Abstract
Purpose of the study
To examine the effect of late-life body mass index (BMI) and rapid weight loss on incident mild cognitive impairment (MCI) and Alzheimer’s disease (AD)
Design
Prospective longitudinal cohort study
Setting
National Alzheimer’s Coordinating Center (NACC) Uniform Data Set, including 34 past and current National Institute on Aging-funded AD Centers across the United States
Participants
6940 older adults (n=5061 normal cognition (NC); n=1879 MCI)
Measurements
BMI (kg/m2) and modified Framingham Stroke Risk Profile (FSRP) score (sex, age, systolic blood pressure, anti-hypertension medication, diabetes mellitus, cigarette smoking, prevalent cardiovascular disease, atrial fibrillation) were assessed at baseline. Cognition and weight were assessed annually.
Results
Multivariable binary logistic regression, adjusting for age, sex, race, education, length of follow-up, and modified FSRP related late-life BMI to risk of diagnostic conversion from NC to MCI or AD and from MCI to AD. Secondary analyses related late-life BMI to diagnostic conversion in the presence of rapid weight loss (>5% decrease in 12 months) and apolipoprotein E (APOE) ε4. During a mean 3.8-year follow-up period, 12% of NC participants converted to MCI or AD and 49% of MCI participants converted to AD. Higher baseline BMI was associated with a reduced probability of diagnostic conversion, such that for each one-unit increase in baseline BMI there was a reduction in diagnostic conversion for both NC (OR=0.977, 95%CI 0.958–0.996, p=0.015) and MCI participants (OR=0.962, 95%CI 0.942–0.983, p<0.001). The protective effect of higher baseline BMI did not persist in the setting of rapid weight loss but did persist when adjusting for APOE.
Conclusions
Higher late-life BMI is associated with a lower risk of incident MCI and AD but is not protective in the presence of rapid weight loss.
Keywords: Dementia, Body Mass Index, Cognition, Alzheimer’s disease, Frailty
INTRODUCTION
Alzheimer’s disease (AD) remains the most common cause of dementia among older adults, and estimates suggest AD will affect over 13 million individuals in the United States by 2050(1). AD has an impact on physical activity (2), quality of life (3), and nutrition (4), and it is frequently associated with weight loss (5). The contributing effect of late-life body weight, and more specifically BMI, on AD has been inconsistent (6, 7). Several studies suggest that a lower late-life BMI is associated with an increased risk of incident dementia (6, 8–11); however, other studies have suggested that late-life obesity is associated with smaller brain volumes (a marker of neurodegenerative disease) in individuals with mild cognitive impairment (MCI) and AD (12), that increased BMI may be associated with an increased risk (6), or that lower late-life BMI does not have an effect on increasing the risk of clinical dementia (13, 14). A meta-analysis pooling data from >13,000 participants yielded null results between late-life BMI and incident dementia (15). This meta-analysis and other studies have been limited by the inconsistent documentation of dementia (10) (e.g., diagnostic codes, medical record documentation, cognitive screening tools, dementia subtype), populations included (MCI, normal cognition) (11), and inconsistent adjustment for comorbid covariates such as cardiovascular (CV) risk factors, time to follow-up, and apolipoprotein E (APOE) ε4 status (a genetic susceptibility risk factor for AD) (10, 16–20).
Frailty, a risk factor for incident dementia that includes unintentional significant weight loss (shrinkage) (21), has added to the complex relations between BMI and incident AD (22). It remains unclear whether a lower BMI is independently associated with incident cognitive impairment or whether a recent decrease in BMI has a greater impact on the risk of AD as compared to individuals who have had a lower but stable BMI. Further, weight loss is a common feature of AD and often occurs prior to the onset of clinical AD symptoms (23), suggesting low BMI may be a clinical feature of the AD pathological process rather than an underlying risk factor.
In this study, we utilize the large National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) (24) to assess whether late-life BMI is associated with diagnostic conversion from normal cognition (NC) or MCI to dementia or conversion from MCI to dementia while statistically adjusting for relevant vascular health variables known to affect cognition (25). We hypothesize that when we control for increased vascular risk, which disproportionately affects obese individuals, lower late-life BMI is associated with an increased risk for conversion to MCI or dementia. We also assess the effects of significant weight loss (shrinkage) in the 12 months preceding diagnostic change and the effects of APOE ε4 status on the relationship between BMI and diagnostic conversion. We anticipate that because APOE ε4 genotype has not been shown to be associated with either increased BMI or vascular risk (17, 18) the relationship between BMI and diagnostic conversion remains in the presence of APOE ε4. Our study’s novelty lies in examining the differential effect of BMI on development and progression of cognitive decline by diagnostic classification, including individuals with NC or MCI, examining associations between BMI and diagnostic conversion in the presence or absence of shrinkage and by utilizing the Framingham Stroke Risk Profile (FSRP) score (26) as a covariate for cerebrovascular risk.
METHODS
Setting and participants
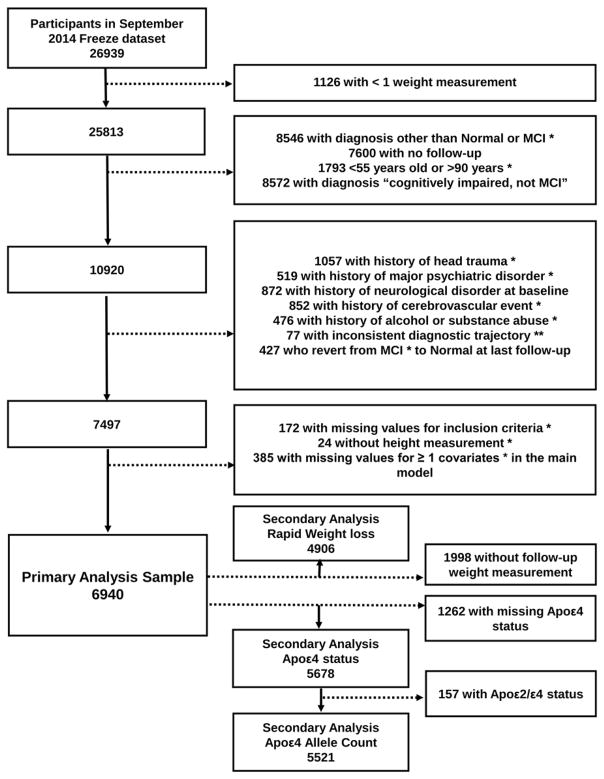
NACC maintains a database of participant information collected from 34 past and present National Institute on Aging-funded Alzheimer’s Disease Centers. In 2005, NACC implemented the UDS, a standard data collection protocol, including clinical, medical history, neurological, and neuropsychological results (24). The current study included participants evaluated between 9/1/2005 and 9/1/2014 age 55 to 90 years diagnosed with NC or MCI at their first UDS visit at which the participant’s weight was recorded. Participant selection and exclusion details (n=6940) are displayed in Figure 1. The study was approved by the local Institutional Review Board prior to data access and analysis.
Figure 1.
Consort diagram displaying derivation of study sample for primary and secondary analyses with source of exclusion. MCI=Mild Cognitive Impairment, Apoε4= Apolipoprotein ε4.
Cognitive diagnostic classification at baseline
Cognitive diagnosis for each participant is based upon clinician judgment or a multi-disciplinary consensus team using information from the comprehensive UDS work-up, definitions below were specific for this study and includes:
NC is defined by (a) Clinical Dementia Rating (CDR)=0 (no dementia), (b) no deficits in activities of daily living directly attributable to cognitive impairment, and (c) no evidence of cognitive impairment. No evidence of cognitive impairment is defined as standard scores falling 1.5 standard deviations within the age-adjusted normative mean on neuropsychological tests assessing language, attention, memory, and executive functioning.
MCI determinations are based upon Albert et al., criteria (27) and defined as (a) a CDR≥0.5 (reflecting mild severity of impairment), (b) relatively spared activities of daily living, (c) objective cognitive impairment in at least one cognitive domain (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean in memory, language, attention, or executive functioning) or a significant decline over time on the neuropsychological evaluation, (d) Mini Mental Status Examination (MMSE) ≥23 (e) report of a cognitive change by the patient or informant or as observed by a clinician, and (f) absence of a dementing syndrome (defined below).
Dementia included meeting criteria for AD (28) or other cause of dementia (29–35) defined as (a) objective cognitive impairment (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean) in at least two cognitive systems (i.e., memory, language, attention or executive functioning), and (b) cognitive impairment contributes directly to impaired activities of daily living.
Cognitive diagnostic outcome
Cognitive diagnostic outcome was defined by assessing the trajectory of cognitive diagnosis over time. For participants with an unchanged diagnosis from baseline to the last available UDS follow-up visit, the diagnostic conversion was defined as “stable”. For participants with progressive diagnostic changes over time, the diagnostic conversion was defined as “convert”. Individuals who ever received a diagnosis of NC or MCI after a diagnosis of AD or who received a diagnosis of MCI at baseline but had a diagnosis of NC as of their last visit were excluded from the analysis. Although prior literature has suggested participants with this trajectory should be included and classified as normal cognition, our rationale to exclude these participants is due to the concern that the original determination of their cognitive impairment may have been accounted for by an underlying medical condition. The nature of the medical condition may have affected BMI (e.g., hypothyroidism) and confounded our findings.(36)
Thus, among NC participants, diagnostic outcome was defined as (i) stable if they were also classified as NC at their last available visit or (ii) conversion if they were classified as either MCI or dementia at their last available visit. Among MCI participants, diagnostic outcome was defined as (i) stable if they were also classified as MCI at their last available visit or (ii) conversion if they were classified as having dementia at their last available visit.
BMI
Baseline BMI was calculated from first visit height (m) and weight (kg) and coded both as a continuous measure (kg/m2) and as a categorical measure according to National Heart, Lung and Blood Institute recommendations (i.e., <18.5=underweight, ≥18.5 <25 =normal, ≥25 <30=overweight, ≥30 <35=obese, ≥35 morbidly obese). For participants with missing weight at the first visit, the first visit with non-missing weight was used as the baseline visit, with height information obtained from the closest available visit.
Significant weight loss prior to diagnostic conversion (shrinkage)
To determine the effects of significant weight loss (shrinkage) in the 12 months prior to diagnostic conversion we categorized participants as follows:
For participants who were diagnostically stable over the follow-up period, significant weight loss was calculated as >5% BMI decrease between the most recent UDS visit where BMI was available and BMI measured 12 months prior (using a 9–15 month window).
For participants who diagnostically converted over the follow-up period, significant weight loss was calculated as >5% BMI decrease between the BMI recorded at the UDS follow-up visit with the diagnostic status change and BMI measured 12 months prior (again, using a 9–15 month window).
Framingham Stroke Risk Profile (FSRP)
To assess systemic vascular health, we calculated a modified FSRP at baseline which assigns points by sex for age, systolic blood pressure, history of diabetes, current cigarette smoking, prevalent cardiovascular disease (defined as history of myocardial infarction, cardiac arrest, coronary angioplasty, endarterectomy, coronary artery bypass grafting, or congestive heart failure), history of atrial fibrillation, and the use of antihypertensive medications. The standard FSRP calculation is modified here to exclude points assigned for the presence or absence of left ventricular hypertrophy because electrographic or echocardiographic measures are unavailable in UDS (25).
Statistical Analysis
Descriptive statistics for baseline clinical characteristics, including age, sex, race, education, years of follow up, systolic blood pressure, prevalent comorbidities (cardiovascular disease [CVD], diabetes mellitus, heart failure) APOE ε4 status and FSRP score were calculated by baseline BMI categories for the total sample and for each baseline diagnostic group (i.e., NC and MCI). Clinical characteristic comparisons between BMI categories were conducted using Kruskal-Wallis tests (for continuous variables) and Pearson’s chi-square tests (for categorical variables).
For primary analyses assessing the effect of baseline BMI status on the likelihood of cognitive diagnostic conversion by baseline diagnostic group, multivariate logistic regression models were used within each baseline diagnostic group with baseline continuous BMI as the independent variable. Covariates, including age, sex, education (years), race (white vs. non-white), length of follow-up in the study (years) and FSRP were selected a priori due to their known associations with both the predictor and outcome variables. The adjusted relationship between BMI and conversion was examined for non-linearity using restricted cubic splines.
In secondary analyses, we performed a subgroup analysis by the presence or absence of significant weight loss in the 12 months preceding diagnostic outcome to relate the effect of BMI on risk of diagnostic conversion in the presence of rapid weight loss. For all participants with APOE genotyping data available (NC n=4080, MCI n=1441), first we assessed the effect of APOE ε4 status (i.e., positive=ε2/ε4, ε3/ε4, or ε4/ε4 versus negative=ε2/ε2, ε2/ε3, or ε3/ε3) and then the effect of APOE ε4 allele count (0=ε2/ε2, ε2/ε3, or ε3/ε3; 1=ε3/ε4; 2=ε4/ε4) in secondary analyses relating BMI to diagnostic conversion. For the latter analysis ε2/ε4 was excluded due to the possible neuroprotective effects of the ε2 allele (37).
RESULTS
Participant characteristics
Of 6940 study participants, 5061 were classified as NC at baseline and 1871 were classified as MCI. Among NC participants, the mean age was 72.4 years, 81% were White, and 69% were female. For MCI participants the mean age was 74.7 years, 82% were White and 53% were female. Table 1 displays characteristics by baseline BMI category and diagnosis. For both diagnostic groups, participants who were younger and had less years of education had increased BMI (all p-values<0.001). There were more women and White participants in the underweight category for both diagnostic groups (all p-values<0.001). In NC, the prevalence of CVD (p=0.001) and diabetes increased with BMI as did mean systolic blood pressure and mean pulse pressure (all p-values<0.001). Accordingly, there was an increase in mean FSRP score as BMI increased (p<0.001). In comparison, in MCI, the prevalence of diabetes and mean systolic blood pressure was higher across increasing BMI categories (p<0.001), but there was no difference in CVD prevalence, mean pulse pressure or FSRP score by BMI category.
Table 1.
Baseline Characteristics by Baseline BMI Group and by Baseline Diagnosis
| Characteristic | Overall | BMI < 18.5 | 18.5 ≤ BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | P-value |
|---|---|---|---|---|---|---|---|
| Normal Cognition at Baseline | |||||||
|
| |||||||
| N=5061 | N=49 | N=1759 | N=1981 | N=863 | N=409 | P-value | |
| Age, years, mean ± SD | 72.4 ± 8.2 | 75.8 ± 7.9 | 73.1 ± 8.7 | 72.6 ± 8.1 | 71.5 ± 7.8 | 69.6 ± 6.9 | < 0.001c |
| Education, years, mean ± SD | 15.7 ± 3.0 | 16.3 ± 2.5 | 16.1 ± 2.7 | 15.7 ± 3.0 | 15.3 ± 3.2 | 14.8 ± 3.3 | < 0.001c |
| Sex, n (% Female) | 3472 (69) | 44 (90) | 1294 (74) | 1214 (61) | 592 (69) | 328 (80) | < 0.001d |
| Race, n (% White) | 4113 (81) | 43 (88) | 1533 (87) | 1643 (83) | 651 (75) | 243 (59) | < 0.001d |
| Time in studya, years, mean ± SD | 4.1 ± 2.3 | 4.0 ± 2.3 | 4.2 ± 2.4 | 4.1 ± 2.3 | 3.9 ± 2.2 | 3.8 ± 2.2 | < 0.032c |
| Prevalent Co-Morbidities | |||||||
| Cardiovascular Disease, % present | 490 (10) | 7 (14) | 123 (7) | 205 (10) | 106 (12) | 49 (12) | 0.001d |
| Diabetes Mellitus, % present | 510 (10) | 0 (0) | 76 (4) | 179 (9) | 148 (17) | 107 (26) | < 0.001d |
| Congestive Heart Failure, % present | 60 (1) | 2 (1) | 15 (1) | 20 (1) | 13 (2) | 11 (3) | 0.082d |
| Systolic BP, mmHg, mean ± SD | 133 ± 18.0 | 132 ± 20.0 | 130 ± 18.0 | 133 ± 18.0 | 137 ± 17.0 | 139 ± 18.0 | < 0.001c |
| APOEε4 Status, n (% Positive)b | 1273 (30) | 16 (38) | 454 (31) | 497 (30) | 207 (29) | 99 (30) | 0.740d |
| FSRP, mean ± SD | 11.2 ± 4.5 | 12.0 ± 3.6 | 10.7 ± 4.6 | 11.2 ± 4.5 | 11.7 ± 4.5 | 11.9 ± 4.3 | < 0.001c |
|
| |||||||
| Mild Cognitive Impairment at Baseline | |||||||
|
| |||||||
| N=1879 | N=23 | N=721 | N=753 | N=274 | N=108 | P-value | |
|
| |||||||
| Age, years, mean ± SD | 74.5 ± 7.5 | 76.0 ± 6.7 | 75.2 ± 7.8 | 75.0 ± 7.3 | 73.9 ± 6.9 | 70.3 ± 7.3 | < 0.001c |
| Education, years, mean ± SD | 15.3 ± 3.3 | 15.4 ± 3.3 | 15.6 ± 3.2 | 15.4 ± 3.2 | 15.0 ± 3.5 | 13.5 ± 3.5 | < 0.001c |
| Sex, n (% Female) | 994 (82) | 22 (96) | 446 (62) | 319 (42) | 134 (49) | 73 (68) | < 0.001d |
| Race, n (% White) | 1535 (82) | 16 (70) | 623 (86) | 627 (83) | 209 (76) | 60 (56) | < 0.001d |
| Time in studya, years, mean ± SD | 3.3 ± 2.0 | 2.9 ± 2.2 | 3.4 ± 2.0 | 3.2 ± 2.0 | 3.3 ± 2.0 | 3.5 ± 2.0 | 0.120c |
| Prevalent Co-Morbidities | |||||||
| Cardiovascular Disease, % present | 266 (14) | 3 (13) | 92 (13) | 116 (15) | 42 (15) | 13 (12) | 0.580d |
| Diabetes Mellitus, % present | 235 (13) | 1 (4) | 51 (7) | 83 (11) | 57 (21) | 43 (40) | < 0.001d |
| Congestive Heart Failure, % present | 25 (1) | 0 (0) | 9 (1) | 8 (1) | 7 (3) | 1 (1) | 0.750d |
| Systolic BP, mmHg, mean ± SD | 136 ± 19.0 | 130 ± 15.0 | 134 ± 19.0 | 136 ± 19.0 | 139 ± 19.0 | 138 ± 19.0 | < 0.001c |
| APOEε4 Status†, n (% Positive)b | 747 (50) | 10 (62) | 310 (54) | 291 (49) | 98 (45) | 38 (44) | 0.048d |
| FSRP Score, mean ± SD | 12.3 ± 4.3 | 12.5 ± 4.1 | 12.1 ± 4.4 | 12.4 ± 4.2 | 12.8 ± 4.2 | 12.2 ± 4.3 | 0.140c |
Time in study from baseline visit to diagnostic outcome visit,
Percentages are calculated from N with non-missing values for APOE ε4 status that differs from total N in each BMI category,
Kruskal-WallisTest,
Pearson Test,
APOE (Apolipoprotein E) ε4 status, positive= ε2/ε4, ε3/ε4, or ε4/ε4, negative=ε2/ε2, ε2/ε3, or ε3/ε3 BMI=Body Mass Index; SD=Standard Deviation; FSRP=Framingham Stroke Risk Profile
Baseline BMI category and conversion status
Table 2 displays the number and percent of participants who remained stable or converted to MCI or dementia by baseline diagnostic group (NC or MCI) and by baseline BMI category. In the NC group, 634 (12%) participants converted to MCI or dementia over a mean 4.1 year (SD 2.3) follow-up period. In the MCI group, 926 (49%) participants converted to dementia over a mean 3.3 year (SD 2.0) follow-up period. Comparison between BMI categories showed a difference in conversion prevalence by BMI category for NC (P=0.002) and MCI (P=0.001). Table 3 displays the prevalence of rapid weight loss (>5% decrease in BMI in 12 months prior to diagnostic conversion) by BMI category for those individuals included in the secondary analysis that had follow-up BMI values available. The results demonstrate that in the NC group the prevalence of significant weight loss differs by BMI category, but it does not differ by BMI category in the MCI group.
Table 2.
Comparisons of Conversion Status by Baseline BMI category
| BMI < 18.5 | 18.5 ≥ BMI < 25 | 25 ≥ BMI < 30 | 30 ≥ BMI < 35 | BMI ≥ 35 | P-valuea | |
|---|---|---|---|---|---|---|
| Conversion Status, n (%) | ||||||
|
| ||||||
| NC at Baseline | N=49 | N=1759 | N=1981 | N=863 | N=409 | 0.002a |
|
| ||||||
| Stable | 44 (90) | 1506 (86) | 1729 (87) | 771 (89) | 377 (92) | |
| Converters | 5 (10) | 253 (14) | 252 (13) | 92 (11) | 32 (8) | |
|
| ||||||
| MCI at Baseline | N=23 | N = 721 | N = 753 | N=274 | N=108 | 0.001a |
|
| ||||||
| Stable | 12 (52) | 336 (47) | 380 (50) | 154 (56) | 71 (66) | |
| Converters | 11 (48) | 385 (53) | 373 (50) | 120 (44) | 37 (34) | |
P-value calculated using Pearson Test, BMI=Body Mass Index; NC=Normal Cognition; MCI=Mild Cognitive Impairment.
Table 3.
Comparisons of Significant Weight Loss by Baseline BMI category
| BMI < 18.5 | 18.5 ≥ BMI < 25 | 25 ≥ BMI < 30 | 30 ≥ BMI < 35 | BMI ≥ 35 | P-valuea | |
|---|---|---|---|---|---|---|
| Weight Loss ≥ 5% in 12 months prior to diagnostic conversion, n (%)b | ||||||
|
| ||||||
| NC at Baseline | N=34 | N=1269 | N=1424 | N=638 | N=328 | |
|
| ||||||
| No | 31 (91) | 1147 (90) | 1258 (88) | 538 (84) | 232 (82) | < 0.001a |
|
| ||||||
| Yes | 3 (9) | 122 (10) | 166 (12) | 100 (16) | 50 (18) | |
| Converters | 0 (0) | 24 (20) | 28 (17) | 12 (12) | 5 (10) | |
|
| ||||||
| MCI at Baseline | N=16 | N=489 | N=512 | N=178 | N=64 | |
|
| ||||||
| No | 12 (75) | 425 (87) | 433 (85) | 154 (87) | 52 (81) | 0.46a |
|
| ||||||
| Yes | 4 (25) | 64 (13) | 79 (15) | 24 (13) | 12 (19) | |
| Converters | 3 (75) | 37 (58) | 33 (42) | 14 (58) | 5 (42) | |
Multivariable analysis of baseline BMI on conversion status
Table 4 displays the results of the multivariable regression analysis. Higher baseline BMI demonstrated a protective effect on the risk of diagnostic conversion in NC participants (per BMI unit increase, OR=0.977, 95% CI 0.958–0.996, P=0.015). Other covariates associated with increased likelihood of conversion included higher baseline age, lower education, and longer follow-up length. In individuals with MCI at baseline, higher baseline BMI had a protective effect on the risk of diagnostic conversion to dementia (per BMI unit increase, OR=0.962, 95% CI 0.942–0.983, P<0.001). When models were repeated restricting the sample to participants age 65 years and older, results were unchanged (data not shown). Other factors associated with increased risk of conversion included White race (MCI group only) and longer follow-up length.
Table 4.
Binary Logistic Regression of Baseline BMI (Continuous) Predicting Conversion Status
| Primary Analysis of effect of BMI on diagnostic conversion | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline Diagnosis: Normal (N=5061) | Baseline Diagnosis: MCI (N=1879) | |||||
|
| ||||||
| Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-value | |
| BMI (kg/m2) | 0.977 | 0.958–0.996 | 0.015* | 0.962 | 0.942–0.983 | < 0.001* |
| Age (years) | 1.078 | 1.061–1.095 | < 0.001* | 1.011 | 0.993–1.029 | 0.224 |
| Education (years) | 0.937 | 0.910–0.965 | <0.001* | 1.012 | 0.982–1.044 | 0.435 |
| Race, White (referent=non-White) | 1.024 | 0.809–1.296 | 0.843 | 1.530 | 1.178–1.987 | 0.001* |
| Sex, Female (referent=male) | 0.812 | 0.675–0.987 | 0.028* | 0.924 | 0.757–1.127 | 0.435 |
| Length of Follow up (years) | 1.083 | 1.043–1.125 | <0.001* | 1.331 | 1.266–1.398 | < 0.001* |
| FSRP Score | 1.023 | 0.995–1.052 | 0.107 | 1.014 | 0.983–1.045 | 0.386 |
|
| ||||||
| Sub-Group Analysis of effect of BMI on conversion status with and without rapid weight decrease in 12 months prior to outcome | ||||||
|
| ||||||
| Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-value | |
|
| ||||||
| Baseline Diagnosis: Normal (N=441) | Baseline Diagnosis: MCI (N=183) | |||||
|
| ||||||
| BMI (kg/m2) with rapid weight decrease (>5%) | 0.976 | 0.925–1.030 | 0.381 | 0.958 | 0.891–1.029 | 0.237 |
|
| ||||||
| Baseline Diagnosis: Normal (N=3206) | Baseline Diagnosis: MCI (N=1076) | |||||
|
| ||||||
| BMI (kg/m2), without rapid weight decrease (≤5%) | 0.959 | 0.933–0.987 | 0.004* | 0.953 | 0.925–0.981 | 0.001* |
|
| ||||||
| Secondary Analysis of Effect of ApoE4 on conversion status | ||||||
|
| ||||||
| Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-value | |
|
| ||||||
| Baseline Diagnosis: Normal (N=4193) | Baseline Diagnosis: MCI (N=1485) | |||||
|
| ||||||
| BMI (kg/m2) | 0.972 | 0.950–0.993 | 0.010* | 0.970 | 0.947–0.993 | 0.011* |
| APOEε4 Status† | 2.165 | 1.765–2.654 | <0.001* | 1.759 | 1.409–2.195 | <0.001* |
|
| ||||||
| Baseline Diagnosis: Normal (N=4080) | Baseline Diagnosis: MCI (N=1441) | |||||
|
| ||||||
| BMI (kg/m2) | 0.973 | 0.951–0.995 | 0.016* | 0.970 | 0.947–0.994 | 0.014* |
| APOEε4 Allele Count‡ (1 allele) | 2.140 | 1.789–2.561 | <0.001* | 1.611 | 1.354–1.916 | <0.001* |
All models adjusted for Age, Sex, Race, Education, Length of follow up, Framingham Stroke Risk Profile (FSRP) score; Odds Ratio (OR) are interpreted for a one-unit increase,
P-values significant at α = 0.05, CI= Confidence Interval; MCI= Mild Cognitive Impairment; BMI = Body Mass Index;
APOE (Apolipoprotein E) ε4 Status, positive= ε2/ε4, ε3/ε4, or ε4/ε4, negative=ε2/ε2, ε2/ε3, or ε3/ε3;
ApoE4 allele count, ε2/ε2, ε2/ε3, or ε3/ε3 = 0, ε3/ε4 = 1, ε4/ε4 =2,
Effect of BMI on conversion status in the setting of rapid weight loss (shrinkage)
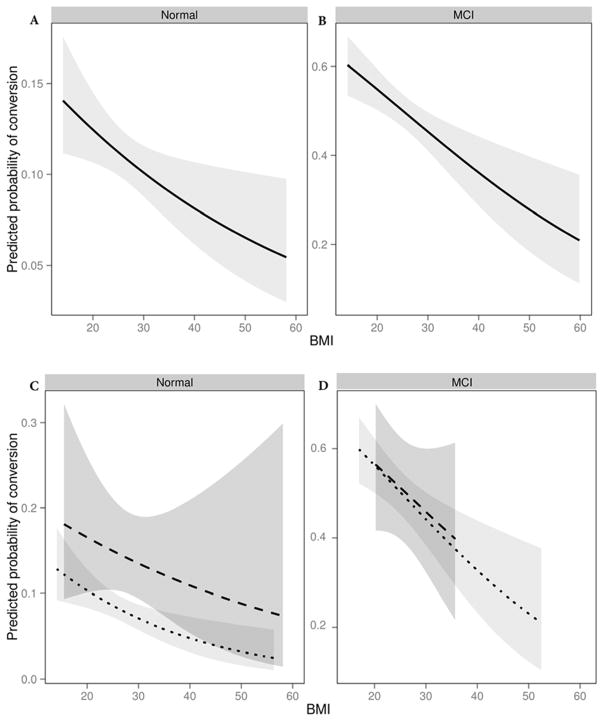
Secondary analyses relating BMI to conversion status by weight shrinkage prior to conversion reveals differing results by shrinkage group (with vs. without rapid weight loss). Compared to individuals who did not lose weight, individuals who lost significant weight were older (significant weight loss mean age of 74.4 years vs no weight loss mean age 72.9 years), more likely to be female (significant weight loss 68% vs no weight loss 63%), had a higher baseline BMI (significant weight loss mean 28.1 kg/m2 vs no weight loss 27.0 kg/m2), and had a greater frequency of cardiovascular disease and diabetes resulting in a higher mFSRP (significant weight loss mean 12.4 vs no weight loss mean 11.4). There were no significant differences between groups for education, race, time in the study, or APOE ε4 status. In individuals with evidence of rapid weight loss (>5% reduction in BMI in the targeted months prior to outcome measure, for NC mean 12.3 months, SD 1.2 months, for MCI mean 12.4 months SD 1.3 months), baseline BMI no longer had an independent protective effect on diagnostic conversion in either baseline diagnostic group (NC OR=0.976, 95%CI 0.925–1.030, p=0.381;, MCI OR=0.958, 95%CI 0.891–1.029, p=0.237), while the opposite finding was observed for individuals without evidence of rapid weight loss (NC OR=0.959, 95%CI 0.933–0.987, p=0.004; MCI OR=0.953, 95%CI 0.925–0.981, p=0.001). Figure 2 shows the effect of baseline BMI on the predicted probability of conversion to MCI or dementia in primary and secondary analyses with Panel A displaying the association in NC and Panel B displaying the association in MCI. Panels C (NC) and D (MCI) display the results of the secondary subgroup analyses of the effect of BMI on diagnostic conversion by participants with (dashed line) and without (dotted line) significant weight loss showing that in NC there is a higher probability of conversion to MCI or dementia in the presence of significant weight loss. This finding was not observed in MCI. Covariates were fixed as follows (median values of MCI participants or most prevalent value of baseline characteristic: age (75), years of education (16), race (white), sex (female), FSRP (12).
Figure 2.
Panel A displays the predicted probability of diagnostic conversion for NC over the spectrum of BMI (kg/m2), showing a decreasing probability of conversion with higher baseline BMI. Panel B displays the corresponding predicted probability of conversion for MCI, showing a similar pattern but with overall greater probability of conversion at every BMI value. Panels C and D display the predicted probability of conversion stratified by the presence or absence of significant weight in the 12 months preceding outcome measure (dotted line representing absence of significant weight loss, dashed line representing significant weight loss). For all panels, data are fitted for a given participant profile (using prevalent level for categorical covariates and median for continuous covariates), including age (75), years of education (16), race (White), sex (female), total years in study (3), modified FSRP (12). Shading reflects 95% confidence interval.
Effect of BMI and APOE ε4 on conversion status
In secondary analyses restricted to participants with APOE ε4 status data (n=5678), BMI retained its protective effect on risk of conversion when APOE ε4 status was included as a covariate in the models (NC OR=0.972, 95% CI 0.950–0.993, P=0.010; MCI OR=0.970, 95% CI 0.947–0.993, P=0.011) but BMI was no longer protective in the presence of rapid weight loss prior to diagnostic conversion (NC OR=0.95, 95% CI 0.89–1.02, P=0.145; MCI OR=1.00, 95% CI 0.92–1.09, P=0.970, data not shown in Table 3). As expected, APOE ε4 presence was associated with higher risk of diagnostic conversion for both baseline diagnostic groups (APOE ε4 presence: NC OR=2.17, 95% CI 1.77–2.65, P<0.001 and MCI OR=1.76, 95% CI 1.41–2.2, P<0.001; per APOE ε4 allele NC OR=2.14, 95%CI 1.79–2.56, P<0.001 and MCI OR=1.61, 95%CI 1.35–1.92, P<0.001).
DISCUSSION
In this study of more than 6900 older adults with normal cognition and MCI, we found that a higher late-life BMI is associated with a lower incidence of conversion to MCI and dementia; but in the presence of rapid weight loss prior to such diagnostic conversion, a higher BMI was no longer protective. Furthermore, we found that in the presence of APOE ε4, higher baseline BMI continued to be associated with a lower incidence of diagnostic conversion to MCI and AD. Again in the presence of rapid weight loss prior to such diagnostic conversion, a higher BMI was no longer protective.
To our knowledge, this study is the largest examination of the association between late-life BMI and clinically measured cognitive trajectory. Additional methodological strengths include our use of the FSRP score to statistically adjust for cerebrovascular risk factor burden, the inclusion of rapid weight loss in our analyses, and the assessment of diagnostic trajectory across the cognitive aging spectrum prior to the onset of dementia.
Our results are consistent with findings in prior smaller studies demonstrating an approximate 3–5% reduced incidence of dementia by each unit increase in late-life BMI (8, 9). Our study differs, however, in that we confirm this prior finding in both NC and MCI diagnostic groups and include both MCI and dementia as diagnostic outcomes. Further, the high prevalence of conversion in our cohort (22% conversion overall with 12% for NC and 49% for MCI participants) yields additional increased power for our analyses compared to prior work.
There are several possible explanations for our findings. First, BMI is known to increase gradually over the adult lifespan, peaking around middle age with a subsequent plateau or decrease (38). Obesity and reduced survival are correlated until approximately 75 years of age (39) and therefore the change in association between BMI and mortality after age 75 may in part be explained by the high mortality rates of younger and middle-aged obese adults leading to a more resilient group of older obese adults in later life (40, 41). Alternatively, our baseline characteristics are consistent with prior studies showing an overall lower BMI with age and a corresponding increase in mortality overall in older adults at the lowest weight (42). Lower BMI may constitute an age-related gradual weight loss that parallels other age-related physiological changes (e.g., decreasing maximal heart rate, reduced creatinine clearance), representing a more accurate biological age.
Regarding AD, a majority of studies have consistently shown that a higher weight or body mass index (BMI) in mid-life is associated with an increased risk for developing AD in late-life (7, 43) and may represent a burden of accumulated vascular and metabolic risk factors over decades. In late life, weight loss and loss of lean body mass occur frequently before the onset of clinical symptoms and as manifestation of the clinical decline as the disease progresses (23). A reduction in weight and change in body composition may occur as a direct result of reducing appetite or physical activity (44, 45) or as an indirect result of shared mechanisms common to AD and sarcopenia (46–48). These associations parallel findings of other late-life risk factors for AD, such as hypertension and total cholesterol (49–51). Although reduced appetite in persons with AD has been suggested to account for weight loss, research suggests this explanation is unlikely as weight loss occurs before the onset of clinical symptoms (23). Mild cognitive changes can occur several years before clinical onset (52), and pathology develops decades before the disease is clinically recognized (52, 53). The association between lower BMI and increased risk of MCI and AD may represent a common early pathological pathway (e.g., increases in inflammation, apoptosis, and senescence) that results in both phenotypes or it may represent an individual loss of overall physiological reserve.
Shrinkage (unintentional weight loss >5% over one year) is a component of the frailty syndrome (21, 46) and an independent risk factor for cognitive impairment and AD (22). Our results do not suggest any protective effect of higher late-life BMI in the presence of shrinkage, so baseline BMI is possibly less relevant to absolute risk of MCI or AD than the trajectory of weight changes over time. Translation of these findings into clinical practice is not straightforward as mechanisms remain unclear; however, in the setting of a patient with a family history or significant cognitive complaints, closer follow-up of memory function and risk factors (e.g., blood pressure (54)) associated with AD may be warranted in individuals with low BMI or significant unintentional weight loss.
Despite the strengths of this study described above, we acknowledge several methodological limitations. Although the NACC cohort is derived from 34 past and current AD centers across the United States, the participants are self-referred or recruited from associated memory clinics, are predominately White and are well educated, which may limit generalizability. The unexpected finding of an increased risk of conversion from MCI to AD in White participants may in part be a result of the limitations of the cohort or possibly due to risk factor differences observed between our White and non-White participants (55). The mean study follow-up time of 3.8 years was shorter than prior studies, but because of the large study sample and prevalence of diagnostic conversion, we had sufficient power to detect our outcomes over the relatively shorter follow-up period. Finally, secondary analyses using APOE ε4 excluded individuals who did not consent to genetic testing, which may bias results (e.g., perhaps toward individuals with a more prevalent family history of dementia).
In conclusion, the current study is among the largest to date to demonstrate the effect of late-life BMI on the trajectory of cognitive impairment. It specifically demonstrates a reduced risk of diagnostic progression with higher baseline BMI in both NC and MCI while adjusting for relevant risk factors, including burden of vascular disease. Further research examining underlying mechanisms that explain the association between BMI and brain health could further advance knowledge regarding causes of AD.
Acknowledgments
FUNDING
Funding Sources: This work was supported by National Institutes of Health [K12-HD043483 to SPB, KAG, TJH; K23AG048347 to SPB; NIRG-13-283276 to KAG; K24-AG046373 to ALJ, R01-AG034962 to ALJ, R01-HL11516 to ALJ]; the Eisenstein Women’s Heart Fund to SPB; Pharmaceutical Research and Manufacturers of America Foundation Fellowship in Translational Medicine and Therapeutics to TJH; and the Vanderbilt Memory & Alzheimer’s Center
The National Alzheimer’s Coordinating Center (NACC) database is funded by National Institute of Aging/National Institutes of Health (NIA/NIH) Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI David Teplow, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), and P50 AG005681 (PI John Morris, MD)
Sponsor’s Role: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, and the U.S. Department of Health and Human Services or any of its agencies.
Footnotes
Conflicts of Interest:
| Elements of Financial/Personal Conflicts | SB | DL | LS | AS | KG | TH | AJ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | X | |||||||
| Grants/Funds | X | X | X | X | X | X | X | |||||||
| Honoraria | X | X | X | X | X | X | X | |||||||
| Speaker Forum | X | X | X | X | X | X | X | |||||||
| Consultant | X | X | X | X | X | X | X | |||||||
| Stocks | X | X | X | X | X | X | X | |||||||
| Royalties | X | X | X | X | X | X | X | |||||||
| Expert Testimony | X | X | X | X | X | X | X | |||||||
| Board Member | X | X | X | X | X | X | X | |||||||
| Patents | X | X | X | X | X | X | X | |||||||
| Personal Relationship | X | X | X | X | X | X | X | |||||||
References
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaffe K, Lindquist K, Vittinghoff E, Barnes D, Simonsick EM, Newman A, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, et al. The effect of maintaining cognition on risk of disability and death. Journal of the American Geriatrics Society. 2010;58(5):889–94. doi: 10.1111/j.1532-5415.2010.02818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffczyk C, Romero B, Jonas C, Lahmeyer C, Muller F, Riepe MW. Generic quality of life assessment in dementia patients: a prospective cohort study. BMC neurology. 2010;10:48. doi: 10.1186/1471-2377-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandman PO, Adolfsson R, Nygren C, Hallmans G, Winblad B. Nutritional status and dietary intake in institutionalized patients with Alzheimer’s disease and multiinfarct dementia. Journal of the American Geriatrics Society. 1987;35(1):31–8. doi: 10.1111/j.1532-5415.1987.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 5.White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. Journal of the American Geriatrics Society. 1998;46(10):1223–7. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 6.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Archives of internal medicine. 2003;163(13):1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 7.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P, Quid PsPA. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60(1):117–9. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 8.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Archives of neurology. 2009;66(3):336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJ, Pocock SJ. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. The lancet Diabetes & endocrinology. 2015 doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- 11.Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus DH, Kukull W, Gustafson DR. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer disease and associated disorders. 2014;28(1):36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Lee S, Hibar D, Dinov ID, Stein JL, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiology of aging. 2010;31(8):1326–39. doi: 10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72(20):1741–6. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45(6):1161–8. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 15.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2011;12(5):e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 16.Harris TB, Launer LJ, Madans J, Feldman JJ. Cohort study of effect of being overweight and change in weight on risk of coronary heart disease in old age. Bmj. 1997;314(7097):1791-. doi: 10.1136/bmj.314.7097.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petkeviciene J, Smalinskiene A, Luksiene DI, Jureniene K, Ramazauskiene V, Klumbiene J, Lesauskaite V. Associations between apolipoprotein E genotype, diet, body mass index, and serum lipids in Lithuanian adult population. PloS one. 2012;7(7):e41525. doi: 10.1371/journal.pone.0041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Andrade M, Thandi I, Brown S, Gotto A, Jr, Patsch W, Boerwinkle E. Relationship of the apolipoprotein E polymorphism with carotid artery atherosclerosis. American journal of human genetics. 1995;56(6):1379–90. [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura K, Ansai T, Awano S, Hamasaki T, Akifusa S, Takehara T, Abe I, Takata Y. Association of body mass index with blood pressure in 80-year-old subjects. Journal of hypertension. 2001;19(12):2165–9. doi: 10.1097/00004872-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 22.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. Journal of the American Geriatrics Society. 2010;58(2):248–55. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Archives of neurology. 2006;63(9):1312–7. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 24.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer disease and associated disorders. 2006;20(4):210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 25.Jefferson AL, Hohman TJ, Liu D, Haj-Hassan S, Gifford KA, Benson EM, Skinner JS, Lu Z, Sparling J, Sumner EC, et al. Adverse vascular risk is related to cognitive decline in older adults. Journal of Alzheimer’s disease: JAD. 2015;44(4):1361–73. doi: 10.3233/JAD-141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke; a journal of cerebral circulation. 1991;22(3):312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 30.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 31.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 32.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Movement disorders: official journal of the Movement Disorder Society. 2003;18(5):467–86. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 34.Mesulam MM. Primary progressive aphasia. Annals of neurology. 2001;49(4):425–32. [PubMed] [Google Scholar]
- 35.Association AP. Diagnostic and statistical manual of mental disorders (DSM-IV) American Psychiatric Association; 1994. [Google Scholar]
- 36.Abner EL, Kryscio RJ, Cooper GE, Fardo DW, Jicha GA, Mendiondo MS, Nelson PT, Smith CD, Van Eldik LJ, Wan L, et al. Mild cognitive impairment: statistical models of transition using longitudinal clinical data. Int J Alzheimers Dis. 2012;2012:291920. doi: 10.1155/2012/291920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–56. [PubMed] [Google Scholar]
- 38.Rissanen A, Heliovaara M, Aromaa A. Overweight and anthropometric changes in adulthood: a prospective study of 17,000 Finns. International journal of obesity. 1988;12(5):391–401. [PubMed] [Google Scholar]
- 39.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. The New England journal of medicine. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 40.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. The New England journal of medicine. 1995;333(11):677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 41.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. The New England journal of medicine. 1999;341(15):1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 42.Cornoni-Huntley JC, Harris TB, Everett DF, Albanes D, Micozzi MS, Miles TP, Feldman JJ. An overview of body weight of older persons, including the impact on mortality. The National Health and Nutrition Examination Survey I--Epidemiologic Follow-up Study. Journal of clinical epidemiology. 1991;44(8):743–53. doi: 10.1016/0895-4356(91)90126-t. [DOI] [PubMed] [Google Scholar]
- 43.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of neurology. 2005;62(10):1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 44.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Archives of neurology. 2010;67(4):428–33. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkins CH, Roe CM, Morris JC, Galvin JE. Mild physical impairment predicts future diagnosis of dementia of the Alzheimer’s type. Journal of the American Geriatrics Society. 2013;61(7):1055–9. doi: 10.1111/jgs.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. Journal of cellular and molecular medicine. 2009;13(9B):3103–9. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty - a mini-review. Gerontology. 2012;58(2):99–106. doi: 10.1159/000330064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGeer EG, McGeer PL. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. Journal of Alzheimer’s disease: JAD. 2010;19(1):355–61. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- 49.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 50.Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension. 2011;58(1):22–8. doi: 10.1161/HYPERTENSIONAHA.110.163055. [DOI] [PubMed] [Google Scholar]
- 51.Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64(10):1689–95. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 52.Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D’Agostino RB. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Archives of neurology. 1995;52(5):485–90. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 53.Musiek ES, Holtzman DM. Origins of Alzheimer’s disease: reconciling cerebrospinal fluid biomarker and neuropathology data regarding the temporal sequence of amyloid-beta and tau involvement. Current opinion in neurology. 2012;25(6):715–20. doi: 10.1097/WCO.0b013e32835a30f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42(9):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]