Abstract
Severe acute graft-versus-host disease (aGVHD) remains a major source of morbidity and mortality following mismatched unrelated donor hematopoietic cell transplantation (HCT). Through a retrospective analysis, we investigated the efficacy of GVHD prophylaxis with rabbit anti-thymocyte globulin (ATG) 7.5 mg/kg (1 mg/kg given on day −3, then 3.25 mg/kg/day on days −2 and −1 before stem cell infusion) followed by standard tacrolimus plus methotrexate in a consecutive series of 45 HLA partially matched unrelated donor HCT recipients. The cumulative incidence of grade III–IV aGVHD was 11% by 100 days (95% confidence interval [CI] 5%–25%). Moderate to severe chronic GVHD (per NIH consensus criteria) was 19% (95% CI 10%–36%) at 1 year, and 28% (95% CI 16%–48%) at 2 years. With a median follow-up time for surviving patients of 12 months (range: 5–39 months), overall survival was 55% (95% CI 39%–71%) at 1 year, and 45% (95% CI 27%–63%) at 2 years. Nonrelapse mortality was 11% (95% CI 5%–25%) by 100 days post-HCT, 26% (95% CI 16%–44%) by 1 year, and 30% (95% CI 18%–50%) by 2 years. The cumulative incidence of primary disease relapse was 23% (95% CI 13%–41%) at 1 year, and 33% (95% CI 20%–56%) by 2 years after HCT. Cytomegalovirus (CMV) infection or reactivation varied according to recipient and donor CMV serostatus. Epstein-Barr Virus (EBV) reactivation occurred in 54% (95% CI 40%–71%) of patients. Preemptive rituximab therapy was administered for EBV reactivation, however, posttransplant lymphoproliferative disorder was diagnosed in 5 (11%) cases, and was fatal in 1. A regimen of ATG 7.5 mg/kg total ending on day −1 effectively decreased the occurrence of grade III–IV aGVHD and severe chronic GVHD.
Keywords: Acute graft-versus-host disease, Antithymocyte globulin, Mismatched unrelated donor transplantation
INTRODUCTION
Acute and chronic graft-versus-host disease (aGVHD, cGVHD) remain significant sources of morbidity and mortality following allogeneic donor hematopoietic cell transplantation (HCT). As donor alloreactive T cells are principal mediators of the syndrome, in vivo T cell depletion by antilymphocyte antibodies have been studied in the prevention [1,2], pre-emptive therapy [3,4], and treatment of established GVHD following HCT [5–10].
The efficacy of anti-thymocyte globulin (ATG) in the prevention of both aGVHD and cGVHD is supported by evidence including retrospective comparative data [11–15] as well as randomized and nonrandomized prospective clinical trials [16–28]. Studies have demonstrated reduction in aGVHD, and long-term follow up has demonstrated significantly lower cGVHD, lung dysfunction, and late nonrelapse mortality (NRM) in those treated with ATG [29,30]. Alongside the beneficial reduction in GVHD, the use of ATG delays immune reconstitution [31–34] and confers an increased risk of Ebstein-Barr virus (EBV) reactivation and EBV-associated posttransplant lymphoproliferative disease (PTLD) [35,36]. Risk of PTLD increases with age, T cell depletion, ATG use, and unrelated or HLA-mismatched grafts [34]. Posttransplantation monitoring of EBV viral load and preemptive rituximab therapy is effective in controlling EBV and preventing PTLD in the majority of cases [37–40].
Diverse approaches to the delivery of antilymphocyte antibodies have been pursued to date, including the following: rabbit ATG (Thymoglobulin) 4.5 mg/kg total, divided over 3 days concluding on day 0 [12]; Thymoglobulin 7.5 mg/kg total, divided on days −4 and −3, or Thymoglobulin 15 mg/kg total, divided from days −5 to −2 [16]; and ATG-Fresenius (ATG-F; Fresenius Biotech, Graefelfing, Germany) total dose ranging from 30 to 90 mg/kg, ending on day −1 [11,19,41]. Comparative data suggest that ATG potency varies among formulations, increases with dose and with shorter time interval to HCT [16,41–43]. Here, we report the safety and efficacy of Thymoglobulin 7.5 mg/kg total, administered over 3 days ending on day −1, for the prevention of aGVHD in mismatched unrelated donor HCT.
METHODS
Patient Characteristics
Adult patients who received ATG for GVHD prophylaxis before HLA mismatched, unrelated donor HCT were the subjects of a retrospective study approved by the University of South Florida institutional review board. Consecutive patients between September 2006 and March 2010 were identified by review of existing database records. Inclusion criteria were the use of ATG as GVHD prevention, and a transplant from an HLA mismatched unrelated donor. Recipient-donor pairs with sole mismatch at HLA-DQ, or complete allelic matching at loci A, B, C, and DR were excluded. Additionally, recipients of umbilical cord blood stem cells were excluded from this analysis. The principal aim of the study was to estimate the cumulative incidence of grade III–IV aGVHD.
Baseline characteristics included the following: date of HCT; stem cell product infusion time; recipient and donor HLA typing; recipient and donor age, gender, and cytomegalovirus (CMV) serostatus; disease condition and remission status at time of HCT; conditioning regimen; aGVHD prophylaxis agents; and ATG utilization (start date and time, schedule of delivery, total dose delivered, stop date, and time).
Standardized data abstraction was performed. Neutrophil engraftment was defined by the first of 3 successive days with an absolute neutrophil count of >500/µL. Platelet engraftment was defined by the first of 3 successive days with a nontransfused platelet count of >20,000/µL. Primary graft failure was defined as failure to achieve a neutrophil count of ≥500/µL in patients who survived ≥28 days following transplantation and who have not undergone a second transplant procedure. Secondary graft failure was defined as a decline of neutrophils to <500/µL after having engrafted that is unresponsive to growth factors. Acute GVHD was scored per modified Glucksberg criteria [44]. Chronic GVHD was scored per the proposed NIH consensus criteria [45]. Chimerism was assessed by capillary electrophoresis of single tandem repeats on peripheral blood sorted CD3- and CD33-positive cells and bone marrow cells at days 30, 90, 180, and 360. Absolute lymphocyte counts, and lymphocyte subsets were quantified at 3, 6, and 12 months following HCT. Data collected included CMV reactivation onset date and serum copy number, peak date and copy number, number of discrete episodes of reactivation, the occurrence and site of CMV disease, and CMV therapy delivered. Data also included the date and log copy number of EBV reactivation, peak date, and copy number, number of doses of rituximab delivered, and the occurrence of PTLD. Data were also gathered on the occurrence of adenovirus, HHV-6, and BK virus following HCT. The occurrence of bacterial and invasive fungal infections was also captured.
Conditioning Therapy and ATG Regimen
The conditioning regimen in 41 of the 45 cases consisted of fludarabine, 40 mg/m2 infused over 30 minutes on days −6 to −3, followed by intravenous busulfan, 130 to 145 mg/m2 over 4 hours daily on the same days. Busulfan PK-samples were obtained on day −6 and analyzed by mass spectrometry; on days −4 and-3. The busulfan dose was adjusted to target an average area under the curve (AUC) of 5300 (±10%) µMol*min (n = 36) or 3500 (±10%) µMol*-min (n = 3) for each of the 4 days; the reduced busulfan target AUC was selected according to transplant physician discretion in these 3 cases because of patient age at transplant (range: 62–67 years of age). Two patients were enrolled on a prospective trial examining dose escalation of i.v. pharmacokinetic- targeted busulfan and fludarabine with a target average AUC of 7500 µMol*-min. Three patients (multiple myeloma, n = 2, acute myelogenous leukemia, n = 1) received fludarabine 40 mg/m2 on days −5 through −2 with melphalan 70 mg/m2 on day −2. Finally, 1 patient with aplastic anemia was conditioned with fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 on days −5 through −2, and 200 cGy total-body irradiation (TBI) on day 0.
The ATG regimen provided a total of 7.5 mg/kg rabbit ATG, administered as 1 mg/kg on day −3 (over ≥6 hours), then 3.25 mg/kg/day on days −2 and −1 (over ≥4 hours) prior to the stem cell infusion. The dose of ATG was based on actual body weight; however, if actual body weight was ≥30% ideal body weight, the dose delivered was per kg of adjusted body weight (adjusted body weight = [ideal body weight + actual body weight]/2). Standard supportive care included the following: on day −3, methylprednisolone 2 mg/kg was given i.v. 1 hour prior to ATG infusion. As well, starting 6 hours after the 2 mg/kg loading dose on day −3 through day −1, methylprednisolone 1 mg/kg was given i.v. every 12 hours × 5 total doses. Additional supportive care included acetaminophen, diphenhydramine, and dilaudid. Standardized protocol existed for management of anaphylactic reactions.
Statistical Methods
Baseline characteristics were summarized using descriptive statistics. Accounting for competing risk events, cumulative incidence of primary disease relapse and nonrelapse mortality (NRM) was calculated [46]. Similarly, the cumulative incidence of acute (grade II–IV, and separately, grade III–IV) and cGVHD (any grade cGVHD, and separately, moderate to severe cGVHD) was estimated. Overall survival (OS) was estimated from date of transplantation using the Kaplan-Meier method. The cumulative incidence for each of CMV (according to recipient-donor serostatus), EBV, adenovirus, HHV-6, BK, bacterial, and invasive fungal infections was calculated. All analyses were performed using NCSS 2007 software version 7.1.20 (NCSS.LLC Kaysville, Utah).
RESULTS
Patient Characteristics
A consecutive series of 45 unrelated donor HCT recipients matched at 6/8 or 7/8 HLA-A, -B, -C, or -DRB1 loci received ATG (Thymoglobulin, Genzyme, Cambridge, MA) for GVHD prevention from September 2006 to March 2010 at Moffitt Cancer Center. Analysis and reporting of this data was approved by the University of South Florida institutional review board. Baseline characteristics are described in Table 1. HCT consisted uniformly of peripheral blood mobilized stem cells. ATG delivery was intended to be completed on day −1. In this series, the actual time elapsed between completion of ATG infusion and start time of stem cell infusion was a median of 18 hours (range: 5–39 hours).
Table 1.
Baseline Characteristics of ATG-Treated HCT Recipients
| Age in years Median | 48 (range: 22–67) |
| Year HCT performed | |
| 2007 | 12 |
| 2008 | 9 |
| 2009 | 19 |
| 2010 | 5 |
| Recipient:donor gender match | |
| F:F | 10 |
| F:M | 10 |
| M:M | 18 |
| M:F | 7 |
| Disease | |
| AA | 1 |
| ALL | |
| CR1 | 2 |
| CR2 | 2 |
| CR3 | 1 |
| AML | |
| CR1 | 11 |
| CR2 | 11 |
| CR3 | 2 |
| CLL (relapsed) | 1 |
| CML | |
| CP | 1 |
| AP | 1 |
| CMML/MPD | 2 |
| HD | |
| CR2 | 2 |
| REL1 | 1 |
| MDS | 4 |
| MM | |
| PR | 1 |
| VGPR | 1 |
| NHL (CR3) | 1 |
| Conditioning regimen | |
| Bu/Flu | |
| AUC 3500 | 3 |
| AUC 5300 | 36 |
| AUC 7500 | 2 |
| FluMel | 3 |
| Flu/Cy/TBI | 1 |
| Recipient:donor CMV serostatus | |
| Neg:neg | 7 |
| Neg:pos | 5 |
| Pos:neg | 18 |
| Pos:pos | 15 |
| GVHD prophylaxis | |
| TAC/MTX | 40 |
| TAC/MMF | 2 |
| TAC/RAPA | 3 |
| Recipient:donor HLA disparity | |
| A | |
| Antigen | 11 |
| Allele | 3 |
| A+DRB | 1 |
| B | |
| Antigen | 10 |
| Allele | 4 |
| C | |
| Antigen | 4 |
| Allele | 4 |
| B+C | 1 |
| DRB | |
| Antigen | 7 |
AA indicates aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; CMML/MPD, chronic myelomonocytic leukemia, myeloproliferative disorder; HD, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MM, mulitple myeloma; NHL, non-Hodgkin lymphoma; Bu/Flu, pharmacokinetic-targeted i.v. busulfan and fludarabine; FluMel, fludarabine and melphalan; Flu/cy/TBI, fludarabine, cyclophosphamide, total-body irradiation; CMV, cytomegalovirus; TAC, tacrolimus; MTX, methotrexate; MMF, mycophenolate mofetil; RAPA, sirolimus; HCT, hematopoietic stem cell transplantation; ATG, anti-thymocyte globulin.
Engraftment and Donor Chimerism
Delivery of ATG was well tolerated with no cases of anaphylaxis. ATG did not interfere with the delivery of conditioning chemotherapy or aGVHD prophylaxis agents. There were no cases of primary engraftment failure. Neutrophil (median 15 days, range: 11–25) and platelet (median 16 days, range: 7–31) engraftment was not delayed. Following predominantly myeloablative conditioning, sustained full donor chimerism was promptly established following HCT in the majority of cases. On day 30 after HCT, median donor chimerism for CD3 was 100% (range: 21%–100%), CD33 was 100% uniformly, and unsorted bone marrow chimerism was 100% (range: 90%–100%). By day 90 after HCT, median donor chimerism for CD3 was 100% (range: 0%–100%), CD33 was 100% (range: 97%–100%), and bone marrow was 97% (86%–100%). At 1 year, donor chimerism for CD3 was 97% (0%–100%), CD33 was 100% (44%–100%), and bone marrow was 97% (84%–100%). In 1 case, donor chimerism was lost in the CD3 compartment by 90 days post-HCT; the patient survives and remains free of relapse 35 months post-HCT. One other patient failed to achieve full donor chimerism in the CD3 compartment, with 21% donor CD3 by day 30 post-HCT; serial measures have demonstrated progressive loss of donor chimerism in both CD3 and CD33 compartments. The patient remains alive, and free of relapse with 6 months of follow-up thus far.
GVHD
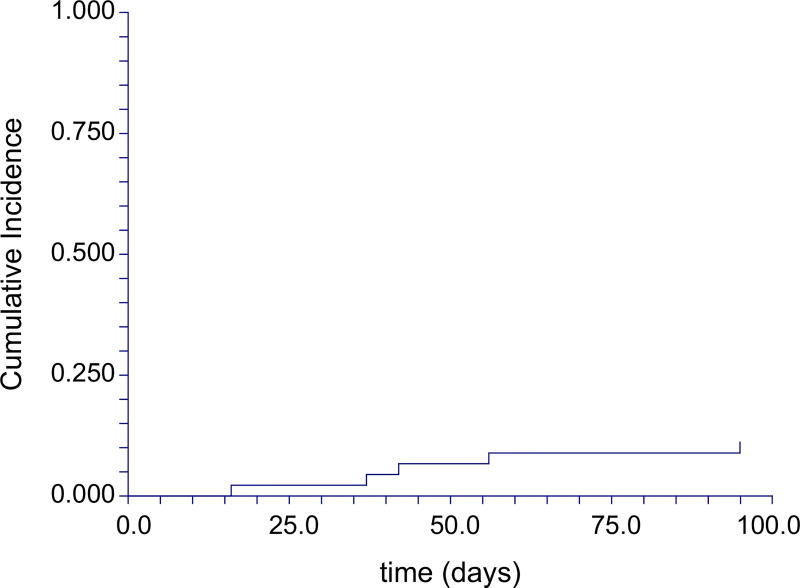
The cumulative incidence of grade II–IV acute GVHD was 64% (95% confidence interval [CI] 52%–80%). There was an overall incidence of severe, grade III–IV aGVHD (Figure 1) of 11% by 100 days (95% CI 5%–25%). Accounting for late aGVHD (without concurrent manifestations of cGVHD), the cumulative incidence of grade III–IV aGVHD by 1 year was 18% (95% CI 10%–34%). Skin was involved in 44% of cases, gastrointestinal (GI) in 78%, and liver in 11%. Of those with skin involvement, severity distribution was the following: grade 1 in 65%, 2 in 25%, and 3 in 10%. Of those with GI involvement, severity distribution was: grade 1 in 74%, 2 in 9%, 3 in 14%, and grade 4 in 3%. Finally, of those with liver involvement, distribution was as follows: grade 1 in 20%, 2 in 20%, 3 in 20%, and 4 in 40%.
Figure 1.
Cumulative incidence of grade III–IV aGVHD from date of HCT.
Initial therapy for aGVHD consisted of systemic glucocorticoids in all cases, with median starting dose of 1 mg/kg/day (range: 0.16–2 mg/kg/day). Secondary therapy was utilized for ongoing GVHD manifestations (n = 15) or steroid dependence (n = 9) according to transplant physician discretion in 24 total patients. Agents utilized were the following: mycophenolate mofetil in 12 cases, rapamycin in 16, infliximab in 3, extracorporeal photopheresis (ECP) in 2, and pentostatin in 1 case.
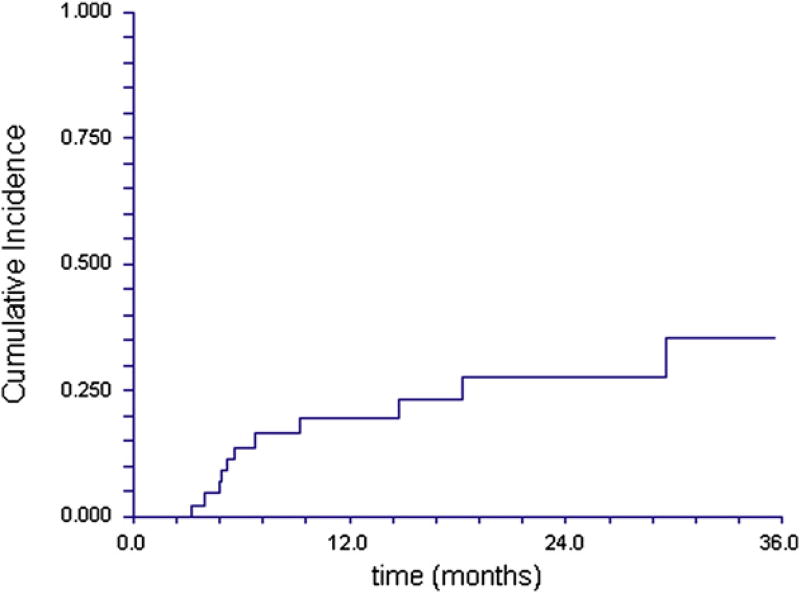
Of those at risk, the overall cumulative incidence of any grade cGVHD was 35% (95% CI 23%–53%) at 1 year, and 43% (95% CI 29%–63%) by 2 years. The cumulative incidence of moderate to severe cGVHD (Figure 2) was 19% (95% CI 10%–36%) at 1 year, and 28% (95% CI 16%–48%) at 2 years.
Figure 2.
Cumulative incidence of moderate to severe cGVHD (per NIH severity criteria) from date of HCT.
Survival, Relapse, and NRM
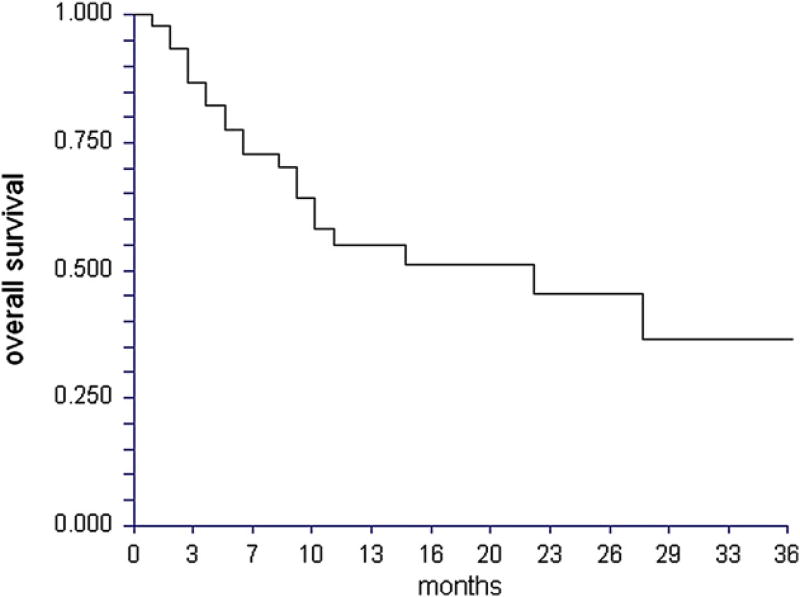
With a median follow-up time for surviving patients of 12 months (range: 5–39 months), OS was 55% (95% CI 39%–71%) at 1 year, and 45% (95% CI 27%–63%) at 2 years (Figure 3). A total of 9 patients died following primary disease relapse posttransplantation. Of these 9 relapse-related deaths, 6 died directly from relapse, 1 died from parainfluenza pneumonia, 1 from refractory aGVHD after manipulation of immune suppression in relapse, and 1 died as a result of EBV reactivation with biopsy-confirmed PTLD leading to multiorgan system failure. A total of 12 patients died in the absence of disease relapse. Of these nonrelapse deaths, causes of death were pneumonia and respiratory failure (n = 2), septic shock and multiorgan failure (n = 1), aGVHD (n = 4), cGVHD (n = 1), invasive fungal infection (n = 2), confirmed CMV pneumonia and respiratory failure (n = 1), and not known (n = 1). The cumulative incidence of NRM was 11% (95% CI 5%–25%) by 100 days post-HCT, 26% (95% CI 16%–44%) by 1 year, and 30% (95% CI 18%–50%) by 2 years. The cumulative incidence of primary disease relapse was 23% (95% CI 13%–41%) by 1 year, and 33% (95% CI 20%–56%) by 2 years post-HCT. These data on primary disease relapse post-HCT should be interpreted with caution given the heterogeneity in disease conditions and remission status represented in this series.
Figure 3.
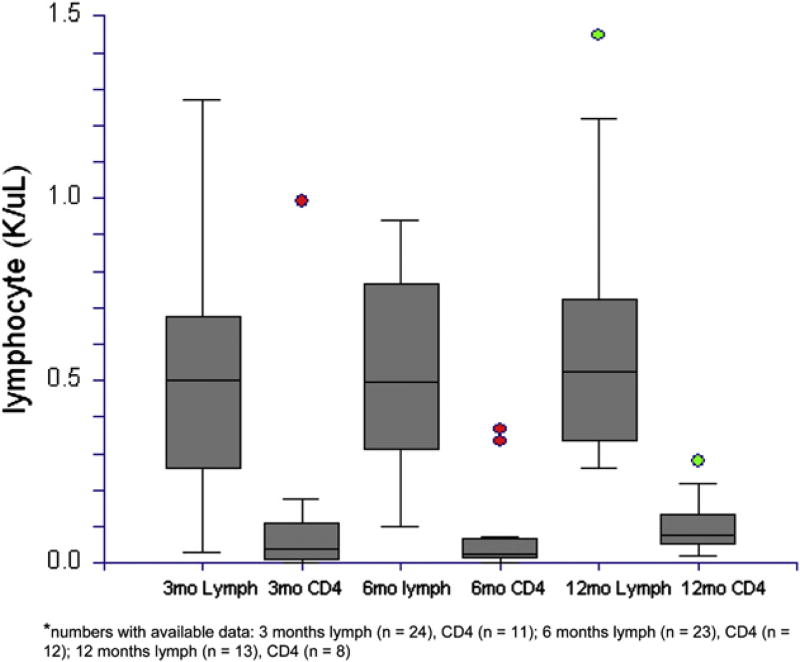
Kaplan-Meier plot of OS from date of HCT. Numbers with available data: 3 months lymph (n = 24), CD4 (n = 11); 6 months lymph (n = 23), CD4 (n = 12); 12 months lymph (n = 13), CD4 (n = 8).
Immune Reconstitution and Infectious Complications
Data on absolute lymphocyte and immune cell subsets were abstracted at 3, 6, and 12 months post-HCT. From those with available data, absolute lymphocyte and CD4 counts are presented in Figure 4. These data demonstrate impaired immune reconstitution. Notably, the median CD4 count remained below 200/µL by 12 months following HCT.
Figure 4.
Absolute lymphocyte and CD4 lymphocyte reconstitution at 3, 6, and 12 months following date of HCT.
CMV viral load by PCR was monitored at least weekly, and preemptive therapy was initiated when copy number surpassed 1000/mL. The overall cumulative incidence of CMV reactivation was 60% (95% CI 47%–76%), which differed according to recipient/ donor CMV serostatus prior to HCT-negative/positive 40% (95% CI 14%–100%); positive/negative 83% (95% CI 68%–100%); and positive/positive 53% (95% CI 33%–86%). Peak copy number was a mean of 22,783 copies/mL, and the median number of discrete episodes (new episodes characterized by resolution of the prior episode, then reactivation of CMV) was 1 (range: 1–4). Confirmed CMV organ involvement was present in 7 total cases (pulmonary 3, gastrointestinal 2, central nervous system 1, and urinary bladder 1). The median time from first detection of CMV to diagnosis of CMV disease in these 7 cases was 106 days (range: 10–284 days). The copy number at first detection of CMV in these 7 cases was a median of 700 copies/mL (range: 500–1350 copies/mL), and progressed to a median peak value of 3000 copies/mL (range: 1350–9950 copies/mL). Therapy with valganciclovir or foscarnet was initiated when copy number exceeded 1000 copies/mL. This therapy successfully managed CMV reactivation and disease in all but 1 patient who died with CMV pneumonia and respiratory failure.
EBV was monitored weekly following HCT for patients who received transplants from 8/1/07 onward (n = 40 total); there were 5 cases prior to this point wherein EBV was not systematically monitored on a weekly basis post-HCT. Of these 5 cases, 1 patient was found to have EBV reactivation approximately 5 months after HCT, but died within a month of this from GVHD and pneumonia. No clinical or pathological evidence of PTLD was present in these 5 cases. Considering only the 40 remaining patients in which EBV was systematically monitored weekly following HCT, the cumulative incidence of EBV reactivation was 54% (95% CI 40%–71%). The median peak value for EBV copy number was 4.1 log (range: 2.9–6.7). Preemptive therapy with rituximab at 375 mg/m2 weekly was administered when EBV copy number exceeded 1000 per mL. Of 23 patients with EBV reactivation, a total of 21 patients received rituximab at a median of 4 doses (range: 1–6). The remaining 2 patients were not treated with rituximab. One had a maximum value of 750 copies/mL and resolved without treatment. The other patient had a peak EBV viremia of 1000 copies/mL, but was not treated in the setting of critical illness with refractory GVHD and pneumonia. PTLD was diagnosed at a median of 3 months (range: 1–9 months) post-HCT in 5 total cases; 2 of these were biopsy confirmed, whereas the others were diagnosed based on radiographic and clinical findings. Of these 5 cases, 1 died with multiorgan system failure in the setting of uncontrolled EBV-associated PTLD. Two others died of sepsis, 1 from GVHD, and 1 from relapsed primary disease.
Other infectious complications examined included the following: adenoviral infection was detected in 4% (95% CI 1%–17%). Human Herpes Virus 6 (HHV6) reactivation was detected in 9% (95% CI 3%–23%). Of these, none had end-organ involvement including pneumonitis or encephalitis. Significant BK virus infection defined by plasma BK virus copy number occurred in 42% (95% CI 30%–60%). Median BK log copy number was 3.9 (range: 2.9–8.6). These cases were successfully treated with either leflunomide or cidofovir. Invasive fungal infections occurred in 28% (95% CI 17%–48%). Fungal species isolated included Candida (n = 7), Cryptococcus (n = 1), Aspergillus (n = 3), and Mucor species (n = 1). Three total patients died from disseminated fungal infection. Finally, 57% (95% CI 44%–75%) experienced bacterial infection following HCT. Organisms identified were a heterogeneous array of Gram- positive and -negative organisms. Of those affected, 3 died from bacterial sepsis.
DISCUSSION
Severe aGVHD remains a significant obstacle to successful unrelated donor HCT. The risk of severe aGVHD and the associated NRM is even greater in the setting of HLA mismatch. To mitigate this risk, we have employed a regimen of rabbit ATG 7.5 mg/kg for the prevention of severe aGVHD after HCT from unrelated donors matched for 6/8 or 7/8 HLA alleles at -A, -B, -C, or -DRB1. We have utilized Thymoglobulin, as allied evidence from solid organ allografting demonstrates that ATG is more immunosuppressive than Atgam (anti-thymocyte globulin [equine];) [47–49]. As well, ATG is associated with no delay in engraftment, in contrast to ATG-Fresenius, which prolongs neutropenia and thrombocytopenia [19]. On the basis of data from the 2 small randomized controlled trials from Bacigalupo et al. [16], we selected the ATG regimen of 7.5 as opposed to the 15 mg/kg total dose to minimize toxicity from opportunistic infections, but decided to delay the schedule of administration to days −3, −2, and −1 before HSCT to optimize efficacy.
The combination of tacrolimus and methotrexate plus ATG 7.5 mg/kg delivered on days −3 to −1 effectively prevented severe (grades III–IV) aGVHD in this series of high-risk patients in all but 11%. In comparison, a large National Marrow Donor Program (NMDP) analysis demonstrated grade III–IV aGVHD in 37% of those with 7/8, and 40% in those with 6/8 recipient-donor mismatch; importantly, only 24% of the 7/8 mismatched unrelated donor HCT recipients in the NMDP analysis had either ex vivo or in vivo T cell depletion by ATG or other antibodies [50]. The cumulative incidence of grade III–IV aGVHD approximates that reported in randomized trials of ATG prevention in completely matched unrelated donor HCT [12,16]. The efficacy of this approach is likely related to the dose and the proximity of ATG delivery to the infusion of the allograft, in keeping with prior literature, which suggests an association between prevention of severe aGVHD and timing of ATG pre-HCT. Direct comparisons to prior series and randomized trials are limited, however, by the disparate pharmacological prophylaxis agents (entirely tacrolimus combination regimens in this series versus cyclosporine/methotrexate in prior trials) utilized in concert with ATG, as well as the divergent stem cell products (uniformly peripheral blood mobilized stem cells in our series). The incidence of any grade cGVHD in this series approximates that reported in other studies, but we have notably observed an overall low incidence of moderate to severe cGVHD. We acknowledge the relatively limited follow-up time in this series, and the likelihood that more mature data will provide a better estimate of the true incidence of moderate to severe cGVHD in this patient series. Further comparisons to existing literature are limited by the use of limited/extensive scoring in prior studies and our use of the recommended NIH consensus severity scoring in this series. Finally, the overall outcomes in this series compare favorably with existing literature. One year OS was 55% in this series, compared to 43% and 33% for 7/8 and 6/8 mismatched unrelated donor transplants in the NMDP analysis, respectively. We have also explored difference in outcomes between those with antigen-versus allele-level mismatch in our series: although moderate to severe cGVHD was significantly lower in those with allele-level mismatch (P = .04), all other major outcomes (grade II–IV aGVHD, III–IV aGVHD, relapse, NRM, OS) did not significantly differ. These conclusions are limited by small sample size.
The regimen demonstrates an overall favorable safety profile. In contrast to published estimates of primary engraftment failure ranging from 13% to 17% for 6/8 and 7/8 HLA locus matched unrelated donor transplants [50], we observed no cases of primary engraftment failure. As well, donor chimerism was promptly achieved and maintained in the majority of these cases following primarily myeloablative conditioning. NRM at 1 year (26% in our series) is lower than the 45% to 55% previously reported for 6/8 or 7/8 HLA locus matched unrelated transplants [50]. Impaired immune reconstitution and infectious complications represent established risks with the use of antilymphocyte antibodies for in vivo T cell depletion. With a strategy of vigilant monitoring and preemptive therapy, CMV reactivation was largely controlled in this series. However, several cases of CMV disease were observed, including 1 death from CMV pulmonary disease. Despite rituximab, PTLD remains a potentially life-threatening complication. Although there is robust evidence in support of surveillance and preemptive therapy for EBV reactivation, primary prevention with prophylactic rituximab post-HCT may attenuate this risk further. Early evidence from a pilot trial conducted by Small et al. [51] suggests marked suppression of EBV reactivation and PTLD with 6 monthly prophylactic doses of rituximab following T cell–depleted HCT. In total, the beneficial reduction in severe aGVHD is balanced by an increased risk of infectious complications, as well as requirement for intensive monitoring and preemptive therapy for CMV and EBV reactivation.
Acknowledging the limitations inherent in single-center retrospective analyses, our data suggest marked activity of ATG 7.5 mg/kg ending on day −1 in the prevention of severe aGVHD in 6/8 or 7/8 matched unrelated donor transplants. Antilymphocyte antibodies appear to decrease the risk of severe aGVHD in unrelated donor HCT. It is also important to acknowledge the evidence to date, which substantiates alternative T cell–depletion strategies, such as ex vivo T cell depletion or alemtuzumab. The evidence available to date in the efficacy of antilymphocyte antibodies for the prevention of severe aGVHD is limited, however, and there is marked heterogeneity in the utilization of these agents before HLA matched or partially matched unrelated donor HCT. Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) showed that, among patients treated for acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and myelodysplastic syndrome (MDS) during 2009, only 395 of 1059 (37%) received ATG for GVHD prophylaxis after HLA matched or partially matched unrelated donor marrow or peripheral blood HCT. Given these limitations, we argue that a definitive randomized trial is needed to discern the true benefit of ATG for the prevention of severe aGHVD in unrelated donor transplantation.
Footnotes
Financial disclosures: The authors have nothing to disclose.
References
- 1.Bacigalupo A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant. 2005;35:225–231. doi: 10.1038/sj.bmt.1704758. [DOI] [PubMed] [Google Scholar]
- 2.Mohty M. Mechanisms of action of antithymocyte globulin:T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 3.Bacigalupo A, Lamparelli T, Milone G, et al. Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant. 45:385–391. doi: 10.1038/bmt.2009.151. [DOI] [PubMed] [Google Scholar]
- 4.Bacigalupo A, Oneto R, Lamparelli T, et al. Pre-emptive therapy of acute graft-versus-host disease: a pilot study with antithymocyte globulin (ATG) Bone Marrow Transplant. 2001;28:1093–1096. doi: 10.1038/sj.bmt.1703306. [DOI] [PubMed] [Google Scholar]
- 5.Macmillan ML, Couriel D, Weisdorf DJ, et al. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood. 2007;109:2657–2662. doi: 10.1182/blood-2006-08-013995. [DOI] [PubMed] [Google Scholar]
- 6.Dugan MJ, DeFor TE, Steinbuch M, Filipovich AH, Weisdorf DJ. ATG plus corticosteroid therapy for acute graft-versus-host disease: predictors of response and survival. Ann Hematol. 1997;75:41–46. doi: 10.1007/s002770050310. [DOI] [PubMed] [Google Scholar]
- 7.Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8:155–160. doi: 10.1053/bbmt.2002.v8.pm11939605. [DOI] [PubMed] [Google Scholar]
- 8.Khoury H, Kashyap A, Adkins DR, et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001;27:1059–1064. doi: 10.1038/sj.bmt.1703032. [DOI] [PubMed] [Google Scholar]
- 9.MacMillan ML, Weisdorf DJ, Davies SM, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:40–46. doi: 10.1053/bbmt.2002.v8.pm11858189. [DOI] [PubMed] [Google Scholar]
- 10.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77:1821–1828. [PubMed] [Google Scholar]
- 11.Ayuk F, Diyachenko G, Zabelina T, et al. Anti-thymocyte globulin overcomes the negative impact of HLA mismatching in transplantation from unrelated donors. Exp Hematol. 2008;36:1047–1054. doi: 10.1016/j.exphem.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Duggan P, Booth K, Chaudhry A, et al. Unrelated donor BMT recipients given pretransplant low-dose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant. 2002;30:681–686. doi: 10.1038/sj.bmt.1703674. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Min WS, Cho BS, et al. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15:704–717. doi: 10.1016/j.bbmt.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Kroger N, Zabelina T, Binder T, et al. HLA-mismatched unrelated donors as an alternative graft source for allogeneic stem cell transplantation after antithymocyte globulin-containing conditioning regimen. Biol Blood Marrow Transplant. 2009;15:454–462. doi: 10.1016/j.bbmt.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Zander AR, Kroger N, Schleuning M, et al. ATG as part of the conditioning regimen reduces transplant-related mortality (TRM) and improves overall survival after unrelated stem cell transplantation in patients with chronic myelogenous leukemia (CML) Bone Marrow Transplant. 2003;32:355–361. doi: 10.1038/sj.bmt.1704157. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay NK, Kersey JH, Robison LL, et al. A randomized study of the prevention of acute graft-versus-host disease. N Engl J Med. 1982;306:392–397. doi: 10.1056/NEJM198202183060703. [DOI] [PubMed] [Google Scholar]
- 18.Weiden PL, Doney K, Storb R, Thomas ED. Antihuman thymocyte globulin for prophylaxis of graft-versus-host disease. A randomized trial in patients with leukemia treated with HLA-identical sibling marrow grafts. Transplantation. 1979;27:227–230. doi: 10.1097/00007890-197904000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 20.Champlin RE, Perez WS, Passweg JR, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toor A, Rodriguez T, Bauml M, et al. Feasibility of conditioning with thymoglobulin and reduced intensity TBI to reduce acute GVHD in recipients of allogeneic SCT. Bone Marrow Transplant. 2008;42:723–731. doi: 10.1038/bmt.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schleuning M, Gunther W, Tischer J, Ledderose G, Kolb HJ. Dose-dependent effects of in vivo antithymocyte globulin during conditioning for allogeneic bone marrow transplantation from unrelated donors in patients with chronic phase CML. Bone Marrow Transplant. 2003;32:243–250. doi: 10.1038/sj.bmt.1704135. [DOI] [PubMed] [Google Scholar]
- 23.Bonifazi F, Bandini G, Rondelli D, et al. Reduced incidence of GVHD without increase in relapse with low-dose rabbit ATG in the preparative regimen for unrelated bone marrow transplants in CML. Bone Marrow Transplant. 2003;32:237–242. doi: 10.1038/sj.bmt.1704138. [DOI] [PubMed] [Google Scholar]
- 24.Meijer E, Bloem AC, Dekker AW, Verdonck LF. Effect of antithymocyte globulin on quantitative immune recovery and graft-versus-host disease after partially T-cell-depleted bone marrow transplantation: a comparison between recipients of matched related and matched unrelated donor grafts. Transplantation. 2003;75:1910–1913. doi: 10.1097/01.TP.0000065737.60591.9D. [DOI] [PubMed] [Google Scholar]
- 25.Finke J, Schmoor C, Lang H, Potthoff K, Bertz H. Matched and mismatched allogeneic stem-cell transplantation from unrelated donors using combined graft-versus-host disease prophylaxis including rabbit anti-T lymphocyte globulin. J Clin Oncol. 2003;21:506–513. doi: 10.1200/JCO.2003.03.129. [DOI] [PubMed] [Google Scholar]
- 26.Remberger M, Storer B, Ringden O, Anasetti C. Association between pretransplant thymoglobulin and reduced non-relapse mortality rate after marrow transplantation from unrelated donors. Bone Marrow Transplant. 2002;29:391–397. doi: 10.1038/sj.bmt.1703374. [DOI] [PubMed] [Google Scholar]
- 27.Tanosaki R, Uike N, Utsunomiya A, et al. Allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning for adult T cell leukemia/lymphoma: impact of antithymocyte globulin on clinical outcome. Biol Blood Marrow Transplant. 2008;14:702–708. doi: 10.1016/j.bbmt.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Bonifazi F, Bandini G, Stanzani M, et al. In vivo T-cell depletion with low-dose ATG is effective in reducing cGVHD after peripheral blood stem cell myeloablative sibling transplants in CML: results from a prospective phase II study. Bone Marrow Transplant. 2005;35:1025–1026. doi: 10.1038/sj.bmt.1704940. [DOI] [PubMed] [Google Scholar]
- 29.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Bacigalupo A, Lamparelli T, Gualandi F, et al. Prophylactic antithymocyte globulin reduces the risk of chronic graft-versus-host disease in alternative-donor bone marrow transplants. Biol Blood Marrow Transplant. 2002;8:656–661. doi: 10.1053/bbmt.2002.v8.abbmt080656. [DOI] [PubMed] [Google Scholar]
- 31.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–2711. [PubMed] [Google Scholar]
- 32.Fehse N, Fehse B, Kroger N, et al. Influence of anti-thymocyte globulin as part of the conditioning regimen on immune reconstitution following matched related bone marrow transplantation. J Hematother Stem Cell Res. 2003;12:237–242. doi: 10.1089/152581603321628377. [DOI] [PubMed] [Google Scholar]
- 33.Juvonen E, Aalto SM, Tarkkanen J, et al. High incidence of PTLD after non-T-cell-depleted allogeneic haematopoietic stem cell transplantation as a consequence of intensive immunosuppressive treatment. Bone Marrow Transplant. 2003;32:97–102. doi: 10.1038/sj.bmt.1704089. [DOI] [PubMed] [Google Scholar]
- 34.Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy N, Rezvani K, Barrett AJ, Savani BN. Strategies to prevent EBV reactivation and post-transplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high risk patients. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Esser JW, van der Holt B, Meijer E, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood. 2001;98:972–978. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad I, Cau NV, Kwan J, et al. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation. 2009;87:1240–1245. doi: 10.1097/TP.0b013e31819f1c49. [DOI] [PubMed] [Google Scholar]
- 38.Blaes AH, Cao Q, Wagner JE, Young JA, Weisdorf DJ, Brunstein CG. Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing nonmyeloablative conditioning for umbilical cord blood transplantation. Biol Blood Marrow Transplant. 16:287–291. doi: 10.1016/j.bbmt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Esser JW, Niesters HG, van der Holt B, et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99:4364–4369. doi: 10.1182/blood.v99.12.4364. [DOI] [PubMed] [Google Scholar]
- 40.Weinstock DM, Ambrossi GG, Brennan C, Kiehn TE, Jakubowski A. Preemptive diagnosis and treatment of Epstein-Barr virus-associated post transplant lymphoproliferative disorder after hematopoietic stem cell transplant: an approach in development. Bone Marrow Transplant. 2006;37:539–546. doi: 10.1038/sj.bmt.1705289. [DOI] [PubMed] [Google Scholar]
- 41.Ayuk F, Diyachenko G, Zabelina T, et al. Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:913–919. doi: 10.1016/j.bbmt.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Hamadani M, Blum W, Phillips G, et al. Improved nonrelapse mortality and infection rate with lower dose of antithymocyte globulin in patients undergoing reduced-intensity conditioning allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2009;15:1422–1430. doi: 10.1016/j.bbmt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meijer E, Cornelissen JJ, Lowenberg B, Verdonck LF. Antithymocyteglobulin as prophylaxis of graft failure and graft-versus-host disease in recipients of partially T-cell-depleted grafts from matched unrelated donors: a dose-finding study. Exp Hematol. 2003;31:1026–1030. doi: 10.1016/s0301-472x(03)00204-2. [DOI] [PubMed] [Google Scholar]
- 44.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 45.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 47.Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 48.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66:29–37. doi: 10.1097/00007890-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 49.Hardinger KL, Rhee S, Buchanan P, et al. A prospective, randomized, double-blinded comparison of thymoglobulin versus Atgam for induction immunosuppressive therapy: 10-year results. Transplantation. 2008;86:947–952. doi: 10.1097/TP.0b013e318187bc67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 51.Small TNKN. Jakubowski A, Young JW, Prockop S, Perales M-A, O’Reilly RJ, Papadopoulos EB. Pilot trial of rituximab for the prevention of Epstein Barr Virus B cell lymphoproliferative disorder (EBV-LPD) following T cell depleted (TCD) unrelated or HLA-mismatched related hemato-poietic stem cell transplantation. Blood. 2006;108:2922. [Google Scholar]