Abstract
Importance
Motor slowing appears in preclinical Alzheimer’s disease (AD), progresses with AD progression and is associated with AD pathology at autopsy. Whether amyloid-beta (Aβ) is associated with gait speed in dementia free elders and whether cognition and apolipoprotein E (APOE) ε4 influence this relationship remains unknown.
Objective
To examine the association between Aβ and gait speed in dementia free older adults and study the influence of cognition and APOE ε4 status on this relationship.
Design
Cross-sectional.
Setting
University center.
Participants
183 elders without dementia enrolled in the Ginkgo Evaluation of Memory (GEM) study, including 144 cognitively normal (CN) subsample (mean age: 85 years in both).
Main Outcomes, Measures
We assessed cerebral Aβ on Pittsburgh B (PiB) PET, gait speed over 15-feet and cognition on the Mini-mental Status Examination (MMSE) and Trail-Making-Test, Parts A and B (TMT-A, TMT-B). We grouped participants into high-Aβ [PiB(+)] and low-Aβ [PiB(−)] on standardized global PiB cutoffs and examined group differences. We studied the influence of cognition and APOE ε4 on the global and regional associations between gait speed and Aβ in the whole sample and in the CN subsample.
Results
PiB(+) were comparable to PiB(−) individuals on demographics, comorbidities, cognition, hippocampal volume and small-vessel disease but not on gait speed (0.85 vs 0.92 m/sec, p=0.012) or proportion of APOE ε4 carriers (29% vs 6%, p<0.001). In this whole sample and in the CN subsample, the association between global PiB retention and slower gait withstood adjustment for covariates (p=0.026 and p=0.042 respectively); however, this relationship was attenuated by MMSE, TMT- A and TMT-B and was rendered statistically nonsignificant by APOE ε4 in both samples (both p≥0.1). Several regional associations between gait speed and PiB uptake withstood relevant adjustments; however, APOE ε4 rendered only the medial and lateral temporal and occipital regions in the whole sample and, the occipital regions in CN subsample statistically significant.
Conclusion
Cerebral Aβ deposition is associated with slower gait speed in older adults without dementia; however, this association is weaker in CN elders. Cognition and APOE ε4 carrier status influence the relationship between Aβ and gait speed in dementia free older adults.
Introduction
Motor slowing appears during the preclinical stages of Alzheimer’s disease (AD),1 accelerates in those who subsequently go on to develop mild cognitive impairment (MCI)/AD2,3 and is associated with severity of AD pathology at death.4 In older adults without dementia, elevated levels of fibrillar amyloid-beta (Aβ) are seen in 30%-65% of adults between 80 and 88 years of age.5–8 Recent evidence suggests that high levels of Aβ increases fall risk in these populations.9 Slowing of gait speed is an important determinant of falls and mortality in older adults10 and is associated with cerebral small-vessel disease and cortical atrophy.11 However, cerebral Aβ may also play a role in gait slowing in older adults without dementia but there is little research to support this association.
Cognitive processes influence gait in cognitively normal (CN) older adults and in those with MCI.11,12 Deficits in these cognitive processes are associated with greater Aβ deposition in these populations.13,14 In addition, APOE ε4 genotype is associated with poor mobility and physical function in older adults15 and in those with MCI16 and presence of an APOE ε4 allele is linked to accelerated motor decline in aging,17,18 with exceptions.19,20 Besides age, APOE ε4 is the largest risk factor for AD and presence of an APOE ε4 allele increases Aβ accumulation in CN older adults,5,21–24 and in prodromal and clinical AD.24–26 Therefore, both cognition and APOE ε4 genotype may influence the association between Aβ and gait speed in dementia free older adults.
We investigated the relationship between cortical and regional Aβ deposition and gait speed in older adults without dementia and assessed whether cognition and APOE ε4 status influence this relationship. We further examined these relationships in a subsample of older adults deemed CN in the parent study5 to understand whether the association between Aβ and gait in the entire cohort was driven by those with MCI. We hypothesized that greater cortical Aβ deposition will be associated with slower gait in the whole sample but its magnitude and statistical significance would be weaker in those without MCI, i.e., the CN individuals. In addition, we posited that cognition and APOE ε4 would independently influence the global and regional relationship between Aβ deposition and gait speed beyond that explained by demographic factors, cardiac risk, cortical atrophy and small-vessel disease, factors known to play a role in age-related motor slowing.11,12
Design and Methods
Population
Gingko Evaluation of Memory study (GEMS), a randomized double-blind, placebo-controlled trial of Ginkgo biloba targeted to prevent dementia, particularly AD, recruited CN older adults and older adults with MCI.27,28 Participants free of dementia at study entry were followed annually from 2000 to 2009 in the GEMS. Approximately 10±3 months following the GEMS closeout visit, 194 dementia free participants enrolled at the University of Pittsburgh site were recruited for the GEMS Imaging Substudy that included a brain MRI and Pittsburgh-B compound (PiB) positron emission tomography (PiB-PET).5,29 Eligibility criteria for the GEMS Imaging Substudy was described previously.5,29 Eleven participants were not included in this analyses due to technical issues relating to their PiB-PET (n=3) and MRI (n=8) scans. This study analyzed data from 183 GEMS participants who had complete brain MRI and PiB-PET data along with physical performance measures.
Cognitive Assessment and Adjudication of MCI
All participants underwent detailed neuropsychological assessments annually as part of the GEMS27,28 a subset of which were included in the GEMS Imaging Substudy.5,29 We assessed global cognitive function on the Mini-mental Status Examination and attention and executive function on the Trail Making Test, parts A (TMT-A) and part B (TMT-B).28 Adjudication of MCI was conducted by the GEMS Cognitive Diagnostic Center, taking into account all neuropsychological assessments from the GEMS parent study and the GEMS Imaging Substudy. Criteria for MCI included a cutoff of 1.5 standard deviations (SD) below age- and education- adjusted norm on 2–3 tests.5
Gait Speed
Time to walk 15-feet was measured using a protocol similar to the one used to assess gait speed in the Short Physical Performance Battery30 and has been described previously.31 Briefly, a 15-foot long traverse was demarcated with tape and participants were instructed to begin walking from standing position at the start line and continue walking past the end line. Time was measured using a stop-watch, which was started after the prompt when one foot started to move across the start line and was stopped when the first foot crossed the 15-foot end mark. Two consecutive 15-foot walks were obtained, the first at usual pace and the second at rapid pace.31 We used the usual paced timed walk measure to derive gait speed in meters per second. The 15-feet walk (4.57 meter) test is a well validated measure of gait speed in older adults with and without dementia with an excellent test-retest reliability (ICC= 0.973–0.977).32 Mean duration between gait speed assessment and brain imaging was 16 months (range: 10 to 25 months).
PiB-PET
PiB-PET imaging methodology was reported previously.5,29 In brief, [C11] PiB ligand (approximately 15 mCi) was injected over 20 seconds and a 10-minute transmission scan was acquired for attenuation correction followed by a 20 minute PiB-PET scan (4 × 5 minute frames) acquired 50 minutes post injection. PiB retention was assessed in the resliced-normalized PET image in the regions of interest (ROIs) encompassing the following bilateral regions:33 anterior cingulate (ACG, pregenual and subgenual), anteroventral striatum (AVS, anterior caudate and putamen), frontal cortex (FRC, dorsal and ventral), lateral temporal cortex (LTC), parietal (PAR) and precuneus (PRC), mesial temporal cortex (MTC, amygdala and hippocampus), occipital cortex (OCC, primary visual cortex), occipital pole (OCP), pons (PON), sensory-motor cortex (SMC), subcortical white matter (SWM) and thalamus (THL). An iterative outlier cutoff method was used to define subjects as PiB(+) if the atrophy corrected PiB standardized uptake value ratio (SUVR, referenced to the cerebellar value) was >1.57 averaged from PiB SUVR of the ACG, AVS, FRC, LTC, PAR and PRC.29 A continuous measure of global PiB SUVR represented values in these six ROIs.
APOE genotyping
APOE genotyping was performed using polymerase chain reaction on DNA isolated from whole blood samples as described previously.34 Participants with at least one APOE ε4 allele (ε2/ε4, ε3/ε4 or ε4/ε4) were identified as APOE ε4 carriers.
Covariates
Several age-related changes are linked to gait and cognitive performance in older adults.11,12 Amongst these, cardiac risk factors are also associated with cerebral Aβ deposition29 and APOE ε4 carrier status.35 Hence, analyses were adjusted for covariates that included demographics (age, gender, race, education), body weight, hypertension, coronary heart disease (presence of a self-reported or chart review based diagnoses angina, myocardial infarction, angioplasty, bypass surgery, pacemaker, valve replacement, heart failure), stroke and MRI measures of cortical atrophy and small-vessel disease (bilateral hippocampal volume and total volume of white matter hyperintensities (WMH), both presented as a proportion of intracranial volume).29
Statistical analyses
We compared PiB(+) and PiB(−) groups on demographic, health and key brain measures using independent samples t-tests. We examined the association between global PiB retention and gait speed using multiple regression adjusting for the above covariates. We included cognitive measures (MMSE, TMT-A and TMT-B) and APOE ε4 carrier status, separately as additional independent variables in the unadjusted and adjusted models. We repeated the analyses using a subsample of participants deemed CN after excluding those with MCI. We included an interaction term in the models to examine whether the association between PiB SUVR and gait speed was different in APOE ε4 carriers and non-carriers, based on our observations in other setting.36 However, recognizing that interaction terms have low statistical power, we a priori planned an exploratory analysis stratified by APOE ε4 carrier status. Finally, we performed an another exploratory analysis of regional association between PiB SUVR and gait speed initially adjusting for all covariates including cognition and then with further adjusting for APOE ε4 status. To assess whether the time between gait assessment and PiB PET scan influenced the overall results we performed a sensitivity analysis by including the duration of time between gait assessment and PiB-PET as a covariate.
Results
Sample characteristics
In the whole sample (N=183, 85.5±3 years, 42% female), the mean MMSE was 28, 20% were APOE ε4 carriers and 54.6% were designated as PiB(+). The characteristics of the whole group and subgroups divided on global PiB SUVR are shown in Table 1. Both PiB(+) and PiB(−) subgroups were similar in terms of their demographic, body weight, comorbidities, MMSE, TMT-A, TMT-B, physical performance measures, number of falls and, hippocampal and WMH volumes. PiB(+) group had greater proportion of APOE ε4 carriers compared to the PiB(−) group (29% vs 6%, p<0.001). Also, we found that the PiB(+) group had a slower gait speed than the PiB(−) group (0.85 vs 0.92 m/sec, p=0.012).
Table 1.
Sample characteristics of the whole sample and differences in PiB(+) and PiB(−) groups.
| Whole sample (N=183) |
PiB(+) (N=100) |
PiB(−) (N=83) |
p-value | |
|---|---|---|---|---|
| Age (years) | 85.5 ± 2.9 | 85.7 ± 3.1 | 85.2 ± 2.5 | 0.26 |
| Women (n, %) | 76 (41.5%) | 44 (44%) | 32 (38.6%) | 0.46 |
| White (n, %) | 177 (97%) | 97 (98%) | 80 (98%) | 0.99 |
| Education (years) | 14.7 ± 2.6 | 14.7 ± 2.5 | 14.6 ± 2.8 | 0.85 |
| MMSE (range: 0 to 30) | 27.6 ± 2.1 | 27.4 ± 2.0 | 27.7 ± 2.1 | 0.36 |
| TMT-A (mean, sec) | 48.7 ± 19.2 | 50 ± 17.4 | 51.9 + 24.1 | 0.5 |
| TMT-B (mean, sec) | 124.6 ± 52.3 | 127 ± 50 | 134 ± 55 | 0.4 |
| Physical Performance Total score (range: 0 to 12) | 8.5 ± 2.3 | 8.2 ± 2.5 | 8.8 ± 2.0 | 0.06 |
| APOE-ε4 carrier status (n, %) | 34 (20.1%) | 29 (29%) | 5 (6%) | <0.001 |
| Standing weight (kg) | 74.5 ± 11.4 | 74.5 ± 11.9 | 74.5 ± 11 | 0.99 |
| Heart Disease (n, %) | 32 (17.6%) | 19 (19%) | 13 (15.9%) | 0.58 |
| Atrial Fibrillation (n, %) | 11 (6.1%) | 6 (6.1%) | 5 (6.2%) | 0.99 |
| Stroke/TIA (n, %) | 8 (4.4%) | 6 (6%) | 2 (2.4%) | 0.3 |
| Hypertension (n, %) | 63 (35.2%) | 39 (39.4%) | 24 (30%) | 0.19 |
| Diabetes Mellitus (n, %) | 10 (5.6%) | 7 (7.1%) | 3 (3.8%) | 0.52 |
| Falls over prior 1 year (n, %) | 18 (9.9%) | 9 (9%) | 9 (11%) | 0.66 |
|
Whole sample (N=183) |
PiB(+) (N=100) |
PiB(−) (N=83) |
p-value | |
| White matter hyperintensities (normalized to ICV) | 0.009085 ± 0.0059 | 0.00894 ± 0.00565 | 0.00925 ± 0.00622 | 0.72 |
| Hippocampal volume (normalized to ICV) | 0.256 ± 0.03 | 0.255 ± 0.03 | 0.257 ± 0.03 | 0.60 |
| PiB SUVR | 1.78 ± 0.48 | 2.136 ± 0.347 | 1.340 ± 0.141 | <0.0001 |
| Gait Speed (m/sec) | 0.88 ± 0.2 | 0.85 ± 0.19 | 0.92 ± 0.2 | 0.012 |
Abbreviations:
PiB SUVR: Pittsburgh B compound standardized value uptake ratio
CN: cognitively normal
MMSE: Mini-mental Status Examination
TMT-A and TMT-B: Part A and Part B of Trail Making Test respectively
APOE ε4 carrier: Presence of at least one ε4 allele on the apolipoprotein-E gene.
ICV: intracranial volume
Sample characteristics of the CN subsample (n=144, 85.4 years) are shown in Table 2. PiB(+) and PiB(−) groups were similar on all above characteristics except on prevalence of hypertension (42% vs 22%, p=0.01), the proportion of APOE ε4 carriers (28% vs 7%, p=0.003) and gait speed (0.87 vs 0.94 m/sec, p=0.036).
Table 2.
Sample characteristics of the sample restricted to CN older adults with differences in PiB(+) and PiB(−) groups within this sub-sample.
| Normal sample (N=144) |
PiB(+) (N=75) |
PiB(−) (N=69) |
p-value | |
|---|---|---|---|---|
| Age (years) | 85.44 ± 2.87 | 85.33 ± 3.08 | 85.55 ± 2.65 | 0.65 |
| Women (n, %) | 82 (56.94%) | 42 (29.17%) | 40 (27.78%) | 0.81 |
| White (n, %) | 142 (97%) | 75 (99%) | 68 (99%) | 0.99 |
| Education (years) | 14.75 ± 2.65 | 14.79 ± 2.61 | 14.71 ± 2.71 | 0.86 |
| MMSE (range: 0 to 30) | 28.03 ± 1.65 | 27.88 ± 1.64 | 28.21 ± 1.66 | 0.25 |
| TMT-A (mean, sec) | 48.7 ± 19 | 51 ± 21.4 | 51 ± 19.8 | 0.99 |
| TMT-B (mean, sec) | 125 ± 52.3 | 129 ± 50 | 130 ± 50 | 0.9 |
| Physical Performance Total score (range: 0 to 12) | 8.66 ± 2.29 | 8.32 ± 2.46 | 9.01 ± 2.04 | 0.07 |
| APOE ε4 carrier status (n, %) | 27 (20%) | 22 (29.3%) | 5 (7.2%) | 0.001 |
| Standing weight (kg) | 75.1 ± 11.1 | 74.2 ± 11.2 | 75.8 ± 10.9 | 0.45 |
| Heart Disease (n, %) | 22 (15.3%) | 11 (14.7%) | 11 (15.9%) | 0.99 |
| Atrial Fibrillation (n, %) | 10 (6.9%) | 5 (6.7%) | 5 (7.2%) | 0.99 |
| Stroke (n, %) | 8 (4.14%) | 4 (5.3%) | 2 (2.9%) | 0.7 |
| Hypertension (n, %) | 46 (32.6%) | 31 (41.9%) | 15 (22.4%) | 0.02 |
| Diabetes Mellitus (n, %) | 7 (5%) | 5 (6.8%) | 2 (3%) | 0.44 |
| Falls over prior 1 year (n, %) | 13 (9.03%) | 5 (6.7%) | 8 (11.6%) | 0.30 |
|
Normal sample (N=144) |
PiB(+) (N=75) |
PiB(−) (N=69) |
p-value |
Normal sample (N=144) |
| White matter hyperintensities (normalized to intracranial volume) | 0.009 ± 0.006 | 0.008 ± 0.005 | 0.009 ± 0.006 | 0.76 |
| Hippocampal volume (normalized to intracranial volume) | 0.26 ± 0.03 | 0.26 ± 0.03 | 0.26 ± 0.03 | 0.82 |
| PiB SUVR | 1.75 ± 0.47 | 2.12 ± 0.34 | 1.35 ± 0.13 | <0.0001 |
| Gait Speed (m/sec) | 0.90± 0.19 | 0.87± 0.18 | 0.94± 0.20 | 0.036 |
Abbreviations:
PiB SUVR: Pittsburgh B compound standardized value uptake ratio
CN: cognitively normal
MMSE: Mini-mental Status Examination
TMT-A and TMT-B: Part A and Part B of Trail Making Test respectively
APOE ε4 carrier: Presence of at least one ε4 allele on the apolipoprotein-E gene.
ICV: intracranial volume
There were no differences in gait speed between the placebo and Gingko biloba arms of the study in the whole sample (0.89 m/sec vs 0.87 m/sec, p=0.6) or in the CN subsample (0.89 m/sec vs 0.92 m/sec, p=0.4).
Association between global PiB SUVR and gait speed
Table 3 shows the association between global PiB binding and gait speed. In the whole sample, greater global PiB SUVR was associated with slower gait (regression coefficient (β)= −0.086, p=0.005) and this association remained significant after adjustment for above covariates (β=−0.068, p=0.026). MMSE correlated with gait speed (r=0.24, p=0.002); TMT-A and TMT-B correlated with both global PiB SUVR (r= 0.3, p=0.005 and r=0.18, p=0.02, respectively) and gait speed (r=−0.3, p=<0.001 and r=−0.19, p=0.01, respectively). The association between PiB SUVR and gait speed was attenuated but tended to persist after adjusting for MMSE (p=0.06), TMT-A (p=0.06) and TMT-B (p=0.08). Accounting for APOE ε4 in the model rendered the association between PiB SUVR and gait speed nonsignificant and contributed to approximately 16% of the additional explained variance in the entire sample (Table 3).
Table 3.
Unadjusted and adjusted associations between global PiB SUVR and gait speed (dependent variable) in the whole sample and in the CN subsample: influence of cognition (MMSE, TMT-A and TMT-B) and APOE ε4 carrier status on these relationships.
| Whole sample (n=183) β (p value), [95%CI] |
CN sample (n=144) β (p value), [95%CI] |
|||
|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| Global PiB SUVR | −0.086 (0.005) [−0.146, −0.027] |
−0.068 (0.026) [−0.127, −0.008] |
−0.072 (0.04) [−0.140,−0.003] |
−0.074 (0.042) [−0.145, −0.003] |
| Global PiB SUVR + MMSE | −0.073 (0.017) [−0.132, −0.013] |
−0.057 (0.056) [−0.115, −0.002] |
−0.059 (0.084) [−0.126, 0.008] |
−0.055 (0.11) [−0.124, 0.013] |
| Global PiB SUVR + TMT-A | −0.061 (0.04) [−0.119, −0.002] |
−0.055 (0.08) [−0.116, −0.006] |
−0.063 (0.07) [0.131, 0.005] |
−0.068 (0.06) [−0.14, 0.004] |
| Global PiB SUVR + TMT-B | −0.071 (0.02) [−0.131, −0.0114] |
0.064 (0.038) [−0.125, −0.004] |
−0.068 (0.06) [−0.137, 0.002] |
−0.077 (0.04) [−0.148, −0.005] |
| Global PiB SUVR + APOE ε4 status | −0.062 (0.057) [−0.126, 0.002] |
−0.055 (0.095) [−0.119, 0.010] |
−0.06 (0.1) [−0.1335, 0.0134] |
−0.058 (0.13) [−0.134, 0.018] |
Footnote:
Covariates included in the adjusted model: age, gender, race, education, weight, hypertension, coronary heart disease, stroke and volume of hippocampal and white matter hyperintensities on MRI normalized to intracranial volume.
PiB SUVR: Pittsburgh B compound standardized value uptake ratio
CN: cognitively normal
MMSE: Mini-mental Status Examination
TMT-A and TMT-B: Part A and Part B of Trail Making Test respectively
APOE ε4 carrier: Presence of at least one ε4 allele on the apolipoprotein-E gene.
In the CN older adults, greater global PiB SUVR was associated with slower gait speed (β=-0.072, p=0.04) and even after adjusting for above covariates (β=-0.074, p=0.042); however, this association was no longer significant after additional adjustments for MMSE, TMT-A, TMT-B or APOE ε4 status. APOE ε4 explained approximately 10% of the additional explained variance in the association between PiB SUVR and gait speed (Table 3).
We did not find a statistically significant interaction with APOE ε4 and global PiB with respect to gait speed. We found no significant relationships between APOE ε4 carrier status and gait speed (eTable 1). The stratified analysis by APOE ε4 carrier status suggested that the associations between gait speed and PiB SUVR, MMSE, TMT-A and TMT-B were stronger in the APOE ε4 non-carriers than carriers in both the samples (eTable 2).
We performed a sensitivity analysis to examine whether the duration between gait assessment and PiB-PET had any bearing on the relationship between Aβ and gait speed. With time period between MRI and gait assessment as a covariate in the regression analysis, the strength of unadjusted relationship between global PiB SUVR and gait speed was unchanged (whole sample: beta = −0.09, p=0.005; CN subsample: beta=−0.072, p=0.04).
Association between regional PiB SUVR and gait speed
Figure 1a depicts an exploratory analysis showing the coefficient of the association between regional PiB SUVR for global PiB SUVR and regional PiB SUVR for the whole group and for the subsample limited to CN individuals adjusted for demographics variables, body weight, hypertension, coronary heart disease, stroke, MMSE and normalized hippocampal and WMH volume but not adjusted for APOE ε4. In the whole sample, slower gait was significantly associated with regional PiB SUVR in AVS (p=0.027), LTC (p=0.012), MTC (p=0.015), PAR (p=0.027), PRC (p=0.019), SMC (p=0.02) and SWM (p=0.031) and, showed a trend with PiB SUVR in ACG, FRC, OCC (all p=0.06) and OCP (p=0.05). However, in CN elders, slower gait was significantly associated with greater regional PiB SUVR in the FRC (p=0.05), AVS (p=0.039), LTC (p=0.023), PRC (p=0.024) and SMC (p=0.021). The association between gait speed and regional PiB SUVR was marginally statistically significant in the ACG (p=0.07) and the MTC, PAR, OCC and OCP (all p=0.05) ROIs (Figure 1a). Gait speed was not significantly associated with regional PiB SUVR in any other ROIs in the whole group or in the subsample of CN older adults.
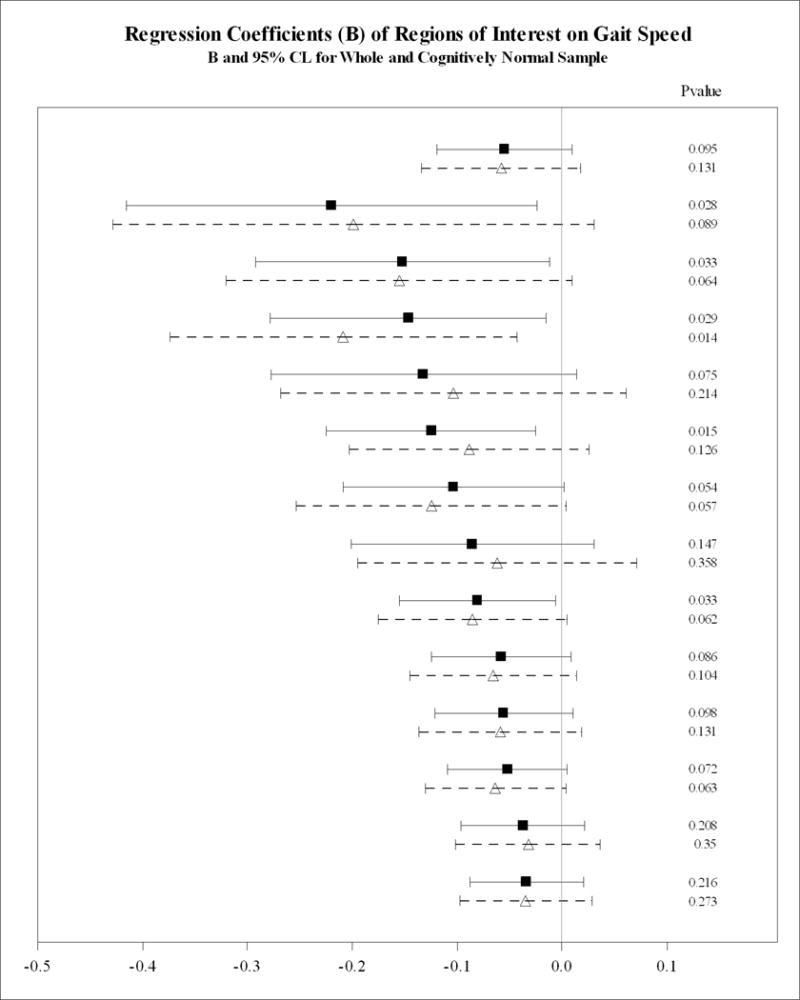
Figure 1.
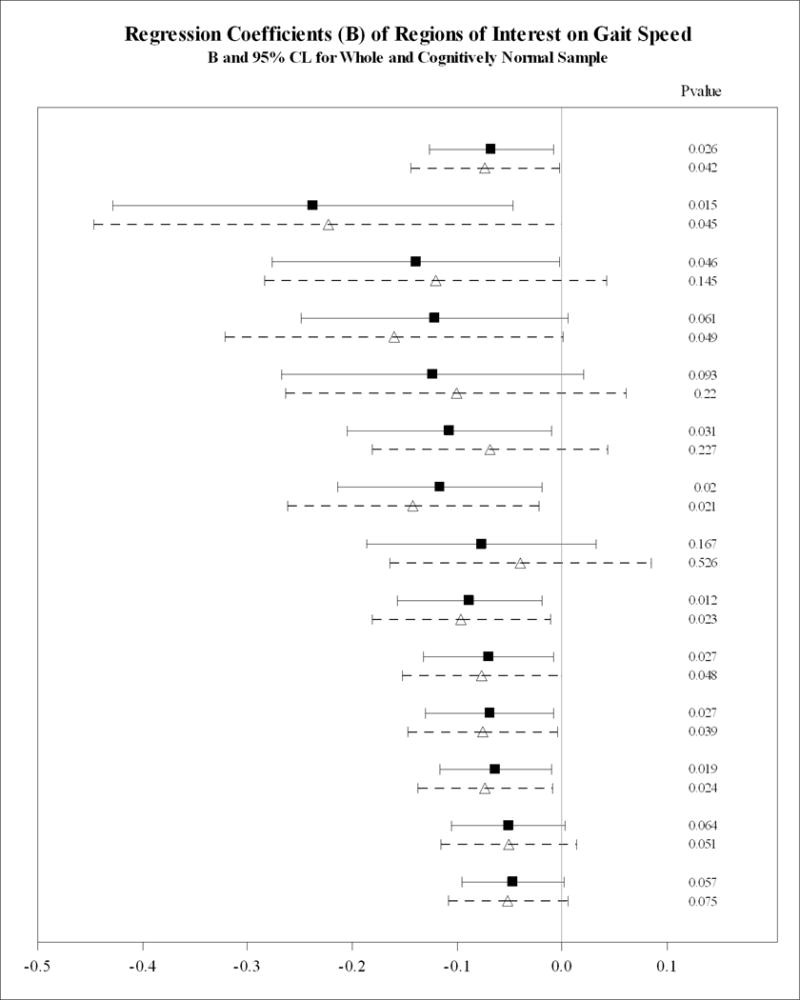
Association between global and regional Aβ and gait speed in the whole sample and in the cognitively normal subsample.
A: Adjusted for covariatesa not APOE ε4 carrier status.
B: Adjusted for covariatesa including APOE ε4 carrier status.
Dementia-free older adults in the whole sample shown in filled square and bolded lines. Cognitive normal subsample shown in open triangles and dotted lines. Regions arranged in descending order of magnitude of regression coefficient (β, 95% CI).
acovariates: age, gender, race, education, weight, cognition (MMSE), hypertension, coronary heart disease, stroke, bilateral hippocampal volume and white matter hyperintensities on MRI normalized to intracranial volume.
Figure 1b shows the associations between regional PiB SUVR and gait speed adjusted for APOE ε4 status. In the models adjusted for the above covariates (Figure 1a), we found that additional inclusion of APOE ε4 rendered the regional association between gait speed and PiB SUVR in the FRC, PRC, AVS and ACG nonsignificant (p>0.1, Figure 1b) in the whole sample and in the subsample of individuals without MCI (the CN individuals) The statistically significant associations between gait speed and PiB SUVR in the MTC, OCC, OCP, SWM, and LTC were retained in the whole sample; however, in the sample without MCI, this association was limited to the OCC.
Discussion
In this sample of dementia free older adults, in the category that we term “oldest old”, brain Aβ deposition was present at high levels in 55% and was associated with slower gait speed, independent of demographic, cardiac risk, hippocampal volume and small-vessel disease. The association between brain Aβ and gait speed was influenced by global cognitive and executive function capabilities as well as APOE ε4 carrier status. Gait speed was associated with regional Aβ in the frontal, striatal, temporal, parietal, anterior cingulate, precuneus and occipital cortices. However, APOE ε4 attenuated the associations between gait speed and global Aβ and the Aβ deposition in several anterior brain regions, particularly in the CN sample. This is the one of the first reports highlighting the influence of cognition and APOE ε4 on the association between global and regional Aβ and gait speed in dementia free and in CN older adults.
Prior studies have linked Aβ pathology to physical performance measures in aging and in neurodegenerative diseases. In population studies, Aβ plaques and neurofibrillary tangles, hallmarks of AD pathology, are associated more strongly with motor performance measures than are other changes in the aging brain such as small cerebral infarcts or Lewy body pathology.17 While both Aβ plaques and NFT are associated weakly with gait speed in cross-sectional studies they are associated more strongly with gait slowing over 6.4 years of longitudinal assessments.37 In dementia free older adults, elevated levels of Aβ are associated with a 5-fold increase in falls.9 These studies support our findings indicating that greater Aβ deposition in the brain is associated with mobility decline in older adults.
APOE ε4 increases Aβ deposition in preclinical AD and in CN individuals.21,24,35,38 APOE ε4 also has other effects on the brain such facilitating tau hyperphosphorylation35 Moreover, APOE ε4 carriers have more severe gait impairments15 and worsened gait speed decline17,18 than APOE ε4 non-carriers. APOE ε4 status is also associated with gait speed in MCI, although not with other physical performance measures such as grip strength, chair stands or cardiorespiratory status.16 This body of literature suggests that APOE ε4 may influence cortical control of gait by influencing both Aβ related and unrelated processes; this may explain why controlling for APOE ε4 in the statistical analyses led to diminution of the magnitude of relationship between Aβ and gait speed. The association between Aβ and gait speed appears to have been driven by 34 and 27 APOE ε4 carriers in the whole sample and the CN subsample respectively – this small sample of APOE ε4 carriers may have also precluded any interaction between APOE ε4 and interpretation of the association between global PiB retention and gait speed within APOE ε4 subgroups. However, our findings complement the growing body of literature showing that APOE ε4 status may play a role in the association between global cortical Aβ deposition and gait speed in dementia free older adults and in CN individuals.
Cognition plays an important role in motor planning and gait control in older adults, including in those without dementia.39 In CN older adults, slower gait is associated with worse attention, executive function, visuospatial processing and memory.39–41 In dementia free elders, Aβ is associated with global cognitive function,13,14,42 memory13,42–44, attention/executive function,14,45 and visual-spatial processing.14,42 We found that both global cognitive function and executive function measures attenuated the relationship between global PiB SUVR and gait speed in the whole sample, and rendered the relationship between Aβ and gait speed statistically nonsignificant in the CN subsample - suggesting that Aβ may influence higher level cognitive processes that play an important role in gait control in these populations. Furthermore, APOE ε4 modulates the association between global Aβ and global cognition, memory and visual-spatial processing14,42 albeit with exceptions.13 Therefore, our findings suggest that APOE ε4 may influence the cognitive processes involved in the control of gait, and influence gait slowing in older adults.
The exploratory regional analysis in the entire cohort, including the 21% with MCI, revealed that Aβ deposition in the AVS, FRC, ACG, MTC, LTC, PAR, SWM, SMC, and PRC was associated with gait speed while in the CN sample, the regional associations were limited to the SMC, AVS, PRC and LTC. These findings are supported by another recent report on the regional associations between Aβ and gait speed.46 The SMC, AVS, PAR, PRC and related networks play an important role in gait control47,48 and our data suggest that Aβ in these areas may affect gait speed in older adults. However, we also found that regional associations in the FRC, ACG, AVS, PAR and PRC were not significant after correction for APOE ε4. APOE ε4 allele influences Aβ deposition in the frontal, cingulate, striatal, parietal and precuneus regions.21,49 In dementia free PiB(+) older adults, regional Aβ distribution is similar to that of AD patients, including nonspecific binding in SWM,8,49,50 and is associated with cognition – medial temporal Aβ with memory43,44 and frontal, temporal and parietal Aβ with global cognition.14 This may explain why APOE ε4 rendered these predominantly anterior regional associations nonsignificant in the entire sample and in the CN subsample. Our findings differ slightly from the recent study on regional Aβ deposition and gait associations in a heterogeneous sample of older adults selected on basis of memory complaints (99.2%), slow gait (11%) and impaired instrumental activities of daily living (6%) that reported that greater regional Aβ in several frontal, temporal and striatal parietal regions was related to slower gait;46 these analysis were adjusted for APOE ε4 status but not for cardiac risk, WMH volume or cortical atrophy.46 The differences in the two studies could relate to varying inclusion criteria, delineation of ROIs, differences in the study samples and the statistical adjustments used.
Our findings show that Aβ is not strongly associated with gait speed in CN individuals suggesting that Aβ per se is not the main driver of slow gait speed in aging or in AD. Aβ may coexist and contribute to other AD-related brain changes such as inflammation, tau aggregation and neurofibrillary tangle pathology, which spreads to the neocortex coinciding with onset of AD symptoms51 that may include gait slowing.2,3 In PiB(+) CN individuals, Aβ deposition may be an early event in the AD process and may be weakly associated with gait speed, nevertheless modified by APOE ε4 allele that favors Aβ deposition over tau aggregation in CN aging.24 Changes in the brain in CN older adults may be independent of Aβ.52 Given the lack of research in this area, we speculate that gait speed in older adults may be affected by both Aβ dependent and Aβ-independent pathways influenced by APOE ε4.
Our study has several limitations. This was an exploratory secondary analysis of data of well-characterized older adults who had PiB PET scans along with physical performance measures. The smaller sample size, especially in the APOE ε4 subgroup analyses, resulted in lower statistical power that is required to show meaningful conclusions. We cannot exclude the possibility of other AD-related pathologies such as tau contributing to gait slowing in our sample. The timing of gait speed assessment was not concurrent with PiB-PET, however, we performed a sensitivity analysis that showed that controlling for the duration of time between gait assessment and PiB-PET scan did not affect the overall results. Lastly, this was a cross-sectional analysis that included an exploratory analysis of regional associations on a well characterized sample; therefore, while our findings are hypotheses generating, we cannot address causality or directionality of these associations.
In summary, this study revealed that in older adults without dementia, gait speed was modestly associated with Aβ deposition independent of cardiac risk, hippocampal volume and small-vessel disease burden, and the relationship between Aβ deposition and gait speed was attenuated by APOE ε4 and cognition.
Acknowledgments
We would like to acknowledge Tao Jiang, PhD, Division of Geriatric Medicine, Department of Medicine, University of Pittsburgh, Pittsburgh, PA for assistance with statistical analysis and figures.
Funding/support: This study was supported by grant U01 AT000162 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements (Drs. DeKosky and Williamson), grant K23 AG049945 from the National Institute on Aging (Dr. Nadkarni), grant P30 AG024827 from the National Institute on Aging (Dr. Perera), grant P50 AG005133 from the National Institute on Aging (Drs. Lopez, Snitz, Mathis) and grant AG047266 from the National Institute on Aging (Dr. DeKosky).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of interest disclosure: GE Healthcare holds a license agreement with the University of Pittsburgh. Drs. Klunk and Mathis are co-inventors of PiB and, as such, have a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. All other authors have no conflicts of interest with this work.
Author contribution: Dr. Neelesh Nadkarni, Assistant Professor, Division of Geriatric Medicine, Department of Medicine, University of Pittsburgh and Dr. Subashan Perera, Associate Professor, Division of Geriatric Medicine, Department of Medicine and Biostatistics, University of Pittsburgh had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Perera conducted and is responsible for the data analysis.
References
- 1.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkins CH, Roe CM, Morris JC, Galvin JE. Mild physical impairment predicts future diagnosis of dementia of the Alzheimer’s type. J Am Geriatr Soc. 2013;61(7):1055–1059. doi: 10.1111/jgs.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71(7):499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathis CA, Kuller LH, Klunk WE, et al. In vivo assessment of amyloid-beta deposition in nondemented very elderly subjects. Ann Neurol. 2013;73(6):751–761. doi: 10.1002/ana.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark SL, Roe CM, Grant EA, et al. Preclinical Alzheimer disease and risk of falls. Neurology. 2013;81(5):437–443. doi: 10.1212/WNL.0b013e31829d8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montero-Odasso M, Oteng-Amoako A, Speechley M, et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69(11):1415–1421. doi: 10.1093/gerona/glu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen RC, Wiste HJ, Weigand SD, et al. Association of Elevated Amyloid Levels With Cognition and Biomarkers in Cognitively Normal People From the Community. JAMA neurology. 2016;73(1):85–92. doi: 10.1001/jamaneurol.2015.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantarci K, Lowe V, Przybelski SA, et al. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012;78(4):232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melzer D, Dik MG, van Kamp GJ, Jonker C, Deeg DJ. The apolipoprotein E e4 polymorphism is strongly associated with poor mobility performance test results but not self-reported limitation in older people. J Gerontol A Biol Sci Med Sci. 2005;60(10):1319–1323. doi: 10.1093/gerona/60.10.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi T, Shimada H, Makizako H, Tsutsumimoto K, Uemura K, Suzuki T. Apolipoprotein E genotype and physical function among older people with mild cognitive impairment. Geriatr Gerontol Int. 2015;15(4):422–427. doi: 10.1111/ggi.12291. [DOI] [PubMed] [Google Scholar]
- 17.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009;23(1):63–69. doi: 10.1097/wad.0b013e31818877b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verghese J, Holtzer R, Wang C, Katz MJ, Barzilai N, Lipton RB. Role of APOE genotype in gait decline and disability in aging. J Gerontol A Biol Sci Med Sci. 2013;68(11):1395–1401. doi: 10.1093/gerona/glt115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan TY, Chang WC, Lan TH, Hurng BS. Apolipoprotein E genotype and risk of developing physical limitations in elderly people. J Am Geriatr Soc. 2009;57(7):1308–1309. doi: 10.1111/j.1532-5415.2009.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blazer DG, Fillenbaum G, Burchett B. The APOE-E4 allele and the risk of functional decline in a community sample of African American and white older adults. J Gerontol A Biol Sci Med Sci. 2001;56(12):M785–789. doi: 10.1093/gerona/56.12.m785. [DOI] [PubMed] [Google Scholar]
- 21.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR, Jr, Wiste HJ, Weigand SD, et al. AGe, sex, and apoe ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA neurology. 2015;72(5):511–519. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annu Rev Neurosci. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- 24.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ossenkoppele R, van der Flier WM, Zwan MD, et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology. 2013;80(4):359–365. doi: 10.1212/WNL.0b013e31827f0889. [DOI] [PubMed] [Google Scholar]
- 26.Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeKosky ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27(3):238–253. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Snitz BE, O’Meara ES, Carlson MC, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302(24):2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez OL, Klunk WE, Mathis C, et al. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83(20):1804–1811. doi: 10.1212/WNL.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- 32.Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89(6):569–579. doi: 10.2522/ptj.20080258. [DOI] [PubMed] [Google Scholar]
- 33.Rosario BL, Weissfeld LA, Laymon CM, et al. Inter-rater reliability of manual and automated region-of-interest delineation for PiB PET. Neuroimage. 2011;55(3):933–941. doi: 10.1016/j.neuroimage.2010.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamboh MI, Aston CE, Hamman RF. The relationship of APOE polymorphism and cholesterol levels in normoglycemic and diabetic subjects in a biethnic population from the San Luis Valley, Colorado. Atherosclerosis. 1995;112(2):145–159. doi: 10.1016/0021-9150(94)05409-c. [DOI] [PubMed] [Google Scholar]
- 35.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebes RD, Pollock BG, Perera S, Halligan EM, Saxton JA. The greater sensitivity of elderly APOE epsilon4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. Am J Geriatr Pharmacother. 2012;10(3):185–192. doi: 10.1016/j.amjopharm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013;80(22):2055–2061. doi: 10.1212/WNL.0b013e318294b462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risacher SL, Kim S, Shen L, et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI) Front Aging Neurosci. 2013;5:11. doi: 10.3389/fnagi.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 41.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164(4):541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 42.Seo EH, Kim SH, Park SH, Kang SH, Choo IH, Alzheimer’s Disease Neuroimaging I Independent and Interactive Influences of the APOE Genotype and Beta-Amyloid Burden on Cognitive Function in Mild Cognitive Impairment. J Korean Med Sci. 2016;31(2):286–295. doi: 10.3346/jkms.2016.31.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(Pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 45.Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1(8–9):371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Campo N, Payoux P, Djilali A, et al. Relationship of regional brain beta-amyloid to gait speed. Neurology. 2016;86(1):36–43. doi: 10.1212/WNL.0000000000002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuyama H, Ouchi Y, Matsuzaki S, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228(3):183–186. doi: 10.1016/s0304-3940(97)00381-9. [DOI] [PubMed] [Google Scholar]
- 48.Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106(4):283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 49.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 50.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 51.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60(4):534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann Neurol. 2012 doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]


