Abstract
Sperm cryopreservation protocols have been developed for live-bearers such as the green swordtail Xiphophorus helleri and the platyfish Xiphophorus couchianus. Despite the high post-thaw motility (~75%) obtained in both species, the requirements of sperm storage within the female reproductive tract coupled with the process of internal fertilization place functional demands upon cryopreserved sperm samples far beyond those of oviparous species. The purpose of this study was to facilitate the artificial insemination process with cryopreserved sperm of X. helleri through evaluation of parameters related to sperm quality after thawing. Specifically, this study evaluated the effects on motility for fresh and thawed sperm samples of centrifugation (for concentration of sperm and washing for removal of cryoprotectant), ionic composition, and additions of glucose and fetal bovine serum (FBS) in extender solutions. Centrifugation at 1000 ×g for 10 min at 4 °C was found to have no adverse effects on sperm motility of fresh samples, and for cryopreserved samples, the removal of glycerol by washing yielded higher and longer post-thaw motility (e.g., 168 h vs. 48 h for the controls). Suspension of fresh sperm samples in magnesium-free Hanks’ balanced salt solution (HBSS) did not affect motility; however, HBSS prepared with the absence of potassium or calcium, and the use of unsupplemented saline (NaCl alone) as extenders significantly reduced sperm motility. The presence of glucose in HBSS yielded higher and longer motility for fresh and thawed samples, but addition of glucose at greater than 2 g/L were unnecessary. Addition of 20% FBS prior to freezing was found to increase the post-thaw motility significantly compared to control treatment with 14% glycerol alone. Also addition of 20% FBS after thawing and centrifugation was found to induce the formation of sperm bundles, which may be beneficial for internal fertilization success. In conclusion, concentration of sperm and the removal of cryoprotectant (through centrifugation), and the addition of 20% FBS in the extender is recommended for future insemination trials with cryopreserved samples.
Keywords: Xiphophorus helleri, Sperm motility, Cryopreservation, Centrifugation, Ionic composition, Glucose, Fetal bovine serum
1. Introduction
The green swordtail Xiphophorus helleri is a viviparous teleost of the family Poecilliidae. Members of this genus are valuable as ornamental fish as well as biomedical research models for cancer genetics (Schartl, 1995; Nairn et al., 1996; Tamaru et al., 2001; Walter and Kazianis, 2001). Due to internal fertilization, the sperm of Xiphophorus are analogous to those of mammalian species, and possess atypical features such as elongated sperm heads (Jamieson, 1991), well-developed mitochondrial sheaths in the midpiece (Stoss, 1983), and glycolytic activity comparable to that of mammalian sperm (Gardiner, 1978). Despite study of sperm cryopreservation in some 200 species of freshwater and marine fishes (Leung and Jamieson, 1991; Rana, 1995; Tiersch, 2000), sperm cryopreservation has just begun in live-bearers. Cryopreservation protocols have been developed to address their small body size and limited sperm volume (e.g., 5–10 µL per fish) (Huang et al., 2004b). In previous studies of X. helleri and Xiphophorus couchianus, sperm were suspended in Hanks’ balanced salt solution at 300 mOsm/kg with 14% glycerol, loaded in 0.25-ml straws, equilibrated for 10 min, cooled at 20–25 °C/min, stored in liquid nitrogen, and thawed at 40 °C in a water bath for 7 s with motility after thawing on average as high as 78% (Huang et al., 2004a,c).
Despite this high post-thaw motility of X. helleri sperm; preliminary artificial insemination trials with cryopreserved sperm did not produce live young (Dong et al., 2005). However, these initial attempts suggested that the collection method (crushing of testis), which was different from routine insemination practice (stripping of sperm by abdominal massage), and the presence of cryoprotectant, per se, were not responsible for reproductive failure because live young were produced in fresh sperm samples collected by crushing of testis and suspended in 14% glycerol (Dong et al., 2005). Other hypotheses included: 1) insufficient numbers of sperm were used for insemination; 2) glycerol may have been toxic to thawed sperm samples; 3) the sperm had reduced motility or viability after cryopreservation that rendered them incapable of internal fertilization.
Thus, the purpose of this study was to facilitate the artificial insemination process with cryopreserved sperm through evaluation of parameters related to sperm quality after thawing, and development of amendment strategies to prepare sperm for insemination. Specifically, this study evaluated the effects on motility for fresh and thawed sperm samples of centrifugation, and extender components including ionic composition, and addition of glucose and fetal bovine serum. Centrifugation was evaluated for the purpose of concentrating sperm as well as for removing cryoprotectants after thawing, and the other factors were studied for the purpose of increasing sperm motility after thawing to improve sperm effectiveness within the female for internal fertilization. Recent artificial insemination trials that utilized sperm concentrated and washed by centrifugation has yielded live young and is reported elsewhere (Tiersch et al., 2005).
2. Materials and methods
2.1. Sperm collection and sample preparation
A total of 22 male X. helleri were used in this study. All fish were from inbred lines maintained by the Xiphophorus Genetic Stock Center, Texas State University, San Marcos, TX 78666 (www.xiphophorus.org). Fish were anesthetized in 0.01% tricaine-methane sulfonate (Western Chemical Inc., Ferndale, WA) for 2 min, and sperm were collected by surgical removal of the testis. Adherent tissue was dissected away and testes were placed into tared, resealable plastic bags (NASCO whirl-pak, MBCOCT, New Haven, CT) and weighed. The number of testes used was based on experimental design considerations and in general 2–4 fish were used for each experiment. Only mature testes (with a creamy white appearance) and samples with an initial motility above 80% for fresh sperm were used for experiments. Hanks’ balanced salt solution (HBSS) was added before crushing of the testis to release sperm. Dilutions with HBSS were based on the testis weight, and a ratio of testis to HBSS (mass:volume) of 1:100 generally yielded a sperm density of ~5 × 107 cells/ml. Based on preliminary research (Huang et al., 2004b,c), HBSS at 300 mOsmol/kg was used for sperm suspension after collection. Unless otherwise specified, the osmolality of the HBSS used in this study was 300 mOsmol/kg.
2.2. Motility estimation
The motility characteristics of Xiphophorus sperm are distinct from those of most other teleosts. The sperm are motile upon collection and remain continuously motile after suspension in HBSS, and therefore activation solutions are not necessary for motility estimates. For estimation, a 5-µl aliquot was removed from each sample and placed on a glass microscope slide. In the cases when the ratio of sperm to HBSS (or HBSS-glycerol mixture) was below 1:100, 2 µl of sperm suspension were diluted 1:100 with HBSS before estimation. Sperm motility was estimated visually at 200× magnification using darkfield microscopy (Optiphot 2, Nikon Inc., Garden City, New York) and was expressed as the percentage of cells moving in a forward direction. Sperm vibrating in place were not considered to be motile. Unless specified, samples were always stored at 4 °C after treatment, but samples were warmed to room temperature (23 °C) prior to motility estimation.
2.3. Freezing procedures
Aliquots (80–100 µl) of sperm suspensions with cryoprotectant (detailed below) were drawn into 0.25-ml French straws (IMV International, Minneapolis, USA) and held (equilibrated) for 8 min at room temperature (23 °C) and 2–7 min at 4 °C before cooling in a controlled-rate freezer (Kryo 16 Series II; Planer Products, Sunbury-on-Thames, UK) at 20 °C per min from 5 °C to −80 °C. The straws were transferred to a liquid nitrogen storage dewar after the temperature reached −80 °C. After a minimum of 12 h, the straws were thawed for 7 s in a 40 °C water bath (Model 1141, VWR Scientific, Niles, Illinois).
2.4. Effect of centrifugation on sperm motility
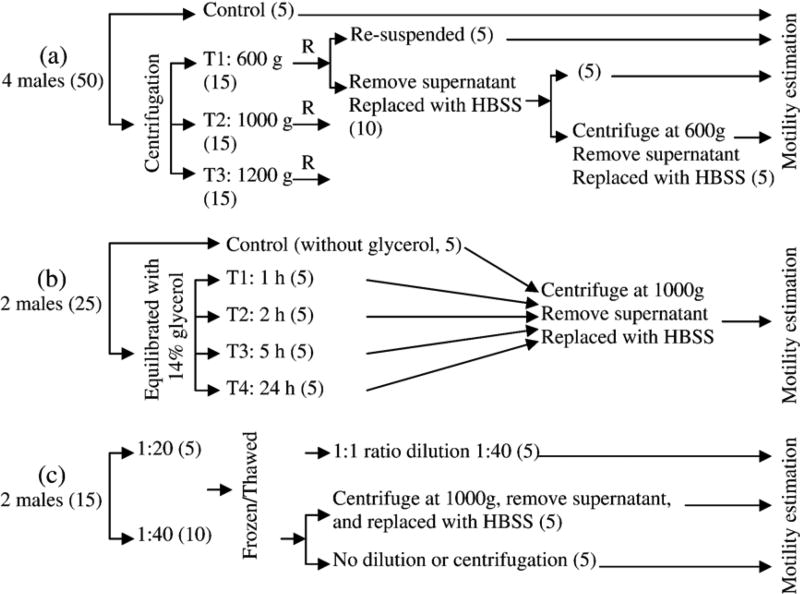
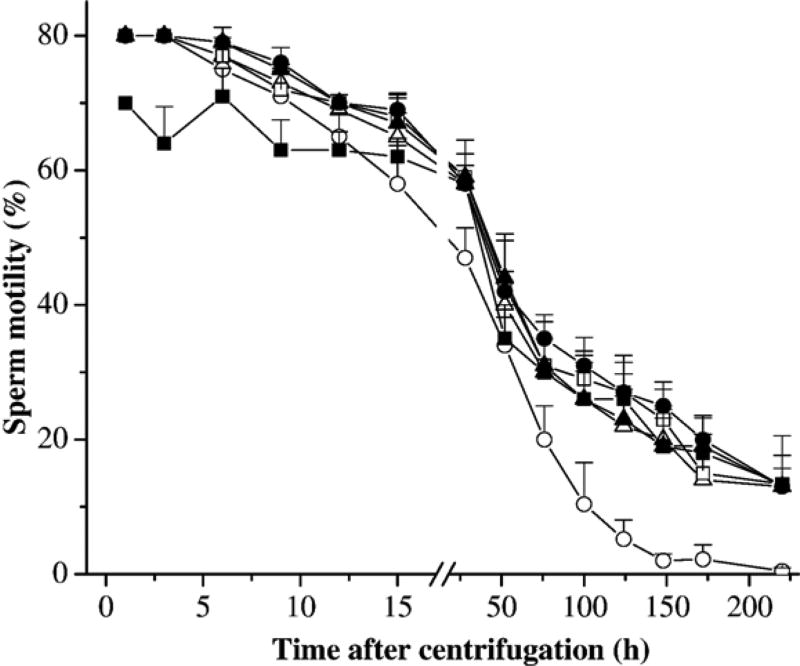
There were three trials in this experiment. In the first trial (Fig. 1a), pooled sperm samples from four males were used to evaluate the effect of centrifuge speeds of 600, 1000, and 1200 ×g on sperm motility of fresh samples. A sperm-extender suspension was prepared from the pooled sperm in HBSS at a ratio of 1:100 (Huang et al., 2004b), and was divided into 50 sub-samples, each containing 60 µl of sperm suspension in 1.5-ml tubes. From 50 tubes, five were used as control without any treatment, and other 45 were divided into three sets of 15 tubes each. The first set of 15 tubes was centrifuged at 600 ×g for 10 min at 4 °C. Immediately after centrifugation, five tubes were gently shaken and sperm were resuspended, and the supernatant in the other 10 tubes was removed and sperm pellets were suspended with fresh HBSS at a volume equal to the removed supernatant. From these 10 tubes, five were centrifuged again at 600 ×g for 10 min at 4 °C, and the supernatants were removed and sperm pellets were resuspended again with fresh HBSS. The second set of 15 tubes was centrifuged at 1000 ×g and the third set at 1200 ×g for 10 min at 4 °C. Immediately after centrifugation, these samples were treated with the same procedures as the first set. Motility was estimated at 2, 16, 48, 96, 168, and 240 h after centrifugation for all samples.
Fig. 1.
Layout of experimental design for the effect of centrifugation on sperm motility. (a) The first trial; (b) the second trial; (c) the third trial. Numbers in the parentheses indicate sub-samples (tubes). T: abbreviation for treatment; R: repetition of step; HBSS: Hanks’ balanced salt solution.
In the second trial (Fig. 1b), sperm samples from two males were used to evaluate the effect of centrifugation at 1000 ×g on sperm motility of samples suspended in 14% glycerol for 1, 2, 5, and 24 h. A sperm-extender suspension was prepared from the pooled sperm in HBSS at a ratio of 1:50, and was divided into 25 sub-samples, each containing 30 µl of sperm suspension in 1.5-ml tubes. Five tubes were diluted with the same volume of HBSS (1:1 ratio dilution), and 20 tubes were diluted with the same volume of HBSS-glycerol (28%), thus with the final glycerol of 14%. The sub-samples with glycerol were divided into four sets, and equilibrated for 1, 2, 5, and 24 h. Immediately after equilibration, the samples were centrifuged at 1000 ×g for 10 min at 4 °C. Supernatants were removed, and sperm pellets were resuspended with fresh HBSS. Motility was estimated immediately after centrifugation and at 48, 120, and 192 h after treatment.
In the third trial (Fig. 1c), sperm samples from two males were used to evaluate the effect of centrifugation at 1000 ×g on sperm motility of thawed samples after cryopreservation. Fifteen sub-samples were prepared from the pooled sperm suspension with 14% glycerol as cryoprotectant, each containing 80 µl of sperm suspension. However, the ratio of sperm to HBSS-glycerol in the five sub-samples was 1:20, and for the remaining 10 sub-samples was 1:40. Immediately after thawing, the sperm suspension with the ratio of sperm to HBSS-glycerol at 1:20 was diluted with the same volume of fresh HBSS. Of the samples with a ratio of sperm to HBSS-glycerol at 1:40, five were centrifuged at 1000 ×g for 10 min at 4 °C, the supernatants were removed, and sperm pellets were resuspended with fresh HBSS at a volume equal to the removed supernatant. The other five were left untreated (without dilution or centrifugation). Motility was estimated at 2, 48, 96, 144, and 172 h after thawing.
2.5. Effect of extracellular ion composition on sperm motility
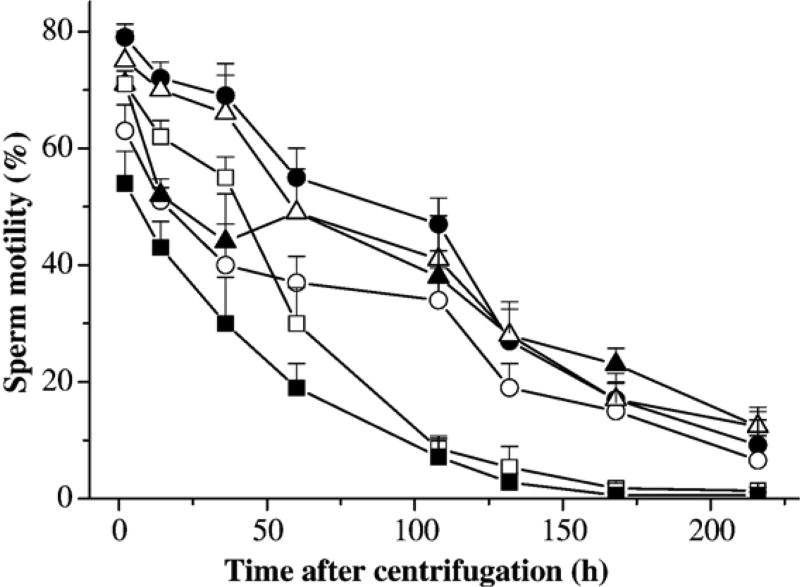
In this experiment, sperm samples from three males were used to evaluate the effect of ionic changes of HBSS on sperm motility. A sperm-extender suspension was prepared from the pooled sperm in HBSS at a ratio of 1:60, and was divided into 30 sub-samples, each containing 80 µl of sperm suspension in 1.5-ml tubes. All samples were centrifuged at 1000 ×g for 10 min at 4 °C. Immediately after centrifugation, supernatants were removed and sperm pellets from each of five tubes were gently resuspended at a volume equal to the removed supernatant with HBSS, HBSS without potassium (K-free HBSS), calcium (Ca-free HBSS), magnesium (Mg-free HBSS), or glucose, and 0.9% saline solution composed of NaCl only. These extenders were adjusted to the same osmolality (300 mOsm/kg, with the addition of NaCl) and pH (from 7.6 to 7.8) prior to the experiment. Centrifugation and re-suspension in the same extenders were repeated two additional times for all samples. Motility was estimated at 2, 14, 36, 60, 104, 128, 168, and 216 h after the final centrifugation.
2.6. Effect of glucose on sperm motility
There were two trials in this experiment. In the first trial, sperm samples from three males were used to evaluate the effect of different concentrations of glucose on sperm motility. A sperm-extender suspension was prepared from the pooled sperm in HBSS without glucose at a ratio of 1:50, and divided into 30 sub-samples, each containing 40 µl of sperm suspension in 1.5-ml tubes. All samples were centrifuged at 1000 ×g for 10 min at 4 °C, supernatants were removed, and sperm pellets were gently resuspended at a volume equal to the removed supernatant with fresh HBSS without glucose. Immediately after re-suspension, each five tubes were double diluted with HBSS with the addition of glucose to achieve the final concentrations of 0, 1, 2, 5, 10, and 25 g/L for glucose. Initially, samples were stored at room temperature and motility was estimated at 30 min and 2, 5, 8, 11 and 14 h after treatment. Samples were stored at 4 °C after 14 h and motility was estimated at 30, 54, 78, 102, 126, 150, 174, and 222 h.
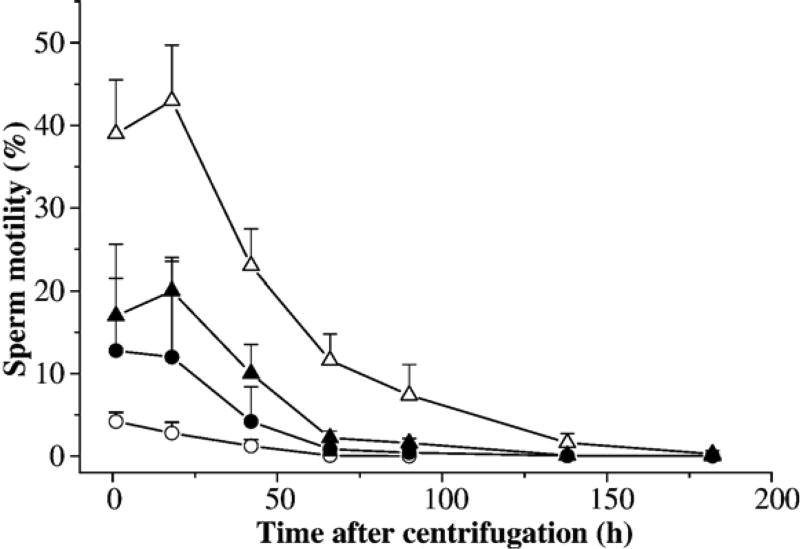
In the second trial, sperm samples from two males were used to evaluate the different combinations of glycerol and glucose on post-thaw sperm motility. A sperm-extender suspension was prepared from the pooled sperm in HBSS at a ratio of 1:50, and divided into 20 sub-samples, each containing 40 µl of sperm suspension in 1.5-ml tubes. The samples were divided into five sets and double diluted with HBSS containing different concentrations of glycerol and glucose. The final concentrations for the first set (5 tubes) were 14% glycerol and 1 g/L glucose, 10% and 25 g/L for the second set, 12% and 25 g/L for the third set, and 14% and 25 g/L for the last set. Immediately after thawing, the samples were all centrifuged at 1000 ×g for 10 min at 4 °C, supernatants were removed, and sperm pellets were gently resuspended at a volume equal to the removed supernatant with fresh HBSS. Motility was estimated at 2, 18, 42, 66, 90, 138, and 182 h after centrifugation.
2.7. Effect of fetal bovine serum (FBS) on sperm motility
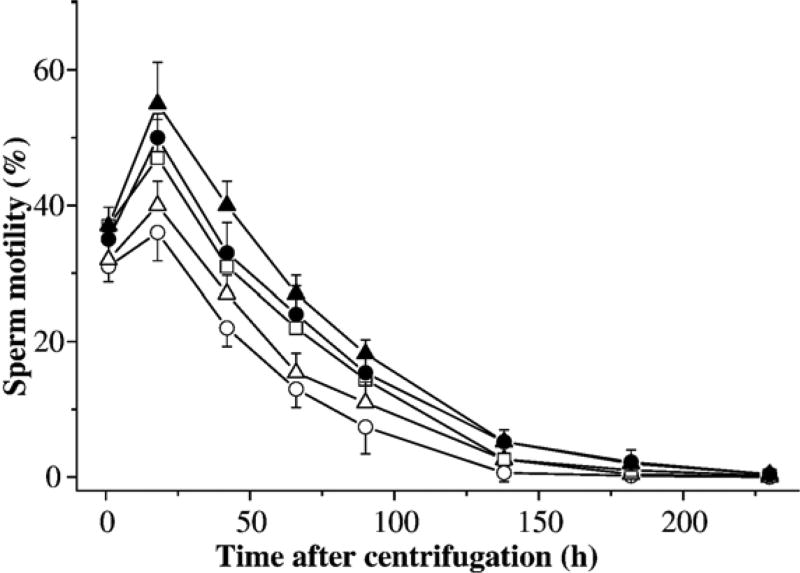
There were two trials in this experiment. In the first trial, sperm samples from three males were used to evaluate the effect of different concentrations of FBS on post-thaw motility. A sperm-extender suspension was prepared from the pooled sperm in HBSS at a ratio of 1:50, and divided into 25 sub-samples, each containing 40 µl of sperm suspension in 1.5-ml tubes. The samples were divided into 5 sets (each with 5 sub-samples) and double diluted with HBSS containing 28% glycerol and 0, 10, 20, 30, and 40% (v:v) FBS. The final concentrations for these five treatments were 14% glycerol with 0, 5, 10, 15, and 20% FBS. Immediately after thawing, all samples were centrifuged at 1000 ×g for 10 min at 4 °C, supernatants were removed, and sperm pellets were gently resuspended at a volume equal to the removed supernatant with fresh HBSS. Motility was estimated at 2, 18, 42, 66, 90, 138, 182, and 230 h after centrifugation.
In the second trial, sperm samples from three males were used to evaluate the FBS at higher concentrations. The same procedures in the first trial were repeated with FBS at final concentrations of 0, 15, 20, 25, and 30% v:v. A difference from the first trial was that thawed sperm pellets were resuspended with fresh HBSS containing 20% FBS after centrifugation. Motility was estimated at 2, 18, 42, 66, 112 h after centrifugation.
2.8. Data analysis
Multiple comparisons of general linear model (GLM) repeated measure analysis was used to test for differences (P=0.05) among results for all experimental treatments. Results were presented as mean±SD. Data for sperm motility were arcsine–square root transformed prior to analysis when heterogeneity of variance was present. The software used was SPSS 10.0 for Windows (1999).
3. Results
3.1. Effect of centrifugation on sperm motility
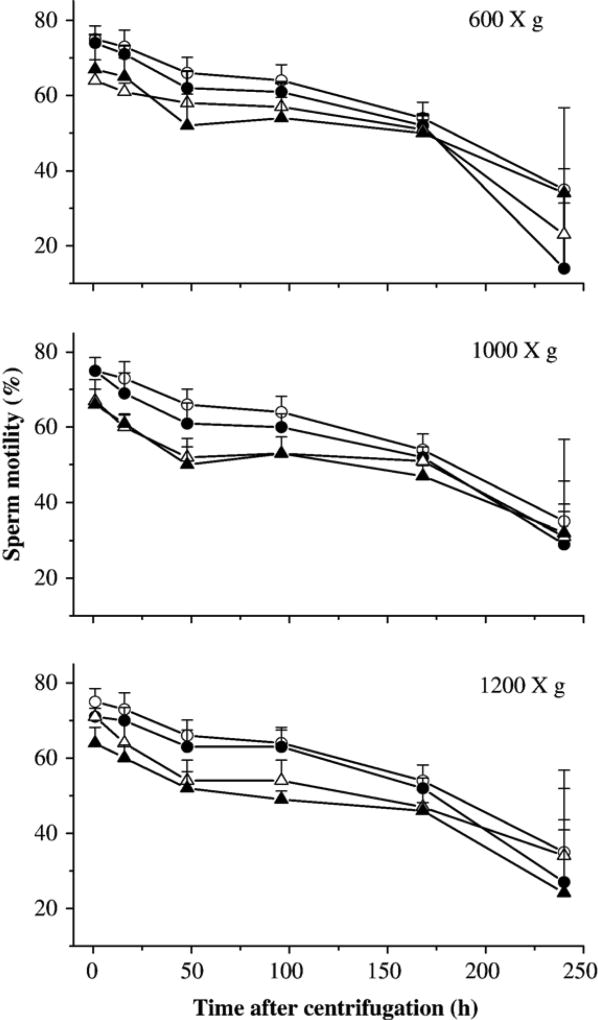
There were three trials in this experiment. The first trial evaluated the effect of centrifuge speeds of 600, 1000, and 1200 ×g on sperm motility of fresh samples (Fig. 2). Compared with the controls (without centrifugation), centrifugation once at 600 and 1000 ×g without removal of supernatant had no effect on motility (P=0.110), but motility was significantly reduced for all other treatments (P< 0.014). Given a particular treatment (e.g., centrifugation once with or without removal of supernatant, and double centrifugation), there was no significant difference (P> 0.289) in motility among the three speeds except for the difference between 600 and 1200 ×g (P=0.043) for samples with double centrifugation. Within the same centrifuge speeds, motility of samples centrifuged once at 600 ×g without removal of supernatant was significantly higher than those centrifuged twice (P<0.001), which in turn was lower than samples centrifuged once with removal of supernatant (P= 0.031). Motility of samples centrifuged once at 1000 ×g without removal of supernatant was significantly higher than those with removal of supernatant (P= 0.017) and those centrifuged twice (P= 0.006), but no significant difference was found between the latter two (P=0.699). There was no significant difference of motility among the treatments of samples centrifuged at 1200 ×g (P>0.127). A gradual decrease of motility over the time was observed for all samples.
Fig. 2.
Motility (mean±SD) of sperm from X. helleri when suspended in Hanks’ balanced salt solution (HBSS) at 300 mOsm/kg and subjected to different treatments: without centrifugation (open circles), centrifuged once without removal of supernatant (filled circles), centrifuged once with removal of supernatant and subsequent replacement of fresh HBSS (open triangles), and centrifuged twice with removal of supernatant and subsequent replacement of fresh HBSS (filled triangles). Samples were centrifuged at the speed of 600 ×g (upper panel), 1000 ×g (middle panel), and 1200 ×g (lower panel). Motility was monitored at 2, 16, 48, 96, 168, and 240 h after treatment.
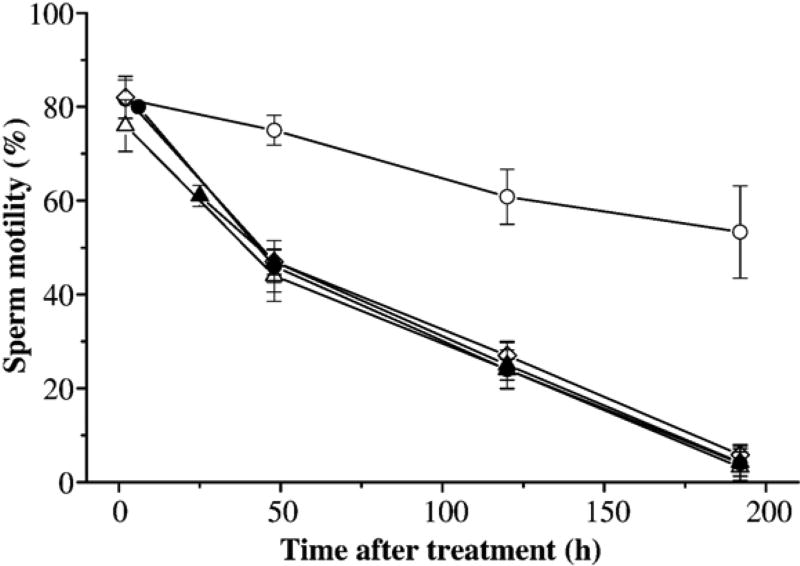
The second trial evaluated the effect of centrifugation at 1000 ×g on sperm motility of samples suspended in 14% glycerol for 1, 2, 5, and 24 h (Fig. 3). Samples suspended in 14% glycerol at each time period had significantly (P<0.001) reduced motility and duration compared to the controls (without glycerol) after centrifugation, removal of supernatant, and replacement with fresh HBSS. However, no significant differences (P>0.065) were observed among samples with 1, 2, 5, and 24 h equilibration in 14% glycerol.
Fig. 3.
Motility (mean±SD) of sperm from X. helleri when suspended in Hanks’ balanced salt solution (HBSS) at 300 mOsm/kg and equilibrated without glycerol (open circles) and with 14% glycerol for 1 h (open triangles), 2 h (open diamonds), 5 h (filled circles), 24 h (filled triangles). After treatment, samples were centrifuged at 1000 ×g for 10 min at 4 °C, and supernatant were removed and replacement of fresh HBSS. Motility was monitored immediately after centrifugation and 48, 120, and 192 h after treatment.
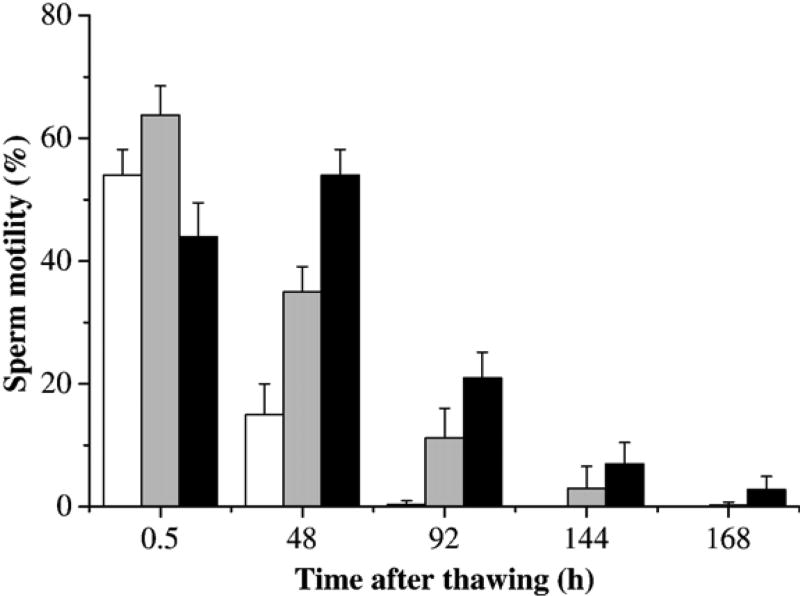
The third trial evaluated the effect of centrifugation at 1000 ×g on sperm motility of thawed samples after cryopreservation (Fig. 4). Samples after thawing were subjected to no treatment (no dilution or centrifugation), 1:1 ratio dilution, and centrifugation with removal of supernatant and replacement with fresh HBSS. Motility of samples without dilution or centrifugation decreased from 54±4% at 2 h after thawing to 15±5% at 2 days, and ceased after 4 days of storage. Samples subjected to 1:1 ratio dilution after thawing, however, retained motility for as long as 6 days. Centrifugation and subsequent removal of supernatant and replacement with fresh HBSS had a significant positive effect on retaining higher post-thaw motility and longer duration. For example, centrifuged samples retained motility 24 h longer than did samples with only the 1:1 ratio dilution (P=0.009), and 120 h longer than samples that received no dilution or centrifugation (P<0.001).
Fig. 4.
Motility (mean±SD) of sperm from X. helleri when suspended in Hanks’ balanced salt solution (HBSS) at 300 mOsm/kg, cryopreserved at 14% glycerol, and subjected to no treatment (white bars), 1:1 ratio dilution (gray bars), and centrifugation at 1000 ×g for 10 min at 4 °C with the removal of supernatant and replacement of fresh HBSS (black bars) after thawing. Motility was monitored at 2, 48, 96, 144, and 172 h after thawing.
3.2. Effect of extracellular ion composition on sperm motility
Sperm retained the highest motility and longest duration in samples suspended in HBSS, which was not significant (P=0.086) from samples suspended in Mg-free HBSS (Fig. 5). Motility decreased significantly in samples suspended in Ca-free HBSS, followed by K-free HBSS, HBSS without glucose, and 0.9% saline solution, which all had significantly lower (P<0.003) motility than those in HBSS. Motility of samples suspended in HBSS without glucose or 0.9% saline solution decreased to less than 10% after 60 h of storage (Fig. 5).
Fig. 5.
Motility (mean±SD) of sperm from X. helleri when suspended in Hanks’ balanced salt solution (HBSS) at 300 mOsm/kg, centrifuged at 1000 ×g for 10 min at 4 °C with the removal of supernatant, and replaced with HBSS (filled circles), HBSS without potassium (open circles), calcium (filled triangles), magnesium (open triangles), and glucose (open squares), and 0.9% saline solution (filled squares). This process was repeated three times, and motility was monitored at 2, 14, 36, 60, 104, 128, 168, and 216 h after the final centrifugation.
3.3. Effect of glucose on sperm motility
The previous experiment showed that motility of samples in HBSS without glucose decreased rapidly (Fig. 5). The first trial of this experiment evaluated the effect of different concentrations of glucose (0, 1, 2, 5, 10, and 25 g/L) on sperm motility (Fig. 6). Similarly, sperm samples suspended in HBSS without glucose had significantly (P<0.001) lower motility and remained motile for a shorter period of time than those in HBSS with the addition of glucose. There were no significant differences in motility (P>0.078) among samples suspended in HBSS with the addition of 0, 1, 2, 5, and 10 g/L glucose. However, the addition of 25 g/L glucose reduced motility significantly (P<0.001).
Fig. 6.
Motility (mean±SD) of sperm from X. helleri when suspended in Hanks’ balanced salt solution (HBSS) at 300 mOsm/kg, centrifuged at 1000 ×g for 10 min at 4 °C with the removal of supernatant, and replaced with HBSS without glucose (open circles), HBSS with the addition of glucose at 1 g/L (open triangles), 2 g/L (open squares), 5 g/ L (filled circles), 10 g/L (filled triangles), and 25 g/L (filled squares). Motility was monitored at 30 min, 2, 5, 8, 11, 14, 30, 54, 78, 102, 126, 150, 174, and 222 h after treatment.
The second trial evaluated the different combinations of glycerol and glucose on post-thaw sperm motility (Fig. 7). The purpose of using high concentrations of glucose (25 g/L) was to test whether the extracellular cryoprotection afforded by glucose could reduce the amount of glycerol with equal protection from freezing and thawing. The highest motility (43±7%) at 18 h after thawing was obtained with 14% glycerol in HBSS prepared with the normal amount of glucose (1 g/L). This combination was also found to retain motility significantly (P<0.001) longer than others. For all combinations with 25 g/L glucose, motility after thawing increased significantly (P<0.001) with the increase of glycerol from 10 to 14%.
Fig. 7.
Motility (mean±SD) after thawing of X. helleri sperm cryopreserved with combined glycerol and glucose at different concentrations: 14% glycerol and 1 g/L glucose (open triangles), 10% and 25 g/L (open circles), 12% and 25g/L (filled circles), and 14% and25g/L (filled triangles), and centrifuged at 1000 ×g for 10 min at 4 °C with the removal of supernatant, and replacement of fresh HBSS. Motility was monitored at 2, 18, 42, 66, 90, 138, and 182 h after centrifugation.
3.4. Effect of fetal bovine serum (FBS) on sperm motility
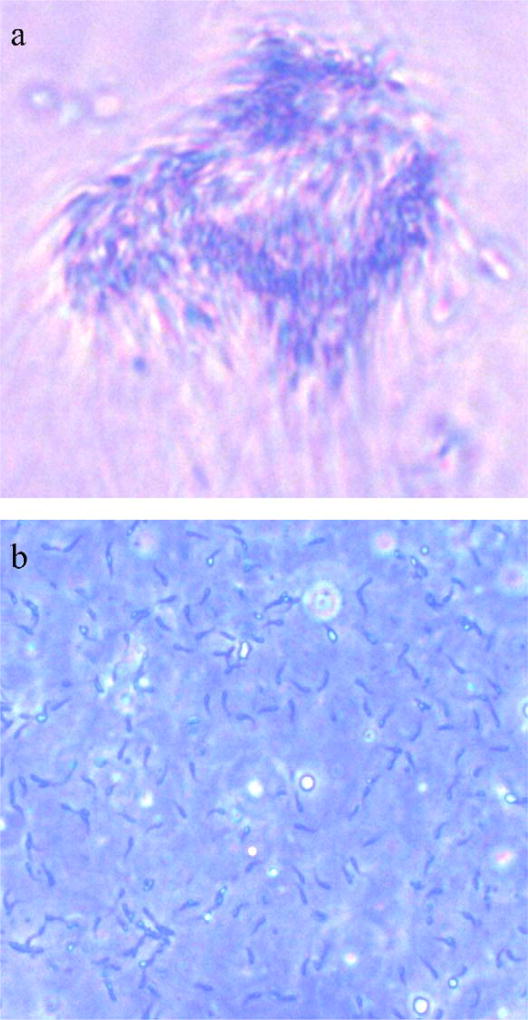
In the first trial (Fig. 8), the addition of FBS was found to increase post-thaw motility significantly (P<0.001) when compared to treatment without FBS. Samples in 20% FBS yielded the highest motility (55±6%) at 18 h after thawing, which was significantly (P<0.001) higher than those cryopreserved with 5, 10, and 15% FBS. However, no significant differences were observed between samples cryopreserved in 10 and 15% FBS (P=0.166). The second trial evaluated FBS at higher concentrations ranging from 15% to 30% (Table 1), and after centrifugation, samples were resuspended with fresh HBSS containing 20% FBS. However, sperm were found to cluster into sperm bundles (Fig. 9a) after storage for 18 h at 4 °C, which rendered motility estimation impossible. In contrast to free-swimming sperm (Fig. 9b), these sperm bundles tended to flow together with their heads parallel to one another, and their tails aligned in the same direction. These bundles were capable of fast forward movement, although they were of irregular shapes with undetermined sizes. Bundles were found to dissociate after 66 h and motility estimation was resumed, but all samples showed limited motility (<5%).
Fig. 8.
Motility (mean±SD) after thawing of X. helleri sperm cryopreserved with 14% glycerol alone (open circles), and 14% glycerol combined with fetal bovine serum at 5% (open triangles), 10% (open squares), 15% (filled circles), and 20% (filled triangles), and centrifuged at 1000 ×g for 10 min at 4 °C with the removal of supernatant, and replacement of fresh HBSS. Motility was monitored at 2, 18, 42, 66, 90, 138, 182, and 230 h after centrifugation.
Table 1.
Motility (mean±SD) after thawing of Xiphophorus helleri sperm cryopreserved with 14% glycerol, different concentrations of fetal bovine serum (FBS), and centrifuged at 1000 ×g for 10 min at 4 °C with the removal of supernatant, and replacement of fresh Hanks’ balanced salt solution containing 20% FBS
| FBS (%, v/v) |
Time after thawing (h)
|
||||
|---|---|---|---|---|---|
| 2 | 18 | 42 | 66 | 112 | |
| 0% | 31±2 | 46±4 | 34±2 | 13±6 | 1±1 |
| 15% | 39±4 | –* | – | 2±2 | 1±1 |
| 20% | 38±3 | – | – | 3±1 | 1±1 |
| 25% | 36±4 | – | – | 3±1 | 1±1 |
| 30% | 41±5 | – | – | 3±1 | 1±1 |
—, motility could not be estimated due to the formation of bundles.
Fig. 9.
Sperm bundles (a, 400×) of X. helleri observed after thawing in samples cryopreserved at 14% glycerol with fetal bovine serum (FBS), and centrifuged at 1000 ×g for 10 min at 4 °C with the removal of supernatant, and replacement of fresh HBSS containing 20% FBS (note association of sperm heads); compared to free swimming sperm (b, 400×).
4. Discussion
Unlike oviparous fishes, viviparous teleosts (live-bearers) employ internal fertilization, internal embryonic development, and give birth to live young. Thus, specialized devices and technical skill are required for artificial insemination, and embryonic development in the female is not easily monitored (Tiersch, 2001). These problems would be exacerbated when cryopreserved sperm are used for insemination. Optimized protocols for sperm cryopreservation of X. helleri have yielded average post-thaw motility above 75% (Huang et al., 2004c). The failure of insemination trials utilizing sperm of high post-thaw motility was unexpected (Dong et al., 2005), and subsequent re-evaluation of the preliminary insemination trials suggested that dilute suspensions had been used for cryopreserved sperm compared to fresh sperm. In routine practice with fresh samples, sperm are extruded by abdominal massage and are used for insemination without further dilution. For cryopreserved samples, however, sperm are collected by crushing of the testis, and diluted to a 1:100 ratio of sperm to HBSS-glycerol prior to freezing (Huang et al., 2004c). Therefore, an estimated 100 times less sperm than the fresh controls were used in preliminary insemination trials with cryopreserved samples. Given the fact that concentrated sperm is necessary for successful fertilization for fresh sperm (our unpublished observations), the use of diluted sperm for cryopreserved samples was suspected to be one of the main reasons for the failure of fertilization. In addition, to obtain fertilization comparable to fresh sperm, a more concentrated sperm density is generally required for thawed samples of aquatic species (Lahnsteiner et al., 1996; Warnecke and Pluta, 2003; He and Woods, 2004). Centrifugation, thus, was evaluated for the purpose of concentrating sperm density after thawing as well as for reducing cryoprotectant toxicity by replacement of supernatant after centrifuging.
Centrifugation has long been routinely used in sperm cryopreservation of mammalian species for concentrating sperm density prior to freezing or for cleaning and collecting high quality sperm after thawing. In aquatic species, it is advisable to use sperm for fertilization immediately after thawing as sperm motility can diminish rapidly (Stoss, 1983), and therefore centrifugation after thawing can be unnecessary, impractical, or detrimental and is not frequently used. In the present study, sperm motility of fresh samples centrifuged at speeds of 600 and 1000 ×g for 10 min at 4 °C were comparable to samples without centrifugation. To maximize the sperm numbers harvested in pellets, 1000 ×g was chosen for future use. In addition, centrifuging at 1000 ×g has been found to have no adverse effect on sperm motility of other aquatic species such as ocean pout, Macrozoarces americanus, an oviparous fish with internal fertilization (Yao et al., 1999). However, removal of supernatant and subsequent replacement of fresh HBSS 300 decreased sperm motility significantly. It is possible that the original supernatant contained substances (e.g., seminal plasma proteins) that are helpful in retaining sperm motility (Chauvaud et al., 1995; Yao et al., 1999). These substances may be derived from crushing of the testis during sperm collection, and were removed with the discarded supernatant after centrifugation. Sperm motility for samples with repeated centrifugation (twice) at 1000 ×g remained the same as those only centrifuged once, which confirms the observation that centrifugation alone at this speed had minimal impact on sperm motility.
Previous insemination attempts using fresh sperm samples suspended in 14% glycerol (final concentration) produced live young (Dong et al., 2005), which suggested that 14% glycerol was not detrimental to fresh sperm or females. However, the present study showed that motility decreased significantly for samples suspended in 14% glycerol compared to the controls (without glycerol) although there were no significant difference among the samples equilibrated with glycerol from 1 to 24 h. It is possible that glycerol has adverse effects on thawed sperm, and renders them incapable of fertilizing eggs after introduction to the female reproductive tract. Our findings also showed that sperm retained higher and longer post-thaw motility after the removal of glycerol by centrifugation. Similar findings were found in another study of X. helleri including sperm samples cryopreserved with hypertonic extenders (500 mOsmol/kg) (Yang et al., 2006). These findings suggest that centrifugation can be used to concentrate sperm density after thawing, and offer an additional advantage of reducing cryoprotectant concentration and toxicity when used in subsequent insemination. In fact, recent insemination trials using cryopreserved sperm concentrated through centrifugation did yield live young and the above-mentioned benefits of centrifugation were suspected to be among the main reasons for insemination success (Tiersch et al., 2005).
Controversy exists in the literature of sperm cryopreservation about how well motility correlates to fertility (e.g., Kerby, 1983; Billard, 1988; Graham, 2001; Warnecke and Pluta, 2003). Unlike fishes with external fertilization, sperm in viviparous fishes have a highly specialized midpiece with plentiful glycogen stores (Grier, 1981; Billard, 1986), and thus can exhibit lengthy motility (36 h when stored at 23 °C and 10 d at 4 °C, Huang et al., 2004b). Sperm motility of oviparous fishes typically lasts no longer than 2–3 min after activation in freshwater species (Zhang, 2004), which provides presumptive evidence that prolonged motility is essential in fishes with internal fertilization much like the situation observed in mammals (Fuller et al., 2002). Considering this unique reproductive mode, which has evolved in the family Poeciliidae from fishes with external fertilization (Reznick et al., 2002), it is possible that vigorous and prolonged sperm motility will lead to a higher probability of insemination success in X. helleri as approaches aimed at retaining prolonged motility after thawing have led to recent fertilization success (Tiersch et al., 2005). Sperm motility can be affected by the ionic composition of the diluent as previously reported (Erdahl and Graham, 1987; Erdahl et al., 1987; Goodall et al., 1989; Gatti et al., 1990; Billard and Cosson, 1992). Findings in the present study with changes in ionic composition of HBSS were similar to those obtained with the freshwater fish ayu, Plecoglossus altivelis, in which sperm incubated in sodium chloride alone lost motility rapidly, and potassium was found to be essential for acquisition of the potential for motility, while the absence of magnesium and calcium in artificial seminal plasma had no effect on sperm motility (Ohta et al., 2001). However, the response of sperm to ionic effects is species-specific in aquatic organisms (Dong et al., 2002). Magnesium was found to correlate with sperm motility for the seasonal variation in ocean pout (Wang and Crim, 1997), and calcium was required for initiation of motility in salmonids (Cosson et al., 1989; Morisawa and Morisawa, 1990; Billard and Cosson, 1992). A high concentration of potassium was reported to inhibit sperm motility in salmonids (Morisawa et al., 1983; Billard et al., 1995), but not in other aquatic species such as ayu (Ohta et al., 2001), and Pacific oyster, Crassostrea gigas (Faure et al., 1995).
Sugars have been used extensively as supplements to extenders to improve sperm motility by serving as exogenous energy substrates, or effective cryoprotectants, or both. Glucose is one of the most widely used monosaccharide in extenders for mammalian sperm (e.g., Tris-glucose-egg yolk) and aquatic species (e.g., HBSS), and for example has been used to cryopreserve sperm of yellowfin seabream, Acanthopagrus latus (Gwo, 1994), cobia, Rachycentron canadum (Caylor et al., 1994), burbot, Lota lota (Lahnsteiner et al., 2002), and various salmonid species (Stoss and Reftsie, 1983; McNiven et al., 1993; Piironen, 1993; Gwo et al., 1999; Babiak et al., 2001; Kusuda et al., 2005). The present study found that the presence of glucose in HBSS was essential for retention of higher and longer motility for sperm of X. helleri. However, glucose at concentrations of >2 g/L was not necessary to improve motility either for fresh or thawed samples, and the addition of glucose did not reduce the amount of glycerol necessary for satisfactory cryoprotection. The results of the present study agreed with those of a previous study (Huang et al., 2004c), in which a high concentration of glycerol (14%) retained higher and longer post-thaw motility. The same result was also obtained in another study with comparisons among 5, 10, and 15% glycerol suspended in isotonic and hypertonic HBSS for sperm of X. helleri (Yang et al., 2006). Glucose at a concentration of 25 g/L was found to negatively affect sperm motility, which could be due to the increased osmolality or viscosity at this concentration. Although sperm of species with internal fertilization were found to metabolize sugars to sustain prolonged viability within the female (Gardiner, 1978), sperm of X. helleri requires a minimal supplementation of glucose, which was in agreement with previous findings that sperm of this species could retain motility for as long as 8 days after thawing when stored at 4 °C in HBSS 300 prepared with 1 g/L glucose (Huang et al., 2004c).
Fetal bovine serum is routinely used in cell and tissue culture, and has been used in sperm cryopreservation of milkfish Chanos chanos (Hara et al., 1982), medaka Oryzias latipes (Aoki et al., 1997), and Sakhalin taimen Hucho perryi (Kusuda et al., 2005). In the present study, 14% glycerol solutions with the addition of 20% FBS were found to increase the post-thaw motility of sperm from X. helleri compared to the control treatments with 14% glycerol alone. Interestingly, the addition of 20% FBS in our secondary trial led to the formation of sperm bundles of thawed samples after centrifugation. These bundles, although in irregular shape and size, were capable of forward movement. In addition, similar sperm bundles were also formed in concentrated (~109 cells/ ml) fresh samples without FBS (viewed at 200× magnification). It is possible that some kind of affinity occurs when sperm are in close contact, or with the aid of FBS.
Formation of sperm bundles, either encapsulated (spermatophore) orun-encapsulated (spermatozeugmata), has been related to the evolutional adaptations for fishes with internal fertilization for the purpose of efficiently transferring spermatozoa in an aqueous medium as in the members of the Atheriniformes (including the Poeciliidae) (Grier, 1981) and the Glandulocaudinae (Burns et al., 1995). Therefore, it is possible that this bundling of sperm serves some purpose within the female reproductive tract under natural insemination conditions. In fact, sperm in some members of Glandulocaudinae have been found to occur as sperm bundles within the ovarian cavity in females (Burns et al., 1995). It is possible that fertilization success in Xiphophorus may respond to the maintenance or formation of bundles, and consequently with the aid of FBS, or by concentrating the sperm, the reconstituted bundles formed in cryopreserved samples may facilitate insemination. It is still unclear how the sperm bundles form in response to FBS addition or concentrated sperm density, and whether these bundles would be beneficial for internal fertilization. In the future, examination of the fate of cryopreserved sperm in the female reproductive tract may reveal factors important for improving fertilization success.
In summary, centrifugation can be used to concentrate sperm density and to remove cryoprotectant for thawed samples without compromising motility. The ionic composition of HBSS was sufficient to retain sperm motility for fresh samples or thawed samples cryopreserved with 14% glycerol. The presence of glucose at 1 g/L in HBSS was essential to retain higher and longer motility for fresh and thawed samples, but additions of glucose at greater than 2 g/L were unnecessary. The addition of 20% FBS prior to freezing was found to increase the post-thaw motility significantly compared to samples with 14% glycerol alone, and high concentrations (>20%) of FBS induced formation of sperm bundles. Post-thaw amendment of cryopreserved sperm (obtained from crushing of testis) through centrifugation to concentrate the cells and remove cryoprotectant is a feasible method for use with artificial insemination of Xiphophorus females and may be of benefit for use with other viviparous fishes. The addition of FBS to facilitate reformation of sperm bundles also merits future study.
Acknowledgments
We thank L. Hazlewood, R. Bowers, and R. Walter at the Xiphophorus Genetic Stock Center for the assistance and provision of fish. This work was supported by the USPHS grants, P40-RR-17072 from the National Center for Research Resources, and additional support provided by the U.S. Department of Agriculture, and the Louisiana Sea Grant College Program. This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 06-11-0253.
References
- Aoki K, Okamoto K, Tatsumi K, Ishikawa Y. Cryopreservation of medaka spermatozoa. Zool. Sci. 1997;14:641–644. [Google Scholar]
- Babiak I, Glogowski J, Goryczko K, Dobosz S, Kuzminski H, Strzezek J, Demianowicz W. Effect of extender composition and equilibration time on fertilization ability and enzymatic activity of rainbow trout cryopreserved spermatozoa. Theriogenology. 2001;56:177–192. doi: 10.1016/s0093-691x(01)00553-2. [DOI] [PubMed] [Google Scholar]
- Billard R. Spermatogenesis and spermatology of some teleost fish species. Reprod. Nutr. Dev. 1986;26:877–920. [Google Scholar]
- Billard R. Artificial insemination and gamete management in fish. Mar. Behav. Physiol. 1988;14:3–21. [Google Scholar]
- Billard R, Cosson MP. Some problems related to the assessment of sperm motility in freshwater fish. J. Exp. Zool. 1992;261:122–131. [Google Scholar]
- Billard R, Cosson J, Crim LW, Suquet M. Sperm physiology and quality. In: Bromage NR, Roberts RJ, editors. Broodstock Management and Egg and Larval Quality. Cambridge University Press; Cambridge: 1995. pp. 25–52. [Google Scholar]
- Burns JR, Weitzman SH, Grier HJ, Menezes NA. Internal fertilization, testis and sperm morphology in Glandulocaudine fishes (Teleostei: Characidae: Glandulocaudinae) J. Morph. 1995;224:131–145. doi: 10.1002/jmor.1052240203. [DOI] [PubMed] [Google Scholar]
- Caylor RE, Biesiot PM, Franks JS. Culture of cobia (Rachycentron canadum): cryopreservation of sperm and induced spawning. Aquaculture. 1994;125:81–92. [Google Scholar]
- Chauvaud L, Cosson J, Suquet M, Billard R. Sperm motility in turbot, Scophthalmus maximus: initiation of movement and changes with time of swimming characteristics. Environ. Biol. Fishes. 1995;43:341–349. [Google Scholar]
- Cosson MP, Billard R, Letellier L. Rise of internal Ca2+ accompanies the initiation of trout sperm motility. Cell Motil. Cytoskelet. 1989;14:424–434. [Google Scholar]
- Dong Q, Eudeline B, Allen SK, Jr, Tiersch TR. Factors affecting sperm motility of tetraploid Pacific oysters. J. Shellfish Res. 2002;21:719–723. [Google Scholar]
- Dong Q, Huang C, Hazlewood L, Walter RB, Tiersch TR. Attempted artificial insemination using cryopreserved sperm of live-bearing fishes (genus Xiphophorus); 42nd Meeting of the Society for Cryobiology; 24–27 July; Minneapolis, USA. 2005. abstract only. [Google Scholar]
- Erdahl AW, Graham EF. Fertility of teleost semen as affected by dilution and storage in a seminal plasma-mimicking medium. Aquaculture. 1987;60:311–321. [Google Scholar]
- Erdahl AW, Cloud JG, Graham EF. Fertility of rainbow trout (Salmo gairdneri) gametes: gamete viability in artificial media. Aquaculture. 1987;60:323–332. [Google Scholar]
- Faure C, Devauchelle N, Girard JP, Cossson J. Gametes as physiological references and parameters of sperm activation in scallop Pecten maximus and Japanese oysters Crassostrea gigas. The Natural and Controlled Reproduction of Cultivated Bivalves in France: Symposium Report IFREMER/Nantes/France. 1995:61–67. [Google Scholar]
- Fuller B, Paynter SJ, Watson P. Cryopreservation of human gametes and embryos. In: Fuller BJ, Lane N, Benson EE, editors. Life in the Frozen State. CRC press; Boca Raton: 2002. pp. 505–539. [Google Scholar]
- Hara S, Canto JT, Almendras JME. A comparative study of various extenders for milkfish, Chanos chanos (Forsskal), sperm preservation. Aquaculture. 1982;28:339–346. [Google Scholar]
- He S, Woods C. Changes in motility, ultrastructure, and fertilization capacity of striped bass Morone saxatilis spermatozoa following cryopreservation. Aquaculture. 2004;236:677–686. [Google Scholar]
- Huang C, Dong Q, Tiersch TR. Sperm cryopreservation of a live-bearing fish, the platyfish Xiphophorus couchianus. Theriogenology. 2004a;62:971–989. doi: 10.1016/j.theriogenology.2003.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Dong Q, Walter RB, Tiersch TR. Initial studies on sperm cryopreservation of a live-bearing fish, the green swordtail Xiphophorus helleri. Theriogenology. 2004b;62:179–194. doi: 10.1016/j.theriogenology.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Dong Q, Walter RB, Tiersch TR. Sperm cryopreservation of green swordtail Xiphophorus helleri, a fish with internal fertilization. Cryobiology. 2004c;48:295–308. doi: 10.1016/j.cryobiol.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner DM. Utilization of extracellular glucose by spermatozoa of two viviparous fishes. Comp. Biochem. Physiol. 1978;59A:165–168. [Google Scholar]
- Gatti JL, Billard R, Christen R. Ionic regulation of the plasma membrane potential of rainbow trout (Salmo gairdneri) spermatozoa: role in the initiation of sperm motility. J. Cell. Physiol. 1990;143:546–554. doi: 10.1002/jcp.1041430320. [DOI] [PubMed] [Google Scholar]
- Goodall JA, Blackshaw AW, Carpa MF. Factors affecting the activation and duration of motility of the spermatozoa of the summer whiting (Sillago ciliate) Aquaculture. 1989;77:243–250. [Google Scholar]
- Graham J. Assessment of sperm quality: a flow cytometric approach. Anim. Reprod. Sci. 2001;68:239–247. doi: 10.1016/s0378-4320(01)00160-9. [DOI] [PubMed] [Google Scholar]
- Grier HJ. Cellular organization of the testis and spermatogen-esis in fishes. Am. Zool. 1981;21:345–357. [Google Scholar]
- Gwo JC. Cryopreservation of yellowfin seabream (Acanthopagrus latus) spermatozoa (teleost, perciforme, Sparidae) Theriogenology. 1994;41:989–1004. doi: 10.1016/s0093-691x(05)80022-6. [DOI] [PubMed] [Google Scholar]
- Gwo JC, Ohta H, Okuzawa K, Wu HC. Cryopreservation of sperm from the endangered Formosan landlocked salmon (Oncorynchus masou formosanus) Theriogenology. 1999;51:569–582. doi: 10.1016/s0093-691x(99)00011-4. [DOI] [PubMed] [Google Scholar]
- Jamieson BGM. Fish Evolution and Systematics: Evidence from Spermatozoa. Cambridge University Press; Cambridge: 1991. p. 295. [Google Scholar]
- Kerby JH. Cryogenic preservation of sperm from striped bass. Trans. Am. Fish. Soc. 1983;112:86–94. [Google Scholar]
- Kusuda S, Koide N, Kawamula H, Teranishi T, Nakajima J, Yamaha E, Arai K, Ohta H. Cryopreservation diluents for spermatozoa of Sakhalin taimen Hucho perryi. Fish. Sci. 2005;71:293–298. [Google Scholar]
- Lahnsteiner F, Berger B, Weismann T, Patzner RA. Changes in morphology, physiology, metabolism, and fertilization capacity of rainbow trout semen following cryopreservation. Prog. Fish-Cult. 1996;58:149–159. [Google Scholar]
- Lahnsteiner F, Mansour N, Weismann T. The cryopreservation of spermatozoa of the burbot, Lota lota (Gadidae, Teleostei) Cryobiology. 2002;45:195–203. doi: 10.1016/s0011-2240(02)00140-2. [DOI] [PubMed] [Google Scholar]
- Leung LK-P, Jamieson BGM. Fish Evolution and Systematics: Evidence From Spermatozoa. Cambridge University Press; Cambridge: 1991. Live preservation of fish gametes; pp. 231–244. [Google Scholar]
- McNiven MA, Gallant RK, Richardson GF. Dimethyl-acetamide as a cryoprotectant for rainbow trout spermatozoa. Theriogenology. 1993;40:943–948. doi: 10.1016/0093-691x(93)90362-9. [DOI] [PubMed] [Google Scholar]
- Morisawa M, Morisawa S. Acquisition and initiation of sperm motility. In: Gagnon C, editor. Controls of Sperm Motility: Biological and Clinical Aspects. CRC Press; Boca Raton: 1990. pp. 137–151. [Google Scholar]
- Morisawa M, Suzuki K, Morisawa S. Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. J. Exp. Biol. 1983;107:105–113. doi: 10.1242/jeb.107.1.105. [DOI] [PubMed] [Google Scholar]
- Nairn RS, Kazianis S, McEntire BB, Della CL, Walter RB, Morizot DC. A CDKN2-like polymorphism in Xiphophorus LG V is associated with UV-B-induced melanoma formation in platyfish–swordtail hybrids. Proc. Natl. Acad Sci. U. S. A. 1996:13042–13047. doi: 10.1073/pnas.93.23.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Unuma T, Tsuji M, Yoshioka M, Kashiwagi M. Effects of bicarbonate ions and pH on acquisition and maintenance of potential for motility in ayu, Plecoglossus altivelis Temminck et Schlegel (Osmeridae), spermatozoa. Aquac. Res. 2001;32:385–392. [Google Scholar]
- Piironen J. Cryopreservation of sperm from the brown trout (Salmo trutta m. lacustris L.) and Arctic charr (Salvelinus alpinus L.) Aquaculture. 1993;116:275–285. [Google Scholar]
- Rana KJ. Preservation of Gametes. In: Bromage NR, Roberts RJ, editors. Broodstock Management and Egg and Larval Quality. Cambridge University Press; Cambridge: 1995. pp. 53–75. [Google Scholar]
- Reznick DN, Mateos M, Springer MS. Independent origins and rapid evolution of the placenta in the fish genus Poeciliopsis. Science. 2002;298:1018–1020. doi: 10.1126/science.1076018. [DOI] [PubMed] [Google Scholar]
- Schartl M. Platyfish and swordtails: A genetic system for the analysis of molecular mechanisms in tumor formation. Trends Genet. 1995;11:185–189. doi: 10.1016/S0168-9525(00)89041-1. [DOI] [PubMed] [Google Scholar]
- Stoss J. Fish gamete preservation and spermatozoan physiology. In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish Physiology, vol. 9, Part B, Behavior and Fertility Control. Academic Press; San Diego: 1983. pp. 305–350. [Google Scholar]
- Stoss J, Reftsie T. Short term storage and cryopreservation of milt from Atlantic salmon and sea trout. Aquaculture. 1983;30:229–236. [Google Scholar]
- Tamaru CS, Cole B, Bailey R, Brown C, Ako H. A manual for commercial production of the swordtail, Xiphophorus helleri. Honolulu: CTSA Publication Number; 2001. p. 128. http://www.soest.hawaii.edu/SEAGRANT. [Google Scholar]
- Tiersch TR. Introduction. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge: 2000. pp. xix–xxvi. [Google Scholar]
- Tiersch TR. Cryopreservation in aquarium fishes. Mar. Biotechnol. 2001;3:212–223. doi: 10.1007/s10126001-0044-z. [DOI] [PubMed] [Google Scholar]
- Tiersch TR, Yang H, Dong Q, Huang C, Hazlewood L, Walter RB. Artificial insemination using cryopreserved sperm of live-bearing fishes (genus Xiphophorus); Aquatic Animals of Human Disease Conference; October 30; Georgia, USA. 2005. abstract only. [Google Scholar]
- Walter RB, Kazianis S. Xiphophorus interspecies hybrids as genetic models of induced neoplasia. J. Inst. Lab Anim. Res. 2001;42:299–322. doi: 10.1093/ilar.42.4.299. [DOI] [PubMed] [Google Scholar]
- Wang Z, Crim LW. Seasonal changes in biochemistry of seminal plasma and sperm motility in the ocean pout, Macrozoarces americanus. Fish Physiol. Biochem. 1997;16:77–83. [Google Scholar]
- Warnecke D, Pluta HJ. Motility and fertilizing capacity of frozen/thawed common carp (Cyprinus carpio L.) sperm using dimethyl-acetamide as the main cryoprotectant. Aquaculture. 2003;215:167–185. [Google Scholar]
- Yang H, Hazlewood L, Walter RB, Tiersch TR. Effect of osmotic immobilization on refrigerated storage and cryopreservation of sperm from a viviparous fish, the green swordtail Xiphophorus helleri. Cryobiology. 2006;52:209–218. doi: 10.1016/j.cryobiol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Richardson GF, Crim LW. A diluent for prolonged motility of ocean pout (Macrozoarces americanus L.) sperm. Aquaculture. 1999;174:183–193. [Google Scholar]
- Zhang T. Cryopreservation of gametes and embryos of aquatic species. In: Fuller BJ, Lane N, Benson EE, editors. Life in the Frozen State. CRC press; Boca Raton: 2004. pp. 415–436. [Google Scholar]