Abstract
Objective
To evaluate the health effectiveness of community health workers among three groups (intervention, attentional control and control groups) of Hispanic adults with uncontrolled (HbA1c >8%) type 2 diabetes mellitus.
Methods
This was a randomized clinical trial involving 180 English- and Spanish-speaking Hispanic individuals with uncontrolled type 2 diabetes mellitus, 40–74 years of age, who received diabetes care at an outpatient, public, urban hospital. Repeated-measures analysis of variance was used to evaluate the effect of time and group on the primary outcome measure and secondary outcomes. Group differences in the percentage of participants achieving at least 1% reduction in HbA1c levels were assessed using chi-square tests.
Results
Patients’ ages ranged from 44 to 74 years, 40% were male, 97% preferred Spanish and seven Spanish-speaking countries were identified as country of origin. Relative to the control and attentional control groups, the intervention group showed greater HbA1c reduction from baseline to 12 months and was the group with the highest percentage of participants showing 1% or more HbA1c reduction.
Conclusion
Integration of community health workers improved disease control for patients with type 2 diabetes mellitus during the intervention phase. Peer-driven/interactive ways to sustain diabetes control need to be explored.
Keywords: Diabetes, community health workers, diabetes self-management, Hispanics
Introduction
Diabetes, a major public health concern, is the fifth leading cause of death for US Hispanics,1 the seventh leading cause of death in the United States overall [Centers for Disease Control and Prevention (CDC)]2 and the fourth leading cause of death in New York City (NYC).3 Hispanics are one of the fastest growing populations in the United States;4 in NYC, Hispanics comprise 28.6% of the population5 and are the third largest ethnic group in NYC with type 2 diabetes mellitus (T2DM). Thus, disease prevention and management strategies clearly are a priority for the Hispanic population in NYC.
As a result of these escalating NYC diabetes rates, the NYC Department of Health and Mental Hygiene (NYC-DOHMH) developed a registry to track and provide surveillance of HbA1c levels6 as a mechanism to monitor glycaemic control and track the diabetes epidemic. The registry became a NYC-DOHMH mandate in 2006, requiring only major laboratories with a New York State license, servicing NYC residents, and with reporting capacity, to electronically report HbA1c values to the NYC-DOHMH.7 In 2007, the registry was implemented as a pilot to major laboratories and medical practices in the South Bronx, a neighbourhood in the Bronx borough of NYC, as a result of its high rates of diabetes; then in 2008, the registry mandate of major laboratories and medical practices was expanded throughout all of NYC.8
Diabetes in the South Bronx continues to be at staggeringly high rates.9 The South Bronx, sometimes called the epicentre of diabetes in the United States, with a 13.7% diabetes rate, has a higher rate of diabetes than NYC (9.2%).9 More than 20% of those with diabetes in the South Bronx have an HbA1c level >75 mmol/mol, well above the recommended <53 mmol/mol.9 Within NYC, the South Bronx also has the highest diabetes-related mortality rates (134.2/1000)7 and hospitalizations rates from diabetes as a principal diagnosis (692.2/100,000).10
Literature review
Hispanics, one of the fastest growing and largest minority groups in the United States, have critical documented levels of diabetes. Thus, it is essential to develop and examine culturally and linguistically appropriate strategies and interventions for Hispanics with diabetes.
Studies involving community health workers (CHWs), also known as Promotoras de Salud or Peer Leaders, have shown that these peers help to address the health and linguistic needs of different ethnic groups with chronic diseases (e.g. diabetes) and improve health outcomes.11–13 Given that CHWs are ‘lay members of communities … who usually share ethnicity, language, socioeconomic status, and life experiences with the community members they serve’,14 they are essentially public health workers who have the ability to build individual and community capacity outreach and community education activities.15
A literature review and meta-analysis12 of studies on CHWs included eight randomized clinical trial (RCT) articles16–23 examining CHW interventions. Of the eight, only three16,17,22 were about a CHW intervention delivered in English- and Spanish-speaking Hispanics with diabetes. A second literature review and meta-analysis found 12 RCTs.24 These articles examined the impact of a CHW intervention on HbA1c levels of Hispanics with diabetes.25–36 These two literature reviews and meta-analyses showed different findings on the effectiveness of a CHW intervention in improving HbA1c levels among Hispanics with diabetes. Of those 12 RCT diabetes studies, seven showed significant (p < 0.05) HbA1c changes between the intervention group (IG) and control group (CG).27,28,30,32–34,36
For the seven RCT studies that showed significant findings, the mean HbA1c level for the IG ranged from 50 to 91 mmol/mol and the CG was from 57 to 89 mmol/ mol,27,28,30,32–34,36 showing similarities between groups. Overall, among these seven studies, the sample sizes ranged from 30 to 352 Hispanic adults with diabetes.27,28,30,32–34,36 The sample participants for four of the articles were only of Mexican Americans,27,28,30,36 while the others were of different Hispanic subgroups. Although all seven studies used a CHW model, there were variations among the studies: (1) delivery of the intervention (individual vs group sessions); (2) type of CHW intervention (telephone follow-up after the group sessions vs individualized home visits); (3) setting of the delivery of the intervention (in clinics, homes or a combination of both); (4) total number of intervention sessions (ranged from 6 to 36 sessions); (5) timeframe of the intervention (ranged from 4 months to 2 years); and (6) intervals the intervention outcomes were measured for follow-up (ranging from 6 to 24 months). Four of the seven studies were conducted weekly and lasted 1–2.5 h long.30,33,34,36 Only three studies achieved a mean 1% decrease in the IG compared to the CG.27,30,33
For the five studies25–26,29,31,35 which reported non-significant findings in the CHW intervention, there are a number of potential reasons for such outcomes. Some reasons include differences in the specific intervention (e.g. format, duration); study design; delivery method (i.e. group vs individual); CHW background, training and diabetes education on content for the intervention; and support or supervision for the CHWs while implementing the intervention. In an analysis of the 12 articles, the requirement of the CHW varies. Of the 12 studies, only one reported that recruitment of the CHWs was from a community health centre,30 while the CHWs of a second study were either from a community health centre, clinic or community organization;33 only one article reported the CHWs educational background, in which the CHWs had a high school diploma and were certified CHWs;32 few studies required CHWs to have diabetes or be a resident of the community of the study site; most of the studies provided training to the CHWs, but the total duration of the training ranged from 4 days to 6 weeks;25,27–31,33–36 and in only half of the studies did the CHWs have some type of supervision.28–31,35,36
Although CHWs have shown some success in promoting health education to Hispanic communities37 and being an effective bridge between community members and health care providers,38 most studies have been conducted on Mexican Americans in California and Texas. Hence, studies on different Hispanic subgroups and conducted in other large states that have large Hispanic populations are needed. While CHW interventions have been conducted for years, RCTs of these interventions on Hispanics with diabetes are limited and are rather recent.24 Given these reasons and the variations among patient population, study designs (e.g. intervention) and CHW characteristics (e.g. background and training), more RCTs on CHWs are needed to evaluate the impact or lack of impact of CHW interventions as an effective or ineffective strategy to improving diabetes outcomes among US Hispanics.
The purpose of this study was to evaluate the health effectiveness of CHWs by comparing pre- and post-intervention physiologic measures [HbA1c levels, systolic blood pressure (SBP), diastolic blood pressure (DBP), triglyceride and weight] in Hispanic adults with diabetes who participated in the diabetes educational intervention delivered by CHWs, in one of two control groups [attention control group (ACG) and CG]. This study was one of the first studies using CHWs leading diabetes educational classes in a public urban hospital primary care clinic.
Methods
The study, entitled ‘The Hispanic Pilot Program: Community Health Workers (HIPP-CHW)’, conducted from 2012 to 2014 was funded by the Clinical Translational Science Center at Weill Cornell. The study was a single-site RCT involving 180 South Bronx Hispanic adults with uncontrolled T2DM (HbA1c ≥64 mmol/mol) who were receiving usual diabetes care at an outpatient primary care clinic at a public urban hospital [Lincoln Medical and Mental Health Center (LMMHC)]. LMMHC is a large public hospital, at which 75% of the clinic population is either uninsured or publicly insured (e.g. Medicaid). An average of 1094 adults with diabetes are seen at the LMMHC clinic each month, 60% of whom are Hispanic, 45% have HbA1c <53 mmol/ mol, 25% have HbA1c of 53–63 mmol/mol, 13% have HbA1c of 64–74 mmol/mol and 18% have HbA1c ≥75 mmol/mol.
Although there is a lack of published data on the National Diabetes Educational Program (NDEP), the diabetes educational materials used by the CHWs, having two CGs (CG and ACG) enabled an examination of whether or not the NDEP educational materials significantly impacted physiological measures [monitoring A1c, blood pressure (BP) and lipid levels]. For these reasons, three groups were included in this study (IG, CG and ACG).
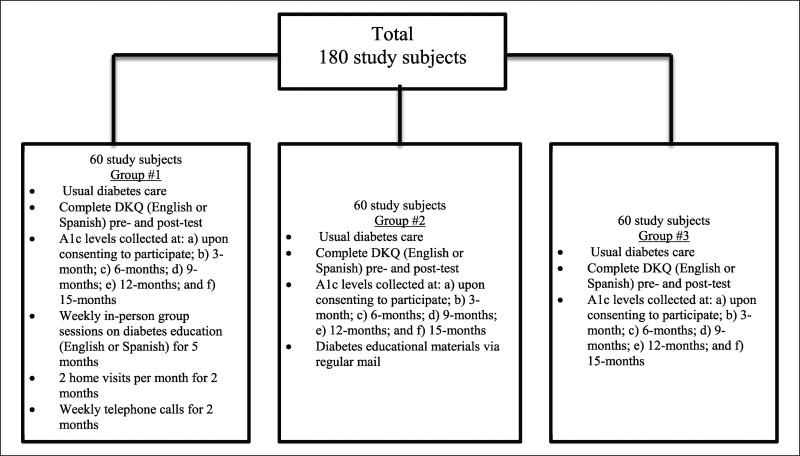
The IG received the CHW intervention, CG participants received usual diabetes care from their primary care provider (PCP) and the ACG participants received usual diabetes care and NDEP educational materials by mail. For this study, each group (IG, CG and ACG) consisted of 60 participants. Each participant was randomly assigned to the IG, CG or ACG. The CHW intervention included usual care plus 5 months of weekly in-person group diabetes education in English or Spanish, two home visits per month for 2 months and weekly follow-up phone calls from the CHW for another 2 months.
Sample
Inclusion criteria (N = 180) were (1) Hispanic adult with T2DM, (2) 40–74 years of age, (3) English or Spanish speaking, (4) receiving care at the LMMHC clinic and (5) an HbA1c level of ≥64 mmol/mol, in the past 6 months. Exclusion criteria were (1) HbA1c of ≤63 mmol/mol, (2) current participation in another study, (3) women who are pregnant, (4) diagnosis of cognitive dysfunction, (5) taking any medications that elevate glucose levels (e.g. steroids) or (6) acute, critical or surgical illness and/or expectation of admission to a critical care unit or to undergo surgery during the proposed study. Only patients of the LMMHC study site investigator, a clinical PCP at LMMHC, were also excluded from the sample pool. All patients of other PCPs at LMMHC were eligible as potential participants.
Recruitment
Of the 236 Hispanic potential participants with diabetes contacted on the phone and invited to participate in the study, 180 were recruited through convenience sampling. Each participant was randomly assigned to the IG, ACG or CG (Figure 1), using the Statistical Package for the Social Sciences (SPSS) for randomization. Usual care for patients with HbA1c ≥64 mmol/mol (participants in this study) included quarterly LMMHC provider visits, which included HbA1c measurement. All research team members except the principal investigator (PI) were blinded to study group assignments, making the study partially blinded. PCPs were not aware of which participants participated in the study and were not allowed to directly refer patients to the research team.
Figure 1.
Sample of intervention, attention control and control groups.
All inclusion criteria except spoken language are programmed into the hospital electronic medical record system (EMRS). The hospital-based research assistant (RA), who was bilingual (English/Spanish) and familiar with the EMRS, entered the criteria into the EMRS and created a list of all patients who fulfilled the criteria. The list included the patients’ name (in alphabetical order), medical record number, date of birth, gender, phone number and clinic provider’s name. The hospital RA then called potential participants, invited them to participate in the study, confirmed and then ensured eligibility (e.g. spoken language) by asking questions from an eligibility questionnaire, which included the inclusion and exclusion criteria. If an interested patient was ineligible, the hospital RA informed that individual and thanked them for their time. If an interested patient was eligible, the hospital RA explained the study, and if the patient agreed to participate, scheduled an appointment at a convenient day and time. During that in-person appointment, the hospital RA obtained written informed consent of all participants in English or Spanish and queried baseline diabetes knowledge using the English or Spanish version of the Diabetes Knowledge Questionnaire (DKQ). Once these data were collected, the hospital RA removed a cover sticker from the list generated by the SPSS randomization program, which provided a participant unique identification number (IDN).
Participants with an even IDN were assigned to one group, either the IG or CG, and those with odd numbers were assigned to the ACG. The even IDNs alternated between IG and CGs. The SPSS randomization program was used to place each participant into the three groups. Each participant’s unique IDN was noted in all questionnaires and was used to link the data but not identify the individual. Although the hospital RA conducted recruitment and collected participant data, they were unaware of the meaning of the even or odd IDNs, allowing them to remain blinded. The PI was the person who notified all participants of the group they were randomly assigned to and kept a list of the IDNs that identified information. This list was kept in a locked cabinet, in a private room, to maintain confidentiality and security. For this reason, the PI was not blinded to the participants and their assigned groups.
Investigators obtained approval from the CUNY Institutional Review Board (IRB) and LMMHC IRB prior to study initiation.
Intervention
The IG received 5 months of weekly in-person group diabetes education, two home visits per month for 2 months and weekly phone calls for 2 months from a CHW. Each weekly class was on a different diabetes-related topic (e.g. kidney disease, eye disease, differences between Type 1 and Type 2 diabetes) using the NDEP educational materials. The home visits were conducted to address any questions the participants had and review with them the foods in their home and how to read a nutritional facts label. The follow-up calls kept the participants engaged and addressed any additional questions they had.
A total of three CHWs (one male and two females) were hired. Each CHW delivered and led two diabetes educational (English or Spanish) classes per week to a group of 10, totalling a caseload of 20 people. Even though the average CHW’s caseload is approximately 30 people,39 each had a smaller than average caseload because this was their first position as a CHW, since they were new graduates of an accredited CHW certificate program.40 Among the three CHWs, each one was partnered with a peer-CHW to support and assist one another in task (e.g. making reminder phone calls to participants).
Before each CHW delivered the intervention, they received 2.5 months of comprehensive diabetes training, which is described in detail in a published article.40 The training was provided by the PI of this study, who is a registered nurse (RN) and certified diabetes educator. The CHWs were not members of the community, did not have diabetes and were not employees of the LMMHC clinic, which were not job requirements of the CHWs. Although the participants of the study did not have the same PCP, the CHWs did not interact with the participants’ PCPs.
Three Hispanic bilingual (English/Spanish) RAs/RNs who were graduate nursing students with at least 2 years of nursing experience and experience in teaching Hispanics with diabetes and fluent (reading/writing) in Spanish were hired. These RAs/RNs were hired as RAs for two reasons: (1) to supervise, evaluate and ensure adherence to the study intervention protocols and integrity of the intervention throughout the study and among the three CHWs and (2) to address any clinical nursing needs of the participants (e.g. teaching insulin administration). The RNs/RAs were asked to keep reflection journals evaluating the CHWs delivering the educational classes. The journals were reviewed on a regular basis among the CHWs, the RNs/ RAs and the PI.
Data collection
Diabetes outcomes were measured by monitoring HbA1c, BP, triglyceride and weight. As the intervention was 9 months long, and all participants are patients of the LMMHC clinic who have blood drawn for HbA1c and have their BP monitored at every quarterly diabetes visit (i.e. every 3 months), these data were retrieved from the LMMHC EMRS for all study participants: at pre-intervention (the most recent results upon consenting to participate); at 3, 6 and 9 months during the intervention; and at 3 and 6 months post-intervention, for a total of 15 months. Due to the amount of missing data for HbA1c, SBP, DBP, triglyceride and weight, for the 15-month time point, these data were dropped for all subjects (IG, CG and ACG) and for this reason not recorded in the tables. In addition, due to missing data of triglyceride for the 3-, 6- and 9-month time point, only baseline and 12-month data for triglyceride were analysed. An assessment of the participants’ change in diabetes knowledge and improvements in identifying healthier foods with pre- and post-study administration of the English or Spanish version of the DKQ was conducted. Each participant completed the DKQ the same day they consented to participation and again approximately 2 weeks after the usual 12-month diabetes care visit, administered by the hospital RA both times.
All participants (CG, ACG, IG) were compensated US$20 in cash: US$10 upon completing the DKQ and consenting (pre-test) to participate in the study, and another US$10 when completing the DKQ for the second time (post-test), approximately 2 weeks after the 12-month usual diabetes care visit. In addition, during the first week of each month, all IG participants received a US$20 Metrocard as reimbursement for the roundtrip public transportation cost for attending the 4-weekly educational session(s) (US$100 total for each participant).
Instrument
The DKQ, available in English and in Spanish and comprising 24 questions,41 is a valid and reliable tool that assesses overall diabetes knowledge as recommended by the National Standards for Diabetes Patient Education Programs.42,43
Data analysis
SPSS was used for all statistical analyses. Descriptive statistics were used for age, gender, language spoken, and Hispanic subgroups, gathered from the eligibility form, and physiologic variables (e.g. HbA1c, BP and triglyceride levels). Univariate analysis of variance (ANOVA) was used with least significant difference (LSD) post hoc tests and chi-square tests with Bonferroni correction, to compare demographic variables and baseline physiologic measures between groups. Repeated-measures analysis of variance (RM-ANOVA) models were conducted to evaluate the effect of time and group on the primary outcome measure (HbA1c) and all secondary measures [SBP and DBP, triglycerides, weight and diabetes knowledge (DKQ)]. Interaction models included time (baseline, 3, 6, 9 and 12 months); two times (baseline and 12 months) for triglycerides and (baseline and 12 months) diabetes knowledge (DKQ) variables, group (IG, CG, ACG) and time × group terms; these were evaluated using F-statistics with Green–Geisser correction. LSD post hoc tests were used to assess within-group changes over time (relative to baseline), as well as between-group differences during study intervention (3-, 6- and 9-month) and the follow-up period (12-month). In addition, group differences in the percentage of participants achieving at least 1% reduction in HbA1c levels were compared at each assessment using chi-square tests with Bonferroni correction. All analyses adhered to the intent-to-treat principle; however, study participants with missing data at any of study assessments were excluded from analyses. In order to assess attrition bias, participants with missing data were compared to those with complete data on baseline characteristics using chi-square analyses for categorical variables and independent samples t-tests for continuous variables. Secondary analyses were not adjusted for the effect of multiple comparisons.
Results
Demographics
Study participants were randomly assigned to either the CHW intervention group (IG, n = 60) or one of the two control groups: usual diabetes care delivered by a PCP (CG, n = 60) or usual diabetes care and National Diabetes Education Program (NDEP) educational materials by mail (ACG, n = 60). The Hispanic participants were ethnically diverse, primarily comprising Puerto Ricans (33.3%), Dominicans (28.3%), Mexicans (16.7%), Ecuadorians (6.7%), Hondurans (11.6%) and Other (Guatemalans and Salvadorians, 3.4%). Given the overall diversity of Hispanics in the South Bronx and the small sample of different subgroups among study participants, all participants were examined as a single Hispanic group.
The mean age of study participants was 59.62 years [standard deviation (SD) = 8.93; range, 42–77 years] and the mean number of years living with diabetes was 15.71 (SD = 10.05; range, 2–40 years of age). There were an equal number (97%) of participants who preferred Spanish among the three groups (IG, CG and ACG). In the IG, 80% were female; in the CG, 65% were female; and the ACG had 45% female participants. There were no significant differences between groups in age and years living with diabetes (p > 0.05). The distribution of ethnicity and gender were unequal among groups (Table 1). A primary reason for differences in the number of female versus male participants could be that more Hispanic men (17%) than women (36%)44 lack a provider, and given that this study was based on participants who received care from a provider, fewer men were involved. Although according to the CDC,45 there is a minimal difference in diabetes rates among men (6.6%) and women (5.9%), studies show that reasons for refusing to participate in studies is the same among men and women.46–48
Table 1.
Demographic and baseline variables by group.
| Demographic | Group
|
p-value | ||
|---|---|---|---|---|
| IG n = 60 |
CG n = 60 |
ACG n = 60 |
||
| M (SD) | M (SD) | M (SD) | ||
| Age (years) | 58.80 (9.10) | 58.55 (8.17) | 61.50 (9.32) | 0.133 |
| Range (years) | 44–73 | 42–70 | 45–77 | |
| Years with diabetes | 15.68 (10.94) | 17.05 (8.45) | 14.40 (10.58) | 0.354 |
| Range | 2–40 | 2–35 | 2–35 | |
| Ethnicity | n (%) | n (%) | n (%) | <0.001 |
| Puerto Rican | 18 (30) | 12 (20) | 30 (50) | |
| Mexican | 12 (20) | 12 (20) | 6 (10) | |
| Dominican | 12 (20) | 24 (40) | 15 (30) | |
| Hondurans | 15 (30) | 6 (10) | 0 (0) | |
| Ecuadorian | 0 (0) | 3 (10) | 9 (20) | |
| Other (Guatemalan or El Salvadorian) | 3 (10) | 3 (10) | 0 (0) | |
| Gender | n (%) | n (%) | n (%) | <0.001 |
| Female | 48 (80) | 39 (65) | 27 (45) | |
| Male | 12 (20) | 21 (35) | 33 (55) | |
| Preferred language | n (%) | n (%) | n (%) | 1.000 |
| Spanish | 58 (97) | 58 (97) | 58 (97) | |
| English | 2 (3) | 2 (3) | 2 (3) | |
| Baseline assessment | M (SD) | M (SD) | M (SD) | |
| A1c (%) | 9.48 (1.11) | 9.29 (1.27) | 9.65 (1.39) | 0.306 |
| Systolic blood pressure (mmHg) | 131.17 (15.82) | 140.98 (17.60) | 137.78 (17.88) | 0.007 |
| Diastolic blood pressure (mmHg) | 76.37 (6.91) | 80.45 (7.15) | 78.36 (7.74) | 0.010 |
| Triglyceride (mg/dL) | 198.18 (127.20) | 211.44 (105.02) | 174.89 (99.92) | 0.260 |
| Weight (kg) | 86.75 (19.22) | 81.97 (17.65) | 81.56 (13.82) | 0.182 |
IG: intervention group; CG: control group; ACG: attention control group; SD: standard deviation; ANOVA: analysis of variance; LSD: least square difference.
Significance tests used were ANOVA with LSD post hoc tests and chi-square tests with Bonferroni correction for between-group pairwise comparisons.
Participants of this study were recruited, engaged and retained; there was an 84.4% retention rate for the entire 12 months of the study, with only 15.6% lost to follow-up. Retention rates were similar in the IG and CG groups (90% and 88%, respectively) and slightly lower in the ACG group (75%; Table 2). Completers did not differ from non-completers on any baseline characteristics (p > 0.05).
Table 2.
Physiologic outcomes: model estimated means and between-group comparisons.
| IG | CG | ACG | Group difference IG versus CG |
p-value | Group difference IG versus ACG |
p- value |
Group difference CG versus ACG |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Ale (mmol/mol) | n = 54 | n = 53 | n = 45 | ||||||
| Baseline | 80 ± 1.14 | 79 ± 1.29 | 81 ± 1.32 | 1 (−0.34, 0.62) | 0.561 | −1 (−0.52, 0.47) | 0.924 | −2 (−0.67, 0.34) | 0.516 |
| Intervention-month 3 | 76 ± 1.41 | 77 ± 1.28 | 77 ± 1.02 | −1 (−0.63,0.33) | 0.526 | −1 (−0.66, 0.34) | 0.527 | −0 (−0.5 1, 0.50) | 0.979 |
| Intervention-month 6 | 72 ± 1.44 | 78 ± 1.27 | 78 ± 1.01 | −6 (−1.01,−0.04) | 0.034 | −6 (−1.06, −0.04) | 0.033 | −0 (−0.53, 0.48) | 0.919 |
| Intervention-month 9 | 70 ± 1.26 | 76 ± 1.34 | 74 ± 1.3 1 | −6 (−1.07,−0.07) | 0.026 | −4 (−0.85,0.19) | 0.210 | 2 (−0.29, 0.76) | 0.327 |
| Follow-up-month 12 | 68 ± 1.51 | 76 ± 1.28 | 73 ± 1.29 | −8 (−1.28,−0.23) | 0.005 | −5 (−1.01,0.08) | 0.094 | 3 (−0.26, 0.84) | 0.295 |
| Systolic blood pressure (SBP; mmHg) | n = 49 | n = 52 | n = 42 | ||||||
| Baseline | 131.08 ± 16.73 | 142.25 ± 17.62 | 138.21 ± 17.90 | −1 1.17 (−18.02,−4.32) | 0.002 | −7.13 (−14.37,0.10) | 0.053 | 4.04 (−3.10, 11.18) | 0.266 |
| Intervention-month 3 | 133.53 ± 13.35 | 141.83 ± 16.21 | 138.95 ± 16.56 | −8.30 (−14.36,−2.24) | 0.008 | −5.42 (−1 1.82,0.98) | 0.096 | 2.87 (−3.44, 9.19) | 0.370 |
| Intervention-month 6 | 131.57 ± 12.92 | 141.40 ± 1 1.85 | 139.14 ± 14.99 | −9.83 (−15.03,−4.64) | <0.001 | −7.57 (−1 3.06, −2.09) | 0.007 | 2.26 (−3.15,9.67) | 0.410 |
| Intervention-month 9 | 132.8 ± 12.44 | 142.73 ± 14.04 | 139.19 ± 16.973 | −9.94 (−15.63,−4.24) | 0.001 | −6.40 (−12.41,−0.38) | 0.037 | 3.54 (−2.39, 9.47) | 0.240 |
| Follow-up-month 12 | 132.69 ± 16.65 | 142.77 ± 16.24 | 141.76 ± 16.37 | −10.08 (−16.54,−3.61) | 0.002 | −9.08 (−15.89, −2.24) | 0.010 | 1.01 (−5.73,7.74) | 0.768 |
| Diastolic blood pressure (DBP; mmHg) | n = 49 | n = 52 | n = 42 | ||||||
| Baseline | 76.57 ± 6.90 | 80.15 ± 6.91 | 78.3 1 ± 67.93 | −3.58 (−6.42, −0.74) | 0.014 | −1.74 (−4.74, 1.26) | 0.254 | 1.84 (−1.12,4.81) | 0.220 |
| Intervention-month 3 | 78.86 ± 5.56 | 79.29 ± 6.5 1 | 78.57 ± 7.49 | −0.43 (−3.00,2.13) | 0.740 | 0.29 (−2.42, 2.99) | 0.835 | 0.72 (−1.96,3.39) | 0.597 |
| Intervention-month 6 | 75.18 ± 7.62 | 77.96 ± 8.58 | 79.6 ± 5.55 | −2.78 (−5.72,0.16) | 0.064 | −4.41 (−7.52,−1.31) | 0.006 | −1.63 (−4.70, 1.43) | 0.294 |
| Intervention-month 9 | 75.08 ± 7.45 | 79.17 ± 7.96 | 78.93 ± 6.79 | −4.09 (−7.03,−1.16) | 0.007 | −3.85 (−6.95, −0.75) | 0.015 | −0.24 (−2.81, 3.30) | 0.875 |
| Follow-up-month 12 | 74.39 ± 6.21 | 79.33 ± 7.77 | 78.93 ± 7.88 | −4.94 (−7.82, −2.06) | 0.001 | −4.54 (−7.58, −1.50) | 0.004 | 0.40 (−2.60, 3.40) | 0.793 |
| Weight (kg) | n = 46 | n = 48 | n = 44 | ||||||
| Baseline | 87.95 ± 18.28 | 82.53 ± 18.04 | 8I.I7± 1 1.97 | 5.42 (−1.29, 12.13) | 0.1 12 | 6.78 (−0.07, 1 3.64) | 0.052 | 1.36 (−5.42,8.15) | 0.692 |
| Intervention-month 3 | 87.92 ± 18.19 | 83.22 ± 18.39 | 80.65 ± 12.54 | 4.70 (−2.10, 1 1.51) | 0.174 | 7.27 (0.31, 14.22) | 0.041 | 2.57 (−4.32, 9.45) | 0.462 |
| Intervention-month 6 | 87.62 ± 18.48 | 83.38 ± 18.29 | 80.53 ± 13.06 | 4.24 (−2.65, 1 1.12) | 0.226 | 7.09 (0.06, 14.13) | 0.048 | 2.85 (−4.11,9.82) | 0.419 |
| Intervention-month 9 | 88.98 ± 18.29 | 84.15 ± 18.91 | 81.30 ± 12.22 | 4.84 (−2.03, 1 1.71) | 0.166 | 7.68 (0.66, 14.70) | 0.032 | 2.84 (−4.1 1,9.79) | 0.420 |
| Follow-up-month 12 | 92.60 ± 23.65 | 83.72 ± 19.58 | 82.30 ± 12.17 | 8.88 (1.06, 16.70) | 0.026 | 10.30 (2.31, 18.29) | 0.012 | 1.42 (−6.49,9.33) | 0.724 |
| Triglycerides (mg/dL) | n = 34 | n = 31 | n = 26 | ||||||
| Baseline | 196.06 ± 121.98 | 209.52 ± 94.54 | 171.77 ± 96.99 | 1 3.46 (−65.92, 39.00) | 0.661 | 24.30 (−30.75, 79.33) | 0.383 | 37.75 (−18.43,93.93) | 0.185 |
| Intervention-month 12 | 173.88 ± 1 10.13 | 248.65 ± 1 14.09 | 179.15 ± 84.15 | 74.76 (−126.52,−23.01) | 0.005 | −5.27 (−59.57, 49.02) | 0.847 | 69.49(14.07, 124.91) | 0.015 |
IG: intervention group; CG: control group; ACG: attention control group; RM-ANOVA: repeated-measures analysis of variance; LSD: least square difference.
Significance tests used were RM-ANOVA with LSD post hoc tests for between-group pairwise comparisons.
1% HbA1c reduction
A 1% HbA1c reduction is associated with a 37% decrease in the risk of microvascular complications (e.g. retinopathy) and a 21% reduction in diabetes-related mortality.49 Among participants in all groups (CG, ACG and IG), at the 6-month assessment, the IG had the highest percentage (41.7%) of individuals with at least 1% HbA1c reduction, as compared to 20.0% in the CG group and 30.5% in the ACG group (Table 3). At the end of the study (12 months), the percentages of individuals with at least 1% reduction in HbA1c levels were significantly higher in the IG and ACG groups, as compared to the CG group (IG, 56.6%; CG, 20.8%; and ACG, 45.7%; p < 0.05).
Table 3.
Percentage in each group with a 1% or more A1c reduction.
| IG
|
CG
|
ACG
|
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| A1c | ||||||
| Baseline | ||||||
| Intervention–month 3 | 17 | 28.3a | 4 | 6.7b | 14 | 23.3a |
| Intervention–month 6 | 25 | 41.7a | 13 | 22.0b | 18 | 30.5ab |
| Intervention–month 9 | 24 | 40.0ab | 13 | 23.2a | 26 | 48.1b |
| Follow-up–month 12 | 30 | 56.6a | 11 | 20.8b | 21 | 45.7a |
IG: intervention group; CG: control group; ACG: attention control group.
Significance tests used were chi-square tests with Bonferroni correction for between-group pairwise comparisons. Comparisons with different alphabetical subscripts differ significantly, p < 0.05.
Secondary outcomes (SBP and DBP, triglycerides, weight)
Table 2 provides data on changes in secondary physiologic outcomes for each group over the study period. Despite random assignment, the IG had lower SBP and DBP compared to the CG and ACG. The IG had a lower BP at baseline that was maintained throughout the study and showed the largest reductions in DBP over the study period (Table 2).
Similarly, the IG had reduced triglyceride levels during the intervention period (6-month intervention mean change −22.18), whereas the CG had significantly higher triglycerides levels (12-month intervention) compared to the IG and ACG groups (p < 0.05).
A significant time × group interaction was observed for the IG and ACG weight variables F(4.06, 274.29) = 0.97, p < 0.05. The IG showed significant weight gains during the follow-up period (12 months) compared to the CG and ACG (Table 2).
Diabetes knowledge
All groups showed significant improvements in diabetes knowledge post-intervention (p < 0.005; Table 4). Although gains were similar in each group, a trend for slightly greater knowledge increase was observed in the IG compared to ACG (p = 0.058).
Table 4.
Change in diabetes knowledge.
| Group | Diabetes knowledgea
|
Statistic
|
||
|---|---|---|---|---|
| Baseline | Follow-up– month 12 |
Mean change |
95% CI | |
| IG | 13.15 (2.75) | 14.35 (2.71) | 1.20 | (−1.83, −0.57) |
| CG | 12.70 (3.47) | 13.90 (2.76) | 1.20 | (−1.83, −0.57) |
| ACG | 12.50 (2.29) | 13.45 (2.24) | .95 | (−1.58, −0.32) |
IG: intervention group; CG: control group; ACG: attention control group; CI: confidence interval.
Total score on the Diabetes Knowledge Questionnaire (DKQ).
Discussion
In this study, the CHW intervention led a larger proportion of patients in the IG to achieve and maintain a 1% decrease in HbA1c at 6 and 12 months compared to the CG. There was a significantly greater decrease in HbA1c among participants in the IG compared to CG at 6, 9 and 12 months and the ACG at 6 months. These study outcomes potentially occurred for another reason, for example, individuals assigned to the IG reported being extremely satisfied with the CHW intervention and found it to be useful, that may have been a factor. Although these outcomes were not tracked in this study, individuals in the IG were taught and assisted to address challenges by the CHW. The CHW intervention was provided by a Hispanic peer who was fluent in English and Spanish and provided the patients with strategies (e.g. requesting a Spanish-speaking provider, requesting a timely follow-up appointment) to improve their self-advocacy. Patients were also provided with information in English or in Spanish on the importance of follow-up and how to improve self-management practices (e.g. taking medications). The CHWs received comprehensive training allowing them to have the foundation and knowledge base to deliver the intervention. They also had the support and supervision of the nurses and of others that they could refer to if needed. Hence, these strategies may have contributed to HbA1c reduction among the IG individuals. Although the IG had the highest HbA1c reduction rate (56.6%) compared to the ACG (45.7%), the HbA1c reduction in the ACG could be attributed to the NDEP educational materials. The primary outcome measure (HbA1c) showed the most improvement.
The CG and IG had the same diabetes knowledge mean change result of 1.20, from baseline to 12-month follow-up. A potential explanation to the increase in diabetes knowledge for the CG can be, in part, due to the Hawthorne effect. This gain in knowledge by the CG participants could possibly be a result of them taking better care of themselves (e.g. communicating with their provider, asking more questions and being more engaged in their care) than they would under normal circumstances, since they knew their diabetes data were being collected via the EMRS.27,50 Another explanation could be a result of the study being a single-site study where all of the participants were from the same clinic. Participants from the IG or ACG could have possibly shared the NDEP diabetes educational information with the CG participants, allowing all participants to gain diabetes knowledge as a result of the NDEP educational materials. Although there may be other explanations, these are the primary two reasons identified.
Upon further inquiry, regarding missing data, it was identified that approximately 30% of study participants do not see their provider on a quarterly basis. Reasons patients identified for lack of provider visits included long delays in availability to see a provider during a scheduled appointment, and language or cultural discordance with the providers. Additional reasons for these self-management practices identified by the CHWs based on their interactions with the participants included the following: lack of understanding of the importance of quarterly provider follow-up, lack of understanding of the relationship between laboratory blood work done prior to provider visit and the visit itself and utilizing the emergency room as the way to get prescription refills resulting in improper timely prescription refill practice. Reasons for these practices were reported as being due to high transportation costs and loss of wages (e.g. to get blood work done, to go to the provider visit or to get prescription refilled).
Studies showed that any reduction in HbA1c is considered to be clinically significant as it is likely to reduce the risk of diabetic complications. The Diabetes Control and Complications Trial (DCCT), Epidemiology of Diabetes Interventions and Complications (EDIC)51 and United Kingdom Prospective Diabetes Study (UKPDS)49 studies showed that a reduction in HbA1c reduces microvascular complications and death. By the end of this study (12 months), the IG group had 56.6% individuals with greater than 1% decrease in HbA1c levels, indicating that these participants had a decrease in risk of microvascular or macrovascular complications and mortality. This large percentage of the IG with an HbA1c reduction makes the CHW intervention a noteworthy success because HbA1c control can delay diabetes progression and diabetes-related complications.
Limitations
Limitations of this study include the following: this study was conducted at a single urban site on a diverse group of Hispanic adults, thereby limiting the generalizability of the findings; possible information/study contamination between groups could have occurred, since participants were from a particular clinic and may have known each other; a Hawthorne effect could have occurred within a group since all participants knew their HbA1c, BP, triglyceride and weight were being collected via their EMRS; a cost analysis of the CHW intervention was not conducted, although it could have allowed for further analysis and evaluation of the cost benefits of a CHW; medications or changes of medications and dosage were not tracked, which may have contributed to weight gain in the participants, particularly in the IG; and individual group (IG, CG, ACG) data were not examined by language (i.e. Spanish or English speakers) or by gender (female vs male). Although there is no reason for differences to exist between language and gender, these variables should be examined in future research.
Conclusion
The HIPP-CHW study was a culturally and linguistically successful intervention. Although 97% of each group (IG, CG and ACG) preferred the Spanish language, the study addressed the language preferences for Hispanics by delivering the CHW intervention in either English or Spanish. The CHWs met the needs of study participants by providing diabetes information, self-management strategies and support. As the Affordable Care Act has identified CHWs as an integral and important part of a health care team and a viable workforce,52 the results of this study support for-malization of the role of CHWs.
Acknowledgments
Funding
This study was funded by a Clinical Translation Science Center award from Weill Cornell Medical College, funded through grant UL1 TR000457-06 from the National Center for Advancing Translational Sciences, National Institutes of Health; and by the Moving from Associate to Full Professor award from the City University of New York.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Heron M Deaths: leading causes for 2008. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital Stat Syst. 2012;60:1–94. [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 3.Kim M, Berger D, Matte T. Diabetes in New York City: public health burden and disparities. New York: New York City Department of Health and Mental Hygiene; 2006. [Google Scholar]
- 4.Kirk JK, Passmore LV, Bell RA, et al. Disparities in HBA1C levels between Hispanic and non-Hispanic white adults with diabetes a meta-analysis. Diabet Care. 2008;31:240–246. doi: 10.2337/dc07-0382. [DOI] [PubMed] [Google Scholar]
- 5.US Census Bureau. QuickFacts. New York: https://www.census.gov/quickfacts/table/PST045215/3651000. [Google Scholar]
- 6.New York City Department of Health and Mental Hygiene. Notice of adoption to amend article 13 of the New York city health code. https://www1.nyc.gov/assets/doh/downloads/pdf/public/notice-adoption-a1c.pdf.
- 7.New York City Department of Health and Mental Hygiene. Epi data brief. Diabetes-related mortality in New York city. 2013;(28) http://www1.nyc.gov/assets/doh/downloads/pdf/epi/databrief28.pdf.
- 8.Chamany S, Silver LD, Bassett MT, et al. Tracking diabetes: New York City’s HbA1c registry. Milbank Q. 2009;87:547–570. doi: 10.1111/j.1468-0009.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.New York City Department of Health and Mental Hygiene. Epi data brief. Diabetes and its complications. 2013;(36) http://www1.nyc.gov/assets/doh/downloads/pdf/epi/data-brief36.pdf.
- 10.Walker EA, Silver LD, Chamany S, et al. Baseline characteristics and Latino versus Non-Latino contrasts among Bronx HbA1c study participants. West J Nurs Res. 2014;36:1030–1052. doi: 10.1177/0193945913517947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balcazar H, Alvarado M, Hollen ML, et al. Salud para su corazon-NCLR: a comprehensive promotora outreach program to promote heart-healthy behaviors among Hispanics. Health Promot Pract. 2006;7:68–77. doi: 10.1177/1524839904266799. [DOI] [PubMed] [Google Scholar]
- 12.Norris SL, Chowdhury FM, Van Le K, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabet Med. 2006;23:544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- 13.Parker EA, Israel BA, Robins TG, et al. Evaluation of community action against asthma: a community health worker intervention to improve children’s asthma-related health by reducing household environmental triggers for asthma. Health Educ Behav. 2008;35:376–395. doi: 10.1177/1090198106290622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin K, Tobler L Community health workers. Communities. 2008;(1) http://www.ncsl.org/print/health/chwbrief.pdf.
- 15.American Public Health Association. Support for community health workers to increase health access and to reduce health inequities. 2009 http://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2014/07/09/14/19/support-for-community-health-workers-to-increase-health-access-and-to-reduce-health-inequities.
- 16.Brown SA, Hanis CL. A community-based, culturally sensitive education and group-support intervention for Mexican Americans with NIDDM: a pilot study of efficacy. Diabetes Educ. 1995;21:203–210. doi: 10.1177/014572179502100307. [DOI] [PubMed] [Google Scholar]
- 17.Corkery E, Palmer C, Foley ME, et al. Effect of a bicultural community health worker on completion of diabetes education in a Hispanic population. Diabet Care. 1997;20:254–257. doi: 10.2337/diacare.20.3.254. [DOI] [PubMed] [Google Scholar]
- 18.Gary TL, Bone LR, Hill MN, et al. Randomized controlled trial of the effects of nurse case manager and community health worker interventions on risk factors for diabetes-related complications in urban African Americans. Prev Med. 2003;37:23–32. doi: 10.1016/s0091-7435(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 19.Hawthorne K, Tomlinson S. One-to-one teaching with pictures - flashcard education for British Asians with diabetes. Br J Gen Pract. 1997;47:301–304. [PMC free article] [PubMed] [Google Scholar]
- 20.Holtrop JS, Hickner J, Dosh S, et al. ‘Sticking to it - diabetes mellitus’: a pilot study of an innovative behavior change program for women with type 2 diabetes. Am J Health Educ. 2002;33:161–166. [Google Scholar]
- 21.Keyserling TC, Samuel-Hodge CD, Ammerman AS, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabet Care. 2002;25:1575–1583. doi: 10.2337/diacare.25.9.1576. [DOI] [PubMed] [Google Scholar]
- 22.Lorig KR, Ritter PL, Gonzalez VM. Hispanic chronic disease self-management: a randomized community-based outcome trial. Nurs Res. 2003;5:361–369. doi: 10.1097/00006199-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 23.McDermott R, Tulip F, Schmidt B, et al. Sustaining better diabetes care in remote indigenous Australian communities. BMJ. 2003;327:428–430. doi: 10.1136/bmj.327.7412.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little TV, Wang ML, Castro EM, et al. Community health worker interventions for Latinos with type 2 diabetes: a systematic review of randomized controlled trials. Curr Diab Rep. 2014;14:1–16. doi: 10.1007/s11892-014-0558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forjuoh SN, Bolin JN, Huber JC, Jr, et al. Behavioral and technological interventions targeting glycemic control in a racially/ethnically diverse population: a randomized controlled trial. BMC Public Health. 2014;14:14–71. doi: 10.1186/1471-2458-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmas W, March D, Darakjy S, et al. Community health worker interventions to improve glycemic control in people with diabetes: a systematic review and meta-analysis. J Gen Intern Med. 2015;30:1004–1012. doi: 10.1007/s11606-015-3247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prezio EA, Cheng D, Balasubramanian BA, et al. Community diabetes education (CoDE) for uninsured Mexican Americans: a randomized controlled trial of a culturally tailored diabetes education and management program led by a community health worker. Diabetes Res Clin Pract. 2013;100:19–28. doi: 10.1016/j.diabres.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Rothschild SK, Martin MA, Swider SM, et al. Mexican American trial of community health workers: a randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus. Am J Public Health. 2014;104:1540–1548. doi: 10.2105/AJPH.2013.301439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hargraves JL, Ferguson WJ, Lemay CA, et al. Community health workers assisting patients with diabetes in self-management. J Ambul Care Manage. 2012;35:15–26. doi: 10.1097/JAC.0b013e31822cbe35. [DOI] [PubMed] [Google Scholar]
- 30.Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, et al. Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a project Dulce promotora randomized trial. Diabet Care. 2011;34:1926–1931. doi: 10.2337/dc10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosal MC, Ockene IS, Restrepo A, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income Latinos: Latinos en control. Diabet Care. 2011;34:838–844. doi: 10.2337/dc10-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryabov I. The impact of community health workers on behavioral outcomes and glycemic control of diabetes patients on the US-Mexico border. Int Q Commun Health Educ. 2011;31:387–399. doi: 10.2190/IQ.31.4.f. [DOI] [PubMed] [Google Scholar]
- 33.Babamoto KS, Sey KA, Camilleri AJ, et al. Improving diabetes care and health measures among Hispanics using community health workers: results from a randomized controlled trial. Health Educ Behav. 2009;36:113–126. doi: 10.1177/1090198108325911. [DOI] [PubMed] [Google Scholar]
- 34.Lorig K, Ritter PL, Villa F, et al. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabet Care. 2008;31:408–414. doi: 10.2337/dc07-1313. [DOI] [PubMed] [Google Scholar]
- 35.Sixta CS, Ostwald S. Texas-Mexico border intervention by promotores for patients with type 2 diabetes. Diabetes Educ. 2008;34:299–309. doi: 10.1177/0145721708314490. [DOI] [PubMed] [Google Scholar]
- 36.Lujan J, Ostwald SK, Ortiz M. Promotora diabetes intervention for Mexican Americans. Diabetes Educ. 2007;33:660–670. doi: 10.1177/0145721707304080. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Addressing chronic disease through community health workers: a policy and systems-level approach. 2011 http://www.cdc.gov/dhdsp/docs/chw_brief.pdf.
- 38.Brownstein JN, Hirsch GR, Rosenthal EL, et al. Community health workers ‘101’ for primary care providers and other stakeholders in health care systems. J Ambul Care Manage. 2011;34:210–220. doi: 10.1097/JAC.0b013e31821c645d. [DOI] [PubMed] [Google Scholar]
- 39.Ro MJ, Treadwell HM, Northridge M. Community health workers and community voices: promoting good health. 2003 http://chwcentral.org/sites/default/files/Community%20Health%20Workers%20and%20Community%20Voices%20-%20Promoting%20Good%20Health_0.pdf.
- 40.Aponte J. Diabetes training for community health workers. J Community Med Health Educ. 2015;5:2161–0711. doi: 10.4172/2161-0711.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzgerald JT, Funnell MM, Hess GE, et al. The reliability and validity of a brief diabetes knowledge test. Diabet Care. 1998;21:706–710. doi: 10.2337/diacare.21.5.706. [DOI] [PubMed] [Google Scholar]
- 42.Aponte J, Panora E. Interdisciplinary diabetes management: hybrid course. J Diabet Metabol. 2013;4:2. [Google Scholar]
- 43.Garcia AA, Villagomez ET, Brown SA, et al. The Starr County diabetes education study development of the Spanish-language diabetes knowledge questionnaire. Diabet Care. 2001;24:16–21. doi: 10.2337/diacare.24.1.16. [DOI] [PubMed] [Google Scholar]
- 44.Livingston G, Minushkin S, Cohn D. Hispanics and the health care in the United States: access, information and knowledge: a Joint Pew Hispanic Center and Robert Wood Johnson Foundation Research Report. Pew Hispanic Center. 2008 http://www.pewhispanic.org/files/reports/91.pdf.
- 45.Centers for Disease Control and Prevention. Diabetes Public Health Resource. Age-adjusted rates of diagnosed diabetes per 100 civilian, non-institutionalized population, by sex, United States. 2015:1980–2014. http://www.cdc.gov/diabetes/statistics/prev/national/figbysex.htm.
- 46.Mansyur CL, Rustveld LO, Nash SG, et al. Social factors and barriers to self-care adherence in Hispanic men and women with diabetes. Patient Educ Couns. 2015;98:805–810. doi: 10.1016/j.pec.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Freedberg KA, Sullivan L, Georgakis A, et al. Improving participation in HIV clinical trials: impact of a brief intervention. HIV Clin Trials. 2015;2:205–212. doi: 10.1310/PHB6-2EYA-GA06-6BP7. [DOI] [PubMed] [Google Scholar]
- 48.Harrison JM, Jung M, Lennie TA, et al. Refusal to participate in heart failure studies: do age and gender matter? J Clin Nurs. 2016;25:983–991. doi: 10.1111/jocn.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller NP, Amouzou A, Hazel E, et al. Assessing the quality of sick child care provided by community health workers. PLoS ONE. 2015;10(11):e0142010. doi: 10.1371/journal.pone.0142010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Diabet Care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wennerstorm A, Johnson L, Gibson K, et al. Community health workers leading the charge on workforce development: lessons from New Orleans. J Commun Health. 2014;39:1140–1149. doi: 10.1007/s10900-014-9869-z. [DOI] [PubMed] [Google Scholar]