Abstract
Purpose
Traditional and complementary medicine (T&CM) strategies are commonly used in pediatric oncology. Patterns may vary based on country income. We systematically reviewed published studies describing T&CM use among pediatric oncology patients in low-income countries (LIC/LMIC), middle-income countries (UMIC), and high-income countries (HIC). Objectives included describing estimated prevalence of use, reasons for use, perceived effectiveness, modalities used, rates of disclosure, and reporting of delayed or abandoned treatment.
Methods
MEDLINE, EMBASE, Global Health, CINAHL, PsycINFO, Allied and Complementary Medicine Database, Cochrane Database of Systematic Reviews, and ProceedingsFirst were searched. Inclusion criteria were primary studies involving children younger than the age of 18 years, undergoing active treatment of cancer, and any T&CM use. Exclusion criteria included no pediatric oncology–specific outcomes and studies involving only children off active treatment. Data were extracted by two reviewers using a systematic data extraction form determined a priori.
Results
Sixty-five studies published between 1977 and 2015 were included, representing 61 unique data sets and 7,219 children from 34 countries. The prevalence of T&CM use ranged from 6% to 100%. Median rates of use were significantly different in LIC/LMIC (66.7% ± 19%), UMIC (60% ± 26%), and HIC (47.2% ± 20%; P = .02). Rates of disclosure differed significantly by country income, with higher median rates in HIC. Seven studies reported on treatment abandonment or delays.
Conclusion
The use of T&CM in pediatric oncology is common worldwide, with higher median prevalence of use reported in LIC/LMIC. Further research is warranted to examine the impact on treatment abandonment and delay.
INTRODUCTION
Traditional and complementary medicine (T&CM) strategies encompass health care practices and traditions that exist outside of the dominant biomedical health perspective, known as conventional medicine. Also termed Western medicine or biomedicine, conventional medicine is considered to include proven and experimental therapies that have arisen from an evidence-based paradigm or longstanding, commonly accepted medical practice. Traditional and complementary therapies often predate this paradigm, and in addition to health practices may represent essential cultural practices, particularly to indigenous peoples. The United Nations Declaration on the Rights of Indigenous Peoples codifies the right of indigenous peoples to “maintain their health practices”1(p9) and the WHO has recently published a 10-year strategy to encourage member states to develop policy to incorporate T&CM in health systems planning.2
In pediatric oncology, T&CM strategies are widely used3 but can present medical, legal, and ethical challenges to health practitioners. The use of T&CM may lead to uncertain interactions with conventional medicine (particularly when the use of T&CM is not disclosed), may delay therapies with proven effectiveness, and may be associated with abandonment of conventional therapy,4,5 potentially increasing mortality risk. Ethical concerns include cost, effectiveness, and availability of T&CM.6,7
Previous reviews3,8-12 have looked at the prevalence of T&CM and attempted to synthesize and critically appraise the quality of T&CM research in pediatric cancer. Reported prevalence of use has ranged from 6% to 91%.3,8-12 A recent review by Bishop et al3 identified heterogeneous study quality as a major barrier to research in T&CM. No studies to date have examined the use of T&CM in pediatric oncology globally by region, in relation to country income level or indicators of health system functioning. In areas where access to conventional medical treatments may be limited by availability of practitioners, cost, or distance, T&CM practices may be the primary health system used.13
The primary objectives of this systematic review were to describe estimated prevalence of T&CM use by country income group, types of T&CM used, reasons for use, perceived effectiveness, and disclosure of T&CM use to the oncology treating team. Secondary objectives included documenting treatment delays or abandonment related to T&CM use, assessing family characteristics associated with higher rates of T&CM use, and analyzing T&CM use in each country relative to national indicators of health expenditure per capita and physician availability.
METHODS
Data Sources and Searches
We adhered to standard protocols for preparing and reporting this systematic review.14,15 This review was registered a priori with the PROSPERO registry (CRD42015017295). We searched Medline, Embase, CINAHL, Global Health, PsycInfo, Allied and Complementary Medicine Database (AMED), the Cochrane Database of Systematic Reviews, and FirstSearch ProceedingsFirst from the date of inception of each database through February 20, 2016. Attempts were made to contact authors for additional information where appropriate. Keyword and text word searching were performed with all relevant terms, controlling for different spellings, synonyms, and truncations. The search strategy was reviewed by all authors as well as a library scientist. Bibliographies of included articles were reviewed for additional references. The search strategies can be viewed in the Data Supplement.
Study Selection and Data Abstraction
Inclusion criteria defined a priori comprised studies concerning children younger than age 18 years in at least 10% of participants, active treatment of cancer, and use of any type of T&CM as defined by the National Center for Complementary and Alternative Medicine, with or without the use of conventional therapy.16 Prayer was included as a T&CM because of its often integral role in traditional medicine strategies. Exclusion criteria defined a priori comprised a lack of pediatric oncology–specific outcomes reported, children not on active treatment in > 50% of participants, and case reports. All other study types were included. There were no language exclusions.
Two reviewers (C.D. and S.M.) independently reviewed all references. Potentially relevant publications were retrieved for full-text review. Final inclusion was determined by consensus.
Data were independently abstracted by two reviewers (C.D. and S.M.) using a standardized form.17 Papers not in English had data abstracted by pediatric oncology health care professionals whose native language was the language of the study.
Outcomes
The primary objective of our study was to describe T&CM use stratified by country income level. The prevalence of T&CM use, types of T&CM used, reasons for use, perceived effectiveness of T&CM, and prevalence of disclosure of T&CM use to the treating oncology team were measured by self-report or parental proxy report in included studies. All modalities and reasons for T&CM use reported in the primary studies were recorded in data abstraction, but only the top five were reported. In addition, any reported use of T&CM for cancer cure was specifically noted. Any papers that reported on abandonment of therapy, delay in diagnosis, or initiation of therapy were recorded. Characteristics associated with T&CM use as captured in the primary studies were listed.
Countries were stratified based on country income into four strata using the World Bank country income classification18: low-income countries (LIC), lower-middle income countries (LMIC), upper-middle income countries (UMIC), and high-income countries (HIC).
Secondary analyses related T&CM use in each country to total (public and private) health expenditure per capita per year (in current US dollars) and physicians per 1,000 people.18 Average per capita spending on health expenditure is $1,008 as per the WHO19; therefore, health expenditures were categorized as < $1,008 per capita versus ≥ $1,008 per capita. A second analysis was done using a cutoff of $44 per capita, which represents the minimum spending per person per year needed to provide basic, life-saving services as per the WHO.19 With regard to physicians per capita, a cutoff of 2.3 physicians per 1,000 people was used in keeping with the WHO Millennium Development Goals physician benchmark.20 Values published for each country were examined, and the most recent value available was used.21,22
Data Synthesis and Analysis
We determined a priori, on the basis of anticipated heterogeneity of data, that a meta-analysis would not be appropriate3; in lieu, it was determined that providing weighted mean estimates per country would allow a meaningful quantification of estimates. A weighted mean prevalence by number was used to synthesize data from countries with more than one published estimate of T&CM use. Significance between prevalence of use and income levels, physicians per capita, and health expenditure, as well as between median rates of T&CM use as cure for cancer and proportion reporting disclosure relative to country income levels, were determined using Kruskal-Wallis one-way analysis of variance. All statistical analyses were performed using the SAS statistical program (SAS-PC, version 9.4; SAS Institute, Cary, NC). All tests of significance were two-sided. Statistical significance was defined as P < .05.
Quality Assessment
Study quality was assessed using the T&CM in pediatric oncology–specific Quality Assessment Tool (QAT).3 We measured agreement in QAT scores using a weighted Cohen κ and percentage of agreement.24 QAT scores were not completed for articles in languages other than English.
RESULTS
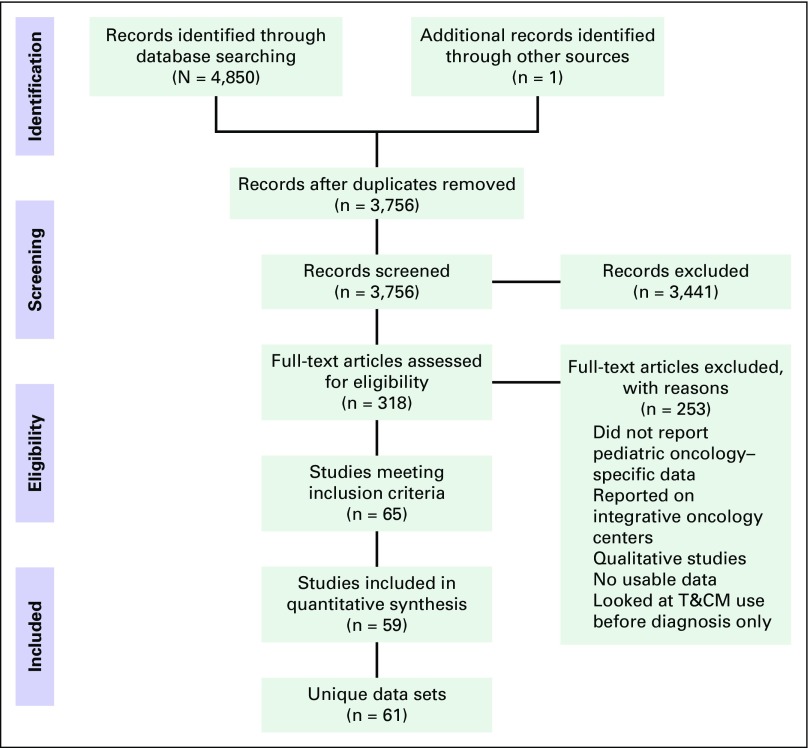
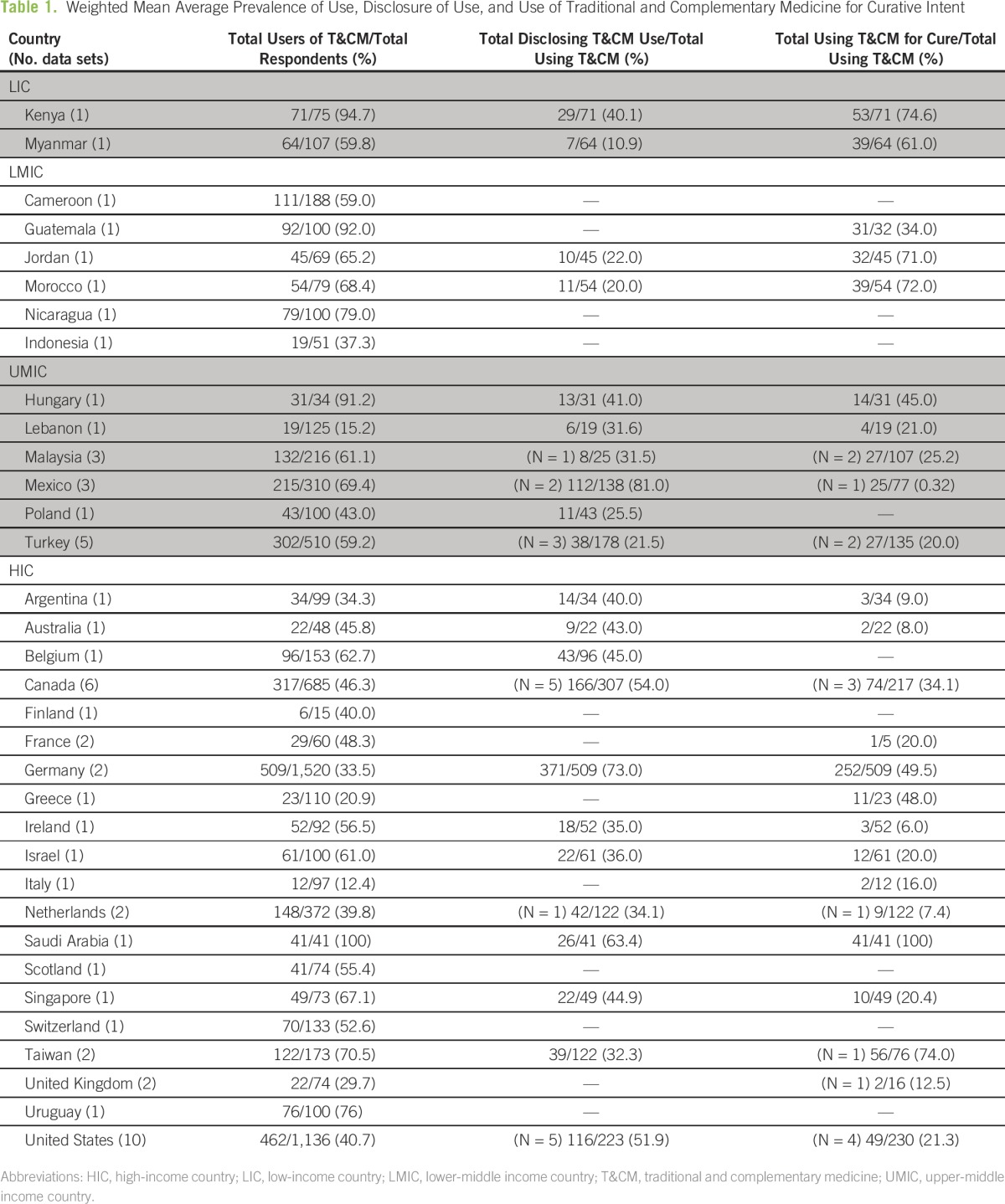
Figure 1 illustrates the flow diagram of trial identification and selection on the basis of the PRISMA template. A total of 3,756 studies were screened. Sixty-five studies met inclusion criteria, but only 59 studies were included in the quantitative synthesis, because several studies published multiple papers examining the same data set,25-30 and there were two studies where conference abstracts had not been identified in screening to contain the same material as the eventual paper.31-34 A total of 61 data sets were included, because one study35 published data from an LMIC, UMIC, and HIC, and these were handled as separate data sets in the analysis. One study was identified through bibliographic reviews.32 Thirty-four different countries were represented within the data set, with a range of one to 10 studies for each country. There were two data sets from LIC, seven from LMIC, 13 from UMIC, and 39 from HIC. Table 1 presents the prevalence and characteristics of use across countries. Most studies were published in English, with three studies in French and one each in Polish, Hungarian, Chinese, and German.
Fig 1.
– Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of study identification and selection. T&CM, traditional and complementary medicine.
Table 1.
– Weighted Mean Average Prevalence of Use, Disclosure of Use, and Use of Traditional and Complementary Medicine for Curative Intent
Prevalence Estimates of the Use of T&CM
Prevalence of use of T&CM ranged from 6% to 100%. The lowest (6%, N = 66) was reported in the United States in 1983.36 Since the first study published on T&CM use in pediatric oncology in 1977, prevalence of use in the United States has ranged from 6% to 84%, with no consistent trend over time.36-45 The highest prevalence of use of T&CM (100%, N = 41) was reported in Saudi Arabia in 2011.46 There are no other published estimates from Saudi Arabia meeting inclusion criteria for comparison; however, in other Middle Eastern countries prevalence of T&CM use ranged from 15% to 65%.33,34,47
The median estimated prevalence of use was 77% (± 25%) in LIC, 67% (± 19%) in LMIC, 60% (± 26%) in UMIC, and 47% (± 20%) in HIC. Because there were only two LIC studies, the LIC and LMIC were considered together for the purposes of this analysis; median prevalence of use in LIC/LMIC was 67% (± 19%). A significant difference was observed between the LIC/LMIC, UMIC, and HIC median rates of use (P = .02).
Median prevalence of use of T&CM was significantly higher among countries that spent < $1,008 per capita at 63% (± 21%) than among those who spent $1,008 or more at 46% (± 19%; P = .01). Median prevalence of use of T&CM in countries where the per capita health expenditure was < $44 was 60% (± 8%, N = 3), and it was 56% (± 22%, N = 31) in those where spending was ≥ $44 per capita (P = .55). Among countries with fewer than 2.3 physicians per 1,000 citizens, median prevalence of use was 61% (± 16%) compared with 46% (± 24%) among nations with at least 2.3 physicians per 1,000 citizens (P = .02).
Type of T&CM Used
Fifty-seven out of 61 data sets (93%) reported on the types of T&CM used. Commonly used modalities included oral supplements and dietary changes, alternative medical systems (including traditional healers), and spiritual treatments including faith healing, prayer, and religious practices. Traditional healers were used in all income groups, reported in Kenya (LMIC),48 Cameroon (LMIC),49 Malaysia (UMIC),50 and Israel and Canada (HIC).51,52 Homeopathy use was reported exclusively in UMIC and HIC, most commonly in European countries. A detailed list of T&CM treatments used is presented in the Data Supplement.
Reasons for T&CM Use
Thirty-nine data sets (64%) reported on reasons for T&CM use. Common reasons cited for the use of T&CM included curing the cancer, doing everything possible for their child, boosting the immune system, improving general well-being, and treating adverse effects of conventional therapy. The median rate of T&CM use for cure of cancer reported in LIC/LMIC was 53% (± 25%), in UMIC was 25% (± 10%), and in HIC was 20% (± 27%), although these differences did not reach significance (P = .23).
Perceived Effectiveness of T&CM
Effectiveness was reported in 29 out of 61 (48%) of the data sets, with most users reporting T&CM as being effective or very effective (Data Supplement). Effectiveness was measured as perceived effectiveness by users/parents; no studies reported objective measures. Most studies reported very high overall effectiveness of T&CM, with only four studies reporting general rates < 50%.36,55,59,60 In particular, one American36 study with four T&CM users noted that none reported that the T&CM treatments were effective.
Disclosure of T&CM Use
Disclosure of T&CM use to the oncology treating team ranged from 8% (Turkey)59 to 78% (Germany)29,30 and was reported in 37 of 61 data sets (61%). Median rates of disclosure differed significantly between LIC/LMIC, UMIC, and HIC, with rates of 21% (± 7%), 32% (± 22%), and 45% (±15%), respectively (P = .03). Reasons for nondisclosure were reported by eight studies,45,48,60-65 including four HIC,45,60,64,65 two UMIC,61,62 and two LIC.48,63 Examples of reasons for nondisclosure included belief that T&CM was nontoxic,64 fear of the physician’s reaction,48,61-63,65 belief that the medical staff lacked knowledge of T&CM,45,60 and the physician did not ask.45,61
T&CM and Delay in Diagnosis or Treatment or Abandonment of Treatment
Three studies reported abandonment of conventional therapy related to T&CM use. In a study from British Columbia, Canada, 5% (eight of 156) of T&CM users chose alternative medicine instead of conventional care.66 Similarly, 4% (two of 46) of T&CM users in a Taiwanese study abandoned conventional treatment of folk remedies.67 A study from Malaysia reported three patients abandoning conventional medicine for T&CM (unspecified if these patients were adults or children).61 Outcomes were reported only for the Taiwanese study, in which both patients relapsed and were rehospitalized.
Four studies reported on delays in conventional treatment to pursue T&CM: one of 92 patients from Quebec, Canada,68 7% (three of 41) in Saudi Arabia,46 9% (four of 45) in Jordan,33 and 13% (three of 13) in Malaysia.69 None of the included studies reported specific outcome data for patients who delayed conventional treatment of T&CM.
Associations With T&CM Use
Details of the family characteristics associated with reported T&CM use are listed in the Data Supplement. Associations with T&CM use were reported almost exclusively by studies from HIC. Several studies reported higher rates of T&CM use associated with increasing parental education29,31,70,71,72 and higher socioeconomic status.73 Only one study from Guatemala reported an analysis of this association in an LMIC.31 Similarly, only studies from HIC reported a statistically significant association between T&CM use and dissatisfaction with conventional treatments (United States40 and Singapore64) and parental T&CM use (United States,43,44 Canada,74 and the Netherlands75). One study from Lebanon reported higher rates of T&CM use associated with a family history of leukemia.47
Quality Assessment Tool Scores
QAT scores determined by consensus for the included studies are listed in the Data Supplement. Scores ranged from 6 to 15, with a median score of 11.5 out of 18 (± 2.5). Interrater reliability demonstrated excellent agreement76 by the weighted Cohen κ statistic of 0.89 (95% CI, 0.81 to 0.97), with 72% agreement in raw scores.
DISCUSSION
The use of T&CM in pediatric oncology is common and widespread. We demonstrate significantly different reported rates of use of T&CM among patients in LIC/LMIC, UMIC, and HIC, and as related to measures of health system resources. We also describe differences in frequency of disclosure of T&CM use to oncology treating teams among patients in LIC/LMIC, UMIC, and HIC; T&CM types used; and reasons for use. Among the limited studies that reported on outcomes of T&CM use, T&CM was associated with abandonment of treatment and delay in diagnosis or treatment of cancer in seven out of 61 data sets in a range of countries. These important characteristics of T&CM use must be used to help guide advocacy and intervention strategies for children with cancer worldwide.
In LIC/LMIC median reported rates of use are significantly higher than in UMIC and HIC. Evidence to explain this difference is limited. In many LIC/LMIC, patients must pay to access conventional health care, and in one study from Cameroon the lower cost of seeking care from a traditional healer was reported as a reason for use.49 Beyond this, other hypothesized reasons include other elements of cost, health beliefs, and trust. Families’ comfort with T&CM practitioners may be higher than with hospitals because of the importance practitioners have in the local community.77-79 Oncologic care in LIC/LMIC is typically delivered at larger centers that may be a significant distance from the homes of patients; as such, travel may be limited by access to vehicles and associated costs, including lost family income.80 Furthermore, in areas where punitive practices such as hospital detention exist, families may be deterred from presenting to hospitals.81 In contrast, the prevalence of use of T&CM in HIC was associated with measures of higher socioeconomic status (including parental education), dissatisfaction with conventional treatment, and parental T&CM use. Further research into context-specific rates and reasons for use of T&CM is required.
Our results demonstrated several reports of abandonment of conventional therapy for T&CM. Although a recent meta-analysis reported rates of abandonment of 29% for children with acute leukemia in LMIC,82 abandonment can also affect patients in UMIC and HIC with prevalence of as high as 2%82 to 5%.66 Physicians in all settings must be aware of the potential for patients to prefer T&CM over conventional therapy and of the local legal, ethical, and moral norms, particularly as these may be in evolution.6,83 The rates of abandonment and delay of treatment due to T&CM in LIC/LMIC are likely higher than what is currently reported in the literature, as there may be a large number of patients with oncologic conditions who are being managed solely by traditional practitioners, who never present to an oncology treating team.13,84 Unfortunately, few studies reported on this important issue. Future research should attempt to quantify the rates of nonpresentation, abandonment (including up-front refusal of therapy), and/or delay of conventional therapy for T&CM.
Awareness of the high rates of T&CM use is essential to guide clinical practice. Pediatricians and oncology providers must learn to work alongside T&CM practitioners across all settings. This is particularly true in LIC and LMIC, where the use of traditional medicines is widespread and embedded in cultural practices.2 Success has been demonstrated in the treatment of HIV/AIDS in sub-Saharan Africa by integrating traditional health care practitioners in the treatment team.85 Harnessing the existing health systems that exist through T&CM paradigms in all resource settings could lead to greater patient satisfaction through the delivery of family-centered, culturally accessible care. Moreover, it is plausible that T&CM may provide relief for symptom management, particularly in areas with limited or inconsistent access to supportive care medications.
Our study has several limitations. Despite the fact that the highest prevalence of use may exist in LIC and LMIC, there is a paucity of data available from these settings. We attempted to address this anticipated shortcoming by not including a language limitation in our search criteria and including data from conference abstracts. We were not able to assess the quality of data in non-English studies, limiting our ability to comment on study quality. Almost all studies published are cross-sectional studies and do not describe the use of T&CM throughout the continuum of care. As previously reported by Bishop et al,3 there is a wide range in the quality of data available. In addition, there is intracountry variability in estimates over time that limits our ability to make accurate estimates of prevalence. Many countries have only single-site estimates that do not necessarily present a representative estimate of true practices in the country. UMIC and HIC prevalence of use may be under-represented, particularly in countries where a T&CM strategy may be part of the dominant health system. For example, there were no reports meeting our study inclusion criteria for estimates of prevalence published from China (UMIC), where traditional Chinese medicine is embedded within the dominant medical system.86 A further limitation is the lack of validated surveys and consistent methodology. Data reported represent heterogeneous patient inclusion with different diagnoses, prognoses, and time points represented. All data available were obtained from patients who at least sought out and received some conventional medical care, and therefore the generalizability is limited.
The use of T&CM in pediatric oncology is common worldwide, with higher median prevalence estimates in lower-income countries. Further research is needed to define the cultural and regional influences on T&CM use and attempt to prospectively assess the impact of T&CM on patient outcomes. Treatment planning and delivery in all income settings must attempt to integrate an awareness of T&CM practices to better provide holistic care to patients and to work to prevent abandonment and delay of therapy.
ACKNOWLEDGMENT
We thank the International Society of Pediatric Oncology (SIOP) Pediatric Oncology in Developing Countries (PODC) Traditional and Complementary Medicine Task Force for their gracious support. We also thank John Wiernikowski, PharmD, and Alexandra Bartal, PharmD, for their assistance.
Footnotes
Presented at the International Society of Pediatric Oncology International Congress, Capetown, South Africa, October 8-11, 2015.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Stacey Marjerrison
Collection and assembly of data: Caroline Diorio, Catherine G. Lam, Stacey Marjerrison
Data analysis and interpretation: Caroline Diorio, Catherine G. Lam, Elena J. Ladas, Stacey Marjerrison
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Caroline Diorio
No relationship to disclose
Catherine G. Lam
Consulting or Advisory Role: Baxalta (I), Sanofi (I), Sigma Tau Pharmaceuticals (I), Jazz Pharmaceuticals (I)
Elena J. Ladas
No relationship to disclose
Festus Njuguna
No relationship to disclose
Glenn M. Afungchwi
No relationship to disclose
Katherine Taromina
No relationship to disclose
Stacey Marjerrison
No relationship to disclose
REFERENCES
- 1. United Nations: United Nations Declaration on the Rights of Indigenous Peoples. http://www.un.org/esa/socdev/unpfii/documents/DRIPS_en.pdf.
- 2. World Health Organization: WHO Traditional Medicine Strategy 2014-2023. Geneva, Switzerland, World Health Organization, 2013.
- 3.Bishop FL, Prescott P, Chan YK, et al. Prevalence of complementary medicine use in pediatric cancer: A systematic review. Pediatrics. 2010;125:768–776. doi: 10.1542/peds.2009-1775. [DOI] [PubMed] [Google Scholar]
- 4.Borker AS, Chaudhary N. Pattern of refusal to treat and abandonment in a new pediatric oncology unit in Southern India. Pediatr Blood Cancer. 2012;59:1100. [Google Scholar]
- 5.Friedrich P, Lam C, Kaur G, et al. Determinants of treatment abandonment: Preliminary results from a global survey by the SIOP PODC working group on treatment abandonment. Pediatr Blood Cancer. 2012;59:1126. [Google Scholar]
- 6.Hord JD, Rehman W, Hannon P, et al. Do parents have the right to refuse standard treatment for their child with favorable-prognosis cancer? Ethical and legal concerns. J Clin Oncol. 2006;24:5454–5456. doi: 10.1200/JCO.2006.06.4709. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MH. Legal and ethical issues relating to use of complementary therapies in pediatric hematology/oncology. J Pediatr Hematol Oncol. 2006;28:190–193. doi: 10.1097/01.mph.0000210401.28685.57. [DOI] [PubMed] [Google Scholar]
- 8.Kelly KM. Complementary and alternative medical therapies for children with cancer. Eur J Cancer. 2004;40:2041–2046. doi: 10.1016/j.ejca.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Myers C, Stuber ML, Bonamer-Rheingans JI, et al. Complementary therapies and childhood cancer. Cancer Contr. 2005;12:172–180. doi: 10.1177/107327480501200305. [DOI] [PubMed] [Google Scholar]
- 10.Gottschling S, Längler A, Tautz C, et al. Complementary and alternative medicine in pediatric oncology [in German] Klin Padiatr. 2006;218:157–164. doi: 10.1055/s-2006-933400. [DOI] [PubMed] [Google Scholar]
- 11.McLean TW, Kemper KJ. Complementary and alternative medicine therapies in pediatric oncology patients. J Soc Integr Oncol. 2006;4:40–45. [PubMed] [Google Scholar]
- 12.Sencer SF, Kelly KM. Complementary and alternative therapies in pediatric oncology. Pediatr Clin North Am. 2007;54:1043–1060, xiii. doi: 10.1016/j.pcl.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Merriam S, Muhamad M. Roles traditional healers play in cancer treatment in Malaysia: Implications for health promotion and education. Asian Pac J Cancer Prev. 2013;14:3593–3601. doi: 10.7314/apjcp.2013.14.6.3593. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, et al: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264-269, W64 2009. [DOI] [PubMed]
- 16. National Center for Complementary and Integrative Health: Complementary, Alternative or Integrative Health: What’s In a Name? https://nccih.nih.gov/sites/nccam.nih.gov/files/Whats_In_A_Name_08-11-2015.pdf.
- 17. Li T, Vedula SS, Hadar N, et al: Innovations in data collection, management, and archiving for systematic reviews. Ann Intern Med 162:287-294, 2015. [DOI] [PubMed]
- 18. The World Bank: World Bank Open Data. http://go.worldbank.org/U9BK7IA1J0.
- 19. World Health Organization: WHO Global Health Expenditure Atlas. Geneva, Switzerland, WHO Press, 2014. [Google Scholar]
- 20. World Health Organization: Achieving the health-related MDGs. It takes a workforce! http://www.who.int/hrh/workforce_mdgs/en/
- 21. The World Bank. Health Expenditure per Capita. 2013. http://data.worldbank.org/indicator/SH.XPD.PCAP?end=2013&start=1995.
- 22. The World Bank. Physicians (per 1000 people). 2013. http://data.worldbank.org/indicator/SH.MED.PHYS.ZS.
- 23. Reference deleted.
- 24.McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 25.Längler A, Spix C, Gottschling S, et al. Parents-interview on use of complementary and alternative medicine in pediatric oncology in Germany [in German] Klin Padiatr. 2005;217:357–364. doi: 10.1055/s-2005-872522. [DOI] [PubMed] [Google Scholar]
- 26.Laengler A, Spix C, Seifert G, et al. Complementary and alternative treatment methods in children with cancer: A population-based retrospective survey on the prevalence of use in Germany. Eur J Cancer. 2008;44:2233–2240. doi: 10.1016/j.ejca.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Läengler A, Spix C, Edelhäuser F, et al. Anthroposophic medicine in paediatric oncology in Germany: Results of a population-based retrospective parental survey. Pediatr Blood Cancer. 2010;55:1111–1117. doi: 10.1002/pbc.22523. [DOI] [PubMed] [Google Scholar]
- 28.Längler A, Spix C, Edelhäuser F, et al. Use of homeopathy in pediatric oncology in Germany. Evid Based Complement Alternat Med. 2011;2011:867151. doi: 10.1155/2011/867151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschling S, Meyer S, Längler A, et al. Differences in use of complementary and alternative medicine between children and adolescents with cancer in Germany: A population based survey. Pediatr Blood Cancer. 2014;61:488–492. doi: 10.1002/pbc.24769. [DOI] [PubMed] [Google Scholar]
- 30.Laengler A, Zuzak T, Meyer S, et al. Use of complementary and alternative medicine in pediatric cancer patients: Differences between children and adolescents. J Altern Complement Med. 2014;20:A114–A115. [Google Scholar]
- 31.Ladas EJ, Rivas S, Ndao D, et al. Use of traditional and complementary/alternative medicine (TCAM) in children with cancer in Guatemala. Pediatr Blood Cancer. 2014;61:687–692. doi: 10.1002/pbc.24791. [DOI] [PubMed] [Google Scholar]
- 32.Ladas EJ, Guillermo F, Klussmann A, et al. Use of dietary supplements among children with cancer in Guatemala. J Soc Integr Oncol. 2010;8:185. [Google Scholar]
- 33.Khateib ALA, Al-Quidemat M, Farhan N. Family strategies for managing childhood cancer: Using complementary and alternative medicine in Jordan. Support Care Cancer. 2009;17:886–887. doi: 10.1111/j.1365-2648.2010.05517.x. [DOI] [PubMed] [Google Scholar]
- 34.Al-Qudimat MR, Rozmus CL, Farhan N. Family strategies for managing childhood cancer: Using complementary and alternative medicine in Jordan. J Adv Nurs. 2011;67:591–597. doi: 10.1111/j.1365-2648.2010.05517.x. [DOI] [PubMed] [Google Scholar]
- 35.Ladas EJ, Lin M, Antillion F, et al. Improving our understanding of the use of traditional complementary/alternative medicine in children with cancer. Cancer. 2015;121:1492–1498. doi: 10.1002/cncr.29212. [DOI] [PubMed] [Google Scholar]
- 36.Copeland DR, Silberberg Y, Pfefferbaum B. Attitudes and practices of families of children in treatment for cancer. A cross-cultural study. Am J Pediatr Hematol Oncol. 1983;5:65–71. [PubMed] [Google Scholar]
- 37.Faw C, Ballentine R, Ballentine L, et al. Unproved cancer remedies. A survey of use in pediatric outpatients. JAMA. 1977;238:1536–1538. doi: 10.1001/jama.238.14.1536. [DOI] [PubMed] [Google Scholar]
- 38.Friedman T, Slayton WB, Allen LS, et al. Use of alternative therapies for children with cancer. Pediatrics. 1997;100:E1. doi: 10.1542/peds.100.6.e1. [DOI] [PubMed] [Google Scholar]
- 39.Kelly KM, Jacobson JS, Kennedy DD, et al. Use of unconventional therapies by children with cancer at an urban medical center. J Pediatr Hematol Oncol. 2000;22:412–416. doi: 10.1097/00043426-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Neuhouser ML, Patterson RE, Schwartz SM, et al. Use of alternative medicine by children with cancer in Washington state. Prev Med. 2001;33:347–354. doi: 10.1006/pmed.2001.0911. [DOI] [PubMed] [Google Scholar]
- 41.Gagnon EM, Recklitis CJ. Parents’ decision-making preferences in pediatric oncology: The relationship to health care involvement and complementary therapy use. Psychooncology. 2003;12:442–452. doi: 10.1002/pon.655. [DOI] [PubMed] [Google Scholar]
- 42.McCurdy EA, Spangler JG, Wofford MM, et al. Religiosity is associated with the use of complementary medical therapies by pediatric oncology patients. J Pediatr Hematol Oncol. 2003;25:125–129. doi: 10.1097/00043426-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Nathanson I, Sandler E, Ramírez-Garnica G, et al. Factors influencing complementary and alternative medicine use in a multisite pediatric oncology practice. J Pediatr Hematol Oncol. 2007;29:705–708. doi: 10.1097/MPH.0b013e31814fb7fc. [DOI] [PubMed] [Google Scholar]
- 44.Post-White J, Fitzgerald M, Hageness S, et al. Complementary and alternative medicine use in children with cancer and general and specialty pediatrics. J Pediatr Oncol Nurs. 2009;26:7–15. doi: 10.1177/1043454208323914. [DOI] [PubMed] [Google Scholar]
- 45.Hsu J, Gordon C, Megason G. Complementary and alternative medicine use in pediatric hematology/oncology patients at the University of Mississippi. Pediatr Blood Cancer. 2013;60:S84. [Google Scholar]
- 46. Al Sudairy R, Al Omari A, Jarrar M, et al: Complementary and alternative medicine use among pediatric oncology patients in a tertiary care center, Riyadh, Saudi Arabia. J Clin Oncol 29, 2011 (suppl; abstr e20003) [Google Scholar]
- 47.Naja F, Alameddine M, Abboud M, et al. Complementary and alternative medicine use among pediatric patients with leukemia: The case of Lebanon. Integr Cancer Ther. 2011;10:38–46. doi: 10.1177/1534735410384591. [DOI] [PubMed] [Google Scholar]
- 48.Njuguna F, Mostert S, Seijffert A, et al. Parental experiences of childhood cancer treatment in Kenya. Support Care Cancer. 2015;23:1251–1259. doi: 10.1007/s00520-014-2475-x. [DOI] [PubMed] [Google Scholar]
- 49.Hesseling P, Kouya F, Kiga M, et al. Burkitt lymphoma (BL) in Cameroon: The role of traditional healers. A prospective study in 188 patients. Pediatr Blood Cancer. 2010;55:869. [Google Scholar]
- 50.Hamidah A, Rustam ZA, Tamil AM, et al. Prevalence and parental perceptions of complementary and alternative medicine use by children with cancer in a multi-ethnic Southeast Asian population. Pediatr Blood Cancer. 2009;52:70–74. doi: 10.1002/pbc.21798. [DOI] [PubMed] [Google Scholar]
- 51.Bold J, Leis A. Unconventional therapy use among children with cancer in Saskatchewan. J Pediatr Oncol Nurs. 2001;18:16–25. doi: 10.1177/104345420101800103. [DOI] [PubMed] [Google Scholar]
- 52.Weyl Ben Arush M, Geva H, Ofir R, et al. Prevalence and characteristics of complementary medicine used by pediatric cancer patients in a mixed western and middle-eastern population. J Pediatr Hematol Oncol. 2006;28:141–146. doi: 10.1097/01.mph.0000210404.74427.10. [DOI] [PubMed] [Google Scholar]
- 53. Reference deleted.
- 54. Reference deleted.
- 55.Duleba K, Wysocki M, Styczyński J. Complementary and alternative medicine treatment in children with cancer. Preliminary report [in Polish] Med Wieku Rozwoj. 2008;12:1155–1160. [PubMed] [Google Scholar]
- 56. Reference deleted.
- 57. Reference deleted.
- 58. Reference deleted.
- 59.Karadeniz C, Pinarli FG, Oğuz A, et al. Complementary/alternative medicine use in a pediatric oncology unit in Turkey. Pediatr Blood Cancer. 2007;48:540–543. doi: 10.1002/pbc.21012. [DOI] [PubMed] [Google Scholar]
- 60.Watt L, Gulati S, Shaw NT, et al. Perceptions about complementary and alternative medicine use among Chinese immigrant parents of children with cancer. Support Care Cancer. 2012;20:253–260. doi: 10.1007/s00520-010-1063-y. [DOI] [PubMed] [Google Scholar]
- 61.Dhanoa A, Yong TL, Yeap SJ, et al. Complementary and alternative medicine use amongst Malaysian orthopaedic oncology patients. BMC Complement Altern Med. 2014;14:404. doi: 10.1186/1472-6882-14-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leal-Leal C, Isaac G, Robles C. Alternative medicine, a prospective study about its use in Mexico. Pediatr Blood Cancer. 2013;60(S3):171–172. [Google Scholar]
- 63.Sandar K, Win Lai M, Han W, et al. Use of complementary and alternative medicine (CAM) in children with cancer at Yangon Children’s Hospital. Myanmar Health Sciences Research Journal. 2013;25:183–188. [Google Scholar]
- 64.Lim J, Wong M, Chan MY, et al. Use of complementary and alternative medicine in paediatric oncology patients in Singapore. Ann Acad Med Singapore. 2006;35:753–758. [PubMed] [Google Scholar]
- 65.Suen YI, Gau BS, Chao SC. Survey of parents of children with cancer who look for alternative therapies [in Chinese] Hu Li Za Zhi. 2005;52:29–38. [PubMed] [Google Scholar]
- 66.Fernandez CV, Stutzer CA, MacWilliam L, et al. Alternative and complementary therapy use in pediatric oncology patients in British Columbia: Prevalence and reasons for use and nonuse. J Clin Oncol. 1998;16:1279–1286. doi: 10.1200/JCO.1998.16.4.1279. [DOI] [PubMed] [Google Scholar]
- 67.Yeh CH, Tsai JL, Li W, et al. Use of alternative therapy among pediatric oncology patients in Taiwan. Pediatr Hematol Oncol. 2000;17:55–65. doi: 10.1080/088800100276668. [DOI] [PubMed] [Google Scholar]
- 68.Martel D, Bussières JF, Théorêt Y, et al. Use of alternative and complementary therapies in children with cancer. Pediatr Blood Cancer. 2005;44:660–668. doi: 10.1002/pbc.20205. [DOI] [PubMed] [Google Scholar]
- 69.Ariffin H, Abdullah WA, de Bruyne J, et al. Belief in traditional healers amongst Malaysian parents of children with cancer. J Trop Pediatr. 1997;43:375–376. doi: 10.1093/tropej/43.6.375. [DOI] [PubMed] [Google Scholar]
- 70.Clerici CA, Veneroni L, Giacon B, et al. Complementary and alternative medical therapies used by children with cancer treated at an Italian pediatric oncology unit. Pediatr Blood Cancer. 2009;53:599–604. doi: 10.1002/pbc.22093. [DOI] [PubMed] [Google Scholar]
- 71.Chantrain C, Servais N, Zech F, et al. Use of complementary and alternative medicine in children with cancer: Report of a Belgian center for pediatric hematology oncology. Haematologica. 2011;96(S2):676. [Google Scholar]
- 72.O’Connor N, Graham D, O’Meara A, et al. The use of complementary and alternative medicine by Irish pediatric cancer patients. J Pediatr Hematol Oncol. 2013;35:537–542. doi: 10.1097/MPH.0b013e31829f408a. [DOI] [PubMed] [Google Scholar]
- 73.Revuelta Iniesta R, Wilson ML, White K, et al. Potential impacts on nutritional management in cancer: Complementary and alternative therapy usage in a Scottish paediatric cohort. Ann Nutr Metab. 2013;63(S1):1271. [Google Scholar]
- 74.Valji R, Adams D, Dagenais S, et al. Complementary and alternative medicine: A survey of its use in pediatric oncology. Evid Based Complement Alternat Med. 2013;2013:527163. doi: 10.1155/2013/527163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singendonk M, Kaspers GJ, Naafs-Wilstra M, et al. High prevalence of complementary and alternative medicine use in the Dutch pediatric oncology population: A multicenter survey. Eur J Pediatr. 2013;172:31–37. doi: 10.1007/s00431-012-1821-6. [DOI] [PubMed] [Google Scholar]
- 76.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 77. Muhamad M, Merriam S, Suhami N: Why breast cancer patients seek traditional healers. Int J Breast Cancer 2012:689168, 2012. [DOI] [PMC free article] [PubMed]
- 78.Struthers R, Eschiti VS. Being healed by an indigenous traditional healer: Sacred healing stories of Native Americans. Part II. Complement Ther Clin Pract. 2005;11:78–86. doi: 10.1016/j.ctnm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Ross E. Traditional healing in South Africa: Ethical implications for social work. Soc Work Health Care. 2008;46:15–33. doi: 10.1300/j010v46n02_02. [DOI] [PubMed] [Google Scholar]
- 80.Magrath I, Steliarova-Foucher E, Epelman S, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol. 2013;14:e104–e116. doi: 10.1016/S1470-2045(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 81. Mostert S, Lam CG, Njuguna F, et al: Hospital detention practices: Statement of a global taskforce. Lancet 386:649, 2015. [DOI] [PMC free article] [PubMed]
- 82. Gupta S, Yeh S, Martiniuk A, et al: The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: A systematic review and meta-analysis. Eur J Cancer 49:2555-2564, 2013. [DOI] [PubMed]
- 83.Mitchell I, Guichon JR, Wong S. Caring for children, focusing on children. Paediatr Child Health. 2015;20:293–295. doi: 10.1093/pch/20.6.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112:461–472. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 85.Homsy J, King R, Balaba D, et al. Traditional health practitioners are key to scaling up comprehensive care for HIV/AIDS in sub-Saharan Africa. AIDS. 2004;18:1723–1725. doi: 10.1097/01.aids.0000131380.30479.16. [DOI] [PubMed] [Google Scholar]
- 86.Hesketh T, Zhu WX. Health in China. Traditional Chinese medicine: One country, two systems. BMJ. 1997;315:115–117. doi: 10.1136/bmj.315.7100.115. [DOI] [PMC free article] [PubMed] [Google Scholar]