SUMMARY
The Drosophila pharyngeal taste organs are poorly characterized despite their location at important sites for monitoring food quality. Functional analysis of pharyngeal neurons has been hindered by the paucity of molecular tools to manipulate them, as well as their relative inaccessibility for neurophysiological investigations. Here, we generate receptor-to-neuron maps of all three pharyngeal taste organs by performing a comprehensive chemoreceptor-GAL4/LexA expression analysis. The organization of pharyngeal neurons reveals similarities and distinctions in receptor repertoires and neuronal groupings compared to external taste neurons. We validate the mapping results by pinpointing a single pharyngeal neuron required for feeding avoidance of L-canavanine. Inducible activation of pharyngeal taste neurons reveals functional differences between external and internal taste neurons and functional subdivision within pharyngeal sweet neurons. Our results provide road maps of pharyngeal taste organs in an insect model system for probing the role of these understudied neurons in controlling feeding behaviors.
INTRODUCTION
In Drosophila, taste neurons located in sensilla in several body regions sense and distinguish nutritive substances such as sugars, amino acids, and low salt, and potentially harmful ones such as high salt, acids, and a diverse variety of bitter compounds (Freeman and Dahanukar, 2015, Liman et al., 2014). Hair-like sensilla on the labellum, the distal segments of the legs (tarsi), the anterior wing margins, and the ovipositor have access to chemicals in external substrates. Pit-like sensilla (taste pegs) on the oral surface have access only once the fly extends its proboscis and opens the labellar palps; similar sensilla in the pharynx have access only when food intake is initiated. Based on its anatomical position, the pharynx is considered to act as a gatekeeper to control ingestion, promoting the intake of appetitive foods and blocking that of toxins.
Three distinct internal taste organs are present in the adult fly pharynx: the labral sense organ (LSO), and the ventral and dorsal cibarial sense organs (VCSO, DCSO). The VCSO and DCSO are paired on opposite sides of the rostrum, whereas the LSO is located in the haustellum (Figure 1A,B). The organization and neuronal composition of all three organs has been described in detail, based on both light and electron microscopy data (Gendre et al., 2004, Nayak and Singh, 1983, Stocker and Schorderet, 1981). Nine separate sensilla are present in the LSO, of which #1–6 are innervated by a single mechanosensory neuron each. The remaining three, named #7–9, are uniporous sensilla, a feature that ascribes chemosensory function to them. Sensillum #7 is the largest one with eight chemosensory neurons. Sensilla #8 and #9 have two neurons, one mechanosensory and one chemosensory, each. Although one study reported two sensilla in the VCSO (Nayak and Singh, 1983), we, and two others (Stocker and Schorderet, 1981, Gendre et al., 2004), observed three sensilla in the VCSO, innervated by a total of 8 chemosensory neurons. The DCSO has two sensilla, each containing three chemosensory neurons. Notwithstanding the availability of detailed anatomical descriptions of pharyngeal taste organs, little is known about their function. The internal location of these organs poses challenges for electrophysiological analysis of taste neurons located within them. Additionally, few molecular tools are currently described to manipulate the function of selected pharyngeal taste neurons.
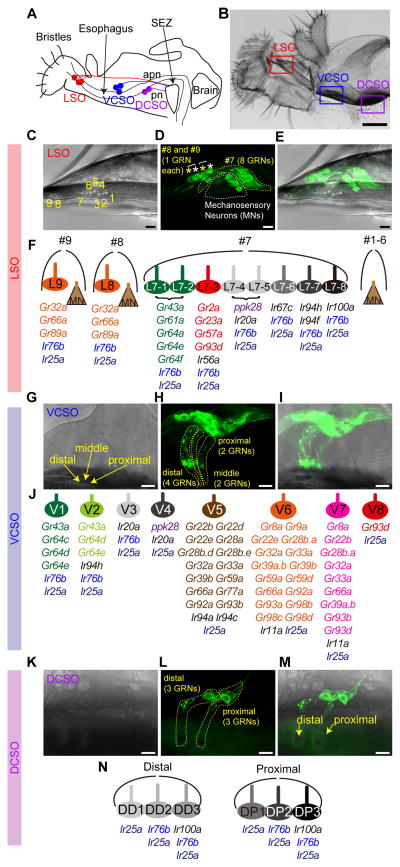
Figure 1. Receptor-to-neuron maps of three pharyngeal taste organs.
Schematic (A) and bright field image of proboscis (B) and three pharyngeal taste organs in wild type flies. Taste neurons from the LSO and VCSO project to the subesophageal zone (SEZ) via the accessory pharyngeal nerve (apn); DCSO neurons project via the pharyngeal nerve (pn). Scale bar: 100 μm (C–E) MJ94-GAL4 driven UAS-mCD8-GFP labeled neurons in LSO. Numbers in (C) indicate the nine LSO sensilla with a linear organization. In (D) white dotted line and asterisks indicate mechanosensory neurons; yellow dotted line and asterisks indicate gustatory receptor neurons (GRNs). Scale bar: 10 μm. (F) Schematic summary of a receptor-to-neuron map of the LSO as defined by reporter gene expression. MN, Mechanosensory neurons. (G–I) Ir25a-GAL4 driven UAS-mCD8-GFP labeled neurons in the VCSO. Yellow arrows mark three VCSO chemosensory sensilla with non-linear organization, which precluded sensillar assignment of individual neurons in the following mapping analysis. Yellow dotted lines delineate groups of GFP labeled neurons in each sensillum. Note that these representative images are the same as shown in Fig. 3A. Scale bar: 10 μm. (J) Schematic summary of a receptor-to-neuron map of the VCSO as defined by reporter gene expression. (K–M) MJ94-GAL4 driven UAS-mCD8-GFP labeled neurons in the DCSO. Yellow arrows mark two DCSO chemosensory sensilla. Yellow dotted lines delineate groups of GFP labeled neurons in each sensillum. Scale bar: 10 μm. (N) Schematic summary of a receptor-to-neuron map of the DCSO as defined by reporter gene expression.
The expression and function of members of several chemosensory receptor gene families such as Gustatory receptors (Grs), Ionotropic receptors (Irs), Pickpocket (Ppk), and Transient receptor potential channels (Trps) have been found in external gustatory receptor neurons (GRNs) of the labellum and the tarsal segments (Freeman and Dahanukar, 2015). A number of Gr- and Ir-GAL4 drivers are also shown to label pharyngeal organs (Kwon et al., 2014, Koh et al., 2014), however only a few, including Gr43a and members of sweet Gr clade, Gr2a, Ir60b, and TrpA1, have been mapped to specific taste neurons (LeDue et al., 2015, Kang et al., 2010, Miyamoto et al., 2012, Kim et al., 2017, Joseph et al., 2017).
Here, we generate receptor-to-neuron maps for three pharyngeal taste organs by a systematic expression analysis of chemoreceptor reporter lines that represent Gr, Ir and ppk receptor families. The maps reveal a large and diverse chemoreceptor repertoire in the pharynx. Some receptors are expressed in combinations that are predictive of neuronal sweet or bitter taste function based on analysis of external GRNs. By contrast, some pharyngeal taste neurons express receptor combinations that are distinct from any that have been reported in other organs, leaving open questions about their functional roles. We validate the receptor-to-neuron maps derived from reporter gene expression by assessing roles of pharyngeal GRNs predicted to detect L-canavanine, a bitter tastant for which a complete receptor repertoire has been reported (Shim et al., 2015). Interestingly, a systematic activation analysis of different classes of pharyngeal taste neurons reveals functional differences between external and internal taste neurons for bitter avoidance and functional subdivision within pharyngeal sweet neurons for sweet acceptance. Together, our study provides a molecular map of pharyngeal taste organs, which will serve as a resource for future studies of the roles of pharyngeal taste neurons in food evaluation.
RESULTS
Chemoreceptor reporter expression in pharyngeal taste organs
Although adult Drosophila pharyngeal taste organs have been anatomically characterized, little is known about receptor expression in sensory neurons housed within these organs. In a previous study, we described neurons in the LSO and VCSO that co-express multiple Grs belonging to the sweet clade (LeDue et al., 2015). However, sweet neurons account for a small fraction (4 of 24) of pharyngeal gustatory receptor neurons (GRNs). We therefore systematically analyzed 43 Gr-GAL4 drivers reported to label afferents in the pharyngeal nerve and terminate in the SEZ (Kwon et al., 2014). We mapped expression of 36 Gr-GAL4 lines, which still showed strong expression in the pharynx (Table S1). We also examined a number of Ir-GAL4 drivers, focusing on 8 members of the Ir20a clade along with Ir11a and Ir100a, whose expression was reported in the pharynx (Koh et al., 2014, Croset et al., 2010). We included drivers for two broadly expressed Ir co-receptors, Ir25a and Ir76b, which are expressed in GRNs of external organs (Hussain et al., 2016, Zhang et al., 2013, Croset et al., 2010), and ppk28-GAL4, which marks water-sensing neurons in the labellum (Cameron et al., 2010). For most receptors, we tested two independent transgenic lines using UAS-mCD8-GFP. First, we identified the number of cells that were GFP positive in the pharynx. Next, we traced labeled dendrites to specific sensilla within each of the three pharyngeal taste organs. By using MJ94-Gal4, which labels most if not all chemosensory and mechanosensory neurons in the pharynx (Gendre et al., 2004), we were able to visualize one mechanosensory neuron each in LSO sensilla #1–6, eight GRNs in LSO sensillum #7, and one mechanosensory neuron and one GRN each in LSO sensilla #8 and #9 (Figure 1C–F). In addition, we observed a total of eight GRNs in three VCSO taste sensilla (Figure 1G–J), and a total of six GRNs in two DCSO taste sensilla (Figure 1K–N) using Ir25a-GAL4 and MJ94-GAL4, respectively. The three taste sensilla in the LSO, named #7, #8 and #9, are easily distinguishable from each other, as are the two proximal and distal sensilla in the DCSO. We were therefore able to map expression of each driver to one or more identified neurons within each sensillum of the LSO and DCSO (Figure 1F and 1N). The cuticular pores of the three sensilla in the VCSO, however, are clustered together in a non-linear manner that precluded unambiguous mapping of labeled dendrites to their particular locations (Figure 1G). Thus, we mapped expression to specific neurons of the VCSO, but did not attest sensillar assignments (Figure 1J). We introduced a new nomenclature for pharyngeal GRNs, abbreviating location and assigning numbers as follows: L7-1 through L7-8 in the LSO sensillum #7, L8 and L9 in the LSO sensilla #8 and #9; V1 – V8 in the VCSO; DD1 – DD3 in the distal sensillum of the DCSO, and DP1 – DP3 in the proximal sensillum of the DCSO.
In general, we found a total of 12 Gr-GAL4 lines expressed in the LSO (Figure 2), including the 5 sweet Gr-GAL4 drivers that we reported previously (LeDue et al., 2015), and 28 in the VCSO (Figure 3A–H). A vast majority of the drivers labeled 1–3 neurons in the VCSO; several showed expression in 1– 2 neurons in the LSO. We found Gr-GAL4 lines that were expressed in the LSO alone, in the VCSO alone, as well as in both. Interestingly, the DCSO appeared to exclude Gr-expressing neurons (Figure 3I–K). ppk28-GAL4 also labeled cells exclusively in the LSO and VCSO. By contrast, we found Ir-expressing neurons in all pharyngeal taste organs. Ir25a-GAL4, in particular, labeled all GRNs in the LSO, VCSO and DCSO, whose expression was validated with an Ir25a antibody (Figure 2C, 3A and I, Supplemental movies 1,3,4). Ir76b-GAL4 also showed broad expression (Figure 2D, 3B and J). All other drivers were expressed in smaller subsets of neurons (Figure 2E, 3F and J). To further identify GRNs that express each driver, we performed a series of double-driver and double-labeling analyses. For the double-driver analyses, we examined selected pairwise combinations of drivers and compared the number of GFP positive neurons for two GAL4 drivers with those observed for a single GAL4 driver alone (Figure 2H–J and 3D,G,H). We also took advantage of several LexA drivers to perform two-color analyses to confirm co-expression of drivers in the same neurons (Figure 2B,F,G, 3B,C,E,F,K). Details of the mapping procedure are described in the following sections. Receptor-to-neuron maps generated from analyses of reporter lines are summarized in Figure 1F, 1J and 1N.
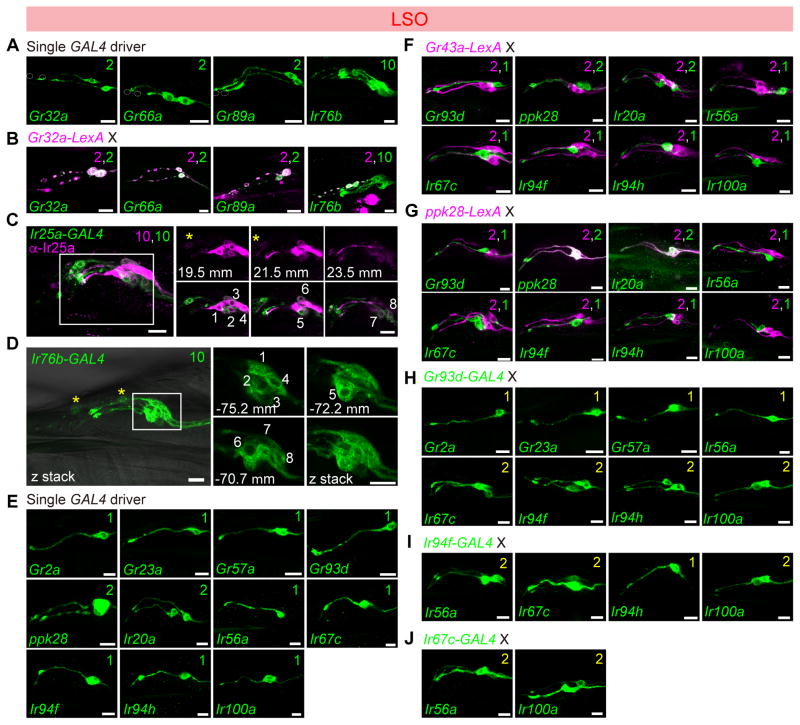
Figure 2. Chemoreceptor-GAL4/LexA reporter mapping in the labral sense organ (LSO).
Expression of Gr-GAL4 and Ir-GAL4 lines in the LSO, tested with UAS-mCD8-GFP alone (green) (A, D, E); co-stained with anti-Ir25a antibody (magenta) (C); tested in combination with LexAop2-mCherry-HA (magenta) in co-labeling experiments with Gr32a-LexA (B), Gr43a-LexA (F), ppk28-LexA (G); and in double-driver experiments with indicated GAL4 driver (H–I). Numbers in panels in (C) and (D) are used to label different cells visualized in different optical planes; positions along the z-axis are indicated in μm for the extracted slices. See also Supplemental movie 1 and 2. Numbers in top right corners indicate total numbers of green and magenta cells labeled by corresponding GAL4/LexA drivers. Numbers in yellow in top right corners in panels (H), (I) and (J) indicate total numbers of GFP+ cells observed with double GAL4 driver analysis. Scale bar: 10 μm.
All panels show compressed z-stacks, with the exception of those labeled with μm in (C) and (D), which represent single optical slices.
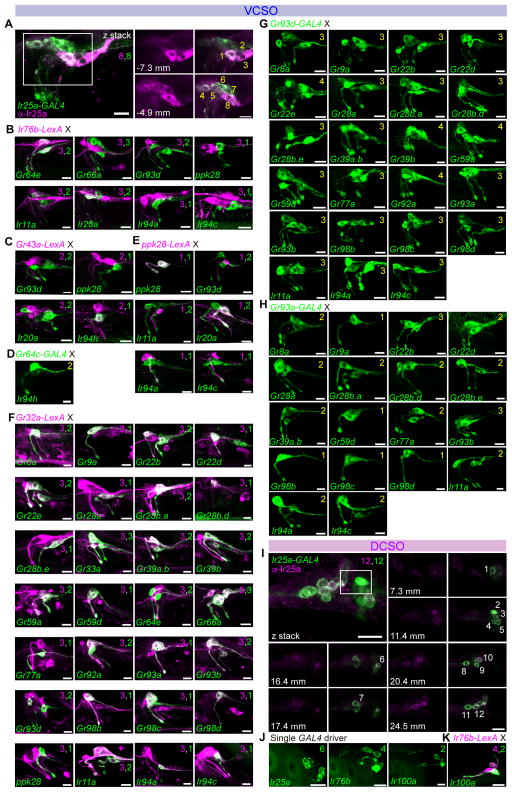
Figure 3. Chemoreceptor-GAL4/LexA reporter mapping in ventral and dorsal cibarial sense organs (VCSO/DCSO).
(A–H) GFP expression (green) driven by indicated Gr-GAL4 and Ir-GAL4 lines in the VCSO co-stained with anti-Ir25a antibody (magenta) (A); tested in combination with LexAop2-mCherry-HA (magenta) in co-labeling experiments with Ir76b-LexA (B), Gr43a-LexA (C), ppk28-LexA (E), and Gr32a-LexA (F); and in double-driver experiments with indicated GAL4 driver (D, G, H). Numbers in panels in (A) are used to label different cells visualized in different optical planes; positions along the z-axis are indicated in μm for the extracted slices. See also Supplemental movie 3. Numbers in top right corners indicate total numbers of green or magenta cells labeled with corresponding GAL4/LexA reporters. Numbers in yellow in the top right corners in panels (D), (G) and (H) indicate total numbers of GFP+ cells observed with double GAL4 driver analysis.
(I) GFP expression (green) driven by Ir25-GAL4 in the DCSO co-stained with anti-Ir25a antibody (magenta). Numbers in panels are used to label different cells visualized in different optical planes; positions along the z-axis are indicated in μm for the extracted slices. See also Supplemental movie 4. Scale bar: 10 μm.
(J–K) GFP expression (green) driven by indicated Ir-GAL4 lines in the DCSO (J) or tested in combination with LexAop2-mCherry-HA (magenta) in co-labeling experiments with Ir76b-LexA (K). Numbers in the top right corners indicate total numbers of green and magenta cells labeled by corresponding GAL4/LexA reporters. See also Figure S1.
All panels show compressed z-stacks, with the exception of those labeled with μm in (A and I), which represent single optical slices. Scale bar: 10 μm.
Chemoreceptor reporter mapping in the labral sense organ (LSO)
In the LSO, we found that L8 and L9 expressed 3 Gr-GAL4 lines (Gr32a, Gr66a and Gr89a) (Figure 2A,B) representing commonly expressed receptors that are broadly expressed in external bitter taste neurons (Ling et al., 2014, Weiss et al., 2011). Ir76b- and Ir25a-GAL4 labeled all taste neurons of the #7–9 sensilla (Figure 2C,D and Supplemental movies 1,2). The expression of Ir25a-GAL4 matched with the anti-Ir25a antibody staining (Figure 2C). In the #7 sensilum of LSO, the L7-1 through L7-8 neurons could be grouped into six classes based on GAL4 expression patterns (Figure 2E–J). As previously described, two neurons, L7-1 and L7-2, expressed Gr43a along with other members of the sweet Gr clade (LeDue et al., 2015). Double labeling experiments with Gr43a-LexA and selected GAL4 drivers that labeled 1–2 neurons of the #7 sensillum revealed that cells expressing Gr93d/Ir56a (L7-3), ppk28/Ir20a (L7-4 and L7-5), Ir67c (L7-6), Ir94f/Ir94h (L7-7) and Ir100a (L7-8) were distinct from those expressing Gr43a (Figure 2F). A similar series of experiments with ppk28-LexA showed overlap with GAL4 lines of Ir20a but not Gr93d, Ir56a, Ir67c, Ir94f, Ir94h and Ir100a (Figure 2G). Mapping of Gr93d, Ir67c, Ir94f and Ir100a GAL4 lines to separate neurons was confirmed by examining pairwise combinations of the four drivers – in all cases animals with two drivers showed two labeled neurons, whereas each driver alone labeled only a single neuron. Additional double-driver experiments with either Gr93d- or Ir94f-GAL4 indicated co-expression of four other receptors, Gr2a, Gr23a, Gr57a and Ir56a, in the L7-3 neuron, and Ir94h in the L7-7 neuron (Figure 2H–J). We mapped Ir100a expression along with Ir25a and Ir76b in the L7-8 neuron, since Ir100a showed no co-expression with driver lines for Gr43a, Gr93d, ppk28, Ir67c and Ir94f. A receptor-to-neuron map for LSO generated from these results is shown in Figure 1F.
Chemoreceptor reporter mapping in the ventral cibarial sense organ (VCSO)
In the VCSO, we found Ir25a-GAL4 to be expressed in all eight neurons of the VCSO as confirmed by an Ir25a antibody (Figure 3A and Supplemental movie 3). By contrast, Ir76b was expressed in only 3 of the 8 GRNs. Two Ir76b+ neurons were identified as V1 and V2, due to co-expression with Gr64e-GAL4. The third Ir76b+ neuron was identified as V3 – it showed partial overlap with Ir20a, but not ppk28 (V4), Gr66a (V5 – V7), Gr93d (V7 – V8), Ir11a, Ir94a, or Ir94c (Figure 3B). Consistent with our previous observations with Gr43a-GAL4 (LeDue et al., 2015), we found that two neurons, V1 and V2, expressed Gr43a-LexA. Double labeling experiments with Gr43a-LexA showed overlap with Ir94h-GAL4 but not with GAL4 lines for Gr93d, ppk28, or Ir20a (Figure 3C). Subsequently, we found that Ir94h-GAL4 and Gr64c-GAL4 independently marked each of the two Gr43a+ neurons, identifying them as Gr64c+ (V1) and Ir94h+ (V2) (Figure 3D). We mapped ppk28 expression to V4, because it was positive for one Ir20a-GAL4 neuron but not another (V3), nor did it overlap with drivers for Gr43a, Ir76b, Gr32a, Gr93d (Figure 3B,C,E,F).
One of the two cells labeled by Gr93d-GAL4 overlapped with Gr32a-LexA, which was expressed in three cells (Figure 3F). Thus, Gr32a and Gr93d together accounted for four additional neurons: V5 (Gr32a+), V6 (Gr32a+) and V7 (Gr32a+, Gr93d+) and V8 (Gr32a−, Gr93d+). We next systematically inspected overlap of Gr32a-LexA expression with GAL4 drivers (Figure 3F). The three Gr32a+ neurons also expressed the Gr33a and Gr66a. The molecular identity of the three Gr32a+ neurons could be further categorized by Gr93a- and Gr93d-GAL4 (Table S2). 11 additional Gr-GAL4 were mapped to V5 by virtue of overlap with Gr32a-LexA but exclusion from Gr93a- and Gr93d-GAL4 cells. A single Gr93a+ neuron was identified as V6, because Gr93a-GAL4 expression overlapped with Gr32a-LexA but not with Gr93d-GAL4. Analysis of driver combinations with Gr93a- and Gr93d-GAL4 mapped a group of 12 additional Gr-GAL4 to V6 (Figure 3F–H). The third Gr32a+ neuron, identified as V7, was characterized as Gr93a–, Gr93d+. Double-driver analyses with Gr93a- and Gr93d-GAL4 placed 5 additional Gr-GAL4 in V7 (Figure 3F–H). V8 was marked solely by expression of Gr93d and no other Gr-GAL4 drivers were co-expressed in this neuron. This series of experiments resolved mapping of all Gr-GAL4 drivers expressed in the VCSO. We next turned to other Ir-GAL4 drivers. Both Ir94a-GAL4 and Ir94c-GAL4 were mapped to V5 because of co-expression with Gr32a-LexA but not Gr93d-GAL4 and Gr93a-GAL4, and confirmed by double-driver analysis that showed three GFP+ neurons in animals that carried Gr93d-GAL4 with either Ir94a-GAL4 or Ir94c-GAL4 (Figure 3G) and two GFP+ neurons in animals that carried Gr93a-GAL4 with either Ir94a-GAL4 or Ir94c-GAL4 (Figure 3H). Ir11a-GAL4 was mapped to V6 and V7 because it overlapped with Gr32a-LexA, Gr93a-GAL4 and partially overlapped with Gr93d-GAL4 (Figure 3F–H). A receptor-to-neuron map for VCSO generated from these results is shown in Figure 1J.
Chemoreceptor reporter mapping in dorsal cibarial sense organ (DCSO)
In the DCSO, we found Ir25a-GAL4 expression in all six neurons in the proximal and distal sensilla (Figure 3I and Supplemental movie 4). In addition, Ir76b-GAL4 marked two of the three neurons in each sensillum (Figure 3J). Notably, Ir100a-GAL4 showed expression in the one taste neuron in each DCSO sensillum, which overlapped with Ir76b-LexA (Figure 3K). Gr-GAL4 expression appears to be excluded from the DCSO, although we inconsistently observed expression of drivers for Gr22b and Gr93d (Figure S1). A receptor-to-neuron map for DCSO generated from these results is shown in Figure 1N.
A pharyngeal taste representation map in the subesophageal zone (SEZ)
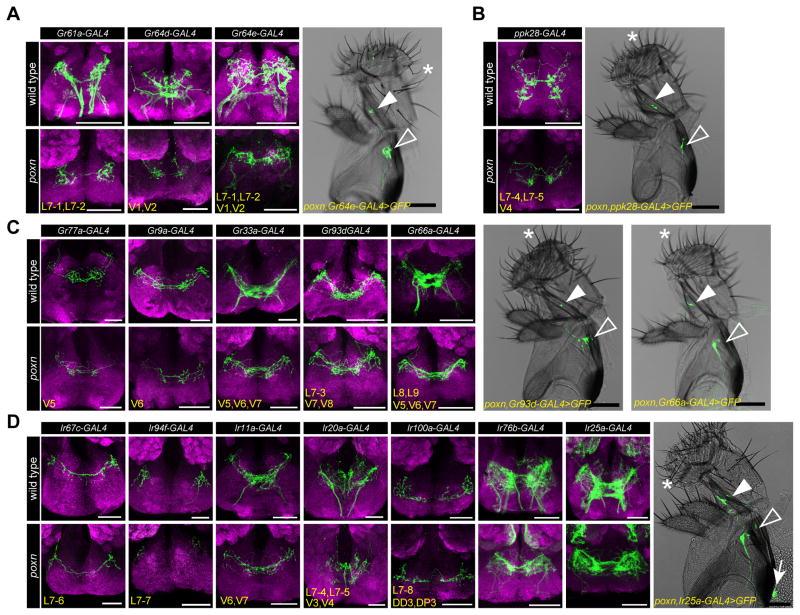
Previous studies have shown that axons of taste neurons in pharyngeal taste organs travel via the pharyngeal and accessory pharyngeal nerves and terminate in the dorso-anterior region of the primary taste center, the subesophageal zone (SEZ) (LeDue et al., 2015, Kwon et al., 2014, Stocker and Schorderet, 1981). Our receptor-to-neuron maps gave us an opportunity to examine axonal termini of bilaterally symmetrical pairs of taste neurons utilizing drivers that label single, or a small subset of, identified neurons. We tested all chemosensory receptor-GAL4 drivers that label every neuronal class identified by mapping analysis, including four main classes of Gr/Ir-expressing pharyngeal GRNs: 1) sweet pharyngeal GRNs labeled by Gr61a-/Gr64d-/Gr64e-GAL4 (Figure 4A); 2) putative water pharyngeal GRNs labeled by ppk28-GAL4 (Figure 4B); 3) putative bitter pharyngeal GRNs labeled by Gr77a-/Gr9a-/Gr33a-/Gr93d-/Gr66a-GAL4 (Figure 4C); 4) Ir-expressing pharyngeal GRNs labeled by Ir67c-/Ir94f-/Ir11a-/Ir20a-/Ir100a-/Ir76b-/Ir25a-GAL4 lines (Figure 4D). Since most of these drivers also showed expression in external GRNs, we examined labeled projections both in wild type and in a pox-neuro (poxn) mutant background, in which all external taste bristles are transformed into mechanosensory bristles (Awasaki and Kimura, 1997, Nottebohm et al., 1992). As expected, in poxn mutants UAS-mCD8-GFP driven by chemosensory receptor-GAL4 drivers showed expression in internal GRNs and their corresponding axonal projections in the SEZ (Figure 4A–D). As reported previously (LeDue et al., 2015), poxn mutants also retained labeling in a few taste pegs present on the oral surface of the labellum; axons of these neurons terminate in characteristic, bilaterally symmetric S-shaped patterns in the SEZ. Axonal termini of pharyngeal GRNs were all found in the expected dorso-anterior region, with some differences in patterns of axonal arborization. We noticed that neurites of GRNs that are predicted to sense aversive tastants (e.g. Gr77a-/Gr9a-/Gr33a-/Gr93d-/Gr66a-GAL4) had extensive projections at the midline, whereas those predicted to sense appetitive tastants (e.g. Gr61a-/Gr64d-/Gr64e-/ppk28-/Ir94f-GAL4) were present in discrete regions on each ipsilateral side.
Figure 4. Axonal projections of different classes of pharyngeal GRNs in the subesophageal zone (SEZ).
(A–D) Images of the SEZ showing axonal termini (green) labeled by indicated Gr- or Ir-GAL4 drivers in wild type (w1118, top) and poxn (poxnΔM22-B5/poxn70, bottom) flies. Four main classes of pharyngeal neuronal projections are presented: sweet pharyngeal GRNs (A), putative water pharyngeal GRNs (B), putative bitter pharyngeal GRNs (C), and Ir-GAL4 expressing pharyngeal GRNs (D). Bright field images of the proboscis show GFP cells (green) labeled by indicated GAL4 driver in the LSO (closed arrowhead), VCSO (open arrowhead) and DCSO (arrow) in poxn mutants. Asterisks point to representative long, bent mechanosensory bristles, which are present in place of external taste hairs in poxn mutants. Scale bar: 100 μm. Neuropil is stained with anti-nc82 (magenta). Subsets of pharyngeal GRNs labeled by GAL4 drivers named in bottom left corners. Also see Figure 1F, J, N for nomenclature. Note the presence of a small subset of taste peg projections labeled by Gr64e-/Ir76b-/Ir25a-GAL4 in the SEZ, and Ir76b-/Ir25a-GAL4 labeled olfactory projections to antennal lobes in poxn mutants.
Pharyngeal taste projections can be separated by neurons and organs
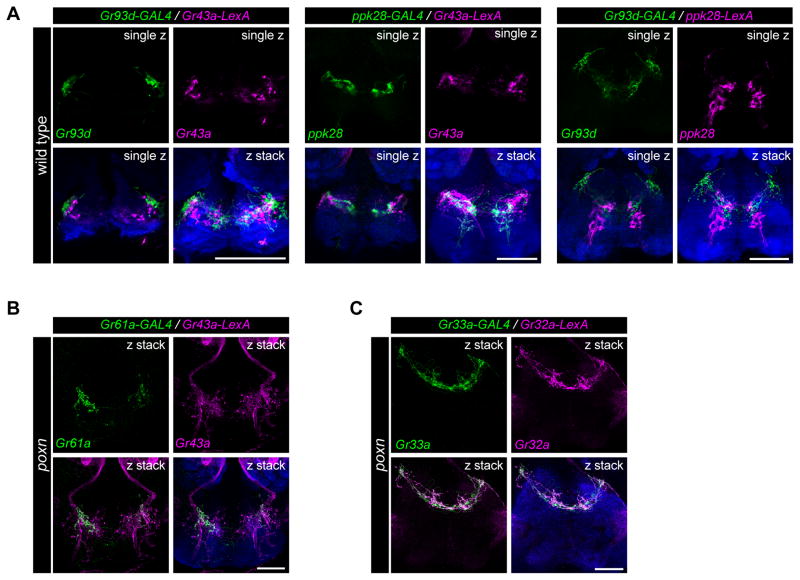
To characterize the projections of different classes of pharyngeal GRNs in the SEZ, we examined the overlap of Gr43a, ppk28, and Gr93d projections by testing combinations of GAL4/LexA drivers. We visualized single optical sections of fluorescence images, which revealed little overlap between Gr43a, ppk28 and Gr93d-labeled termini (Figure 5A), consistent with the idea that these GRNs, which are likely to sense different categories of tastants, have distinct representations in the SEZ. To examine whether GRNs of the same taste category originating in different pharyngeal taste organs target discrete areas of the SEZ, we compared axonal projections labeled by Gr43a-LexA (LSO and VCSO) and Gr61a-GAL4 (LSO alone) in poxn mutants, using two-color analysis. We note that Gr43a-LexA projections of olfactory neurons were also visualized via labial nerves in the antennal lobes of poxn mutants. We found Gr43a-LexA taste projections distributed in an anterior zone of the SEZ, labeling neurites in medial and lateral regions. Overlapping Gr61a-GAL4 projections were found in the lateral areas, but were limited or absent in the medial region, suggesting the pharyngeal sweet neuronal projections from the VCSO terminate medially as compared to those from the LSO (Figure 5B). The separations between putative bitter pharyngeal GRNs of the LSO and VCSO were less obvious when we compared Gr32a-LexA (LSO and VCSO) and Gr33a-GAL4 (VCSO alone) in labeling in poxn flies (Figure 5C), mainly due to the extensive projections at the midline in these putative bitter pharyngeal GRNs. Overall, these results suggest that pharyngeal GRNs of different classes and/or different pharyngeal taste organs target distinct areas of the SEZ and may represent distinct neural circuits, and possibly distinct functional roles.
Figure 5. Pharyngeal neurons of different categories or location show distinct patterns of axonal projections in the subesophageal zone (SEZ).
(A) Axonal projections of pharyngeal GRNs labeled by different GAL4/LexA drivers in the SEZ in wild type (w1118) flies. Annotations in top right corners of each image indicate a single optical section (single z) to examine reporter co-localization, or compressed z-stacks (z stack) for comparison. Neuropil is stained with anti-nc82 (blue). In all panels, scale bar: 50 μm.
(B) Axonal projections labeled by Gr61a-GAL4 (green) and Gr43a-LexA (magenta) in the SEZ in poxn (poxnΔM22-B5/poxn70) flies. Note the presence of intact olfactory projections to antennal lobes through labial nerves labeled by Gr43a-LexA in poxn mutants. Neuropil is stained with anti-nc82 (blue). scale bar: 50 μm.
(C) Axonal projections labeled by Gr33a-GAL4 (green) and Gr32a-LexA (magenta) in the SEZ in poxn (poxnΔM22-B5/poxn70) flies. Neuropil is stained with anti-nc82 (blue). scale bar: 50 μm.
Functional validation of pharyngeal taste receptor-to-neuron maps
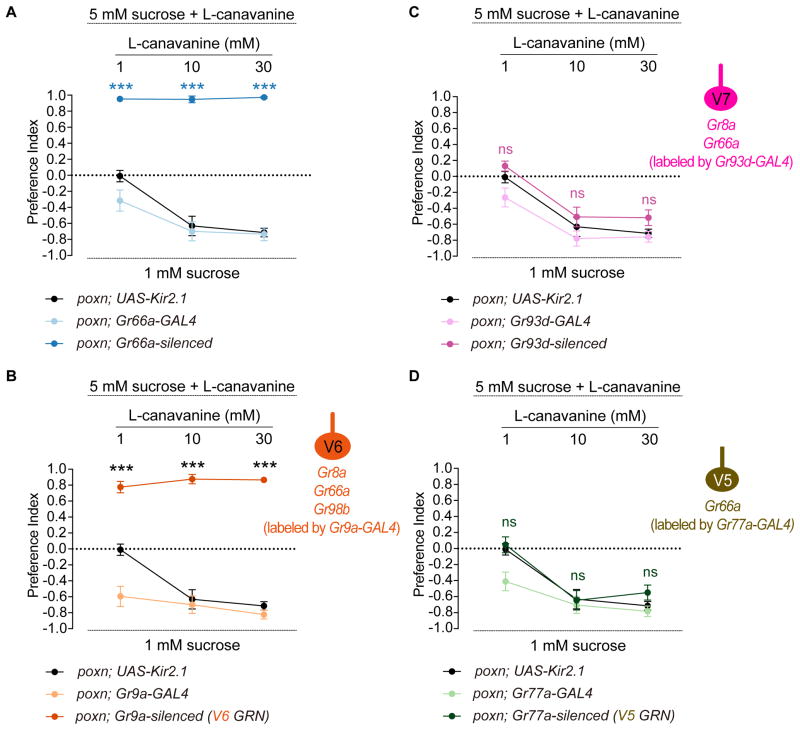
We next wished to validate the results of our receptor-to-neuron maps, given the caveat that transgenic drivers, which we used to assess receptor expression patterns, may not always reflect endogenous expression patterns of receptors. In a previous study, we confirmed that Gr64e-GAL4 does in fact label pharyngeal sweet GRNs using calcium imaging and behavior assays (LeDue et al., 2015). We therefore decided to focus on a different taste category to validate other neuronal identities. Specifically, we elected to test a bitter compound, L-canavanine for two reasons. First, L-canavanine is known to activate bitter GRNs but it does not inhibit sweet GRNs (French et al., 2015, Jeong et al., 2013), which can otherwise confound interpretations of feeding assays using sugar/bitter mixtures. Second, L-canavanine is the only bitter compound for which a complete receptor repertoire has been described (Shim et al., 2015). A recent study reported a high affinity complex comprising Gr8a/Gr66a/Gr98b for detection of L-canavanine (Shim et al., 2015). Perusal of our receptor-to-neuron maps implicated a single Gr66a neuron, the V6 neuron in the VCSO, as a high affinity sensor of L-canavanine.
We first silenced all Gr66a pharyngeal GRNs using the inwardly rectifying channel, Kir2.1, and tested behavioral responses to various concentrations of L-canavanine mixed with 5 mM sucrose. Experiments were carried out in poxn mutants to exclude any contribution from external GRNs. As predicted, control flies showed avoidance of L-canavanine in a dose dependent manner, which was completely abolished in Gr66a-silenced flies (Figure 6A). We then assessed the role of the V6 neuron in sensing L-canavanine using Gr9a-GAL4, which is expressed exclusively in this neuron. Notably, in the absence of a functional V6 neuron, flies lost the ability to avoid L-canavanine at all concentrations tested (Figure 6B), similar to Gr66a-silenced flies. This result provides functional evidence for L-canavanine receptor expression in the V6 neuron.
Figure 6. Genetic silencing experiments support receptor-to-neuron maps.
Mean preference index values from binary choice experiments with sucrose tested against a mixture of sucrose and L-canavanine at indicated concentrations. All genetics manipulations with Gr66a-GAL4 (A), Gr9a-GAL4 (B), Gr93d-GAL4 (C), and Gr77a-GAL4 (D) were performed in a poxn mutant background (poxnΔM22-B5/poxn70). Schematics of identified VCSO neurons derived from Figure 3 indicating expression of Gr8a-/Gr66a-/Gr98b-GAL4 are shown on the right of Figure 6B, C, D. n=10–30. Error bars = SEM. ***P < 0.0001 versus UAS control, two-way ANOVA with post-hoc Tukey test. ns, not significant.
The previous study suggested that Gr8a and Gr66a together may be sufficient for a weak response to L-canavanine (Shim et al., 2015). We therefore tested the role of the Gr8a/Gr66a-labeled V7 neuron. Since a GAL4 driver that is exclusively expressed in V7 is not available, we expressed Kir2.1 with Gr93d-GAL4, which would silence V7 along with two other neurons in the LSO (L7-3) and VCSO (V8). The resulting flies exhibited no difference in feeding avoidance of L-canavanine as compared to the UAS control at all concentrations of L-canavanine (Figure 6C). As an additional control, we silenced the V5 neuron specifically by Gr77a-GAL4, which does not express either Gr8a or Gr98b according to our reporter analysis. As predicted, this manipulation caused no reduction in L-canavanine avoidance (Figure 6D). We note that Gr93d- and Gr77a-silenced flies showed a significant difference in feeding avoidance of 1 mM L-canavanine as compared to the corresponding GAL4 control, but not the UAS control, suggesting that the difference is likely due to the background effect of UAS-Kir2.1. Taken together, we identified V6 as functional L-canavanine-sensing pharyngeal neurons, with V5 or V7 playing little if any role in sensing L-canavanine, on the basis of their molecular signatures.
Inducible activation of different pharyngeal taste neurons identifies functional differences between and within different taste organs
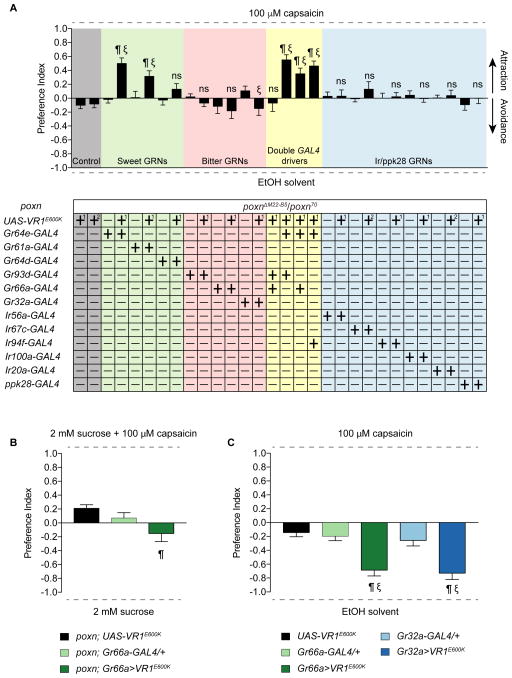
We next wished to identify the valence that each class of pharyngeal taste neurons might carry in our feeding choice assay. The use of cognate tastants may not be ideal for such experiments for two main reasons. First, cross-modality interactions between tastants and GRNs (French et al., 2015, Jeong et al., 2013) confound interpretation of individual GRN valence. Second, a recent report has found sugar-sensing pharyngeal GRNs that have a negative effect on consumption (Joseph et al., 2017), suggesting that tastants defined as “appetitive” or “aversive” may not activate predictable sets of pharyngeal GRNs. Therefore, we expressed the mammalian capsaicin receptor (UAS-VR1E600K) (Marella et al., 2006) under the control of selected GAL4 drivers in poxn mutants and measured feeding preference for capsaicin (Figure 7A). Flies were tested in binary choice assays with 100 μM capsaicin and ethanol solvent as the two alternatives. All GAL4 and UAS controls were tested, and none showed a preference for capsaicin. We found that Gr64e>VR1E600K flies had a significant preference for capsaicin, demonstrating that activation of Gr64e+ neurons in the LSO and VCSO is sufficient to trigger taste acceptance and ingestion. One caveat is that a few taste pegs would also be activated in Gr64e>VR1E600K flies, and activation of taste pegs has been shown to be sufficient for feeding acceptance (Fischler et al., 2007). We next decided to activate Gr64e+ neurons only in the LSO alone (via Gr61a-GAL4) or in the VCSO alone (via Gr64d-GAL4). The former resulted in preference for capsaicin whereas the latter did not, suggesting some functional subdivision of pharyngeal sweet GRNs in driving feeding attraction to sugars.
Figure 7. A systematically inducible activation of different classes of pharyngeal neurons.
(A) Mean preference index values from binary choice experiments with capsaicin tested against ethanol solvent. Genetic manipulations were performed in poxn mutant (poxnΔM22-B5/poxn70). Two different UAS-VR1E600K controls are shown; [1] is a recombinant of UAS-VR1E600K with the poxnΔM22-B5 allele; [2] is a recombinant with the poxn70 allele. n=19–30. Error bars indicate SEM. ¶ indicates significant difference from the corresponding UAS control; ξ indicates significant difference from the corresponding GAL4 control (for double driver experiments, ξ indicates significant difference from both GAL4 controls); P<0.05, one-way ANOVA with Tukey test. ns, not significant.
(B) Mean preference index values from binary choice experiments with capsaicin-sucrose mixture tested against sucrose alone. Genetic manipulations were performed in poxn mutant (poxnΔM22-B5/poxn70). n=28–32. Error bars indicate SEM. ¶ indicates significant difference from the corresponding UAS control; P<0.05, one-way ANOVA with Tukey test.
(C) Results of binary choice feeding assays performed using flies expressing VR1E600K under the control of the indicated GAL4 drivers in a wild type background with only one poxnΔM22-B5 allele. Tastants used were capsaicin and ethanol solvent. n=10–20. Error bars = SEM. ¶ indicates significant difference from the UAS control; ξ indicates significant difference from the corresponding GAL4 control; P<0.05, one-way ANOVA with Tukey test. ns, not significant.
Surprisingly, we did not observe significant feeding avoidance of capsaicin with the activation of putative bitter taste neurons using either the Gr66a- or Gr93d-GAL4 drivers. To rule out the possibility that expression via Gr66a-GAL4 is too weak to drive functional levels of the capsaicin receptor, we confirmed these results using the Gr32a-GAL4 driver, whose expression overlaps precisely with that of Gr66a-GAL4. The activation of Gr32a neurons also did not elicit significant capsaicin avoidance (Figure 7A). Neither did the combined activation of Gr66a+ and Gr93d+ neurons. A 10-fold greater concentration of capsaicin (1 mM) did not yield conclusive results, because it affected gelling of the agarose droplets and thwarted participation of adequate numbers of flies. We next tested whether an avoidance function for these neurons could be uncovered when the flies were induced to consume capsaicin. We found that poxn flies in which Gr66a+ neurons were activated simultaneously with Gr64e+ showed a non-significant reduction of mean PI for capsaicin compared to activation of Gr64e+ alone (two-tailed t-test, p=0.2931, ns). A similar effect was not observed using Gr64e-GAL4 combined with either Gr93d or Ir94f drivers. Thus, although not statistically significant, the reduction in mean PI was specifically observed with the Gr66a driver. We therefore tested whether activation of Gr66a neurons by capsaicin could induce feeding avoidance of a sugar/capsaicin mixture. This experimental paradigm uncovered activation of weak avoidance in Gr66a>VR1E600K flies as compared to the UAS control (one-way ANOVA with Tukey test, p=0.0079) but not the corresponding GAL4 control (one-way ANOVA with Tukey test, p=0.1012, ns) (Figure 7B). Thus, it appears that VR1E600K-mediated activation of bitter GRNs does not elicit a strong feeding avoidance response. Although we cannot rule out the possibility that VR1E600K expression or function is weaker in bitter neurons, capsaicin-induced activation of external bitter neurons in wild type flies using Gr66a- or Gr32a-GAL4 caused strong feeding avoidance (Figure 7C). The two different outcomes may arise from functional differences between internal and external bitter taste circuits, or from numerical differences in activation of bitter neurons.
No discernible phenotypes were observed upon activation or neurons of unknown function marked by Ir56a, Ir67c, Ir94f, Ir100a or Ir20a, or of neurons expressing the Ppk28 water receptor. It is possible that these pharyngeal taste neurons may be involved in other behaviors, such as choice of oviposition substrate, as has been reported for Gr66a (Joseph and Heberlein, 2012). Alternatively, their roles in feeding behaviors may be dependent on the context, such as prior experience, internal state, or complexity of food substrate.
DISCUSSION
Internal pharyngeal taste organs are the least explored taste organs, despite their obvious importance in insect feeding behaviors, which are crucial drivers for damaging crops and vectoring disease. Here we investigate the organization of pharyngeal taste neurons by generating maps of chemoreceptor-GAL4 expression, which showcase the complex molecular signatures and groupings of these in the pharynx.
The receptor-to-neuron maps of pharyngeal taste organs suggest a high degree of molecular complexity, with co-expression of different chemoreceptor family members in many pharyngeal GRNs. In particular, none of the pharyngeal GRNs were found to express Gr genes alone, rather one or more Ir genes were always found in the same neurons. Gr and Ir genes are also co-expressed in some external sweet and bitter-sensing GRNs (Van Giesen et al., 2016, Croset et al., 2010). Thus, both classes of receptors are likely to contribute to responses of Gr/Ir-expressing neurons in the LSO and VCSO, but whether they interact functionally or act independently remains to be determined. In the LSO, expression of sweet Grs and Ir76b overlaps in pharyngeal sweet GRNs, as observed in tarsi as well (Ganguly et al., 2017). In the pharynx, we also found co-expression of ppk28 with Ir genes, which has not been described for external GRNs. These observations invite explorations of possible crosstalk, and its functional significance, between the two classes of receptors.
Pharyngeal GRNs also exhibit distinctive functional groupings. All external bitter GRNs have always been found grouped with sweet GRNs in taste hairs. By contrast, canonical sweet and bitter GRNs appear to segregate in different sensilla in the LSO, which is most well characterized for this perspective. L8 and L9 may be functionally identical, and house only one Gr66a-expressing bitter GRN each, whereas L7 contains two sweet GRNs (L7-1, L7-2). Moreover, external hairs typically have 2–4 GRNs, each of which has a distinct functional profile. In the LSO we find duplications – L7-1 and L7-2 are identical, as are L7-4 and L7-5 – although differences between these pairs of GRNs may emerge as additional chemoreceptors are mapped in the pharynx. Finally, it is difficult to ascribe putative functions to most pharyngeal GRNs based on existing knowledge of receptor function in external counterparts. The L7-3 Gr-expressing neuron, for example, does not express members of the sweet clade, but neither does it express any of the common bitter Grs (Gr32a, Gr66a and Gr89a) that would corroborate its role as a bitter GRN. Similarly, with the exception of salt neurons that may express Ir76b alone, there are few known functions for GRNs that solely express Ir genes. One possibility is that some of these GRNs possess novel chemoreceptor family-ligand interactions. For example, L7-7 is involved in sensing sucrose but limiting sugar ingestion, representing an Ir neuron that operates in a negative circuit module for sugar intake (Joseph et al., 2017). In addition, another recent study suggests that TRPA1 expression in L8 and L9 of the LSO is involved in feeding avoidance to bacterial endotoxins lipopolysaccharides (LPS) (Soldano et al., 2016). Alternatively, some pharyngeal GRNs may evaluate characteristics other than palatability, such as temperature or viscosity. Ir25a, which is broadly expressed in all 24 pharyngeal GRNs, is required for cool and temperature sensing (Ni et al., 2016, Chen et al., 2015). It will be worth investigating whether one or more pharyngeal GRNs act to integrate information about temperature and chemical quality of food substrates.
Expression analyses also hint at some functional subdivisions between pharyngeal taste organs. The LSO contains a smaller proportion of Gr-expressing neurons as compared to the VCSO, which also expresses a larger number of Gr genes that are co-expressed with Gr66a. Thus, we might expect broader bitter taste function in the VCSO. By contrast, sweet taste function appears to be more dominant in the LSO – its sweet GRNs express more sweet Gr-GAL4 drivers than the ones in the VCSO, and their activation is sufficient to drive feeding preference. VCSO sweet GRNs fail to promote ingestion by themselves, but may contribute to an increase in feeding preference when activated simultaneously with those in the LSO. Thus, there may be synergistic or hierarchical interactions between LSO and VCSO sweet taste circuits, with the latter coming into play only once the former is activated. The finding that Gr and Ir genes are expressed in the LSO and VCSO but only Ir genes in the DCSO is also striking, and raises the possibility that the DCSO, which is present at the most internal location relative to the others, may serve a unique role in controlling ingestion.
Based on its molecular signature, we identify the V5 neuron as an L-canavanine sensing neuron in the pharynx. As predicted, feeding avoidance of L-canavanine is dependent on V5. It was thus unexpected that capsaicin-mediated activation of bitter pharyngeal GRNs, which include V5, did not induce strong feeding avoidance either in the absence or presence of sugar. Since the strength and pattern of pharyngeal neuronal activation by bitter tastants or capsaicin is unknown, it is possible that capsaicin response may be weaker than that of canonical bitter tastants. Alternatively, sweet and bitter inputs from internal and external neurons may be summed differently. It is known that activation of one or few external sweet neurons can lead to proboscis extension (Dethier, 1976, Keene and Masek, 2012), for example, but a larger number of bitter neurons may need to be activated for avoidance.
The afferents of pharyngeal GRNs target regions of the subesophageal zone (SEZ) that are distinct from areas in which afferents from labellar and tarsal GRNs terminate (Kwon et al., 2014). Interestingly, pharyngeal GRN projections between molecularly different classes of neurons, as well as between GRNs of the LSO and VCSO are also distinct. Projections of sugar-sensing GRNs were found in separate ipsilateral regions, whereas those of neurons predicted to detect aversive tastants were found at the midline, suggesting the presence of contralateral termini. These observations may inform future functional studies of pharyngeal GRNs. L7-6 neurons, for example, would be predicted to sense aversive compounds based on the presence of their termini at the midline. Analysis of pharyngeal GRN projections also suggests distinct connectivity to higher order neuronal circuits (Yapici et al., 2016). With the molecular tools described here, future investigations of pharyngeal GRNs and pharyngeal taste circuits will provide insight into how internal taste is integrated with external taste to control various aspects of feeding behavior.
EXPERIMENTAL PROCEDURES
Fly strains
Flies were reared on standard cornmeal-dextrose-agar food at 25°C and 60–70% relative humidity under a 12 h:12 h dark:light cycle. The following fly lines were used: MJ94-GAL4 was a gift from L. Griffith at Brandeis University, Gr-GAL4 (Ling et al., 2014, Weiss et al., 2011), Gr66a-GAL4 (BDSC#28801), Ir-GAL4 (Koh et al., 2014), Ir11a-GAL4 (BDSC#41742), Ir100a-GAL4 (BDSC#41743), Ir76b-GAL4 (BDSC#41730), Ir25a-GAL4 (BDSC#41728), ppk28-GAL4 (Cameron et al., 2010), Gr43a-LexA (Miyamoto and Amrein, 2014), Gr32a-LexA (Fan et al., 2013), Ir76b-LexA (Ganguly et al., 2017), ppk28-LexA (Thistle et al., 2012), UAS-Kir2.1 (Baines et al., 2001), UAS-VR1E600K (Marella et al., 2006), poxnΔM22-B5 (Boll and Noll, 2002), poxn70 (Awasaki and Kimura, 1997), UAS-mCD8-GFP (Weiss et al., 2011), and LexAop2-6XmCherry-HA (BDSC#52271, 52272). For experiments using poxn mutants, we confirmed the poxn mutant background in all sorted flies by observing the transformed long and bent mechanosensory hairs in the labellum, as well as the fused three tarsal segments in the legs.
Immunohistochemistry
At least 50 flies per genotype were anesthetized on ice, and the proboscis and brain tissue were dissected in 1X PBST (PBS with 0.3% Triton X-100) and fixed for 30 min with 4% paraformaldehyde in 1X PBST at room temperature. After three washes with 1X PBST, samples were blocked with 5% normal goat serum (Sigma, #G9023) in 1X PBST. Tissues were incubated in primary antibody solutions for 3 days at 4°C. Primary antibodies were: chicken anti-GFP (1:5000; Abcam, #ab13970), rabbit anti-GFP (1:1000; Invitrogen, #A11122), rabbit anti-DsRed (1:200; Clontech, #632496), rabbit anti-Ir25a (1:500; a gift from L. Vosshall at Rockefeller University), and mouse anti-nc82 (1:20; DSHB). Secondary antibodies (1:400; Invitrogen) were: goat anti-chicken Alexa Fluor 488, goat anti-rabbit Alexa Fluor 488 and 546, and goat anti-mouse Alexa Fluor 568 and 647. Samples were mounted in 80% glycerol in 1X PBST or VECTASHIELD antifade mounting medium (Vector Laboratories, #H-1000) and stored at 4°C. Fluorescent images were acquired using a Leica SP5 confocal microscope with 400 Hz scan speed in 512×512 or 1024×1024 pixel formats. Image stacks were acquired at 1-μm optical sections. Unless otherwise noted, all images were presented as maximum projections of the z stack generated using Leica LAS AF software.
Expression analyses
Expression patterns of Gr/Ir/ppk-GAL4/LexA lines were mapped in the three pharyngeal taste organs by using UAS-mCD8-GFP and LexAop2-6XmCherry-HA reporters. For most of chemosensory receptors, we tested two or more independent reporter lines. Initial analysis was performed through live fluorescence imaging with at least 50 flies per line. The number of pharyngeal GRNs labeled by independent driver lines was consistent, although different signal intensities were observed across individual lines for the same receptor. We selected one representative line with stronger live fluorescence signal for further immunofluorescence mapping and behavioral experiments. For double-driver analysis, the UAS-mCD8-GFP transgene was under the control of two different Gr-GAL4 drivers and the number of GFP-labeled neurons was compared to flies containing a single Gr-GAL4 driver alone. Images were acquired using a Leica SP5 confocal microscope.
Binary choice feeding assays
Feeding preference assays were performed as described previously (Charlu et al., 2013). Sucrose (S7903) and L-canavanine (C1625) were obtained from Sigma-Aldrich and were dissolved in water; capsaicin (M2028) was also obtained from Sigma-Aldrich and was prepared in ethanol. Briefly, flies were sorted into groups of 10 males and 10 females upon eclosion and aged for 5–8 days. Since poxn mutant male flies are sterile, we added 2 heterozygous males with curly wings (poxn/CyO) in each group to ensure that all sorted females were mated. Heterozygous males were discarded during scoring for abdominal color. Flies were starved for 24 hr on water-saturated tissues and then placed in tight-fit Petri dishes (Falcon Cat. #35–1006) with eighteen 10 μL dots of 0.75% agarose that alternated in tastant and color using either 25 mg/mL indigo carmine (Sigma, #I8130) or 50 mg/mL sulforhodamine B (Sigma, #230162). We swapped dyes for each tastant with similar numbers of trials to account for any dye preference. Flies were allowed to feed for 2 hours at 25°C in a dark, humidified chamber, after which they were frozen and scored for abdomen color by dissecting the guts within 24 hours. Trials with participation lower than 50% were excluded. Preference index (PI) was calculated as ((# of flies labeled with the tastant color) – (# of flies labeled with the control color))/(total number of flies that fed). Thus, a PI of 0 would indicate equal preference between the two choices. In all cases, PI values were calculated for mixed populations of males and females.
Experimental design and statistical analysis
All data are presented as mean ± S.E.M. Statistical tests were conducted using Prism 7 (GraphPad Software). All the experiments were performed in parallel with both control and experimental genotypes. Complete genotypes used in this study are listed in Table S3. Complete statistics evaluations with the exact n for each groups are listed in Table S4. The sample size for each experiment was based on previously published reports. All independent trials were performed over 2 days. To improve normality and homogeneity of variances, we arcsine-transformed the square root of preference indices prior to analysis. Differences between means of different groups were evaluated for statistical significance with parametric ANOVA followed by post-hoc Tukey multiple comparisons test.
Supplementary Material
Table S1. Summary of Gr-GAL4 lines showing pharyngeal expression. Related to Figure 1.
Table S2. Summary of Gr/Ir-GAL4 double driver expression in V5–V7 neurons of the VCSO. Related to Figure 3.
Table S3. Complete genotypes of flies used in this study. Related to Experimental Procedures.
Table S4. Statistical analysis summary in this study. Related to Experimental Procedures.
Ir25a-GAL4 (green) expression and anti-Ir25a antibody (magenta) immunosignal in the LSO.
Ir76b-GAL4 (green) expression in #7 sensillum of the LSO.
Ir25a-GAL4 (green) expression and anti-Ir25a antibody (magenta) immunosignal in the VCSO.
Ir25a-GAL4 (green) expression and anti-Ir25a antibody (magenta) immunosignal in the DCSO.
Acknowledgments
This work was funded by grants from the Whitehall Foundation (2010-12-42), National Institutes of Health (R01DC013587, R01DC014092), and National Science Foundation (IOS-1149667). Y.-C.C. is a Howard Hughes Medical Institute International Student Research Fellow. We thank B. Jablonska for technical support and M. Blick, R Joseph, M. Gordon, J. Carlson and members of the Dahanukar lab for helpful comments on the manuscript. We are grateful to H. Amrein, J. Carlson, M. Gordon, L. Griffith, K. Scott, and N. Shah for sharing fly strains, and L. Vosshall for the anti-Ir25a antibody. Stocks were also obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537).
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, Y.-C.C. and A.D.; Methodology, Y.-C.C. and A.D.; Investigation, Y.-C.C.; Validation, Y.-C.C.; Formal Analysis, Y.-C.C.; Writing – Original Draft, Y.-C.C., Writing – Review & Editing, Y.-C.C. and A.D.; Visualization, Y.-C.C.; Supervision, A.D.; Funding Acquisition, A.D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awasaki T, Kimura K. pox-neuro is required for development of chemosensory bristles in Drosophila. J Neurobiol. 1997;32:707–21. doi: 10.1002/(sici)1097-4695(19970620)32:7<707::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–31. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Noll M. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002;129:5667–81. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–5. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlu S, Wisotsky Z, Medina A, Dahanukar A. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat Commun. 2013;4:2042. doi: 10.1038/ncomms3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Buhl E, Xu M, Croset V, Rees JS, Lilley KS, Benton R, Hodge JJ, Stanewsky R. Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature. Nature. 2015;527:516–20. doi: 10.1038/nature16148. [DOI] [PubMed] [Google Scholar]
- Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, Gibson TJ, Benton R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6:e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The hungry fly: a physiological study of the behavior associated with feeding. Cambridge, Mass: Harvard University Press; 1976. [Google Scholar]
- Fan P, Manoli DS, Ahmed OM, Chen Y, Agarwal N, Kwong S, Cai AG, Neitz J, Renslo A, Baker BS, Shah NM. Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell. 2013;154:89–102. doi: 10.1016/j.cell.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–7. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- Freeman EG, Dahanukar A. Molecular neurobiology of Drosophila taste. Current Opinion in Neurobiology. 2015;34:140–148. doi: 10.1016/j.conb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AS, Sellier MJ, Ali Agha M, Guigue A, Chabaud MA, Reeb PD, Mitra A, Grau Y, Soustelle L, Marion-Poll F. Dual mechanism for bitter avoidance in Drosophila. J Neurosci. 2015;35:3990–4004. doi: 10.1523/JNEUROSCI.1312-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Pang L, Duong VK, Lee A, Schoniger H, Varady E, Dahanukar A. A Molecular and Cellular Context-Dependent Role for Ir76b in Detection of Amino Acid Taste. Cell Rep. 2017;18:737–750. doi: 10.1016/j.celrep.2016.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre N, Luer K, Friche S, Grillenzoni N, Ramaekers A, Technau GM, Stocker RF. Integration of complex larval chemosensory organs into the adult nervous system of Drosophila. Development. 2004;131:83–92. doi: 10.1242/dev.00879. [DOI] [PubMed] [Google Scholar]
- Hussain A, Zhang M, Ucpunar HK, Svensson T, Quillery E, Gompel N, Ignell R, Grunwald Kadow IC. Ionotropic Chemosensory Receptors Mediate the Taste and Smell of Polyamines. PLoS Biol. 2016;14:e1002454. doi: 10.1371/journal.pbio.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YT, Shim J, Oh SR, Yoon HI, Kim CH, Moon SJ, Montell C. An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 2013;79:725–37. doi: 10.1016/j.neuron.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Heberlein U. Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics. 2012;192:521–32. doi: 10.1534/genetics.112.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Sun JS, Tam E, Carlson JR. A receptor and neuron that activate a circuit limiting sucrose consumption. Elife. 2017:6. doi: 10.7554/eLife.24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Masek P. Optogenetic induction of aversive taste memory. Neuroscience. 2012;222:173–80. doi: 10.1016/j.neuroscience.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Jeong YT, Choi MS, Choi J, Moon SJ, Kwon JY. Involvement of a Gr2a-Expressing Drosophila Pharyngeal Gustatory Receptor Neuron in Regulation of Aversion to High-Salt Foods. Mol Cells. 2017 doi: 10.14348/molcells.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, Stewart S, Carlson JR. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron. 2014;83:850–65. doi: 10.1016/j.neuron.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. A map of taste neuron projections in the Drosophila CNS. J Biosci. 2014;39:565–74. doi: 10.1007/s12038-014-9448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDue EE, Chen YC, Jung AY, Dahanukar A, Gordon MD. Pharyngeal sense organs drive robust sugar consumption in Drosophila. Nat Commun. 2015;6:6667. doi: 10.1038/ncomms7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81:984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling F, Dahanukar A, Weiss LA, Kwon JY, Carlson JR. The molecular and cellular basis of taste coding in the legs of Drosophila. J Neurosci. 2014;34:7148–64. doi: 10.1523/JNEUROSCI.0649-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–95. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. Diverse roles for the Drosophila fructose sensor Gr43a. Fly (Austin) 2014;8:19–25. doi: 10.4161/fly.27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–25. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SV, Singh RN. Sensilla on the Tarsal Segments and Mouthparts of Adult Drosophila-Melanogaster Meigen (Diptera, Drosophilidae) International Journal of Insect Morphology & Embryology. 1983;12:273–291. [Google Scholar]
- Ni L, Klein M, Svec KV, Budelli G, Chang EC, Ferrer AJ, Benton R, Samuel AD, Garrity PA. The Ionotropic Receptors IR21a and IR25a mediate cool sensing in Drosophila. Elife. 2016:5. doi: 10.7554/eLife.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm E, Dambly-Chaudiere C, Ghysen A. Connectivity of chemosensory neurons is controlled by the gene poxn in Drosophila. Nature. 1992;359:829–32. doi: 10.1038/359829a0. [DOI] [PubMed] [Google Scholar]
- Shim J, Lee Y, Jeong YT, Kim Y, Lee MG, Montell C, Moon SJ. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat Commun. 2015;6:8867. doi: 10.1038/ncomms9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldano A, Alpizar YA, Boonen B, Franco L, Lopez-Requena A, Liu G, Mora N, Yaksi E, Voets T, Vennekens R, Hassan BA, Talavera K. Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila. Elife. 2016:5. doi: 10.7554/eLife.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF, Schorderet M. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 1981;216:513–23. doi: 10.1007/BF00238648. [DOI] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact Chemoreceptors Mediate Male-Male Repulsion and Male-Female Attraction during Drosophila Courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Giesen L, Hernandez-Nunez L, Delasoie-Baranek S, Colombo M, Renaud P, Bruggmann R, Benton R, Samuel AD, Sprecher SG. Multimodal stimulus coding by a gustatory sensory neuron in Drosophila larvae. Nat Commun. 2016;7:10687. doi: 10.1038/ncomms10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–72. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Cohn R, Schusterreiter C, Ruta V, Vosshall LB. A Taste Circuit that Regulates Ingestion by Integrating Food and Hunger Signals. Cell. 2016;165:715–29. doi: 10.1016/j.cell.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–8. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of Gr-GAL4 lines showing pharyngeal expression. Related to Figure 1.
Table S2. Summary of Gr/Ir-GAL4 double driver expression in V5–V7 neurons of the VCSO. Related to Figure 3.
Table S3. Complete genotypes of flies used in this study. Related to Experimental Procedures.
Table S4. Statistical analysis summary in this study. Related to Experimental Procedures.
Ir25a-GAL4 (green) expression and anti-Ir25a antibody (magenta) immunosignal in the LSO.
Ir76b-GAL4 (green) expression in #7 sensillum of the LSO.
Ir25a-GAL4 (green) expression and anti-Ir25a antibody (magenta) immunosignal in the VCSO.
Ir25a-GAL4 (green) expression and anti-Ir25a antibody (magenta) immunosignal in the DCSO.