Abstract
Purpose
One half of the Mexican population lacks comprehensive health care coverage. In 2003, a reform to the General Health Law was approved that led to the creation of the System of Social Protection in Health and made universal health coverage mandatory. The main innovation of this reform was Seguro Popular, which provided coverage for breast cancer. Here we report the outcomes of women with breast cancer treated at a cancer center in Mexico under Seguro Popular.
Materials and Methods
This was a retrospective cohort study that included all patients with breast cancer treated in the Instituto Nacional de Cancerología in Mexico City between January 2007 and December 2013 with Seguro Popular coverage. Demographic and clinical information were collected and survival outcomes were analyzed.
Results
A total of 4,300 women with breast cancer were included in this analysis. Most patients had locally advanced disease at diagnosis (53%, n = 2,293), and 13% (n = 558) presented with stage IV disease. Neoadjuvant chemotherapy was administered to 1,834 patients (52%), with a pathologic complete response in 25.1% (n = 460). Median follow-up was 40.5 months. Five-year survival for the entire cohort was 82% (95% CI, 81% to 84%). Five-year survival was 97% for early-stage disease (95% CI, 95% to 98%), 82% for locally advanced disease (95% CI, 80% to 84%), and 36% for metastatic disease (95% CI, 30% to 42%).
Conclusion
This represents the first description of a cohort of patients with breast cancer treated in Mexico under Seguro Popular. Seguro Popular has allowed our institution, and other Mexican centers, to establish efficient standardized mechanisms to treat patients with breast cancer.
INTRODUCTION
At the beginning of the century, Mexico faced several issues that severely hampered the health care system’s ability to provide services for the entire population: low government spending in health, a predominance of out-of-pocket expenses, an unequal distribution of public resources between social security schemes, and a failing health infrastructure.1 Until 2003, Mexicans who were not covered by social security (those without a formal employer) or without private insurance were not entitled to any benefits or health care plans and faced a high risk of catastrophic expenditure, which affected more than half of the Mexican population.2
In 2001 to 2006, the Programa Nacional de Salud (National Health Program) established the Seguro Popular de Salud (Popular Health Insurance), which aimed to extend health care coverage to the entire Mexican population.3 In April 2003, a reform to the Ley General de Salud (General Health Law) was approved, leading to the creation of the Sistema de Proteccion Social en Salud (System of Social Protection in Health) and making universal health coverage mandatory from a legal standpoint.4 These actions intended to promote a reduction in the catastrophic health spending incurred by families and to create a health care system that incentivized efficient spending and equitable and accessible medical care. In addition to offering attention for multiple primary care–level health problems, the System of Social Protection in Health covers several expensive specialized diseases and interventions through the Fondo de Proteccion contra Gastos Catastroficos (Catastrophic Expenses Protection Fund, FPGC). The FPGC is funded with contributions from three sources: federal government, state governments, and beneficiary families, who contribute according to their income. These resources are managed through a federal trust, which reimburses providers (both public and private) who offer health care for the diseases included in the FPGC, using fixed rates for each intervention.5
The diseases and interventions included in the FPGC were chosen by members from the National Health Council, who selected those diseases that were prevalent and likely to cause catastrophic expenses. In 2004, only 90 interventions and four diseases were covered (HIV/AIDS, cervical cancer, lymphoblastic leukemia in children, and cataracts), and 5.3 million people were affiliated. By 2013, the number of covered interventions had increased to 285, and the number of affiliated individuals to 57.3 million.6 Because of its rising incidence in Mexico, breast cancer was included in FPGC’s covered diseases in 2007, and it currently receives up to 25% of its budget.7-9 One of the main consequences of its inclusion has been the reduction in loss to follow-up of patients with breast cancer, which went from 30% before 2007 to less than 6%.10
Here we describe the clinical characteristics, prognostic factors, and outcomes of patients with breast cancer treated at the Instituto Nacional de Cancerologia (National Cancer Institute, INCan) of Mexico between 2007 and 2013 under FPGC’s coverage.
MATERIALS AND METHODS
This was a retrospective cohort study that included all patients with breast cancer treated at INCan between January 2007 and December 2013 under FPGC’s coverage. The study was approved by INCan’s Institutional Review Board. Individual patient files were reviewed to collect clinical, demographic, histopathologic, and follow-up information. The collected information was then validated and analyzed by a group of epidemiologists and oncologists.
Demographic and clinical information, such as age at diagnosis, menopausal status, body mass index, reproductive risk factors, and comorbidities, were collected. Clinical stage at diagnosis was determined using clinical and radiologic criteria. Patients were categorized into early disease (stages I to IIA), locally advanced disease (stages IIB to IIIC), and metastatic disease (stage IV). Histopathology examinations were undertaken by specialized breast pathologists. Hormone receptor (HR) status was determined using the Allred score by immunohistochemistry (IHC).11 An IHC scale greater than two (1% to 10% of positive cells) was considered positive for both estrogen (ER) and progesterone receptors (PR). Cases with an Allred score of two or less were classified as ER or PR negative. Human epidermal growth factor receptor 2 (HER2) status was determined initially by IHC, followed by fluorescent in situ hybridization (FISH) in cases with 2+ membrane staining. Tumors were considered as HER2 positive in cases reported as 3+ by IHC or with an amplified FISH and negative in cases with 0+, 1+, and 2+ IHC staining with negative FISH amplification.12 HR-negative, HER2-negative tumors were considered to be triple negative (TN).
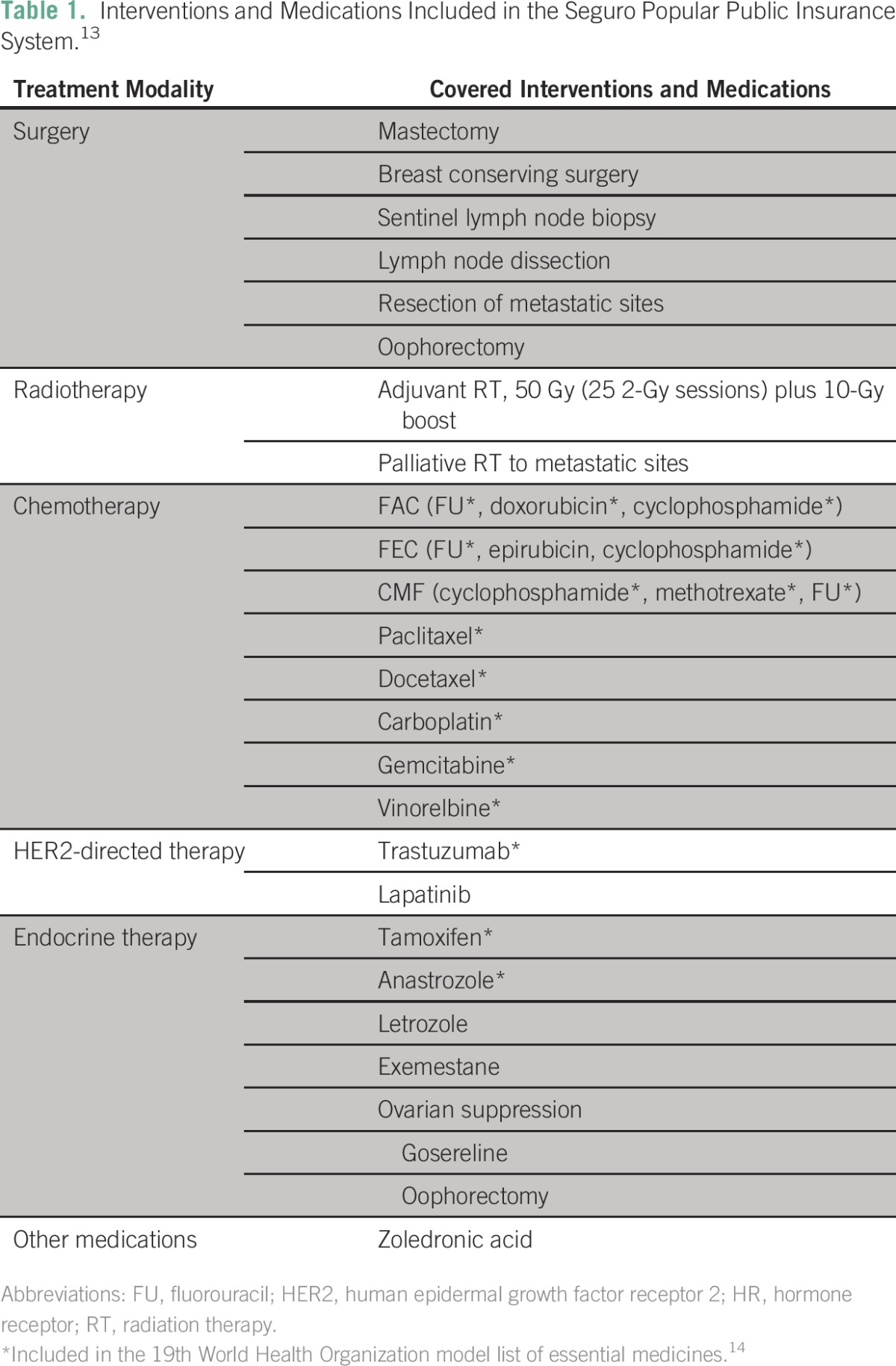
All the patients were covered by the Seguro Popular public insurance scheme and had access to the same treatments and procedures. The choice of treatment was selected from the list of authorized interventions and medications included in Seguro Popular guidelines by each patient’s treating oncologist. The covered interventions are shown in Table 1.
Table 1.
– Interventions and Medications Included in the Seguro Popular Public Insurance System.13
The tumor response to neoadjuvant treatment was also recorded. A pathologic complete response (PCR) was defined as the absence of residual invasive tumor both in the breast and in the axilla (ypT0/is, ypN0). Overall survival (OS) was calculated from the date of diagnosis to the date of last visit or death.
Descriptive statistics were used to analyze the clinical and demographic characteristics of the population. Measures of central tendency and dispersion were used for quantitative variables, and frequencies were used for qualitative variables. OS was determined using the Kaplan-Meier method. Univariate and multivariate analysis using a Cox proportional hazards model were undertaken to determine which clinical and demographic characteristics were associated with worse OS. All statistical analyses were performed using STATA v 12 (StataCorp 2011, Stata Statistical Software: Release 12; StataCorp, College Station, TX) A two-sided P value < .05 was considered statistically significant.
RESULTS
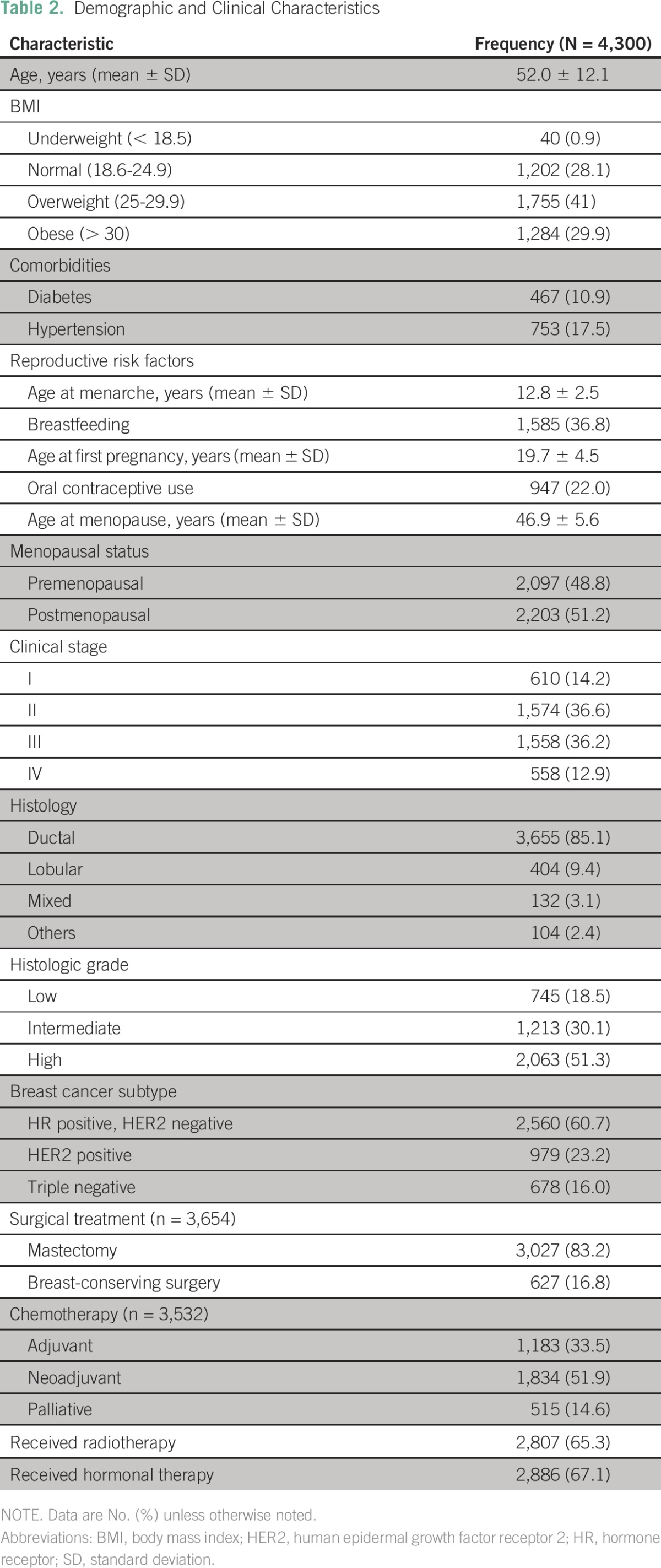
Between 2007 and 2013, 5,500 patients with a diagnosis of breast cancer were treated at INCan, of whom 4,300 had complete clinical records and were included in this analysis. The demographic and clinical characteristics of the patients are shown in Table 1. Mean age at diagnosis was 52 (±12.1) years, with 15.3% (n = 645) younger than 40 years of age. Forty-one percent (n = 1,755) were overweight, and 30% (n = 1,284) were obese. Eleven percent (n = 467) had diabetes, 18% (n = 7,530) had hypertension, and 5.2% (n = 223) had both. Regarding the presence of reproductive risk factors: 37% (n = 1,585) reported breastfeeding, average age at menarche was 12.8 ± 2.5 years, average age at first pregnancy was 19.7 ± 4.5 years, age at menopause was 46.9 ± 5.6 years, and 22% (n = 947) reported using contraceptive pills. Fifty-one percent of included patients (n = 2,203) were postmenopausal.
Clinical characteristics can also be found in Table 1. Most patients had locally advanced disease at the time of diagnosis (53%, n = 2,293), and 13% (n = 558) presented with stage IV disease. The most common histologic subtype was HR-positive, HER2-negative (60.7%; n = 2,560), followed by HER2-positive (23.2%; n = 979) and TN (16%; n = 678). Neoadjuvant chemotherapy was administered to 1,834 patients (52%), with a PCR observed in 25.1% of patients (n = 460). Treatment modalities used are summarized in Table 2.
Table 2.
Demographic and Clinical Characteristics
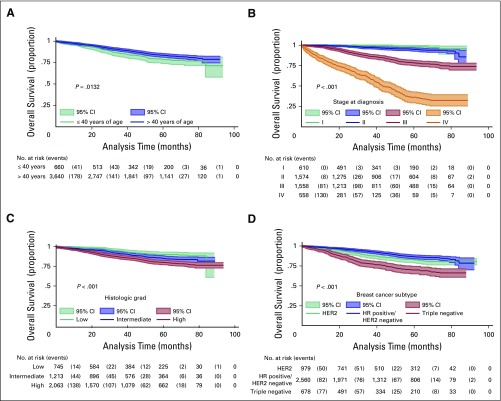
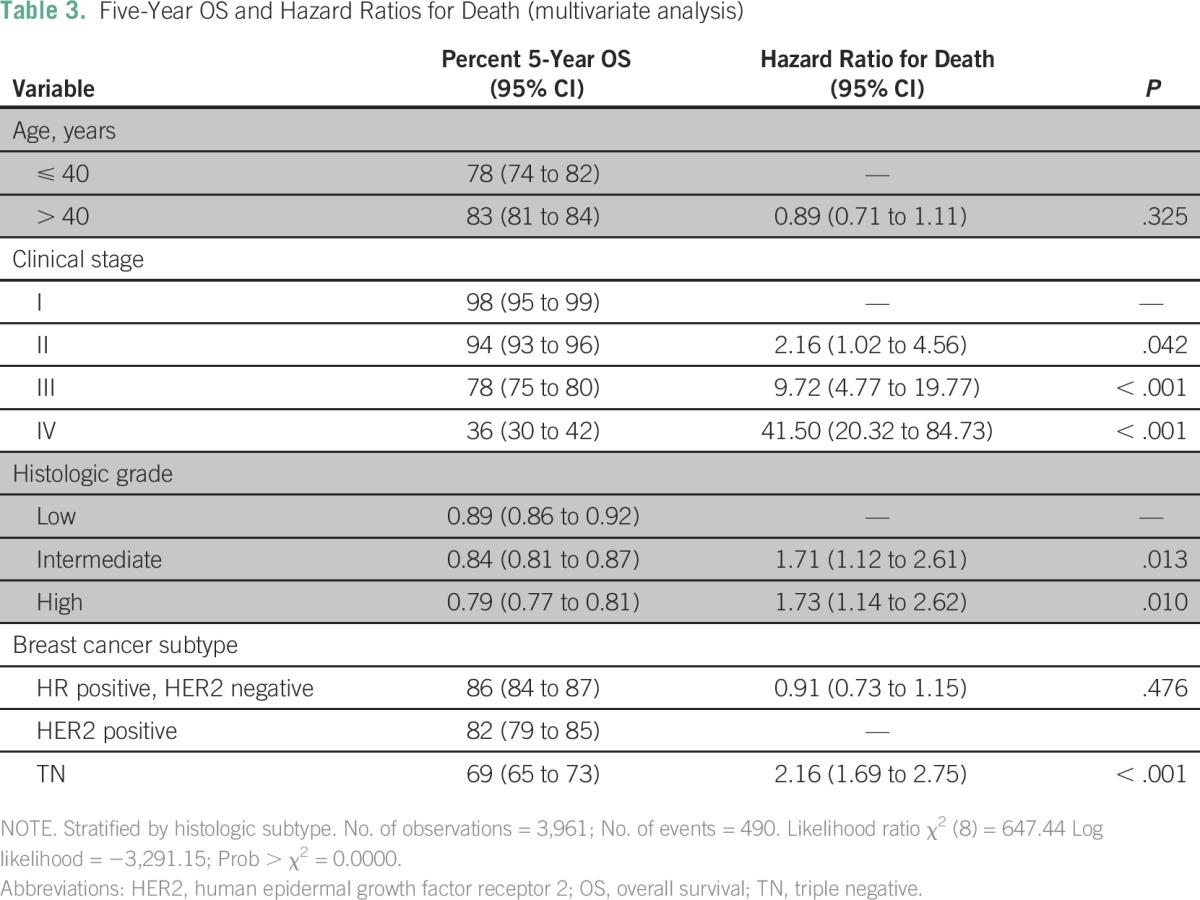
Median follow-up for the entire cohort was 40.5 months. Twenty-four percent of patients (n = 1,040) had recurrent or persistent disease, and 13% (n = 551) died. Five-year OS for the entire cohort was 82% (95% CI, 81% to 84%). The 5-year OS for patients with early-stage disease was 97% (95% CI, 95% to 98%), whereas for those with locally advanced disease it was 82% (95% CI, 80% to 84%), and for those with metastatic disease it was 36% (95% CI, 30% to 42%). Survival curves according to clinical demographic characteristics can be seen in Figure 1. On univariate analysis, the following were shown to be associated with worse survival: age ≤ 40 years, advanced stage at diagnosis, lobular tumors, high histologic grade, and TN tumors. On multivariate analysis (stratified by histologic subtype), the stage at diagnosis, histologic grade, and immunophenotype remained significant after adjusting for treatment variables (Table 3). Age was not a significant predictive factor for survival after multivariate analysis.
Fig 1.
– Overall survival according to relevant clinical and demographic characteristics. (A) Age; (B) clinical stage at diagnosis; (C) histologic grade; and (D) breast cancer subtypes. HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Table 3.
– Five-Year OS and Hazard Ratios for Death (multivariate analysis)
DISCUSSION
This work represents the first description to our knowledge of a large cohort of patients with breast cancer treated in Mexico under Seguro Popular’s FPGC. Our data provide insight into the epidemiologic profile of Mexican patients with breast cancer, which is similar to that found across Latin America. Our patients had a relatively young age at diagnosis, showed a high prevalence of risk factors, and were diagnosed at advanced stages. In spite of that, most of the patients were alive 5 years from diagnosis, with an OS of 82%, which is comparable to that reported in more developed nations of the world.15,16
In Mexico, as in the rest of Latin America, the changing epidemiology of breast cancer is related to a high prevalence of risk factors, such as overweight, obesity, a low rate of breastfeeding, low physical activity, and high hormonal exposure.7,8,17-19 In addition, the lack of high-quality health-related information represents a barrier to the implementation of successful prevention and early detection campaigns, which in turn leads to diagnosis at advanced stages and higher mortality rates.7,20 In this context, and because of the limited availability of personnel, equipment, and resources in the region, better strategies to achieve primary and secondary prevention and downstaging of the disease are desperately needed.21
The creation of the FPGC has provided access to health care to more than half of the previously uninsured population, as proven in this patient cohort, who otherwise would potentially not have received appropriate medical care. The patients included in this analysis were all treated according to the standardized multidisciplinary protocols stipulated by Seguro Popular’s clinical guidelines, which allow for the use of surgery, endocrine therapy, radiotherapy, and several cytotoxic chemotherapy regimens as well as trastuzumab for patients with HER2-positive disease.13 For patients receiving neoadjuvant treatment using these guidelines, the PCR rate was 25%, which is similar to those previously reported in large pooled analyses of clinical trials.22,23 This, along with the high 5-year OS in our series, shows that access to treatment, and not ethnicity or socioeconomic status, is the most important factor leading to good outcomes in breast cancer.
Our 5-year OS of 82% compares favorably to that reported by the public sector in Mexico before the advent of Seguro Popular. For instance, in a retrospective study from Mexico City’s Hospital General, the reported 5-year OS for a cohort of 432 women with breast cancer treated between 1990 and 1999 was only 58.9%.24 Of note, the stage distribution of this cohort was similar to ours, with 10% of women presenting with stage I, 52% with stage II, 34% with stage III, and only 3% with stage IV disease. In those women who presented with stage III disease, for instance, the reported 5-year OS was 45%, compared with 78% in our study, and this holds true for all stages. These striking improvements in outcome do not seem to be dependent on the patients’ characteristics but rather on access to appropriate oncologic and supportive treatments.
One of the key findings in our study is the fact that most of the patients presented with locally advanced or metastatic tumors. In developed countries like the United States or the United Kingdom, < 20% of patients are diagnosed in such advanced stages, whereas in our population, two thirds of patients had advanced disease.15,25 This factor, which has been proven to have a negative impact on survival, is nevertheless modifiable through improvements in the health care system. Improving health-related education, strengthening early detection programs, creating mechanisms for prompt referral to specialized centers, and training health care personnel are of the utmost importance.21 Unfortunately, these aspects have not been improved by Seguro Popular, which is mainly designed to offer medical treatment at specialized centers in metropolitan areas, thus having little impact on downstaging of the disease.19 The large number of patients with metastatic disease translates into higher health care costs, particularly for patients receiving end-of-life care in the inpatient setting.26 Furthermore, the low availability of palliative care resources in Mexico and in Latin America as a whole is a critical problem faced by patients with advanced disease who may be receiving suboptimal care.2 Thus, downstaging the disease would not only improve patient outcomes but also lower spending for the entire health care system and allow for a better distribution of existing resources.
There are still many shortcomings in Seguro Popular and in the FPCGC that should be addressed and improved in the future. Besides the low investment in preventive measures, there is a lack of assigned resources for supportive care, and some medications that have been proven to be effective for the treatment of breast cancer are still not included. In addition, we also found a high mastectomy rate, which could be related to local practices or to patient-related barriers for the receipt of radiotherapy or adequate follow-up. Finally, there are still disparities on the available resources to treat cancer between different regions of the country, with most specialized cancer centers located in large urban areas.
This study has limitations. These data come from a national cancer center located in Mexico City, and, as such, some interventions may not be available in other regions of the country where infrastructure is less developed and specialized health care personnel are lacking. Even though INCan’s patient population is representative of all social, cultural, and demographic backgrounds, only a small proportion of Mexicans receive their care at tertiary cancer centers like INCan. Obtaining data from smaller cancer centers located in the various regions of the country would undoubtedly be of great value to better understand the impact of Seguro Popular and, we hope, to achieve national homogeneity in high-quality cancer care. Another limitation is the fact that our database lacks information on some specific characteristics of treatment, such as completion of planned chemotherapy, dose intensity, adherence to hormonal treatment, and chemotherapy toxicity. Nevertheless, the fact that fewer patients are lost to follow-up, coupled with higher survival rates compared with previous reports, may indicate that patients are in fact able to complete their planned treatments.
The creation of the FPCG has allowed our institution, and other cancer centers in Mexico, to establish efficient and standardized mechanisms to treat patients with breast cancer, reducing the number of patients lost to follow-up to < 10%.10 Before FPCG was instituted, patients were treated erratically, their outcomes were worse, and their information was not collected and reported, which meant that there were no real data regarding the landscape of breast cancer in Mexico. Seguro Popular has been beneficial for many Mexican women with breast cancer, and its databases represent a unique opportunity to study breast cancer (and other malignancies) in Mexico. We believe that our experience in treating breast cancer under this scheme will allow the Mexican health care system, and other systems throughout Latin America, to evaluate their current practice and policies and to find new opportunities to improve the outcomes of patients with breast cancer in the region.
AUTHOR CONTRIBUTIONS
Conception and design: Nancy Reynoso-Noverón, Cynthia Villarreal-Garza, Enrique Soto-Perez-de-Celis, Claudia Arce-Salinas, Abelardo Meneses-García, Fernando Lara-Medina, Enrique Bargalló-Rocha, Alejandro Mohar
Collection and assembly of data: Juan Matus-Santos, María Teresa Ramírez-Ugalde, Alberto Alvarado-Miranda, Paula Cabrera-Galeana
Data analysis and interpretation: Nancy Reynoso-Noverón, Alejandro Mohar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Nancy Reynoso-Noverón
No relationship to disclose
Cynthia Villarreal-Garza
Consulting or Advisory Role: Myriad Genetics, Roche
Travel, Accommodations, Expenses: Roche, Myriad Genetics
Enrique Soto-Perez-de-Celis
Travel, Accommodations, Expenses: Amgen, Bristol-Myers Squibb
Claudia Arce-Salinas
No relationship to disclose
Juan Matus-Santos
No relationship to disclose
María Teresa Ramírez-Ugalde
No relationship to disclose
Alberto Alvarado-Miranda
No relationship to disclose
Paula Cabrera-Galeana
Consulting or Advisory Role: Pfizer
Abelardo Meneses-García
No relationship to disclose
Fernando Lara-Medina
No relationship to disclose
Enrique Bargalló-Rocha
Speakers' Bureau: Genomic Health
Alejandro Mohar
No relationship to disclose
REFERENCES
- 1.Frenk J, González-Pier E, Gómez-Dantés O, et al. Comprehensive reform to improve health system performance in Mexico. Lancet. 2006;368:1524–1534. doi: 10.1016/S0140-6736(06)69564-0. [DOI] [PubMed] [Google Scholar]
- 2.Strasser-Weippl K, Chavarri-Guerra Y, Villarreal-Garza C, et al. Progress and remaining challenges for cancer control in Latin America and the Caribbean. Lancet Oncol. 2015;16:1405–1438. doi: 10.1016/S1470-2045(15)00218-1. [DOI] [PubMed] [Google Scholar]
- 3. Programa Nacional de Salud 2001-2006. Secretaria de Salud, Mexico. http://www.salud.gob.mx/unidades/cdi/nom/compi/pns20012006.pdf.
- 4. Cámara de Diputados del H. Congreso de la Unión: Sistema de Protección en Salud: Hacia un sistema universal de salud. Firma del decreto por el que se reforma y adiciona la Ley General de Salud, Mexico. http://dof.gob.mx/nota_detalle.php?codigo=695626&fecha=15/05/2003.
- 5. Comité Técnico del Fideicomiso del Sistema de Protección Social en Salud: Reglas de Operación del Fideicomiso del Sistema de Protección Social en Salud, Mexico. http://seguropopular.guanajuato.gob.mx/archivos/compilacion_juridica/ROP_Fideicomiso_2014.pdf. [Google Scholar]
- 6. Comisión Nacional de Protección Social en Salud: Informe de resultados Enero-Junio 2015. Secretaría de Salud, Mexico. http://www.seguro-popular.gob.mx/images/Contenidos/informes/Informe%20de%20Resultados%20Ene-Jun%202015.pdf. [Google Scholar]
- 7.Soto-Perez-de-Celis E, Chavarri-Guerra Y. National and regional breast cancer incidence and mortality trends in Mexico 2001-2011: Analysis of a population-based database. Cancer Epidemiol. 2016;41:24–33. doi: 10.1016/j.canep.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Chávarri-Guerra Y, Villarreal-Garza C, Liedke PE, et al. Breast cancer in Mexico: A growing challenge to health and the health system. Lancet Oncol. 2012;13:e335–e343. doi: 10.1016/S1470-2045(12)70246-2. [DOI] [PubMed] [Google Scholar]
- 9.Knaul F, Arreola-Ornelas H, Méndez O, et al. Fair health financing and catastrophic health expenditures: potential impact of the coverage extension of the popular health insurance in Mexico [in Spanish] Salud Publica Mex. 2005;47(suppl 1):S54–S65. [PubMed] [Google Scholar]
- 10.Arce-Salinas C, Lara-Medina FU, Alvarado-Miranda A, et al. Evaluation of breast cancer treatment at a tertiary-level institution with Popular Health Insurance in Mexico [in Spanish] Rev Invest Clin. 2012;64:9–16. [PubMed] [Google Scholar]
- 11.Harvey JM, Clark GM, Osborne CK, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Consejo de Salubridad G: Identificación de Tratamientos y Medicamentos Asociados a Gastos Catastróficos. PROTOCOLO TÉCNICO. Enfermedad CIE.10: C50 Tumor maligno de la mama. Mexico, 2011. [Google Scholar]
- 14.World Health Organization: WHO Model List of Essential Medicines (ed 19). http://www.who.int/medicines/publications/essentialmedicines/en/
- 15. Howlader N, Noone AM, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/
- 16.Hamy-Petit AS, Belin L, Bonsang-Kitzis H, et al. Pathological complete response and prognosis after neoadjuvant chemotherapy for HER2-positive breast cancers before and after trastuzumab era: Results from a real-life cohort. Br J Cancer. 2016;114:44–52. doi: 10.1038/bjc.2015.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villarreal-Garza C, Aguila C, Magallanes-Hoyos MC, et al. Breast cancer in young women in Latin America: An unmet, growing burden. Oncologist. 2013;18(suppl):26–34. doi: 10.1634/theoncologist.18-S2-26. [DOI] [PubMed] [Google Scholar]
- 18.Goss PE, Lee BL, Badovinac-Crnjevic T, et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013;14:391–436. doi: 10.1016/S1470-2045(13)70048-2. [DOI] [PubMed] [Google Scholar]
- 19.Justo N, Wilking N, Jönsson B, et al. A review of breast cancer care and outcomes in Latin America. Oncologist. 2013;18:248–256. doi: 10.1634/theoncologist.2012-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigenda G, Caballero M, González-Robledo LM. Access barriers in early diagnosis of breast cancer in the Federal District and Oaxaca [in Spanish] Salud Publica Mex. 2009;51(suppl 2):s254–s262. [PubMed] [Google Scholar]
- 21.Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: Executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12:387–398. doi: 10.1016/S1470-2045(11)70031-6. [DOI] [PubMed] [Google Scholar]
- 22.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 23.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 24.Flores-Luna L, Salazar-Martínez E, Duarte-Torres RM, et al. Prognostic factors related to breast cancer survival [in Spanish] Salud Publica Mex. 2008;50:119–125. doi: 10.1590/s0036-36342008000200005. [DOI] [PubMed] [Google Scholar]
- 25. Cancer Research UK. Statistics by Cancer Type: Breast Cancer. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/survival#heading-Zero.
- 26.Chastek B, Harley C, Kallich J, et al. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8:75s–80s. doi: 10.1200/JOP.2011.000469. [DOI] [PMC free article] [PubMed] [Google Scholar]