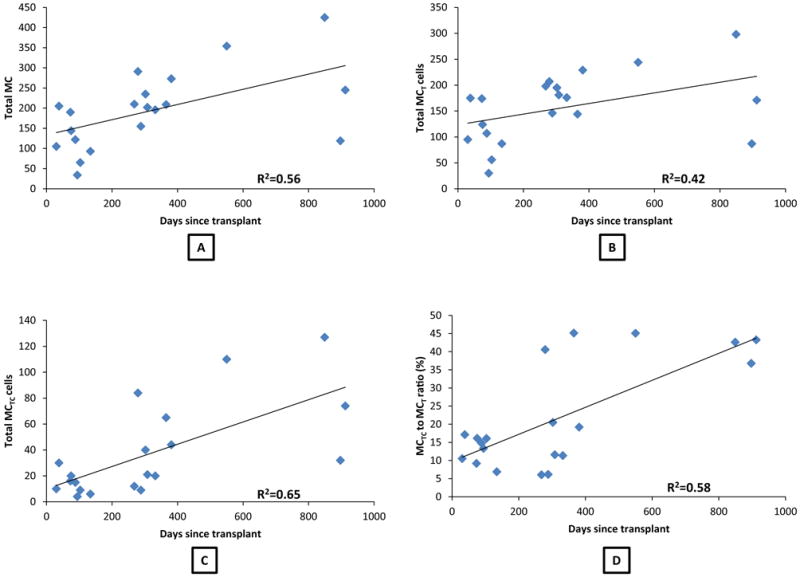
(A) Scatter plot of total MC vs number of days since transplantation (Spearman’s correlation coefficient=.56, P=.009); (B) Scatter plot of MCT phenotype vs number of days since transplantation (Spearman’s coefficient=.42, P=.07; (C) Scatter plot of MCTC phenotype vs number of days since transplantation (Spearman’s coefficient=.65, P=.002); (D) Scatter plot of the ratio of MCTC to MCT cells vs number of days since transplantation (Spearman’s coefficient=.58, P=.008).
The overall difference in total MC was significant between the four groups (P=.02). In comparison with early normal group (11.4±6.7 cells per HPF), total MC appeared to be increased with ACR (17.5±7.2 cells per HPF) (see Table 2) although the difference did not reach statistical significance. Nevertheless, total MC were significantly increased among TBLB with CLAD (27±10.8 cells per HPF, P=.018, Fig. 3A). On analyzing MC phenotypes, mean MCT cells per HPF were again lowest in early normal group with 9.8±6.4 cells per HPF and increased in biopsies with ACR (14.8±7.3 cells per HPF) and further increased in late normal group (19±6.1 cells per HPF) as well as those with CLAD (18.9±10.5 cells per HPF). Despite the overall difference in MCT being significant (P=.04), the intergroup comparison reached statistical significance only between early normal and late normal groups (9.8±6.4 vs 19±6.1 cells per HPF, P=.04, Figs. 2 and 3B).
FIGURE 3.
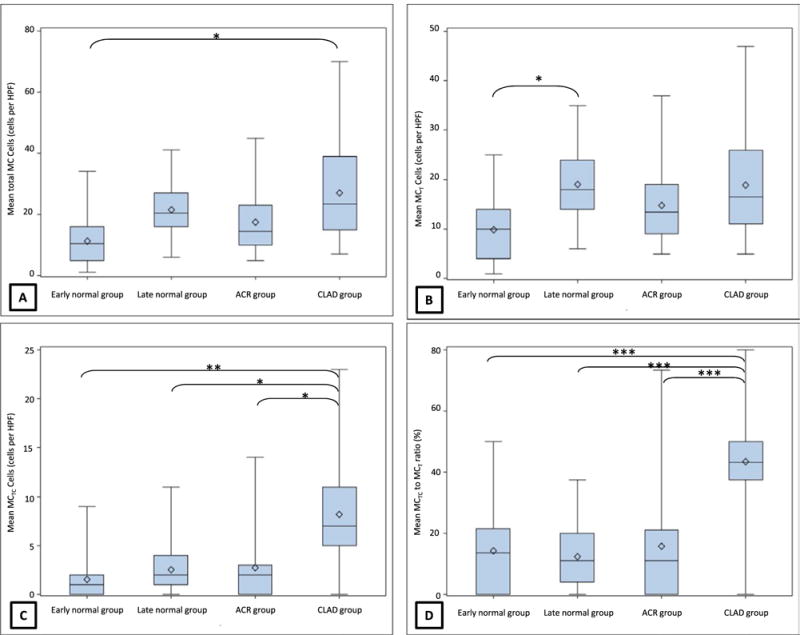
(A) Box plot of total MC among the four groups; intergroup difference between early normal group and CLAD group was statistically significant (*P<.05); (B) Box plot of MCT among the four groups; intergroup difference between early normal group and late normal group was statistically significant (*P<.05); (C) Box plot of MCTC among the four groups; intergroup difference between CLAD group and all other groups was statistically significant (*P<.05, **P<.01); (D) Box plot of ratio of MCTC to MCT among the four groups; intergroup difference between CLAD group and all other groups was statistically significant (***P<.001). ACR, acute cellular rejection; CLAD, chronic lung allograft dysfunction.
FIGURE 2.
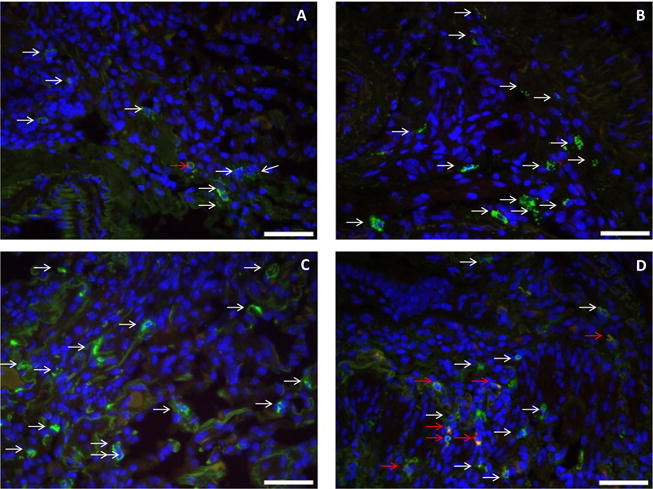
Immunofluorescence staining for tryptase, chymase, and DAPI nuclear staining (40×) forali the four groups (A: early normal allograft, B: late normal allograft, C: acute cellular rejection, D: chronic lung allograft dysfunction) for patient #3. DAPI nuclear staining shows as blue color indicating any nucleated cell. Tryptase only positive cells (MCT cells) show green-colored granules (white arrows) which appear to increase from figure A to D. MCTC cells show dual staining (red and green, red arrows) which appear to be significantly increased in (D) representing the CLAD group.
In contrast, the absolute number of MCTC and the ratio of MCTC to MCT were similar on biopsies from early normal, late normal, and ACR group and significantly increased on biopsies from CLAD group (Table 2). This was confirmed on GLM analysis where difference in both MCTC (P=.002) as well as the ratio of MCTC to MCT (P<.001) between the four groups was statistically significant, with CLAD group having the highest number of MCTC cells compared to the other three groups (Tables 3 and 4 and Fig. 3C, D).
TABLE 3.
Intergroup differences in the total burden of MCTC cells among the four groups (overall difference between the groups significant at P=.002)
| Group | Group | Estimate | Standard error | df | t Value | Pr>|t| | Adj P |
|---|---|---|---|---|---|---|---|
| Early normal | Late normal | −0.4718 | 0.4849 | 171 | −0.97 | .3320 | .7651 |
| Early normal | ACR | −0.3231 | 0.4877 | 171 | −0.66 | .5086 | .9110 |
| Early normal | CLAD | −1.7007 | 0.4772 | 171 | −3.56 | .0005 | .0026 |
| Late normal | ACR | 0.1487 | 0.4815 | 171 | 0.31 | .7578 | .9897 |
| Late normal | CLAD | −1.2290 | 0.4708 | 171 | −2.61 | .0098 | .0480 |
| ACR | CLAD | −1.3777 | 0.4736 | 171 | −2.91 | .0041 | .0212 |
ACR, acute cellular rejection; CLAD, chronic lung allograft dysfunction. Bold indicates P values <0.05.
TABLE 4.
Intergroup differences in the ratio of MCTC to MCT cells among the four groups (overall difference between the groups significant at P<.001)
| Group | Group | Estimate | Standard error | df | t Value | Pr>|t| | Adj P |
|---|---|---|---|---|---|---|---|
| Early normal | Late normal | 2.1184 | 2.7680 | 186 | 0.77 | .4450 | .8700 |
| Early normal | ACR | −1.1059 | 2.8718 | 186 | −0.39 | .7006 | .9805 |
| Early normal | CLAD | −28.8924 | 2.8118 | 186 | −10.28 | <.0001 | <.0001 |
| Late normal | ACR | −3.2243 | 2.7858 | 186 | −1.16 | .2486 | .6544 |
| Late normal | CLAD | −31.0108 | 2.7550 | 186 | −11.26 | <.0001 | <.0001 |
| ACR | CLAD | −27.7865 | 2.7536 | 186 | −10.09 | <.0001 | <.0001 |
ACR, acute cellular rejection; CLAD, chronic lung allograft dysfunction. Bold indicates P values <0.05.
4 | DISCUSSION
To the best of our knowledge, this is the first human study evaluating MC phenotypes in the allograft at different clinicopathologic stages after LT. The current analysis suggests that the total burden of MC in the allograft increases over time after LT, and TBLB specimens with CLAD have an increased burden of total MC that is driven by a change in MC phenotype toward MCTC (Tables 3 and 4, Fig. 3).
Correlation analysis of time since transplant and number of MC appears to suggest a progressive increase in MC infiltration with time (Fig. 1). It is unclear whether this process is exacerbated by episodes of ACR. The trends in total MC and MCT cell counts seemed to favor an increase with ACR (Table 2). However, the intergroup comparisons on GLM analysis did not show a statistically significant difference in the total MC or MCT populations between biopsies with and without ACR. This could indeed be a β error given the small sample size. Earlier studies examining the role of MC in ACR reported conflicting results. The only human study in LT patients demonstrated a positive association between MC burden and ACR,10 as did other human studies of non-pulmonary solid organ transplantation.8,9 In contrast, an animal study did not find an increase in MCT cells with ACR.24 Interestingly, in the current study, all biopsies with ACR were performed prior to the biopsies from late normal group and the intergroup difference in the MCT population was statistically significant between early normal and late normal group. These data may suggest a gradual increase in total MC and MCT populations occurring as a time-dependent variable, perhaps driven by a variety of environmental influences.
The burden of MC infiltration was the highest among biopsies with CLAD which could reflect a time-dependent increase as these biopsies were farthest from the time of transplantation. This finding is in agreement with the earlier human study among LT patients where patients with OB had the highest burden of MC in comparison with normal allograft or ACR.10 However, it is noteworthy that the pattern of MC infiltration among CLAD patients was dominated by the MCTC phenotype. It appears plausible that an increase in allograft MC burden, dominated initially by MCT phenotype cells with potentially immunomodulatory effects, evolves into a profibrotic MCTC phenotype which heralds a progressive decline in lung function culminating in the development of CLAD. This is in consonance with the phenotypic switch of MCT to MCTC and its association with allograft fibrosis that has been reported post-renal transplantation.21,22 Nevertheless, whether MCTC phenotype is independently linked to development of CLAD, or more significantly, plays a pathologic role in CLAD remains to be investigated.
Identifying a phenotypic switch of MCT to MCTC may have important clinical and therapeutic implications. Immunostaining of TBLB specimens is technically straightforward and may be a useful diagnostic tool. Finding of increased MCTC phenotype may favor a diagnosis of CLAD which is helpful given the current diagnostic criteria is purely based on spirometry and requires waiting for 3 months. Further, if MCTC phenotype is indeed a precursor to CLAD, it is pertinent to design mechanistic studies aimed at understanding pathways that lead to this switch. In this regard, IL-4 has been identified as one of the mediators that may promote “maturation” of MCT to the MCTC phenotype.25 Interestingly, in a rat model animal study, prophylactic use of cromolyn sodium, a mast cell stabilizing agent, has been shown to attenuate development of OB lesions.26 Further, in a study of MC phenotypes among asthma patients, MCTC was increased among patients with severe and poorly controlled asthma.27 These patients had poor corticosteroid responsiveness, which may partly explain the lack of response to many of the treatment approaches among CLAD patients.
The current study has several limitations. The lack of a control arm (normal allograft) that was matched with the CLAD group for time since transplant was a major limitation. Although the inclusion of a control arm was considered, TBLB specimens among patients with normal allograft function are generally not available beyond 1 year after LT. This limitation is difficult to circumvent even with a prospective design due to ethical reasons with regard to sampling asymptomatic patients. This was a pilot study with small sample size and possibility of β error that may have contributed to lack of differences among some of the groups. We also did not look at different severity grades of ACR or CLAD phenotypes, all of which could be possibly characterized with different degrees of MC inflammation. Finally, the current study did not assess for activation of MC phenotypes in the allograft which may be difficult to assess on archival TBLB specimens. Future studies may include measurement of cytokines in concurrently collected bronchoalveolar lavage fluids to address this deficiency.
In conclusion, there appears to be a time-dependent increase in MC infiltration in the allograft after LT. More significantly, there appears to be a phenotypic switch from MCT to MCTC cells that may be associated with a progressive and potentially irreversible decline in allograft function. Future studies need to confirm these preliminary findings and evaluate the potential use of therapeutics for manipulating MC-mediated inflammation in an effort to improve the long-term outcome among patients with lung transplantation.
Acknowledgments
The authors would like to acknowledge Dr Serpil Erzurum, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic for guidance, input, and helpful discussions regarding the study design. We would also like to thank Dr Carol Farver from the Department of Anatomic Pathology, Cleveland Clinic for assisting with the paraffin embedded TBLB specimens. This work was supported by NIH HL119321 and by a Lerner Research Institute Research Program Committee grant.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHORS’ CONTRIBUTIONS
AB involved in study design, chart review, immunostaining of slides, and the preparation of the manuscript. YH involved in immunostaining of slides and the preparation of the manuscript. XW contributed to data management and analysis and the preparation of the manuscript. FH designed the study, analyzed the data, and prepared the manuscript.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Burke CM, Theodore J, Dawkins KD, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest. 1984;86:824–829. doi: 10.1378/chest.86.6.824. [DOI] [PubMed] [Google Scholar]
- 3.Glanville AR, Baldwin JC, Burke CM, Theodore J, Robin ED. Obliterative bronchiolitis after heart-lung transplantation: apparent arrest by augmented immunosuppression. Ann Intern Med. 1987;107:300–304. doi: 10.7326/0003-4819-107-2-300. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 5.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan JB, Hart JP, Pizzo SV, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 7.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 8.Li QY, Raza-Ahmad A, MacAulay MA, et al. The relationship of mast cells and their secreted products to the volume of fibrosis in posttransplant hearts. Transplantation. 1992;53:1047–1051. doi: 10.1097/00007890-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Lajoie G, Nadasdy T, Laszik Z, Blick KE, Silva FG. Mast cells in acute cellular rejection of human renal allografts. Mod Pathol. 1996;9:1118–1125. [PubMed] [Google Scholar]
- 10.Yousem SA. The potential role of mast cells in lung allograft rejection. Hum Pathol. 1997;28:179–182. doi: 10.1016/s0046-8177(97)90103-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries In regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 12.Boerma M, Fiser WP, Hoyt G, et al. Influence of mast cells on outcome after heterotopic cardiac transplantation in rats. Transpl Int. 2007;20:256–265. doi: 10.1111/j.1432-2277.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Jungraithmayr W. The putative role of mast cells in lung transplantation. Am J Transplant. 2015;15:594–600. doi: 10.1111/ajt.13126. [DOI] [PubMed] [Google Scholar]
- 14.O’Keeffe C, Baird AW, Nolan N, McCormick PA. Mast cell hyperplasia in chronic rejection after liver transplantation. Liver Transpl. 2002;8:507. doi: 10.1053/jlts.2002.30343. [DOI] [PubMed] [Google Scholar]
- 15.Pardo J, Diaz L, Errasti P, et al. Mast cells in chronic rejection of human renal allografts. Virchows Arch. 2000;437:167–172. doi: 10.1007/s004280000211. [DOI] [PubMed] [Google Scholar]
- 16.Koskinen PK, Kovanen PT, Lindstedt KA, Lemström KB. Mast cells in acute and chronic rejection of rat cardiac allografts—a major source of basic fibroblast growth factor. Transplantation. 2001;71:1741–1747. doi: 10.1097/00007890-200106270-00007. [DOI] [PubMed] [Google Scholar]
- 17.Walgenbach KJ, Heeckt PF, Stanson JD, Whiteside TL, Bauer AJ. Increased presence of mast cells and interleukin-4 during chronic rejection of rat intestinal allografts. Transplant Proc. 1996;28:2454. [PubMed] [Google Scholar]
- 18.Overed-Sayer C, Rapley L, Mustelin T, Clarke DL. Are mast cells instrumental for fibrotic diseases? Front Pharmacol. 2014;4:174. doi: 10.3389/fphar.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz LB. Analysis of MC(T) and MC(TC) mast cells in tissue. Methods Mol Biol. 2006;315:53–62. [PubMed] [Google Scholar]
- 20.Jahanyar J, Youker KA, Loebe M, et al. Mast cell-derived cathepsin g: a possible role in the adverse remodeling of the failing human heart. J Surg Res. 2007;140:199–203. doi: 10.1016/j.jss.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Yamada M, Ueda M, Naruko T, et al. Mast cell chymase expression and mast cell phenotypes in human rejected kidneys. Kidney Int. 2001;59:1374–1381. doi: 10.1046/j.1523-1755.2001.0590041374.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Hyodo Y, Ishimura T, Takeda M, Hara I, Fujisawa M. Mast cell numbers and protease expression patterns in biopsy specimens following renal transplantation from living-related donors predict long-term graft function. Clin Transplant. 2005;19:817–824. doi: 10.1111/j.1399-0012.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 23.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Minami M, Nakahara K, Matsumura A, et al. Histamine release from pulmonary mast cells after lung transplantation in rats. J Heart Lung Transplant. 1995;14:505–511. [PubMed] [Google Scholar]
- 25.Toru H, Eguchi M, Matsumoto R, Yanagida M, Yata J, Nakahata T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91:187–195. [PubMed] [Google Scholar]
- 26.Chang JC, Leung J, Tang T, et al. Cromolyn ameliorates acute and chronic injury in a rat lung transplant model. J Heart Lung Transplant. 2014;33:749–757. doi: 10.1016/j.healun.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balzar S, Fajt ML, Comhair SA, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


