Abstract
The onset of spermatogenesis occurs in response to retinoic acid (RA), the active metabolite of vitamin A. However, whether RA plays any role during establishment of the spermatogonial stem cell (SSC) pool is unknown. Because designation of the SSC population and the onset of RA signaling in the testis that induces differentiation have similar timing, this study asked whether RA influenced SSC establishment. Whole mount immunofluorescence and flow cytometric analysis using the Id4-eGfp transgenic reporter mouse line revealed an enrichment for ID4-EGFP+ cells within the testis following inhibition of RA Synthesis by WIN 18,446 treatment. Transplantation analyses confirmed a significant increase in the number of SSCs in testes from RA-deficient animals. Conversely, no difference in the ID4-EGFP+ population or change in SSC number were detected following exposure to an excess of RA. Collectively, reduced RA altered the number of SSCs present in the neonatal testis but precocious RA exposure in the neonatal testis did not, suggesting that RA deficiency causes a greater proportion of progenitor undifferentiated spermatogonia to retain their SSC state past the age when the pool is thought to be determined.
Keywords: retinoic acid, spermatogenesis, testis, spermatogonial stem cell
Introduction
Within the neonatal mouse testis, establishment of the spermatogenic lineage initiates when prospermatogonia, the earliest germ cell precursors present at birth, migrate to the basement membrane of the seminiferous cord. After migration, these cells follow one of two fates: 1) transition directly into differentiating spermatogonia to produce the first round of spermatogenesis, or 2) become undifferentiated spermatogonia. However, whether the undifferentiated population is solely SSCs or both SSCs and committed progenitors is unknown. Progenitor spermatogonia are known to contain the cell machinery to respond to RA signaling and proceed through the differentiation process (de Rooij and Russell, 2000), yet there is no published evidence to indicate that the SSCs can respond to RA. The best predictor of stem cell or progenitor capacity during steady state spermatogenesis is inhibitor of DNA binding 4 (ID4) levels, particularly when trying to detect the transitional state during which SSCs transition into progenitors (Chan et al., 2014; Helsel et al., 2017). ID4 is a helix-loop-helix protein that binds other helix-loop-helix transcription factors, acting as a repressor (Benezra et al., 1990; Riechmann et al., 1994). Transplantation from transgenic Id4-eGfp mice demonstrated that the ID4-EGFP+ spermatogonial population contains most of the SSC population (Chan et al., 2014; Helsel et al., 2017). Further analyses of ID4-EGFP+ spermatogonia have revealed that the intensity of the EGFP signal correlates with regenerative capacity, with the ID4-GFPBright population being pure SSCs (Helsel et al., 2017).
Previous work suggested that the SSC pool forms between 0 and 6 dpp (Bellve et al., 1977; Chan et al., 2014; de Rooij and Russell, 2000; Huckins and Clermont, 1968; McLean et al., 2003). This timing correlates with the onset of RA signaling in the testis that occurs between 3 and 5 dpp (Busada et al., 2014; Snyder et al., 2011; Snyder et al., 2010). RA deficiency, induced by diet, genetically, or chemically using the retinaldehyde dehydrogenase inhibitor WIN 18,446, has been shown to block spermatogonial differentiation, producing testes lacking advanced germ cells but enriched with undifferentiated spermatogonia (Griswold et al., 1989; Hogarth et al., 2013; Hogarth et al., 2011; McLean et al., 2002; Mitranond et al., 1979; Unni et al., 1983; Van Pelt and De Rooij, 1990a, b). Conversely, precocious exposure to RA at 1 or 2 dpp resulted in premature expression of stimulated by retinoic acid gene 8 (STRA8) in prospermatogonia and a delay in meiotic entry when compared to vehicle treated controls (Busada et al., 2014; Snyder et al., 2011) suggesting that prospermatogonia may be vulnerable to extrinsic signals prior to the time of normal RA production. However, the effects of aberrant testicular RA levels on the pool of SSCs in the neonatal mouse testis have not been directly examined.
Here we show that reduced RA alters the number of SSCs present in the neonatal testis but precocious RA exposure in the neonatal testis does not, and we present a model for how RA regulates SSC establishment.
Materials and Methods
Animals
Animal experiments were approved by the Washington State University Animal Care and Use Committees and conducted in accordance with the guided principles for the care and use of research animals of the National Institutes of Health. B6;129S- Gt(ROSA)26Sor/J (mice designated ROSA; The Jackson Laboratory, Stock No: 002073), ID4-EGFP transgenic reporter line (Chan et al., 2014), and C57BL/6-129ScvP mouse colonies were maintained in a temperature- and humidity-controlled environment with food and water provided ad libitum. Animals were euthanized by CO2 asphyxiation followed by decapitation (0–10 dpp) or cervical dissociation (10–180 dpp), and their testes dissected.
WIN 18,446 and RA treatments
WIN 18,446 and RA treatments were performed as described (Hogarth et al., 2013; Snyder et al., 2011). From 2 to 8 dpp, 100 μg/g body weight of WIN 18,446, suspended in 1% gum tragacanth, was pipette fed daily to neonatal ID4-GFP or ROSA mice and euthanized at 9 dpp. Vehicle treated control animals received 1% gum tragacanth alone. Some WIN 18,446-treated animals also received an intraperitoneal (IP) injection of 200 μg of RA, diluted in 10 μL of dimethyl sulfoxide (DMSO), to synchronize the onset of spermatogenesis, and were left to recover for either 8 or 48 hours. Precocious RA exposure was induced in 2 dpp mice by subcutaneous injection of 50 μg of RA, diluted in 10 μL of DMSO, and mice were allowed 24 hours to recover prior to euthanasia. Vehicle control mice received a subcutaneous injection of 10 μL of DMSO.
Whole Mount Immunofluorescence
Whole mount immunofluorescence was performed as previously described (Agrimson et al., 2016). ID4-EGFP testes were detunicated and the tubules were gently dissociated manually with forceps under magnification using a dissecting microscope (Olympus Model SZX-ILLD2-100, PA, USA). The dissociated tubules were fixed using 4% paraformaldehyde (P6148, Sigma-Aldrich, MO, USA) with gentle rotation for 2 hours at 4°C and stored in IX PBS (137mM NaCl/2.7mM KCl/10.1mM Na2HP04/1.8mM KH2PO4) at 4°C until use. Primary antibodies were: goat anti-GFP-FITC (2.5 μg/mL, AB6662, Abcam, MA, USA) and rabbit anti-STRA8 (1:2000 dilution, made in-house (Hogarth et al., 2013)). Secondary antibody was Alexa Fluor reagent donkey anti-rabbit 488 (2 (μg/mL, A21206, Invitrogen, CA, USA), diluted in PB (1% bovine serum albumin/PBS). Confocal microscopy (LEICA TCS SP5 II, IL, USA) was used to image whole seminiferous tubules and at least 3 neonatal animals were examined to ensure consistent results. ID4-EGFP and STRA8-copositive spermatogonia were quantified in testis cross sections following RA treatment. At least 250 round tubule cross sections within at least three sections, separated by 25 microns, from three animals per treatment group were examined to determine the percentage of ID4-EGFP+ prospermatogonia expressing STRA8. Paired Student T Tests (Microsoft Excel) were used to determine significance between control and treated samples. A P-value less than 0.05 was deemed significant.
Germ Cell Transplantation
Transplantation was performed as previously described (Helsel and Oatley, 2017). Recipients were C57BL6/JX129S1/svlmJ F1 hybrid mice treated with IP injection of busulfan (50mg/kg of body weight) at 6 weeks of age and recovered at least 6 weeks to ensure germ cell depletion (Brinster and Avarbock, 1994). Donors were Rosa26-LacZ transgenics (obtained from Jackson laboratories, stock no. 002073), treated with either 7 consecutive daily treatments of WIN 18,446-only or a single RA-only injection at 2 dpp. Single cell suspensions of testes were generated by enzymatic disassociation and the germ cell population enriched by Percoll selection as described previously (Oatley and Brinster, 2006) and germ cells were re-suspended in mouse SSC serum-free medium (mSFM) at 106 cells/mL. Approximately 7 to 10 μl of cell suspension was microinjected into seminiferous tubules via the rete testis. For each recipient, one testis received germ cells from WIN 18,446-only or RA-only donors and the contralateral testis received cells from a vehicle control donor. Microinjection of treated or control cell suspensions was varied between the right and left testis. Three transplantation studies were performed for each experimental treatment and 2 to 6 recipient mice were used for each. Recipient mice were given at least 70 days to recover and allow for spermatogenic colony regeneration. Following euthanasia, the recipient testes were detunicated, fixed with 4% paraformaldehyde for 2 hours at 4°C, and washed and stained in bromo-chloro-indolyl-galactopyranoside (X-gal) as previously described (McLean et al., 2002). Dark blue colonies of spermatogenesis were counted using a dissecting microscope by gently separating the seminiferous tubules (Figure 2C). The relative number of SSCs per donor testis was determined by multiplying the number of colonies counted from each testis by the fold-difference in cells per mL isolated from treated testes versus control testes. Paired Student T Tests (Microsoft Excel) were performed to determine significance between control and treated samples. A P-value less than 0.05 was deemed significant. Transplantation analyses were performed at least 3 times from 3 different treated and control donor animals to achieve technical and biological replication.
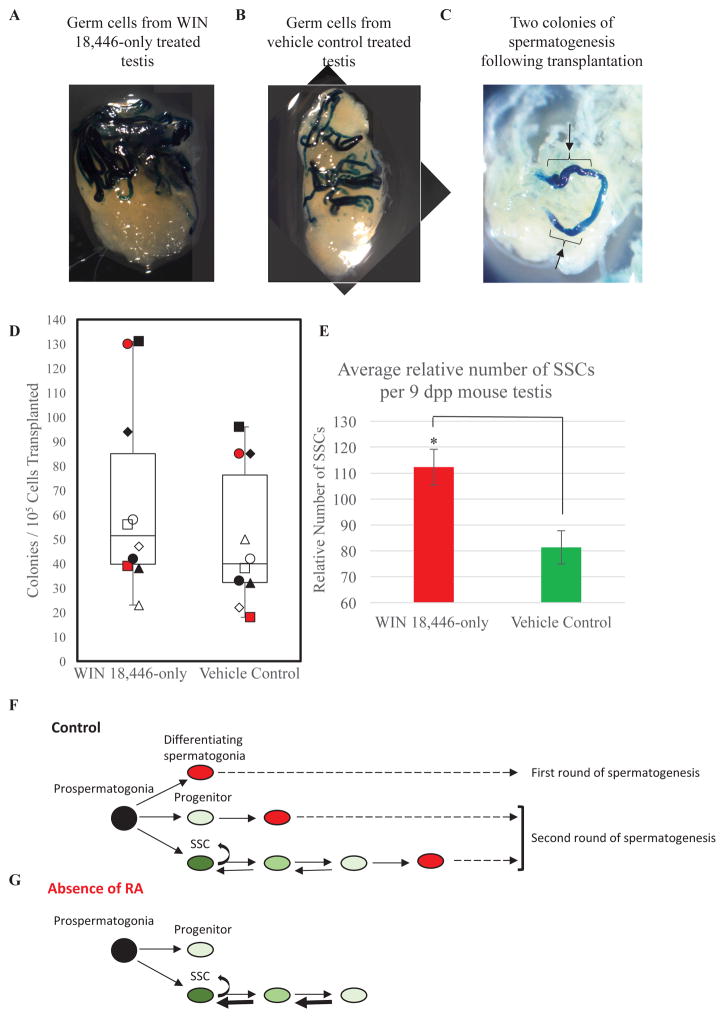
Figure 2. Alteration of SSC content in the RA deficient neonatal mouse testis.
Representative images of recipient testes 70 days following transplantation that have been stained for colonies of donor-derived spermatogenesis from germ cells isolated after treatment with (A) WIN 18,446-only or (B) vehicle control. (C) Image depicts how dark blue colonies of spermatogenesis, shown by the brackets and black arrows, were counted within each recipient testis. (D) Box and whisker plot shows the quantitation of the number of donor-derived colonies from germ cells collected from either WIN 18,446-only treated or vehicle control treated mice. (E) Quantitative comparison of number of SSCs in testes of mice treatment with WIN 18,446-only or the vehicle control. Data are mean±SEM and n=3 different cell preparations and 10 recipient mice (each individual recipient mouse is labeled differently as white, black or red squares, triangles, circles, or diamonds). * denotes significantly different at a p < 0.05. (F) Hypothesized model depicting the derivation of 3 distinct populations (SSCs, progenitors, and differentiating spermatogonia) from prospermatogonia in the neonatal testis. Black oval indicates prospermatogonia. Dark, medium, and light green ovals depict ID4-EGFP bright (SSCs), mid (cells transitioning from SSC to progenitor), and dim (progenitor) cells, respectively. Red ovals mark STRA8-positive differentiating spermatogonia. (G) Hypothesized model depicting the effects RA deficiency on ID4-EGFP + germ cell dynamics during SSC pool establishment. Black oval indicates prospermatogonia. Dark, medium, and light green ovals depict ID4-EGFP bright (SSCs), mid (cells transitioning from SSC to progenitor), and dim (progenitor) cells, respectively.
Flow Cytometric Analysis
Single cell suspensions were prepared using trypsin/EDTA for testis digestion (Oatley and Brinster, 2006) and re-suspended at a concentration of 1 × 106 cells/mL in DPBS-S. Treated and control cell suspensions were then analyzed for EGFP intensity using a Cytoflex flow cytometer (B53000, Beckman Coulter, CA, USA) and the CytExpert Software (Beckman Coulter) to determine the percentage of ID4-EGFP+ and ID4-EGFP- cells. Additionally within the ID4-EGFP+ cells, the percentage of ID4-EGFP Bright, Mid, and Dim cells between treatments was measured as described previously (Helsel et al., 2017). The analysis was conducted at least 3 times from 3 different treated and control donor animals to achieve technical and biological replication.
Results
RA deficiency alters the ratio of ID4-EGFP-bright and -dim spermatogonia and increases SSC number in the neonatal testis
Vitamin A deficiency (VAD) and RA depletion by WIN 18,446 treatment both enrich testes with undifferentiated spermatogonia (Evans et al., 2014; Griswold et al., 1989; Hogarth et al., 2013; McLean et al., 2002; Mitranond et al., 1979; Unni et al., 1983; Van Pelt and De Rooij, 1990a, b). However, the effect of RA deficiency on the ID4-EGFP expressing SSC population has not been examined. We used whole mount immunofluorescence to qualitatively compare the ID4-EGFP populations in seminiferous tubules of control (RA sufficient) and WIN 18,446-treated (RA deficient) mice. An abundance of apparently ID4-EGFPBright SSCs were present in WIN 18,446 treated animals, while these cells were sparse in vehicle treated controls (Figure 1A and 1B). After RA exposure of WIN 18,446 treated animals 3 different populations of spermatogonia emerged within 8 hours: 1) ID4-EGFPBright, 2) STRA8- and ID4-EGFPDim -co-positive, and 3) STRA8 only-positive spermatogonia (Figure 1C). Forty-eight hours after RA the first and third cell populations were only present, consistent with differentiation of the STRA8- and ID4-EGFPDim -co-positive cells (Figure 1D).
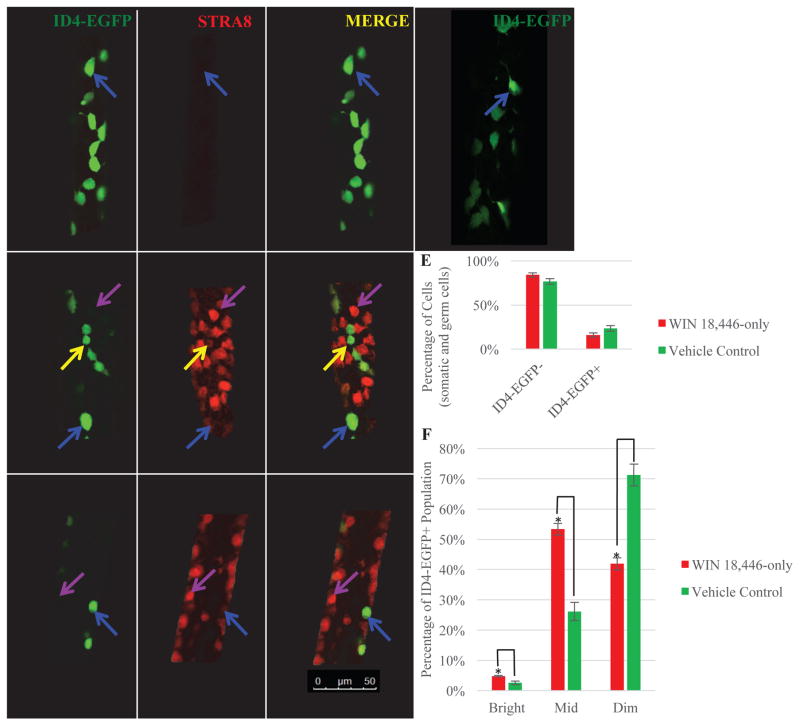
Figure 1. RA deficiency alters the dynamics of the ID4-EGFP+ population in the mouse neonatal testis.
Representative images of whole mount testis tubules from mice treated with WIN 18,446-only (A), vehicle control (B), WIN 18,446 followed RA for 8 hours (C) or 48 hours (D). All tubules were stained for both STRA8 and EGFP. Blue arrows denote what appear to be ID4-EGFPBright+/STRA8- germ cells, yellow arrows indicate ID4-EGFPDim+/STRA8+ germ cells, and purple arrows designate ID4-EGFP-/STRA8+ spermatogonia. n=3 different mice for each treatment. Bars = 50 μm. (E) Quantitative comparison from flow cytometric analysis of the percentage of total ID4-EGFP+ and ID4-EGFP- cells in testes of mice treated with WIN 18,446-only and vehicle control. (F) Quantitative comparison of the percentage of the ID4-EGFP+ population that could be classified as Bright, Mid, or Dim in mice treated with WIN 18,446-only or vehicle control. Data are mean±SEM for three independent experiments. * denotes significantly different at a p-value < 0.05.
To quantify the effects of RA deficiency on the ID4-EGFP+ population in the neonatal testis, we used flow cytometric analysis to assess: 1) the overall percentage of EGFP+ cells in testes from animals treated with WIN 18,446 compared to vehicle control, and 2) the proportion of Bright, Mid, and Dim fluorescent cells within the ID4-EGFP+ population in testes of animals from the two treatment conditions. The percentage of total ID4-EGFP+ cells was unchanged in the absence of RA (Figure 1E). However, the proportions of ID4-EGFPBright and ID4-EGFPMid cells were significantly elevated and the proportion of ID4-EGFPDim cells was significantly decreased in testes from WIN 18,446-only treated animals compared to vehicle treated controls (Figure 1F). Collectively, these results indicate RA deficient testes have increased numbers of ultimate SSCs and daughter cells in transition to a progenitor state.
To functionally test whether the increase in ID4-EGFPBright/Mid spermatogonia in RA deficient animals reflects an increase in SSC number, we performed transplantation analysis. We compared engraftment of germ cells from ROSA donor mice treated with either WIN 18,446 or vehicle (Figure 2A and 2B). Because each donor-derived colony in recipient testes is clonally-derived from a single SSC, quantification of colony number provides a retrospective measure of SSC content in the transplanted donor cell suspension. We found that germ cells from both RA-deficient and RA-sufficient control testes could generate spermatogenic colonies following transplantation (Figure 2A and 2B) and testes of RA deficient mice contained a significantly greater number of SSCs compared to those of controls (Figure 2D and 2E). Taken together, these results demonstrated an increased number of true SSCs when RA is depleted.
Precocious RA exposure induces STRA8 expression within a majority of the ID4-EGFP+ prospermatogonial population but does not alter the number of SSCs in neonatal testes
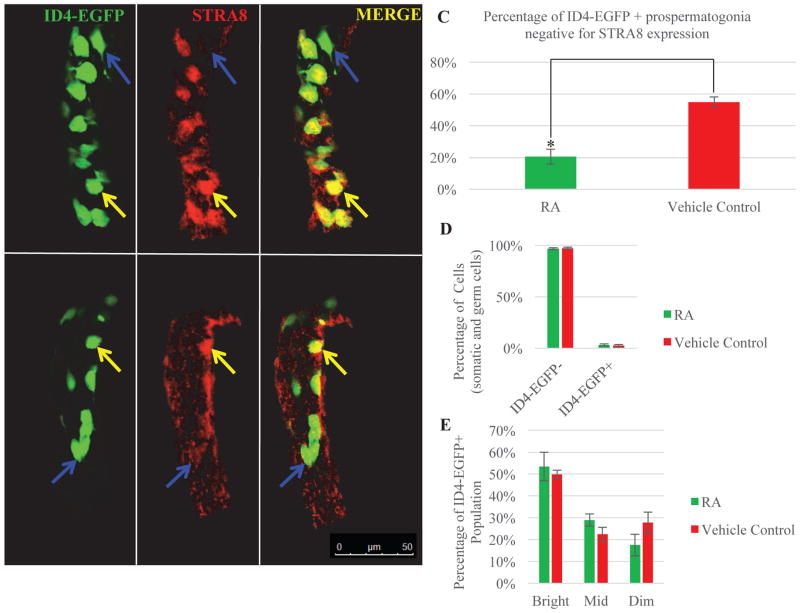
Next, we asked whether exogenous RA treatment can cause premature SSC establishment at 2 dpp. Using whole mount immunofluorescence, we observed that early exposure to RA induced STRA8 expression in 80% of the ID4-EGFP+ prospermatogonia (Figure 3A and 3C), while only 45% of the ID4-EGFP+ spermatogonia in vehicle control mice expressed STRA8 (Figure 3B and 3C). These results indicate that the ID4-EGFP+ population can respond to RA by activating STRA8 expression, but do not address whether this reflects a change in functional capacity. To assess this, we used flow cytometric analysis to quantify ID4-EGFP expression. We found no significant difference in the percentage of germ cells that were ID4-EGFP+ in testes exposed to excess RA compared to controls (Figure 3D) and no change in the abundance of Bright, Mid, or Dim cells in the presence of excess RA exposure (Figure 3E).
Figure 3. Induction of STRA8 expression in ID4-EGFP+ prospermatogonia following exposure to excess RA does not alter the dynamics of the ID4-EGFP+ germ cell population in the neonatal testis.
Representative images of whole mount testis tubules from mice treated with exogenous RA (A) or vehicle control (B) and stained by immunofluorescence for STRA8 (red) and ID4-EGFP (green). Blue arrows denote ID4-EGFP+/STRA8- cells and yellow arrows indicate ID4-EGFP+/STRA8+ cells. Bar = 50 μm. (C) Quantitative comparison of the percentage of ID4-EGFP only-positive prospermatogonia. (D) Quantitative comparison from flow cytometric analysis of the percentage of total ID4-EGFP+ and ID4-EGFP- cells in testes of mice treated with vehicle or exogenous RA. (E) Quantitative comparison of the percentage of the ID4-EGFP+ population that can be classified as Bright, Mid, or Dim in testes of mice treated with vehicle or exogenous RA. Data are mean±SEM and n=3 different mice for each treatment. *denotes significantly different at a p = 0.005.
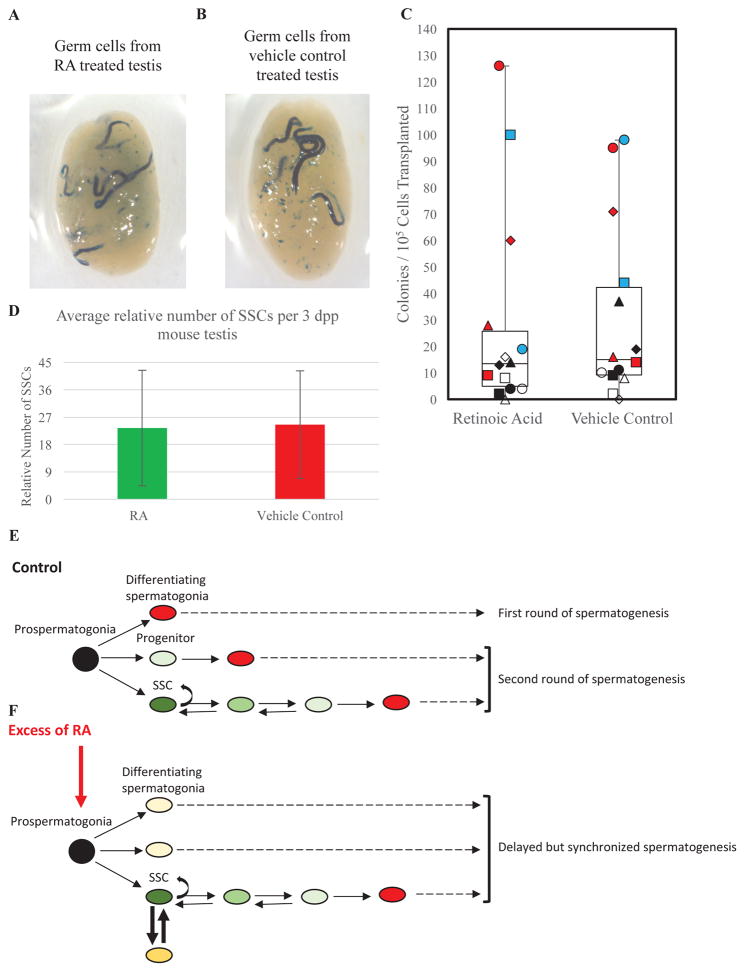
Finally, we asked whether premature expression of STRA8 by ID4-EGFP+ prospermatogonia alters their capacity to regenerate spermatogenesis. We used transplantation analyses to compare the SSC content in testes of mice exposed to excessive RA versus controls. Donor mice at 2 dpp were treated with subcutaneous RA or vehicle injection, germ cell suspensions were isolated 24 hr later, and a standard number of cells was transplantation into recipient testes. Both RA-treated and control donors generated colonies, demonstrating the presence of SSCs (Figure 4A and 4B) and calculation of engraftment efficiency found no difference between RA-treated and control mice (Figure 4C and 4D). Collectively, these data indicate that excessive RA in neonatal testes does not alter SSC abundance.
Figure 4. Excess RA exposure in the neonatal mouse testis does not alter SSC number.
Representative images of recipient testes 70 days post-transplantation that have been stained for colonies of donor-derived spermatogenesis from germ cells isolated after treatment with (A) RA or (B) vehicle control. (C) Quantitation of the number of donor-derived colonies generated by germ cell suspensions from testes of mice treated with exogenous RA or vehicle control. (D) Quantitative comparison of the number of SSCs present in testes of mice treated with exogenous RA or vehicle control. Data are mean±SEM and n=3 different cell preparations and 14 recipient mice (each individual recipient mouse is labeled differently as white, black, blue or red squares, triangles, circles, or diamonds). (E) Hypothesized model depicting the derivation of 3 distinct populations (SSCs, progenitors, and differentiating spermatogonia) from prospermatogonia in the neonatal testis. Black oval indicates prospermatogonia. Dark, medium, and light green ovals depict ID4-EGFP bright (SSCs), mid (cells transitioning from SSC to progenitor), and dim (progenitor) cells, respectively. Red ovals mark STRA8-positive differentiating spermatogonia. (F) Hypothesized model depicting the effects of excess RA exposure on ID4-EGFP + germ cell dynamics during SSC pool establishment. Black oval indicates prospermatogonia. Dark, medium, and light green ovals depict ID4-EGFP bright (SSCs), mid (cells transitioning from SSC to progenitor), and dim (progenitor) cells, respectively. Dark and light yellow ovals show ID4-EGFP and STRA8 co-positive cells. Red ovals mark STRA8-positive differentiating spermatogonia.
Discussion
This study has, for the first time, provided evidence to suggest that RA availability may influence SSC pool formation in the neonatal testis. During the onset of spermatogenesis, germ cells with features of undifferentiated spermatogonia arise between 3 and 5 dpp (Drumond et al., 2011; Kluin and de Rooij, 1981; Yoshida et al., 2006; Yoshida et al., 2004), with the SSC pool eventually forming within this population. The exact timing of SSC pool establishment is not known, but is proposed to occur between 0 and 6 dpp (Bellve et al., 1977; Chan et al., 2014; de Rooij and Russell, 2000; Huckins and Clermont, 1968; McLean et al., 2003). RA is known to trigger spermatogonial differentiation and the onset of spermatogenesis during this period, but whether RA regulates SSC pool establishment has been unknown. Our results suggest that RA deficiency causes a greater proportion of progenitor undifferentiated spermatogonia to retain their SSC state past the age when the pool is thought to be determined.
While genetic models have been used to induce RA deficient testes in juvenile mice (Li et al., 2011; Raverdeau et al., 2012), SSC abundance has not been examined in these models. We recently showed that treatment with the aldehyde dehydrogenase enzyme inhibitor is a highly effective method to eliminate RA production in neonatal testes (Hogarth et al., 2015; Hogarth et al., 2013). Spermatogonial differentiation does not occur in mice treated with WIN 18,446 and consequently the testes become enriched with undifferentiated spermatogonia (Hogarth et al., 2013). Work of Helsel et al., 2017 demonstrated through functional transplantation analyses that the ID4-EGFPBright population is essentially pure SSCs by 8 dpp. Here we found that ID4-EGFPBright spermatogonia are enriched in the testis following WIN 18,446 treatment, suggesting an increased number of spermatogonia with stem cell potential, a possibility we confirmed by transplantation. Taken together, flow cytometry and transplantation analyses suggest that some ID4-EGFPMid spermatogonia still retain regenerative capacity consistent with the proposal by Helsel et al., 2017 that ID4-EGFP levels are proportional to SSC potential.
Based on our findings, we hypothesize that RA signaling in the neonatal testis promotes formation of the SSC pool, either directly or indirectly. Previous work has shown that steady state SSCs do not express RA receptor γ (RAR γ) and the earliest germ cell expression of RARγ occurs at 5 dpp (Gely-Pernot et al., 2012; Vernet et al., 2006), however expression of the receptor has not been fully investigated in the prospermatogonial population between 1 and 5 dpp. To fully understand how RA availability may be fostering SSC pool formation future studies will be necessary. Our data indicate that RA deficiency leads to more undifferentiated spermatogonia retaining the potential to be SSCs. It is unlikely that this increased regenerative potential is due to increased SSC proliferation, as we previously found that the undifferentiated spermatogonial population exits the cell cycle upon WIN 18,446 treatment (Agrimson et al., 2016). Transplantation studies have shown that the number of Sertoli cells in the testis correlates directly to the number of SSCs (Oatley et al., 2011), and the Sertoli cells are known to synthesize the RA required for the onset of spermatogenesis (Raverdeau et al., 2012). We suggest that RA from Sertoli cells may help, at least initially, to coordinate Sertoli cell number with SSC pool size.
It is well known that excess RA exposure has a potent effect on spermatogenesis prior to the time of preleptotene spermatocyte formation (Busada et al., 2014; Davis et al., 2013; Snyder et al., 2011), including early expression of STRA8 in prospermatogonia and delayed progression of spermatogenesis (Busada et al., 2014; Snyder et al., 2011). It has also been suggested that the delay is because excess RA induces RA degrading enzymes, thus slowing differentiation (Busada et al., 2014). We found that 24 hours after RA treatment, ID4-EGFP expressing prospermatogonia also expressed STRA8, suggesting that this population of germ cells are susceptible to the RA trigger in terms of induction of a hallmark gene response. However, we observed no difference in the proportion of ID4-EGFPBright prospermatogonia or the number of regenerative spermatogonia as defined by transplantation. Moreover, excess RA did not shift the populations of ID4-EGFPBright/Mid/Dim prospermatogonia. Thus, RA induction of STRA8 gene expression does not necessarily indicate a functional change in SSC potential.
We found that RA depletion affects SSC abundance in neonates but RA supplementation does not. To explain these results, we suggest that at least three populations of spermatogonia derive from prospermatogonia: differentiating spermatogonia destined to form the first wave of spermatogenesis, SSCs, and progenitor undifferentiated spermatogonia (Figure 2F and 4E). We suggest that the progenitor cells are responsive to RA depletion and retain the capacity to form SSCs (Figure 2G). In contrast, prospermatogonial exposure to excess RA causes a reversible induction of STRA8, via an unknown mechanism that prevents differentiation of the ultimate SSCs (Figure 4F). This response likely coordinates with the delay in timing of meiotic entry (Busada et al., 2014; Snyder et al., 2011). Recent work has shown that within the RA deficient testes neither SSC niche availability nor excessive germ cell proliferation can explain the increased SSC number following WIN 18,446-only treatment (Agrimson et al., 2016). Ultimately, understanding the dynamics of the ID4-EGFP+ population will help reveal how RA availability affects the establishment of the SSC pool.
Acknowledgments
The authors would like to thank Amy Kaucher and Aileen Helsel for their assistance during the germ cell preparations for SSC transplantations, Dr. Qi-En Yang for his technical assistance regarding counting SSC colonies following transplantation, and Dr. David Zarkower for critically reading the manuscript.
Funding: This work was supported by the National Institutes of Health [Grants R01 HD10808 to MDG and R01 HD061665 to JMO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrimson KS, Onken J, Mitchell D, Topping TB, Chiarini-Garcia H, Hogarth CA, Griswold MD. Characterizing the Spermatogonial Response to Retinoic Acid During the Onset of Spermatogenesis and Following Synchronization in the Neonatal Mouse Testis. Biology of reproduction. 2016;95:81. doi: 10.1095/biolreprod.116.141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Kaye EP, Renegar RH, Geyer CB. Retinoic acid induces multiple hallmarks of the prospermatogonia-to-spermatogonia transition in the neonatal mouse. Biology of reproduction. 2014;90:64. doi: 10.1095/biolreprod.113.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan F, Oatley MJ, Kaucher AV, Yang QE, Bieberich CJ, Shashikant CS, Oatley JM. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes & development. 2014;28:1351–1362. doi: 10.1101/gad.240465.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JC, Snyder EM, Hogarth CA, Small C, Griswold MD. Induction of spermatogenic synchrony by retinoic acid in neonatal mice. Spermatogenesis. 2013;3:e23180. doi: 10.4161/spmg.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. Journal of andrology. 2000;21:776–798. [PubMed] [Google Scholar]
- Drumond AL, Meistrich ML, Chiarini-Garcia H. Spermatogonial morphology and kinetics during testis development in mice: a high-resolution light microscopy approach. Reproduction. 2011;142:145–155. doi: 10.1530/REP-10-0431. [DOI] [PubMed] [Google Scholar]
- Evans E, Hogarth C, Mitchell D, Griswold M. Riding the spermatogenic wave: profiling gene expression within neonatal germ and Sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biology of reproduction. 2014;90:108. doi: 10.1095/biolreprod.114.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gely-Pernot A, Raverdeau M, Celebi C, Dennefeld C, Feret B, Klopfenstein M, Yoshida S, Ghyselinck NB, Mark M. Spermatogonia differentiation requires retinoic acid receptor gamma. Endocrinology. 2012;153:438–449. doi: 10.1210/en.2011-1102. [DOI] [PubMed] [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Annals of the New York Academy of Sciences. 1989;564:154–172. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- Helsel AR, Oatley JM. Transplantation as a Quantitative Assay to Study Mammalian Male Germline Stem Cells. Methods in molecular biology. 2017;1463:155–172. doi: 10.1007/978-1-4939-4017-2_12. [DOI] [PubMed] [Google Scholar]
- Helsel AR, Yang QE, Oatley MJ, Lord T, Sablitzky F, Oatley JM. ID4 levels dictate the stem cell state in mouse spermatogonia. Development. 2017 doi: 10.1242/dev.146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Arnold S, Kent T, Mitchell D, Isoherranen N, Griswold MD. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biology of reproduction. 2015;92:37. doi: 10.1095/biolreprod.114.126326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Mitchell D, Kent T, Small C, Amory JK, Griswold MD. Turning a spermatogenic wave into a tsunami: synchronizing murine spermatogenesis using WIN 18, 446. Biology of reproduction. 2013;88:40. doi: 10.1095/biolreprod.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, Small C, Amory JK, Griswold MD. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biology of reproduction. 2011;84:957–965. doi: 10.1095/biolreprod.110.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C, Clermont Y. Evolution of gonocytes in the rat testis during late embryonic and early post-natal life. Arch Anat Histol Embryol. 1968;51:341–354. [PubMed] [Google Scholar]
- Kluin PM, de Rooij DG. A comparison between the morphology and cell kinetics of gonocytes and adult type undifferentiated spermatogonia in the mouse. International journal of andrology. 1981;4:475–493. doi: 10.1111/j.1365-2605.1981.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Li H, Palczewski K, Baehr W, Clagett-Dame M. Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biology of reproduction. 2011;84:336–341. doi: 10.1095/biolreprod.110.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DJ, Friel PJ, Johnston DS, Griswold MD. Characterization of spermatogonial stem cell maturation and differentiation in neonatal mice. Biology of reproduction. 2003;69:2085–2091. doi: 10.1095/biolreprod.103.017020. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Russell LD, Griswold MD. Biological activity and enrichment of spermatogonial stem cells in vitamin A-deficient and hyperthermia-exposed testes from mice based on colonization following germ cell transplantation. Biology of reproduction. 2002;66:1374–1379. doi: 10.1095/biolreprod66.5.1374. [DOI] [PubMed] [Google Scholar]
- Mitranond V, Sobhon P, Tosukhowong P, Chindaduangrat W. Cytological changes in the testes of vitamin-A-deficient rats. L Quantitation of germinal cells in the seminiferous tubules. Acta Anat (Basel) 1979;103:159–168. doi: 10.1159/000145007. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- Oatley MJ, Racicot KE, Oatley JM. Sertoli cells dictate spermatogonial stem cell niches in the mouse testis. Biology of reproduction. 2011;84:639–645. doi: 10.1095/biolreprod.110.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raverdeau M, Gely-Pernot A, Feret B, Dennefeld C, Benoit G, Davidson I, Chambon P, Mark M, Ghyselinck NB. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16582–16587. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann V, van Cruchten I, Sablitzky F. The expression pattern of Id4, a novel dominant negative helix-loop-helix protein, is distinct from Id1, Id2 and Id3. Nucleic acids research. 1994;22:749–755. doi: 10.1093/nar/22.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biology of reproduction. 2011;84:886–893. doi: 10.1095/biolreprod.110.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Small C, Griswold MD. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biology of reproduction. 2010;83:783–790. doi: 10.1095/biolreprod.110.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni E, Rao MR, Ganguly J. Histological & ultrastructural studies on the effect of vitamin A depletion & subsequent repletion with vitamin A on germ cells & Sertoli cells in rat testis. Indian J Exp Biol. 1983;21:180–192. [PubMed] [Google Scholar]
- Van Pelt AM, De Rooij DG. The origin of the synchronization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biology of reproduction. 1990a;42:677–682. doi: 10.1095/biolreprod42.4.677. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biology of reproduction. 1990b;43:363–367. doi: 10.1095/biolreprod43.3.363. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Developmental biology. 2004;269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]