Abstract
Background. Lower breast density (BD) is associated with lower risk of breast cancer and may serve as a biomarker for the efficacy of chemopreventive strategies. This review explores parameters that are thought to be associated with lower BD. We conducted a systematic review of articles published to date using the PRISMA strategy. Articles that assessed change in BD with estrogen-receptor modulators (tamoxifene [TAM], raloxifene [RLX], and tibolone) and aromatase inhibitors (AIs), as well as cross-sectional and longitudinal studies (LSs) that assessed association between BD and physical activity (PA) or diet were reviewed. Results. Ten studies assessed change in BD with TAM; all reported TAM-mediated BD decreases. Change in BD with RLX was assessed by 11 studies; 3 reported a reduction in BD. Effect of tibolone was assessed by 5 RCTs; only 1 reported change in BD. AI-mediated BD reduction was reported by 3 out of 10 studies. The association between PA and BD was assessed by 21 studies; 4 reported an inverse association. The relationship between diet and BD was assessed in 34 studies. All studies on calcium and vitamin D as well as vegetable intake reported an inverse association with BD in premenopausal women. Two RCTs demonstrated BD reduction with a low-fat, high-carbohydrate intervention. Conclusion. TAM induces BD reduction; however, the effect of RLX, tibolone, and AIs on BD is unclear. Although data on association between diet and BD in adulthood are contradictory, intake of vegetables, vitamin D, and calcium appear to be associated with lower BD in premenopausal women.
Keywords: tamoxifene, raloxifene, tibolone, letrozole, anastrozole, exercise
Introduction
Breast cancer (BC) is the most frequently diagnosed female cancer, and the second leading cause of deaths related to cancer in women worldwide.1 Risk factors such as aging, breast density (BD), lifestyle, and genetic parameters have all been implicated in breast carcinogenesis.2,3 Of these risk factors, BD has been shown to be very significant, especially in younger women.4 Established BC risk factors associated with genetics and lifestyle are also confounders for BD through hormonal and genetic pathways and modify the relationship between BD and BC risk.5 Breast density refers to the proportion of the breast that is composed of fibroglandular tissue and is represented by the radiopaque areas on a mammogram.6,7 Although BD is regarded as a strong risk factor for BC, it is still contentious whether it is an independent risk factor or whether it merely reflects opportunities for cancer to develop. Regardless of these contentions, high mammographic BD (MBD) has been shown to be associated with BC risk and interval cancer.4 Importantly, BD is regarded as a modifiable risk factor for BC8,9 and, therefore, may be an important biomarker for the effect of interventions on BC risk.
Identifying the relationship between MBD and interventions that modify BD requires reliable and reproducible methods for MBD assessment. Currently, area-based and volumetric approaches exist for MBD assessment.6,7 The qualitative area-based methods classify MBD into different categories based on subjective opinion, using features such as area covered by dense tissue and ductal prominence. They include the Wolfe, Boyd and Tabẚr methods, along with the Visual Analogue Scale and breast imaging reporting and data system (BI-RADS).6,7 Semiautomated area-based methods such as planimetry, Cumulus, and Madena use thresholding and segmentation techniques to measure percentage mammographic density (PMD). Automated area-based methods use thresholding and/or statistical modeling to estimate PMD and include texture-based approaches, Autodensity, and MedDensity.6 Volumetric approaches use statistical or physical modeling to calculate volumetric BD (VBD). Volumetric approaches include calibration techniques, dual-energy X-ray absorptiometry, Cumulus V, and 3 physics model–based volumetric techniques: Standard Mammographic Form, Volpara, and Quantra.6,7
Many studies have attempted to assess the relationship between BD and specific clinical interventions such as estrogen-receptor modulators,8-11 aromatase inhibitors (AIs),12-17 physical activity (PA),18-23 and diet.24-31 However, the nature and magnitude of the relationship between BD and these interventions is unclear. Furthermore, the category of women (age, ethnicity, body mass index [BMI], and menopausal status) in which these parameters are more effective is unclear. This lack of clarity underscores the need for a review of interventions that are thought to have an impact on BD, given the role of BD as an intermediate and potentially modifiable risk factor for BC.5,8,9 Increasingly, BD notification legislations have been passed in 22 states in the United States,32 and BD details are being made available to screened women. The benefit of BD notification to women will only be accrued when such data are accompanied with clear information about BD and cancer as well as information about parameters that are associated with lower BD and cancer risk. Therefore, this review examines the effect of estrogen-receptor modulators such as tamoxifene (TAM), raloxifene (RLX), and tibolone as well as AIs such as letrozole, anastrozole, and exemestane on BD. It also explores the association between BD and parameters such as PA and diet.
Materials and Methods
Search Strategy
The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) strategy was used to search for articles published to date using MEDLINE, EMBASE, CINAHL (Ebscohost), PubMed, Cochrane library, Web of Science, and Scopus databases. We also conducted a Google search, and reference lists of published articles were examined to identify additional articles not found in the database search. The search was conducted in the English language. To systematically search for literature of interest, a combination of search terms was used; these were thematically related to our hypothesis and also common themes identified through preliminary search of the literature. These were “breast density reduction interventions,” “breast density modifiers,” “breast density and estrogen-receptor modulators,” “breast density and tamoxifene,” “breast density and raloxifene,” “breast density and tibolone,” “breast density and letrozole,” “breast density and anastrozole,” “breast density and exemestane,” “ breast density and aromatase inhibitors,” “breast density and physical activity,” “exercise and breast density,” “breast density and diet.”
Inclusion Criteria
Articles were included if they were randomized controlled trials (RCTs), case-control studies (CCSs), or cohort studies (CSs) that investigated change in BD with interventions. Longitudinal studies (LSs) and cross-sectional studies (CSSs) that assessed association between BD and parameters such as PA and diet were also included. Articles were also included if they were published in the English language. Articles that did not fulfill the above criteria were excluded, as were reviews and case reports.
Data Synthesis
Data extraction was performed independently by 2 reviewers, with differences of opinion resolved by discussion. No article needed to be excluded for reasons of differences of opinion between reviewers; however, had consensus not been reached, articles would have been excluded. For each study, reviewers extracted information using the Participant Intervention Comparator and Outcomes (PICOS) method (Table 1). Studies that assessed BD from RCTs or were a subset of RCTs were considered RCTs in the current review. Studies were qualitatively assessed for quality and risk of bias based on study-specific design (clarity of protocol, assessment and report of compliance, blinding of outcome assessors, and outcome measures); this enabled us to appraise the conduct of each study. Table 1 shows eligibility criteria for inclusion of studies.
Table 1.
Eligibility Criteria for Inclusion of Studies.
| Characteristics | Criteria |
|---|---|
| Study year | Studies published to November 2014 |
| Study design | 1. Randomized controlled trials |
| 2. Case-control studies | |
| 3. Nested case-control studies | |
| 4. Cohort studies | |
| 5. Cross-sectional studies | |
| 6. Longitudinal studies | |
| Population | Women of all ages |
| Intervention | 1. Estrogen-receptor modulators |
| 2. Aromatase inhibitors | |
| 3. Physical activity | |
| 4. Diet | |
| Comparator | Relationship between interventions and breast density |
| Outcomes | Mammographic breast density |
Results
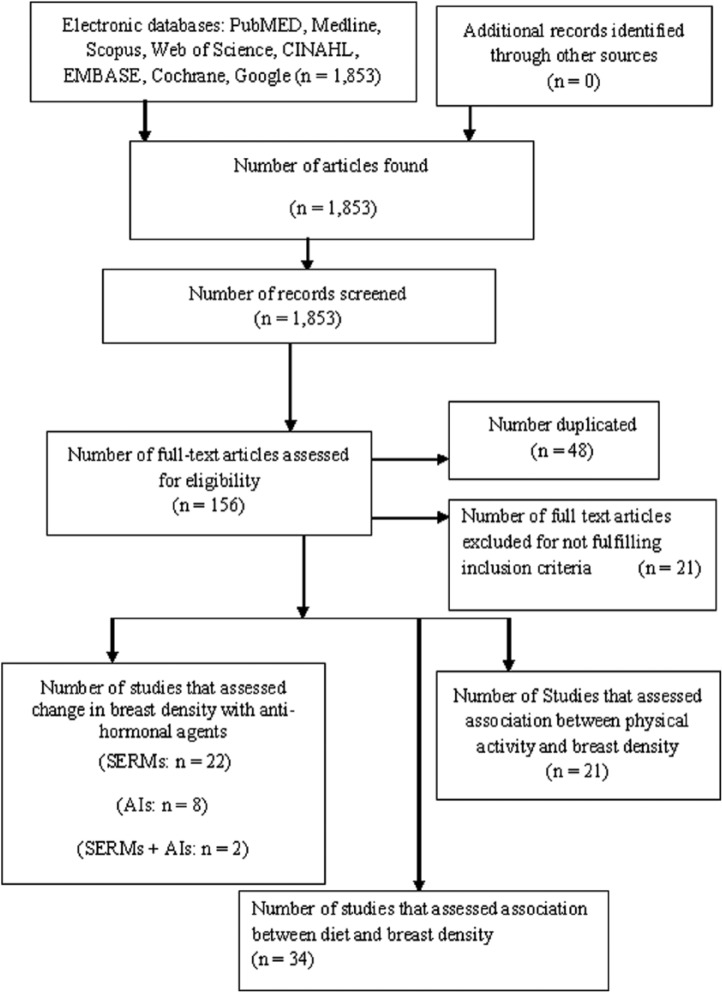
The search strategy identified 1853 publications, from which 156 eligible articles were found. Of these, 48 were duplicates, and 21 did not fulfil the inclusion criteria and were excluded, resulting in 87 articles that fulfilled the inclusion criteria. Of the articles that fulfilled inclusion criteria, 22 were on selective estrogen-receptor modulators (SERMs), 2 were on SERMs and AIs, 8 were on AIs alone, 21 were on PA, and 34 were on diet (Figure 1).
Figure 1.
Chart of studies identified on search.
Abbreviations: AI, aromatase inhibitor; SERMs, selective estrogen-receptor modulators.
Of the studies on estrogen-receptor modulators, 10 assessed change in BD with TAM intervention and included 1 RCT,10 2 nested CCSs,8,9 1 CS,11 and 6 post hoc analyses of RCTs.33-38 The sample sizes of the 10 studies ranged from 16 to 1065 (n = 2877). Among the studies, 7 included premenopausal and postmenopausal women, with heterogeneity in criteria for ascertaining menopausal status. Most of the studies administered 20 mg of TAM per day, and total duration of TAM administration varied from 1.5 to 6 years. Also, 7 studies used area-based qualitative and/or quantitative approaches for MBD assessment, and 1 assessed fibroglandular volume with MRI. Only 4 studies adjusted for confounding factors that affect BD.8-10,35 All studies reported TAM-mediated BD decreases (Table 2).
Table 2.
Characteristics of Studies on the Effects of Estrogen-Receptor Modulators and Aromatase Inhibitors on BD.
| Author, Year | Study Design | Study Population (n) | BD Assessment Age | Type of Intervention | Intervention Assessment | Outcome | Major Significant Result | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Brisson et al,10 2000 | RCT | BCPT; cases: n = 36; controls: n = 33 (Canada) | ≥35 years | Tamoxifen | 20 mg/d for 5 years | Wolfe’s parenchymal pattern and PMD | Mean PMD reduction ● 1.0-3.4 years = −6.9 ± 11.1 ● 3.5-5.0 years = −10.9 ± 12.4 Overall PMD reduction ● TAM: −9.4% ● Placebo: −3.6% P < .01 |
Number of first-degree relatives with BC, AH, nulliparity, age at first live birth, number of breast biopsies, and age at menarche |
| Chen et al,11 2011 | CS | ER+ BC patients treated with TAM as AHT; n = 16 (Taiwan) | 33-51 years | Tamoxifen | 20 mg Oral tablet per 8-26 months | %FV, PMD (MRI: computer-assisted algorithm) | BD reduction after 17 months ● %BD = 5.8% %FV = 8.2% |
Age. |
| Cigler et al,14 2010 | RCT | NCIC CTG; cases: n = 44; controls: n = 23 (Canada, United States) | >55 years | Aromatase inhibitor | Letrozole 2.5 mg/d for 12-24 months | PMD (Cumulus 5, BI-RADS) | Null | BC Hx, age, menopausal status |
| Valdivia et al,52 2004 | RCT | BC negative women; cases: n = 18; controls: n = 19 (Chile) | <65 years Menopausal women | Tibolone | 2.5 mg/d for 1 year | Mean BD (BI-RADS) | Mean BD reduction: from 2.22 to 1.67 (0.84) | Nil |
| Cuzick et al,8 2011 | Nested CCS | IBIS-I; cases: n = 123; controls: n = 942 (United Kingdom) | 30-70 years | Tamoxifen | 20 mg/d for 5 years | PMD (Boyd’s SCC, Cumulus) | Selective BD reduction; 63% BC risk reduction for women with 10% reduction in BD | History of atypical hyperplasia or LCIS |
| Cuzick et al,9 2004 | Nested CCS | IBIS-I; cases: n = 388; controls: n = 430 (United Kingdom) | 35-70 years | Tamoxifen | 20 mg/d For 5 years | PMD (Boyd’s method, visual assessment) | BD reduction: 7.9% (95% CI = 6.9% to 8.9%); 28.2% BD decrease from baseline | Age, menopausal status, BMI, and previous AH, smoking status |
| Eng-Wong et al,40 2008 | RCT | Phase 11 trial of raloxifene; n = 27 (United States) | Premenopausal, 35-47 years | Raloxifene | Raloxifene 60 mg/d for 2 years | PMD; MRIV (thresholding technique) | ● PMD: null MRIV: −17% (95% CI: −28 to −9; P = .0017) |
Age, BMI, duration |
| Eilertsen et al,49 2008 | RCT | RET; n = 177 (Norway) | 45-65 years | Raloxifene + tibolone | 60 mg Raloxifene, 2.5 mg tibolone | VBD (automated physics-based volumetric method) | VBD changes ● Raloxifene = −4.1; P < .0001 Tibolone = 0.7; P ≤ .002 |
BMI, menopausal status, age, smoking status, age at menopause, blood pressure |
| Freedman et al,41 2001 | RCT | OPT; n = 168 (United States) | 45-60 years | Raloxifene | 60 mg/d Or 150 mg/d 3 months for 2 years | PMD (computer-assisted technique) | BD decreases ● For 60 mg/d, 13.3%; P = .002 For 150 mg/d, 19.0%; P < .001 |
Age, years since menopause, BMI, previous HRT, alcohol, smoking, baseline BD |
| Jackson et al,44 2003 | RCT | ccHT: n = 84; raloxifene: n =109 (Latin America) | ≥60 years | Raloxifene | 60 mg/d For 1 year | Mean BD (BI-RADS) | Null | Weight, race, age, smoking status, HRT use, menopausal status |
| Lasco et al,45 2006 | CCS | Postmenopausal women with BMI 24.7 ± 2.8 kg/m2; n = 70 (Italy) | 52.4 ± 4.1 years | Raloxifene | 60 mg/d orally for 2 years | IMI (Image Pro-Plus ad hoc software) | Null | BMI, menopausal status |
| Lundstrom et al,50 2002 | RCT | Healthy women; n = 166 (Sweden) | 50-70 years | Tibolone | 2.5 mg/d For 1 year | Wolfe BD patterns, PMD (visual) | Null | Nil |
| Lundstrom et al,51 2011 | RCT | Healthy women; n = 154 (Sweden) | 50-70 years | Tibolone | 2.5 mg/d For 1 year | PMD (Cumulus) | Null | BMI, years since menopause |
| Vachon et al,55 2007 | RCT | NCIC CTG; n = 104 (United States) | Not specified | Aromatase inhibitor | Letrozole 2.5 mg/d for 1 year | PMD (Cumulus) | Null | Age, BMI, nodal status, number of tumors, time on TAM |
| Harvey et al,43 2013 | RCT | n = 507 (United States) | 55.2-56.3 years | Raloxifene | 60 mg/d for 2years | PMD, Cumulus | Mean BD change: −0.23; 95%CI: −0.54 to 0.08 | Age, BMI, years since menopause |
| Harvey et al,42 2009 | RCT | n = 444 (United States) | ≤62 years | Raloxifene | 60 mg/d For 2 years | PMD (Cumulus) | Mean BD change: −0.4% | Age, BMI, years since menopause |
| Henry et al,15 2013 | RCT | ELPh; n = 273 (United States) | Postmenopausal | Aromatase inhibitor | Letrozole 2.5 mg/d; exemestane 25 mg/d for 2 years | PMD (BI-RADS, MDEST) | PMD decrease from 17.1% to 15.1% (P < .001) | Age, BMI, HRT, prior chemotherapy |
| Mousa et al,54 2008 | CS | n = 46 (Canada) | Postmenopausal | Aromatase inhibitor | Letrozole 2.5 mg 3 times/wk for 2 years | PMD (BI-RADS and ImageQuant) | PMD decrease: P < .05 | Age, BMI, age at menopause, HRT, mammogram interval |
| Vachon et al,56 2013 | CCS | NCIC CTG; n = 387 (United States) | Postmenopausal | Aromatase inhibitor | Anastrozole 1 mg/d; exemestane 25 mg/d for 1 year | PMD (Cumulus) | Null | BMI, age at baseline mammogram, HRT, chemotherapy |
| Cigler et al,13 2011 | RCT | NCIC CTG; n = 98 (United States) | Postmenopausal >50 years | Aromatase inhibitor | Exemestane 25 mg/d for 1 year | PMD (Cumulus, BI-RADS, Boyd’s) | Null | Age, BMI, Hx of benign breast disease, Hx of breast cancer |
| Fabian et al,53 2007 | RCT | BCPT; n = 42 (United States) | Postmenopausal >50 years | Aromatase inhibitor | Letrozole 2.5 mg/d for 6 months | PMD (Cumulus) | Null | Nil |
| Kim et al,99 2012 | CS | n = 1065 (Korea) | 24-77 years) | Aromatase inhibitor | Anastrozole; letrozole for 5 years (dosage not specified) | PMD (Cumulus) | Mean PMD reduction = 5.9% (range, −17.2% to 36.9%). | Age, duration of therapy, nodal status |
| Prowell et al,16 2011 | RCT | n = 54 (United States) | Postmenopausal >60 years | Aromatase inhibitor | Anastrozole 1 mg/d for 1 year | PMD (Cumulus) | BD reduction: −16%, 95% CI: 30-2; P = .08 (null) | BMI, age, race, nodal status |
| Smith et al,17 2012 | RCT | LCHRWC; n = 16 (United States) | Postmenopausal ≥50 years | Aromatase inhibitor | Letrozole 2.5 mg /d for 1 year | PMD (Madena) | Statistically significant decrease in PMD; P = .04 | Nil |
| Silverio et al,47 2007 | RCT | n = 80 | Mean age 61.1 years | Raloxifene | 60 mg/d For 2 years | PMD; BI-RADS and computer-assisted | Null | Age, time of menopause, BMI parity, breastfeeding, HRT |
| Decensi et al,33 2009 | RCT | BC women; n = 235 (Italy) | Premenopausal | Tamoxifene | 5 mg/d For 2 years | PMD (Boyd’s and Cumulus) | 20% Reduction in PMD from baseline (P < .05) | Nil |
| Chow et al35 2000 | RCT | High-risk BC women; n = 32 (United States) | 36-74 years | Tamoxifene | 20 mg/d For 23 months | PMD (Wolfe’s, BI-RADS, Image J Semiquantitative) | Significant decrease in density (P < .005) | Age, menopausal status |
| Atkinson et al,34 1999 | RCT | n = 282 (United Kingdom) | 50-64 years | Tamoxifene | 20 mg/d For 2 years | PMD (Wolfe’s) | Significant decrease in density (P = .0001) | Nil |
| Son and Oh’38 1999 | RCT | n = 102 (United States) | 28-67 years | Tamoxifene | 20 mg/d For 2 years | Visual assessment | Significant decrease in density (P < .005) | Nil |
| Konez et al,36 2001 | RCT | n = 27 (United States, Canada) | 32-81 years | Tamoxifene | 20 mg/d For 5 years | PMD (visual assessment) | Minimal decrease in density | Nil |
| Meggiorini et al,37 2008 | RCT | n = 148 (Italy) | Mean age, 58.5 ± 9.3 years | Tamoxifene | 20 mg/d For >1 year | PMD (BI-RADS, Cumulus) | Significant decrease in density (P < .005) | Nil |
| Nielsen et al,46 2009 | Retroanalysis of RCT | n = 135 (Denmark) | 55-80 years | Raloxifene | 60 mg/d For 2 years | PMD (BI-RADS, computer analysis) | Null | BMI |
| Christodoulakos et al,48 2002 | PS | n = 131 (Greece) | 41-67 years | Tibolone and raloxifene | Tibolone: 2.5 mg/d; raloxifene: 60 mg/d for 1 year | PMD (Wolfe’s) | Null | Nil |
| Cirpan et al,39 2006 | Retroanalysis of RCT | n = 55 (Turkey) | Postmenopausal | Raloxifene | 60 mg/d For 16 months | PMD (BI-RADS) | Null | Nil |
Abbreviations: BD, breast density; RCT, randomized controlled trials; BCPT, Breast Cancer Prevention Trial; PMD, percentage mammographic density; TAM, tamoxifen; BC, breast cancer; AH, atypical hyperplasia; CS, cohort study; ER+, estrogen receptor positive; AHT, adjuvant hormonal therapy; FV, fibroglandular volume; MRI, magnetic resonance imaging; NCIC CTG, National Cancer Institute of Canada Clinical Trials Group; Null, non–statistically significant result; Hx, history; BI-RADS, Breast Imaging Reporting and Data System; CCS, case-control study; IBIS-I, International Breast Cancer Intervention Study; SCC, 6-category classification; LCIS, lobar carcinoma in situ; BMI, body mass index; RET, raloxifene estrogen tibolone; VBD, volumetric BD; OPT, osteoporosis prevention trial; HRT, hormone replacement therapy; ccHT, continuous-combined hormone therapy; IMI, image mean index. ELPh, Exemestane letrazole pharmacogenomics; MDEST, mammographic density estimator; CAD, computer-aided calculation; PS, prospective study; MRIV, magnetic resonance imaging volume; LCHRWC, lynne cohen high risk women’s clinic.
A total of 9 studies assessed change in BD with RLX alone,39-47 and 2 studies assessed tibolone and RLX intervention.48,49 The sample size ranged from 27 to 444 (n = 2005), and 8 of the 9 studies assessed postmenopausal women. Most of the studies administered 60 mg of RLX per day, and the duration of the RLX administration varied from 3 months to 3 years. Area-based methods were used for MBD assessment in a majority of the studies, and 1 study measured fibroglandular volume with MRI. There was a paucity of information on BD confounders in most of the studies. Of all RLX studies reviewed, only 3 reported a significant reduction in BD (Table 2).40,41,49
There were 5 RCTs that assessed change in BD with tibolone intervention.48-52 Ages of participants ranged from 41 to 70 years, and the sample size ranged from 37 to 177 (n = 665). Tibolone administration was 2.5 mg/d, and duration of administration was 1 year. Three studies performed subjective MBD assessment, 1 used Cumulus, and 1 performed VBD assessment. Only the study that measured VBD adjusted for confounders and reported a significant tibolone-mediated BD reduction.49
Changes in BD with AIs (letrozole, 2.5 mg/d; anastrozole, 1 mg/d; exemestane, 25 mg/d) were assessed in 10 studies.13-17,53-56 Four of the studies used letrozole alone; there was 1 study each on anastrozole and exemestane, and others combined 2 of the 3 AIs. Of these, 4 were prospective arm trials and 3 were RCTs. The sample size varied from 16 to 1065 (n = 2110), and the age of participants ranged from 24 to 77 years, with 80% of the studies involving postmenopausal women alone. The duration of administration ranged from 6 months to 2 years. Among the studies, 7 assessed MBD using area-based computer-assisted methods, and the remaining studies used an area-based subjective approach alone or in combination with area-based computer-assisted methods. There was little or no adjustment for BD confounding factors in most of the studies. Only 3 out of the 10 studies reported statistically significant reduction in BD with AIs (Table 2).15,17,54
The association between PA and BD was assessed in 21 studies.18-23,57-71 Of these, 71% were CSSs, and the sample size ranged from 95 to 2720 (n = 20 424). Association between PA and BD was evaluated within 5 years prior to date of mammographic examination in 71% of the studies, and 29% assessed this association more than 5 years prior to mammography date. In all, 5 studies investigated the association of childhood and adolescent PA with BD in adulthood. Also, 11 studies assessed nonoccupational PA; 7 assessed household, occupational, and recreational PA; and 2 assessed life-course PA (Table 3). Qualitative assessment of MBD was performed in 7 studies, and area-based quantitative approaches were used for PMD assessment in 14 studies. A significant percentage (81%) of the studies found no association between PA and BD22,23,57-71; 19% of the studies reported a statistically significant inverse association between PA and BD in perimenopausal and postmenopausal women with BMI >25 kg/m2.18-21
Table 3.
Characteristics of Studies on the Association Between PA and BD.
| Author, Year | Study Design | Population of Study (n) | Age of Participants | Intervention | Intervention Assessment | Outcome | Major Significant Result | Adjustments |
|---|---|---|---|---|---|---|---|---|
| Irwin et al,19 2007 | PCS | HEAL; n = 522 (United States) | ≥55 years | PA | Kaiser Physical Activity Survey (KPAS) questionnaires and interviews | DA, PMD (Cumulus 108) | Lower BD for BMI ≥30 kg/m2
● DA: P for trend =.036 ● PMD: P for trend =.0001 |
Age, BMI, race/ethnicity, study site, education, parity, TAM use, AT, RT, CT, RaCT, BC recurrence, smoking, HRT use |
| Marmara et al,20 2011 | CSS | GPCSP; n = 724 (Greece) | 45-67 years | PA | KPAS questionnaires and interviews | PMD (BI-RADS) | BD association ● All women: OR = −0.10; 95% CI = 0.018, −0.001 ● Older women: OR = −0.036; 95% CI = 0.063, 0.009 (P < .05) |
Age at menarche, age at menopause, BMI, number of live births |
| Masala et al,21 2009 | LS | EPIC-Florence; n = 2000 (Italy) | ≥50.48 years | PA | EPIC-lifestyle questionnaire | PMD (semiquantitative and Wolfe’s classification) | Lower PMD with highest BMI: OR = 0.34; 95% CI = 0.20-0.57 (P < .05) | BMI, PA, HRT, education, age, age at menarche, parity, menopausal status, age at first birth, number of children |
| Peters et al,60 2008 | CSS | EPIC-Norfolk; n = 1394 (United Kingdom) | 40-74 years | PA | Validated questionnaire for occupational and leisure time | PMD (visual assessment using Boyd method) | Null | BMI, age, parity, energy intake, HRT use, alcohol intake, education, smoking status, age at first birth |
| Qureshi et al,61 2012 | CSS | NBCSP (Hofvind 2007); n = 2218 (Norway) | 50-69 years | PA | Physical activity questionnaire | PMD (computer-assisted method, Madena) | Null | Age, BMI, HRT education, age at menarche, number of pregnancies, age at first birth |
| Reeves et al,62 2007 | CSS | MAMS; n = 728 (United States) | ≥18 years | PA | Interview questionnaire | PMD (polar planimeter) | Null | BMI, menopausal status |
| Siozon et al,71 2006 | CSS | CARE; n = 418 (United States) | 35-64 years | PA | Interview questionnaire | PMD (computer-assisted method, Madena) | Null | Ethnicity, age, age at menarche, age at first full-term pregnancy, BMI, menopausal and HRT use status, FH of BC, smoking, alcohol intake, education |
| Suijkerbuijk et al,65 2006 | CSS | Prospect- EPIC- Dutch; n = 620 (Netherlands) | 49-68 years | PA | Self-administered questionnaire | PMD (computer-assisted method) | Null | Age, education, BMI, waist-to-hip ratio, menopausal status, parity, and smoking |
| Wolin et al,22 2007 | CSS | CBHP; n = 95 (United States) | ≥40 years | PA | IPAQ | PMD (ImageJ) | Null | Age, smoking status, BMI |
| Woolcott et al,23 2010 | RCT | ALPHA ● Cases: n = 160 ● Controls: n = 160 (Canada) |
50-74, 1 years | Aerobic exercise | Aerobic exercise of 5 times per week for 45 minutes for 1 year | PMD, PDV (computer-assisted and volumetric software) | Null | BMI. |
| Conroy et al,57 2010 | LS | SWAN; n = 722 (United States) | 42-52 years | PA | KPAS questionnaires and interviews | TBA, ADBT (polar planimeter) | Null | Ethnicity, height, HRT, BMI, education, age at menarche, parity, age, age at first birth, FH of BC, weight, smoking, menopausal status |
| Gram et al,58 1999 | CSS | Tromsø 11 and 111; n = 2720 (Norway) | 40-56 years | PA | Self-administered questionnaire | Tabăr | OR for lower density ● Postmenopausal: OR = 1.3; 95% CI = 0.4-4.2 ● Premenopausal: OR = 0.8; 95% CI = 0.4-1.5); P > .05 |
Age, education, number of children, BMI, age at menarche, alcohol intake, OC use, and menopausal status |
| Oestreicher et al,59 2008 | CSS | SWAN; n = 772 (United States) | 40-50 year and older | PA | KPAS | PMD and TDA | ● PMD: β = −2.62; 95% CI = −5.84 to 0.60 ● TDA: β = −4.75; CI = −10.40 to 0.88 (P > .05) |
Race/ethnicity, menopausal status, parity, past use of hormones, waist circumference, education, and BMI |
| Samimi et al,63 2008 | CSS | NuHS; n = 1398 (United States) | 42-78 years | PA | Self-administered questionnaire | PMD (Cumulus) | Null | Age, BMI, parity, smoking, alcohol use, Hx BC, Hx benign breast disease, menopausal status |
| Lopez et al,67 2003 | CSS | CBHP; n = 294 (United States) | 40-59 years | PA | Interviews and questionnaire | PMD (Image-J) | Null | Age, BMI, parity, smoking, education, HRT, number of live births, physical inactivity |
| Jeffreys et al,66 2004 | CSS | GAC; n = 628 (United Kingdom) | 55.1-68.3 years at screening | PA | Posted questionnaires | Boyd SCC | Null | Age at menarche, birth weight, oral, OC use, height, leg length, BMI, exercise at age 20, smoking, age at first birth |
| Vachon et al,69 2000 | CSS | MBCFS; n = 1900 (United States) | 20-80+ years | PA | Telephone interview | PMD (subjective assessment) | Null | Age at first birth, age at menarche, BMI, alcohol, WHR, menopausal status |
| Tseng et al,68 2011 | CSS | Chinese immigrants; n = 201 (United States) | Mean age 53.1 (10.2) years | PA | Self- administered questionnaire | PMD (BI-RADS) | Null | Age, menopausal status, acculturation, BMI, first-degree BC relative, number of live births, age at first live birth, adult dairy food intake |
| Sellers at al,64 2007 | CSS | MBCFS; n = 1893 (United States) | PA | Self- administered questionnaire | PMD (Cumulus) | Null | Age, age at menarche, HRT, height, weight, adiposity, diet | |
| Sala et al,70 2000 | CCS | EPIC-Norfolk; n = 400 (United Kingdom) | Not specified | PA | Self- administered EPIC Health and Lifestyle questionnaire | PMD (Wolfe’s classification) | OR = 0.93; 95% CI = 0.75-1.50 | BMI, OC use, smoking, Hx of BC, Hx of benign breast disease, HRT, menopausal status, age at menarche, age at first birth, number of children, hysterectomy, breastfeeding |
| Irwin et al,18 2006 | PCS | HEAL; n = 474 (United States) | Not specified | PA | Self- administered Modifiable Activity questionnaire | DA and PMD (Cumulus) | ● DA: P for trend =.046 ● PMD: P for trend =.026 ● Premenopausal BMI <30 kg/m2: PMD (P for trend =.037) |
Age, BMI, ethnicity, HRT use, education, parity, type 2 diabetes, age at menarche, disease stage, study site |
Abbreviations: PA, physical activity; BD, breast density; PCS, prospective cohort study; HEAL, healing emotions after loss; PMD, percentage mammographic density; DA, dense area; BMI, body mass index; TAM, tamoxifen; AT, adjuvant therapy; RT, radiation therapy; CT, chemotherapy; RaCT, radiation and chemotherapy; BC, breast cancer; HRT, hormone replacement therapy; CSS, cross-sectional study; GPCSP, Greek population-based screening program; BI-RADS, Breast Imaging Reporting and Data System; LS, longitudinal study; EPIC, European Prospective Investigation Into Cancer; NBCSP, Norwegian Breast Cancer Screening Program; MAMS, Mammograms and Masses Study; CARE, Contraceptive and Reproductive Experiences; FH, family history; CBHP, Chicago Breast Health Project; IPAQ, International Physical Activity Questionnaire–Long Form; ALPHA, Alberta Physical Activity; PDV, percentage dense volume; SWAN, Study of Women’s Health Across the Nation; TBA, total breast area; ADBT, Area of dense breast tissue; OC, oral contraceptive; TDA, total dense area; NuHS, Nurses’ Health Study; Hx, history; GAC, Glasgow Alumni cohort; MBCFS, Minnesota Breast Cancer Family Study; WHR, waist-to-hip ratio; CCS, case-control study; SCC, six category classification.
The relationship between diet and BD was assessed in 34 studies: 59% were CSSs on the association between diet and BD, and 27% were RCTs that assessed change in BD with dietary interventions (Table 4). The sample size ranged from 30 to 2252 (n = 24 579). Of these, 27 studies assessed diet in adults aged 25 to 79 years,24-26,28,29,31,72-92 and 7 studies assessed the association of childhood and/or adolescent diet (4 to 18 years) with BD in adult life.27,64,68,93-96 Of the studies in adults, 5 evaluated calcium and vitamin D (≥750 mg/d and ≥100 IU/d respectively), 4 assessed circulating vitamin D—25(OH) D—and 6 assessed isoflavone. Four studies assessed dietary fats26,80-82; 3 assessed a low-fat, high-carbohydrate diet25,29,83; and 4 assessed carbohydrates and proteins.80-82,90 Two studies assessed vegetables,26,73 and 2 assessed Mediterranean diets (Med-diets) and multivitamin-multimineral supplements (M-M supplements).24,92 Of the 7 studies that assessed childhood and adolescent diet, 3 were on dietary patterns,64,84,95 and other studies were on calorie restriction,96 alcohol,93 dietary vitamin D and calcium,94 and a low-fat diet.27 A majority of the studies assessed BD with area-based methods such as Cumulus and qualitative approaches. No association was found between childhood or adolescent diet and BD in adulthood.27,64,68,93-95 In adults, all 5 studies on calcium and vitamin D reported an inverse association with BD in premenopausal women but not in postmenopausal women.72,73,76,77,84 All RCTs of isoflavone demonstrated no change in BD,31,86-89 and all CSSs reported no inverse association between dietary fat and BD.26,80-82 The 2 RCTs on low-fat, high-carbohydrate intervention found statistically significant BD decreases,25,83 and no further change was noted in a 4-year post hoc analysis of 1 RCT.29 Studies on protein and carbohydrate intake and BD generated conflicting results, with 2 reporting higher BD,82,90 1 demonstrating an inverse association,80 and 1 reporting no association with BD.81 The 2 studies on vegetable intake in adulthood reported an inverse association with BD.26,73 Intake of Med-diets and M-M supplements also demonstrated conflicting results, with one reporting lower BD for Med-diet and M-M supplements in postmenopausal women alone (P < .05)92 and the other demonstrating higher BD in premenopausal women but not in postmenopausal women (Table 4).24
Table 4.
Characteristics of Studies on the Association Between Diet and BD.
| Author, Year | Study Design | Study Population (n) | Diet/BD Assessment Age | Food Species of Interest | Dietary Assessment | Outcome | Significant Results | Adjustments |
|---|---|---|---|---|---|---|---|---|
| Bérubé et al,72 2005 | CSS | ● Postmenopausal women: n = 783 ● Premenopausal women: n = 777 (Canada) |
● Postmenopausal women: 61.8 years ● Premenopausal women: 46.7 years |
Dietary and supplemental VD and Ca | Food questionnaire (661 items) | PMD (computer-assisted approach | ● Premenopausal: 8.5% BD decreases with 1000 mg and 400 IU intake of Ca and VD (P ≤ .004) Postmenopausal women: null |
Smoking status, alcohol, PA, OC use, age, age at menarche, BMI, education, age at first full-term and number of full-term births, ethnicity, FH of BC (first-degree relatives), previous breast biopsies |
| Masala et al,73 2006 | CSS | Mediterranean women–EPIC Florence section; n = 1668 (Italy) | Premenopausal, perimenopausal, and postmenopausal women | Ca and VD; cheese; vegetables | Food frequency questionnaire (FFQ; 160 items) | Wolfe’s method (P2 + DY vs N1 + P1) | BD inversely associated with vegetables, cheese, VD, and Ca; P < .05 | BMI, age, education, total energy, menopausal status, Ca, and VD |
| Vachon et al,74 2000 | CSS | MBCFSC; n = 1508 (United States, NH-White) | 61.4 years | Polyunsaturated fat, vitamins E and C, saturated fat, total diary intake | FFQ (153 items) | PMD (visual assessment) | Null association for polyunsaturated fat, vitamins E and C; P < .05 for saturated fat, total diary intake | Smoking status, OC use, alcohol, energy, age, age at menarche, BMI, age at first full-term birth and number of full-term births, FH of BC, HRT. |
| Nordevang et al,75 1993 | CSS | BC patients; n = 238 (Sweden) | 57.5 years | Ca | Interview of dietary history with 4 months of BC diagnosis | Wolfe’s method (P2 + DY vs N1 + P1) | Mammographic pattern: low Ca intake is associated with P2 and Dy patterns | ER status, BMI, age |
| Diorio et al,77 2006 | CSS | Premenopausal women; n = 771 (Canada) | <46 years If smoker and <48 years if nonsmoker | Dietary and supplemental Ca and VD | Food questionnaire | PMD (computer-assisted method) | PMD (food and supplement) ● VD: β = −1.4 ● Ca: β = −1.9 PMD (food only) ● VD: β = −1.8 ● Ca: β = −1.8 ● (P = .002) |
Age, age at menarche, age at first full-term birth, number of full-term births, alcohol, total energy, BMI, FH of BC (first- degree relative), breast biopsies, past use of HRT and OC, PA, education |
| Bérubé et al,76 2004 | CSS | Premenopausal and postmenopausal women with extreme densities; n = 543 (United States) | ● PMD ≤30%: 51 years ● PMD ≥70%: 46 years |
Dietary Ca and VD | Food questionnaire (232 items) | PMD (visual assessment) | BD (ORQ4 vs Q1) ● VD: P = .0005 Ca: P = .0006 |
Smoking status, alcohol, PA, OC use age, age at menarche, BMI, education, age at first full-term gestation, number of full-term gestations combined, FH of BC, menopausal status, and use of HRT |
| Bertone-Johnson et al,78 2010 | CSS | MDAS: WHI; n = 808 postmenopausal (United States NH-White, Black, other races) | 50-79 years | Dietary and supplemental Ca and VD | Supplement inventory + food questionnaire (122 items) | PMD (computer-assisted approach) | Null | Smoking, alcohol, PA, OC use and duration of use, previous HRT use and duration, MV use, parity, age at menarche, BMI, age, ethnicity, Gail risk |
| Knight et al,79 2006 | CSS | MBCFSC; n = 487 (United States, NH-White) | 56.4 years | Dietary Ca and VD—25(OH)D | FFQ | TDA, PMD (Cumulus) | Null | Age, BMI, PA, parity, age at first birth |
| Brisson et al,26 1989 | CCS | NBSS ● Cases: n = 290 ● Controls: n = 645 (Canada) |
40-62 years | Dietary fats; vegetables (carotenoid) | FFQ (114 items) | Wolfe’s method (P2 + DY vs N1 + P1) and visual assessment | ● Increased BD with dietary fat (P > .05) Lower BD with carotenoid intake (P < .05) |
Education, age, parity, body weight, energy |
| Tseng et al,84 2007 | CSS | Women with FH of BC and ovarian cancer; n = 157 (United States, NH-White) | 50 years | Ca and VD | FFQ (126 items) | PMD (visual assessment) | PMD; VD intakeT3 vs T1: OR = 0.5; 95% CI = 0.2-1.1 | Age, age at menarche, menopausal status, HRT Hx, FH of category, calorie intake, BMI |
| Sala et al,82 2000 | CCS | EPIC-Norfolk ● Cases: n = 203 Controls: n = 203 |
59 years | Dietary fats, carbohydrates and proteins | Seven-day record | Wolfe’s method (P2 + DY vs N1 + P1) | Null for dietary fats; high BD for carbohydrates and proteins intake (P = .04) | Parity, BMI, menopausal status, HRT |
| Nagata et al,80 2005 | CSS | Japanese women; n = 601 (Japan) | ● Premenopausal women: 42.6 years ● Postmenopausal women: 57.8 years |
Dietary fats; carbohydrates | FFQ (169 items) | PMD (fully automated method) | ● Dietary fats Postmenopausal: positively associated; P > .05 ● Premenopausal: null Carbohydrates: inversely associated with BD; P = .03 |
● Premenopausal: BMI, age, smoking status, number of births, breastfeeding Hx Postmenopausal: education, age, BMI, age at menopause, total energy |
| Qureshi et al,81 2011 | CSS | NBCSP; n = 2252 (Norway) | 58 years | Protein, carbohydrates, dietary fiber, total fat, saturated fat | FFQ (180 items) | AD and PMD; computer-assisted approach | Null for protein, carbohydrates, dietary fiber; high BD with total fat intake (P = .10) and saturated fat (P = .06) | Age at menarche, age at mammography, age at full-term birth, number of pregnancies, BMI, HRT, education, total energy |
| Knight et al,83 1999 | RCT | ● Entry: premenopausal ● Follow-up: postmenopausal n = 78 (Canada) |
● Intervention: 49.5 years ● Controls: 49.2 years |
Low-fat, high CHO interventions vs control (2 years) | 3 Food records | PMD, ADT (automated approach) | ● Mean decrease in DA: −11.0 vs −4.5 cm2; P = .004 Decrease in percentage density: −11.0% vs −5.2%; P = .025 |
Age, FH, age at menarche, parity, age at first birth, OC use, PA, breastfeeding, total energy, weight change |
| Bertone-Johnson et al,85 2012 | RCT | WHI ca + D trial; n = 330 postmenopausal women (United States) | ● Intervention: 61.8 years ● Controls: 62.0 years |
Daily supplementation of 400 IU of VD and 1000 mg of Ca (1 year) | FFQ (122 items) | PMD (computer-assisted approach) | Null | Total VD, age, ethnicity, HT treatment, BMI, residence region, Gail risk score, baseline BD |
| Martin et al,29 2009 | RCT | Women with PMD ≥ 50%; n = 461 (Canada) | ● Intervention: 48.7 years ● Controls: 48.6 years |
Low-fat, high CHO interventions | Food records | PMD, DA, TBA NDA (computer assisted) | Null | FH of BC, HRT use, OC use, dietary fats, and postmenopausal status |
| Boyd et al,25 1997 | RCT | PMD ≥ 50%; n = 817 (Canada) | ● Intervention: 46.5 years ● Controls: 45.9 years |
Low-fat, high CHO diet interventions (2 years) | Food records (3 days) | PMD (automated approach) | Intervention 6.1% vs control (2.1%); P = .01 | Menopausal status, weight, age, grouping |
| Maskarinec et al,88 2003 | RCT | Isoflavone trials; n = 30 (Hawaii) | 35-46 years | 100 mg Of isoflavone mixture/d for 12 months | Tablet counts and urinary isoflavone excretion | PMD (computer-assisted approach) | Null | Race, weight, BD ≥40% |
| Maskarinec et al,31 2004 | RCT | n = 220 (Caucasians, Asians and others) Hawaii | ● Intervention: = 43.2 ± 3.1 years ● Control: 42.8 ± 2.9 years |
2 Daily servings of soy for 2 years | Validated soy questionnaire, urinary isoflavone excretions | PMD (computer-assisted approach | BD reduction of 3.14%/year at least 1 serving/wk (insignificant BD change) | Ethnicity, age, group status, place of birth, number of children, %BD at baseline |
| Atkinson et al,86 2004 | RCT | NHSBSP; n = 205 (United Kingdom) | 49–65 years | Red clover–derived isoflavone tablet/d for 12 months | Urinary isoflavone excretions | PMD (Wolfe classification, visual assessment) | Null | Menopausal status, age at baseline, BMI, genotype |
| Verheus et al,89 2008 | RCT | DPBCSP; n = 202 (Netherlands) | 60-75 years | 99 mg isoflavone/d for 1year | Intake of 36.5 g of soy powder/d | PMD (computer assisted) | Null | Equol status, %BD at baseline |
| Maskarinec et al,87 2009 | RCT | OPUS; n = 406 (United States, Greece) | 40-60 years | 80 Or 120 mg/d of isoflavone for 2 years | FFQ, pill counts, blood isoflavone measurement | PMD (computer-assisted method) | Null | Age, BMI |
| Tseng et al,91 2013 | LS | Chinese immigrants; n = 436 (United States) | 36-58 years | 25 to 30 mg/d of isoflavone for 3 days | 48-Hour dietary recall, urinary isoflavone excretions | PMD (computer-assisted method) | Null; PMD for Equol vs non–equol producers: 31.8 vs 35.3, respectively | Sociodemographic characteristics, dietary intake, equol status, equol dose |
| Jones et al,28 2015 | CSS | DISC; n = 172 (United States) | 25-29 years | Dietary energy density | 24-Hour dietary recalls | %DBV, ADBV (MRI) | 25.9% (95% CI = 6.2% to 56.8%) increase in %DBV (P < .01) | Race, smoking status, education, parity, duration of sex hormone use, whole body percentage fat, childhood BMI, and energy from beverage, fat, and alcohol |
| Masala et al,90 2013 | CSS | EPIC-Florence; n = 1668 (Italy) | Not specified | Carbohydrate intake | Self-administered FFQ | PMD (Wolfe classification, visual assessment) | ● BD increase: OR = 1.73, 95% CI = 1.13-2.67 ● Simple sugar: OR = 1.71; 95% CI = 1.13-2.59 |
Age, education, BMI, menopause, number of children, breastfeeding, physical activity, non–alcohol energy, fibers, saturated fat, and alcohol |
| Voevodina et al,92 2013 | CSS | n = 424 (Germany) | 21-84 years; Premenopausal and postmenopausal | Mediterranean diet and M-M supplements | Self-administered FFQ | PMD (BI-RADS) | OR for lower BD ● Mediterranean diet: 0.95; P < .05 ● M-M supplements: ● Premenopausal: 0.53; P > .05 Postmenopausal: 0.51; P < .05 |
Age, age at menarche, age at first birth, number of live births, PA, alcohol, smoking status, menopausal status, education, HRT, OCs, Hx of BC, breastfeeding |
| Bérubé et al,24 2008 | CSS | Premenopausal and postmenopausal women; n = 1560 (Canada) | ● Mean age Postmenopausal = 61.8 years Premenopausal = 46.7 years |
Diet and multivitamin multimineral, and individual vitamin and mineral supplement use | Self-administered FFQ | PMD, Cumulus | BD ● Increase in premenopausal (P for trend = .04) ● Postmenopausal: null (P for trend = .40) |
Smoking status, alcohol, PA, OC use, age, age at menarche, BMI, education, age at first full-term birth and number of full-term births, ethnicity, FH of BC (first-degree relatives), previous breast biopsies |
| Vachon et al,93 2005 | CSS | MBCFSC; n = 1575 (NH-white, United States) | <18 years/60.4 years | Alcohol | Follow-up questionnaire | PMD (Cumulus) | Null | Age, age at menarche, age at first birth, number of live births, HRT, BMI, smoking status, education, oral OC use, menopausal status, alcohol |
| Sellers at al,64 2007 | MBCFSC; n = 1552(NH-white, United States) | 12-13 years/60.4 years | Chicken and fish, vegetables, fruits, high-fat meats, animal fat, dairy, high-fat foods, high-fat snacks and desserts | Questionnaire (retrospective recall) | PMD (Cumulus) | Null | Age at menarche, parity, age at first birth, smoking history, education, OC use, HRT use, menopausal status, alcohol intake | |
| Mishra et al,94 2008 | PCS | MRC NSHD; n = 979 (Britain) | 4/51.5 years | Dietary VD and Ca | Maternal recall of child’s diet within 1-24 hours | ADT, ANDT, PMD (Cumulus) | Null | BMI, age at menarche, parity, energy, smoking status, adult SES |
| Tseng et al,68 2011 | CSS | Chinese-American immigrant; n = 201 (United States, Asian) | 12-17/53.1 years | Green vegetables, fruits, tofu, beef, pork | Questionnaire (Retrospective recall) | BI-RADS | OR 95% CI for high BD ● Red meat: 3.0%; P = .003 ● Tofu and fruits: 1.6%; P = .39 Vegetables: null |
Age, BMI, level of acculturation, age at first live birth, number of live births, adult dietary intake |
| Mishra et al,95 2011 | PCS | MRC NSHD; n = 792 (Britain) |
4 years/51.5 years | Dietary patterns at age 4: (a) Fried potatoes and fish (b) Breads and fats (c) Milk, biscuits, and fruits |
Maternal recall of child’s diet within 1-24 hours | ADT, ANDT, PMD (Cumulus | Null | BMI at 53 years, age at menarche, parity, energy, age at mammogram, HRT, mammographic view, smoking status, adult SES, PA, social class, dietary pattern |
| Dorgan et al,27 2010 | CSS (RCT follow-up) | DISC; premenopausal women; n = 182 (United States, NH-White | 25-29 years | Low-fat diet long-term effect assessment | 3-24 Hour dietary recalls | VDT and PMD (MRI) | Null | Age at randomization, race, education, BMI-Z score, percentage body fat, age at visit, smoking status, clinic, number of full-term gestations, hormonal contraceptives, PA at age 14-17 years and during the past year |
| Haars et al,96 2010 | CSS | DOM-project; n = 356 (Holland) | 10-18/53 years | Short-term energy restriction | Exposure to hunger, cold, and weight loss (retrospective recalls of 1944-1945 Dutch famine) | PMD, NDT, DT, BS (visual mammographic assessment) | Null | Menopausal status, parity, BMI, and age at mammography |
Abbreviations: BD, breast density; CSS, cross-sectional study; VD, vitamin D; PMD, percentage mammographic density; PA, physical activity; OC, oral contraceptive; BMI, body mass index; FH, family history; BC, breast cancer; EPIC, European Prospective Investigation Into Cancer; MBCFSC, Minnesota Breast Cancer Family Study cohort; NH-Whites, Non-Hispanic Whites; HRT, hormone replacement therapy; ER, estrogen receptor; MDAS WHI, Mammogram Density Ancillary Study of the Women’s Health Initiative; MV, multivitamins; TDA, total dense area; NBSS, National Breast Screening Study; RCT, randomized controlled trial; TBA, total breast area; AD, absolute density; Hx, history; ADT, area of dense tissue; NDA, nondense area; NHSBSP, National Health Service Breast Screening Programme; DPBCSP, Dutch Population-Based Breast Cancer Screening Programme; OPUS, osteoporosis prevention using soy; LS, longitudinal study; MRI, magnetic resonance imaging; M-M, multivitamin-multimineral; BI-RADS, Breast Imaging Reporting and Data System; PCS, prospective cohort study; SES, socioeconomic status; CHO, carbohydrates; NBCSP, Norwegian Breast Cancer Screening Program DISC, dietary intervention study in children; DBV, dense breast volume; ADBV, absolute dense breast volume; MRC NSHD; Medical Research Council National Survey of Health and Development; ADT, area of dense tissue; ANDT, area of non-dense tissue; VDT, volume of dense tissue NDT, non-dense tissue; DT, dense tissue; BS, breast size.
Discussion
One encouraging attribute of BD is that it can be altered.8 Because most determinants of BD and BC are interrelated and interdependent,5 it is logical that parameters that lower BD may lower BC risk. Many parameters are thought to alter BD; however, this review focuses on SERMs, AIs, PA, and diet.
Estrogen plays a critical role in BD and increased BC risk5; therefore, it is intuitive that interventions that decrease estrogen bioavailability may lower BD. A few substances with strong binding affinity for estrogen receptors such as SERMs have been identified to antagonize the action of estrogen on breast tissue through inhibition of 17β-estradiol activity.97 SERMs also decrease insulin-like growth factor (IGF)-1 levels and increase IGF binding protein (IGFBP) and sex hormone binding-globulin.8,9,15 Other substances (AIs) inhibit the production of estrogen by aromatase.15,56 Evidence shows that TAM mediates BD reduction in both premenopausal and postmenopausal women, but no concrete evidence exists for BD reduction with RLX, tibolone, letrozole, anastrozole, or exemestane (Table 2). The BD reduction in TAM studies might be explained by the prevalence of premenopausal women as shown by the stronger BD decreases in premenopausal women. Most of the AIs, tibolone, and RLX studies were performed in postmenopausal women. Because menopause is associated with tissue involution,98 postmenopausal breast tissue may not be responsive to AIs and RLX therapies.
A few limitations are noted in studies on antiestrogen agents. About 80% of the studies on TAM and tibolone did not adjust for BD confounders. Two of the studies on estrogen modulators had very small sample sizes,11,17 and 1 study99 reported duration of treatment but not dosage administered. There was variability in age, populations, and sample size between studies, thus making comparison of studies difficult. A majority of the studies did not assess the consistency of MBD assessments, and 90% of the studies used area-based approaches for MBD assessment, which may not detect change in BD when the quantity of dense tissue changes but the dense area remains unchanged. Significant changes in BD with 3 different estrogen-receptor modulators were demonstrated where VBD assessment was performed11,40,49 and emphasize the need for further studies using volumetric methods to assess the effect of RLX, tibolone, and AIs on BD in premenopausal women. Thus, variability in MBD assessment continues to be a confounding factor in studies assessing the impact of interventions on BD, as demonstrated by the heterogeneity in results obtained in the same patients when different MBD measurement approaches were used.35,40,54
PA alters BMI by reducing adiposity and increasing muscle mass, and the association between BMI, BD, and cancer is well established.100 However, the evidence for the association between PA and BD is conflicting. Of the 21 studies, 81%, including a RCT of aerobic exercise on BD,23 found no association between PA and BD. It is well established that BMI and postmenopausal status are negative confounders for BD.100 BMI was found to attenuate the association between PA and BD, but only 9 studies adjusted for menopausal status; 95% of the studies did not report whether participants were premenopausal or postmenopausal at the time of PA, and 1 study70 did not specify the timing of PA. There was heterogeneity in the type, duration, and intensity of PA, making comparison of results difficult. The only RCT that assessed change in BD with aerobic exercise was in postmenopausal women. Involution of the breast and depletion of sex hormones and growth factors is common in postmenopausal women.5,98 Because exercise acts through these hormonal agents, it is unsurprising that no change was noted in BD with aerobic exercise in postmenopausal women. About 90% of the studies assessed Caucasian women, limiting the generalization of results to other ethnic populations. As yet, there is no evidence to suggest that PA is associated with lower BD. Nonetheless, because PA is inversely related to serum IGF-1, estrogen, and progesterone bioavailability and reduction in their serum concentration reduces cell proliferation and exposure to carcinogens, PA is a controllable, important BC risk mitigation agent.
Although evidence for the association between diet and BD is also contradictory,27,64,68,93-96 intake of vitamin D and calcium; a low-fat, high-carbohydrate diet; and vegetables appears to be associated with lower BD, mostly in premenopausal women (Table 4). Calcium and vitamin D play an important role in the modulation of epithelial cell growth, proliferation, and differentiation.101 The inverse association between these food sources and BD in premenopausal women is stronger at higher threshold consumption and among women with high concentrations of IGF-I or IGFBP-3.77 Lower BD from calcium and vitamin D intake has been attributed to antioxidant activity101 and inhibition of IGFs.77 Premenopausal, compared with postmenopausal, women have more proliferating cells and mitogens implicated in BD increases,5,8,77 which is perhaps the reason for the inverse association between calcium and vitamin D with BD in premenopausal women only. Isoflavone consumption has been shown to be associated with lower cancer incidence102 but not BD.31,86-89 A recent LS shows that soy-product consumers who metabolize daidzein manufactured by intestinal bacteria to equol (a nonsteroidal estrogen) demonstrate slightly lower BD than non–equol producers.91 This suggests that the metabolism of a specific diet may influence its association with BD and needs to be explored. Isoflavones reduce the effects of mitogens and mutagens through increases in antioxidant activity and sex hormone–binding globulin serum bioactivity,102 and these 2 properties reduce cell proliferation associated with BD increases. Isoflavone studies are limited by the area-based assessment of MBD and inadequate adjustment for BD confounders.
The literature on the association between carbohydrates and proteins with BD also presents conflicting outcomes: direct association,82 inverse association,80 and no association.81 However, RCTs on low-fat, high-carbohydrate diet have shown an inverse association with BD in premenopausal and postmenopausal women.25,29 There is evidence that the fiber make-up of carbohydrates influences the IGF/IGFBP sequence and oxidative stress.103 Therefore, varying fiber content in the different sources of carbohydrates may differentially influence growth factors responsible for BD variations, and this may be the reason for the inconsistent relationship between carbohydrate intake and BD.
Vegetables and carotene inhibit cell proliferation and IGF-1,104 and these may be the reasons for their association with lower BD in premenopausal and postmenopausal women.26,73 Although regular use of M-M supplements has generated different outcomes, these supplements contain antioxidants that reduce the activity of mitogens responsible for BD increases24 and, therefore, need to be further investigated.
Generally, the reliance of CSSs on questionnaires whose reliability can be diminished by memory deficiency makes it difficult to measure the extent of exposure to dietary factors.105 The association between a specific diet and BD may also be attenuated if such food is consumed in combination with other counteracting food substances and if intake occurred before mammogenesis. Therefore, RCTs using VBD measurement approaches may provide more accurate evidence for the impact of interventions on BD. The literature demonstrates that antidiabetes agents such as metformin inhibit IGF-1 and insulin/IGF chain and alter metabolic processes.106 These processes are associated with reduction in cell proliferation,106 which may lower BD. Thus, it may be important to assess the effect of antidiabetes agents and changes in glucose homeostasis on BD. Finally and encouragingly, change in BD over time is consistent with change in BC risk,107,108 and reduced BD is associated with a reduced risk of BC108,109 and death from the disease.110 Therefore, BD may have potential utility as a biomarker for the efficacy of chemopreventive interventions.
Conclusion
There is substantial evidence that BD is potentially reducible. Tamoxifen reduces BD; however, the effect of RLX, tibolone, and AIs on BD is still unclear. There is no evidence for association of PA and childhood or adolescent diet with BD. Although data on the association between dietary factors and BD are conflicting, intake of vegetables, vitamin D, and calcium in adulthood is associated with lower BD in premenopausal women. It is hoped that lowering BD with interventions may lower BC risk and improve early detection of BC with mammography. However, these benefits can only be amassed if women are adequately informed about BD and BC risk mitigation strategies. Implementation of these strategies may hold the key to reducing the risk of BC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [DOI] [PubMed] [Google Scholar]
- 2. Cabanes A, Pastor-Barriuso R, Garcia-Lopez M, et al. Alcohol, tobacco, and mammographic density: a population-based study. Breast Cancer Res Treat. 2011;129:135-147. [DOI] [PubMed] [Google Scholar]
- 3. Boyd NF, Martin LJ, Rommens JM, et al. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol. 2009;472:343-360. [DOI] [PubMed] [Google Scholar]
- 4. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236. [DOI] [PubMed] [Google Scholar]
- 5. Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ekpo EU, McEntee MF. Measurement of breast density with digital breast tomosynthesis: a systematic review. Br J Radiol. 2014;(1043):20140460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ekpo EU, Hogg P, Highnam R, McEntee MF. Breast composition: measurement and clinical use. Radiography. 2015;21:324-333. [Google Scholar]
- 8. Cuzick J, Warwick J, Pinney E, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744-752. [DOI] [PubMed] [Google Scholar]
- 9. Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621-628. [DOI] [PubMed] [Google Scholar]
- 10. Brisson J, Brisson B, Cote G, Maunsell E, Bérubé S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9:911-915. [PubMed] [Google Scholar]
- 11. Chen JH, Chang YC, Chang D, et al. Reduction of breast density following tamoxifen treatment evaluated by 3-D MRI: preliminary study. Magn Reson Imaging. 2011;29:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahern TP, Hankinson SE, Willett WC, Pollak MN, Eliassen AH, Tamimi RM. Plasma C-peptide, mammographic breast density, and risk of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1786-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cigler T, Richardson H, Yaffe MJ, et al. A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res Treat. 2011;126:453-461. [DOI] [PubMed] [Google Scholar]
- 14. Cigler T, Tu D, Yaffe MJ, et al. A randomized, placebo-controlled trial (NCIC CTG MAP1) examining the effects of letrozole on mammographic breast density and other end organs in postmenopausal women. Breast Cancer Res Treat. 2010;120:427-435. [DOI] [PubMed] [Google Scholar]
- 15. Henry NL, Chan HP, Dantzer J, et al. Aromatase inhibitor-induced modulation of breast density: clinical and genetic effects. Br J Cancer. 2013;109:2331-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prowell TM, Blackford AL, Byrne C, et al. Changes in breast density and circulating estrogens in postmenopausal women receiving adjuvant anastrozole. Cancer Prev Res (Phila). 2011;4:1993-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith J, Dilawari A, Ursin G, et al. A pilot study of letrozole for one year in women at enhanced risk of developing breast cancer: effects on mammographic density. Anticancer Res. 2012;32:1327-1331. [PubMed] [Google Scholar]
- 18. Irwin ML, Aiello EJ, McTiernan A, et al. Pre-diagnosis physical activity and mammographic density in breast cancer survivors. Breast Cancer Res Treat. 2006;95:171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irwin ML, Aiello EJ, McTiernan A, et al. Physical activity, body mass index, and mammographic density in postmenopausal breast cancer survivors. J Clin Oncol. 2007;25:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marmara EA, Papacharalambous XN, Kouloulias VE, Maridaki DM, Baltopoulos JP. Physical activity and mammographic parenchymal patterns among Greek postmenopausal women. Maturitas. 2011;69:74-80. [DOI] [PubMed] [Google Scholar]
- 21. Masala G, Assedi M, Ambrogetti D, et al. Physical activity and mammographic breast density in a Mediterranean population: the EPIC Florence longitudinal study. Int J Cancer. 2009;124:1654-1661. [DOI] [PubMed] [Google Scholar]
- 22. Wolin KY, Colangelo LA, Chiu BC, Ainsworth B, Chatterton R, Gapstur SM. Associations of physical activity, sedentary time, and insulin with percent breast density in Hispanic women. J Womens Health. 2007;16:1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woolcott CG, Courneya KS, Boyd NF, et al. Mammographic density change with 1 year of aerobic exercise among postmenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2010;19:1112-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bérubé S, Diorio C, Brisson J. Multivitamin-multimineral supplement use and mammographic breast density. Am J Clin Nutr. 2008;87:1400-1404. [DOI] [PubMed] [Google Scholar]
- 25. Boyd NF, Greenberg C, Lockwood G, et al. Effects at two years of a low-fat, high-carbohydrate diet on radiologic features of the breast: results from a randomized trial. Canadian Diet and Breast Cancer Prevention Study Group. J Natl Cancer Inst. 1997;89:488-496. [DOI] [PubMed] [Google Scholar]
- 26. Brisson J, Verreault R, Morrison AS, Tennina S, Meyer F. Diet, mammographic features of breast tissue, and breast cancer risk. Am J Epidemiol. 1989;130:14-24. [DOI] [PubMed] [Google Scholar]
- 27. Dorgan JF, Liu L, Klifa C, et al. Adolescent diet and subsequent serum hormones, breast density, and bone mineral density in young women: results of the Dietary Intervention Study in Children follow-up study. Cancer Epidemiol Biomarkers Prev. 2010;19:1545-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones JA, Hartman TJ, Klifa CS, et al. Dietary energy density is positively associated with breast density among young women. J Acad Nutr Diet. 2015;115:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin LJ, Greenberg CV, Kriukov V, et al. Effect of a low-fat, high-carbohydrate dietary intervention on change in mammographic density over menopause. Breast Cancer Res Treat. 2009;113:163-172. [DOI] [PubMed] [Google Scholar]
- 30. Maskarinec G, Meng L. An investigation of soy intake and mammographic characteristics in Hawaii. Breast Cancer Res. 2001;3:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089-3094. [DOI] [PubMed] [Google Scholar]
- 32. Price ER, Hargreaves J, Lipson JA, et al. The California breast density information group: a collaborative response to the issue of breast density, breast cancer risk, and breast density notification legislation. Radiology. 2013;269:887-892. [DOI] [PubMed] [Google Scholar]
- 33. Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27:3749-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8:863-866. [PubMed] [Google Scholar]
- 35. Chow CK, Venzon D, Jones EC, Premkumar A, O’Shaughnessy J, Zujewski J. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev. 2000;9:917-921. [PubMed] [Google Scholar]
- 36. Konez O, Goyal M, Reaven RE. Can tamoxifen cause a significant mammographic density change in breast parenchyma? Clin Imaging. 2001;25:303-308. [DOI] [PubMed] [Google Scholar]
- 37. Meggiorini ML, Labi L, Vestri AR, Porfiri LM, Savelli S, De Felice C. Tamoxifen in women with breast cancer and mammographic density. Eur J Gynaecol Oncol. 2008;29:598-601. [PubMed] [Google Scholar]
- 38. Son HJ, Oh KK. Significance of follow-up mammography in estimating the effect of tamoxifen in breast cancer patients who have undergone surgery. AJR Am J Roentgenol. 1999;173:905-909. [DOI] [PubMed] [Google Scholar]
- 39. Cirpan T, Akercan F, Itil IM, Gundem G, Bilgen I, Yucebilgin MS. Does raloxifene therapy affect mammographic breast cancer screening in postmenopausal patients? Eur J Gynaecol Oncol. 2006;27:177-178. [PubMed] [Google Scholar]
- 40. Eng-Wong J, Orzano-Birgani J, Chow CK, et al. Effect of raloxifene on mammographic density and breast magnetic resonance imaging in premenopausal women at increased risk for breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1696-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freedman M, San Martin J, O’Gorman J, et al. Digitized mammography: a clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placebo. J Natl Cancer Inst. 2001;93:51-56. [DOI] [PubMed] [Google Scholar]
- 42. Harvey JA, Holm MK, Ranganath R, Guse PA, Trott EA, Helzner E. The effects of bazedoxifene on mammographic breast density in postmenopausal women with osteoporosis. Menopause. 2009;16:1193-1196. [DOI] [PubMed] [Google Scholar]
- 43. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause. 2013;20:138-145. [DOI] [PubMed] [Google Scholar]
- 44. Jackson VP, San Martin JA, Secrest RJ, et al. Comparison of the effect of raloxifene and continuous-combined hormone therapy on mammographic breast density and breast tenderness in postmenopausal women. Am J Obstet Gynecol. 2003;188:389-394. [DOI] [PubMed] [Google Scholar]
- 45. Lasco A, Gaudio A, Morini E, et al. Effect of long-term treatment with raloxifene on mammary density in postmenopausal women. Menopause. 2006;13:787-792. [DOI] [PubMed] [Google Scholar]
- 46. Nielsen M, Raundahl J, Pettersen PC, et al. Low-dose transdermal estradiol induces breast density and heterogeneity changes comparable to those of raloxifene. Menopause. 2009;16:785-791. [DOI] [PubMed] [Google Scholar]
- 47. Silverio CD, Nahas-Neto J, Nahas EAP, Guazeelli MMO, Gomez MA, Dias R. Effect of treatment with raloxifene on mammographic breast density in postmenopausa. Rev Bras Ginecol Obstet. 2007;29:525-531. [Google Scholar]
- 48. Christodoulakos GE, Lambrinoudaki IV, Vourtsi AD, Panoulis KP, Kelekis DA, Creatsas GC. Mammographic changes associated with raloxifene and tibolone therapy in postmenopausal women: a prospective study. Menopause. 2002;9:110-116. [DOI] [PubMed] [Google Scholar]
- 49. Eilertsen AL, Karssemeijer N, Skaane P, Qvigstad E, Sandset PM. Differential impact of conventional and low-dose oral hormone therapy, tibolone and raloxifene on mammographic breast density, assessed by an automated quantitative method. BJOG. 2008;115:773-779. [DOI] [PubMed] [Google Scholar]
- 50. Lundstrom E, Christow A, Kersemaekers W, et al. Effects of tibolone and continuous combined hormone replacement therapy on mammographic breast density. Am J Obstet Gynecol. 2002;186:717-722. [DOI] [PubMed] [Google Scholar]
- 51. Lundstrom E, Hirschberg AL, Soderqvist G. Digitized assessment of mammographic breast density-effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo. Maturitas. 2011;70:361-364. [DOI] [PubMed] [Google Scholar]
- 52. Valdivia I, Campodonico I, Tapia A, Capetillo M, Espinoza A, Lavín P. Effects of tibolone and continuous combined hormone therapy on mammographic breast density and breast histochemical markers in postmenopausal women. Fertil Steril. 2004;81:617-623. [DOI] [PubMed] [Google Scholar]
- 53. Fabian CJ, Kimler BF, Zalles CM, et al. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res Treat. 2007;106:75-84. [DOI] [PubMed] [Google Scholar]
- 54. Mousa NA, Crystal P, Wolfman WL, Bedaiwy MA, Casper RF. Aromatase inhibitors and mammographic breast density in postmenopausal women receiving hormone therapy. Menopause. 2008;15:875-884. [DOI] [PubMed] [Google Scholar]
- 55. Vachon CM, Ingle JN, Suman VJ, et al. Pilot study of the impact of letrozole vs. placebo on breast density in women completing 5 years of tamoxifen. Breast. 2007;16:204-210. [DOI] [PubMed] [Google Scholar]
- 56. Vachon CM, Suman VJ, Brandt KR, et al. Mammographic breast density response to aromatase inhibition. Clin Cancer Res. 2013;19:2144-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Conroy SM, Butler LM, Harvey D, et al. Physical activity and change in mammographic density: the study of women’s health across the nation. Am J Epidemiol. 2010;171:960-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gram IT, Funkhouser E, Tabar L. Moderate physical activity in relation to mammographic patterns. Cancer Epidemiol Biomarkers Prev. 1999;8:117-122. [PubMed] [Google Scholar]
- 59. Oestreicher N, Capra A, Bromberger J, et al. Physical activity and mammographic density in a cohort of midlife women. Med Sci Sports Exerc. 2008;40:451-456. [DOI] [PubMed] [Google Scholar]
- 60. Peters TM, Ekelund U, Leitzmann M, et al. Physical activity and mammographic breast density in the EPIC-Norfolk cohort study. Am J Epidemiol. 2008;167:579-585. [DOI] [PubMed] [Google Scholar]
- 61. Qureshi SA, Ellingjord-Dale M, Hofvind S, Wu AH, Ursin G. Physical activity and mammographic density in a cohort of postmenopausal Norwegian women: a cross-sectional study. Springerplus. 2012;1:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reeves KW, Gierach GL, Modugno F. Recreational physical activity and mammographic breast density characteristics. Cancer Epidemiol Biomarkers Prev. 2007;16:934-942. [DOI] [PubMed] [Google Scholar]
- 63. Samimi G, Colditz GA, Baer HJ, Tamimi RM. Measures of energy balance and mammographic density in the Nurses’ Health Study. Breast Cancer Res Treat. 2008;109:113-122. [DOI] [PubMed] [Google Scholar]
- 64. Sellers TA, Vachon CM, Pankratz VS, et al. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166:456-464. [DOI] [PubMed] [Google Scholar]
- 65. Suijkerbuijk KP, Van Duijnhoven FJ, Van Gils CH, et al. Physical activity in relation to mammographic density in the Dutch prospect-European prospective investigation into cancer and nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:456-460. [DOI] [PubMed] [Google Scholar]
- 66. Jeffreys M, Warren R, Gunnell D, McCarron P, Smith GD. Life course breast cancer risk factors and adult breast density (United Kingdom). Cancer Causes Control. 2004;15:947-955. [DOI] [PubMed] [Google Scholar]
- 67. Lopez P, Van Horn L, Colangelo LA, Wolfman JA, Hendrick RE, Gapstur SM. Physical inactivity and percent breast density among Hispanic women. Int J Cancer. 2003;107:1012-1016. [DOI] [PubMed] [Google Scholar]
- 68. Tseng M, Olufade TO, Evers KA, Byrne C. Adolescent lifestyle factors and adult breast density in U.S. Chinese immigrant women. Nutr Cancer. 2011;63:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control. 2000;11:653-662. [DOI] [PubMed] [Google Scholar]
- 70. Sala E, Warren R, McCann J, Duffy S, Luben R, Day N. High-risk mammographic parenchymal patterns, hormone replacement therapy and other risk factors: a case-control study. Int J Epidemiol. 2000;29:629-636. [DOI] [PubMed] [Google Scholar]
- 71. Siozon CC, Ma H, Hilsen M, Bernstein L, Ursin G. The association between recreational physical activity and mammographic density. Int J Cancer. 2006;119:1695-1701. [DOI] [PubMed] [Google Scholar]
- 72. Bérubé S, Diorio C, Masse B, et al. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14:1653-1659. [DOI] [PubMed] [Google Scholar]
- 73. Masala G, Ambrogetti D, Assedi M, Giorgi D, Del Turco MR, Palli D. Dietary and lifestyle determinants of mammographic breast density: a longitudinal study in a Mediterranean population. Int J Cancer. 2006;118:1782-1789. [DOI] [PubMed] [Google Scholar]
- 74. Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000;9:151-160. [PubMed] [Google Scholar]
- 75. Nordevang E, Azavedo E, Svane G, Nilsson B, Holm LE. Dietary habits and mammographic patterns in patients with breast cancer. Breast Cancer Res Treat. 1993;26:207-215. [DOI] [PubMed] [Google Scholar]
- 76. Bérubé S, Diorio C, Verhoek-Oftedahl W, Brisson J. Vitamin D, calcium, and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2004;13:1466-1472. [PubMed] [Google Scholar]
- 77. Diorio C, Berube S, Byrne C, et al. Influence of insulin-like growth factors on the strength of the relation of vitamin D and calcium intakes to mammographic breast density. Cancer Res. 2006;66:588-597. [DOI] [PubMed] [Google Scholar]
- 78. Bertone-Johnson ER, Chlebowski RT, Manson JE, et al. Dietary vitamin D and calcium intake and mammographic density in postmenopausal women. Menopause. 2010;17:1152-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Knight JA, Vachon CM, Vierkant RA, Vieth R, Cerhan JR, Sellers TA. No association between 25-hydroxyvitamin D and mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15:1988-1992. [DOI] [PubMed] [Google Scholar]
- 80. Nagata C, Matsubara T, Fujita H, et al. Associations of mammographic density with dietary factors in Japanese women. Cancer Epidemiol Biomarkers Prev. 2005;14:2877-2880. [DOI] [PubMed] [Google Scholar]
- 81. Qureshi SA, Couto E, Hilsen M, Hofvind S, Wu AH, Ursin G. Mammographic density and intake of selected nutrients and vitamins in Norwegian women. Nutr Cancer. 2011;63:1011-1020. [DOI] [PubMed] [Google Scholar]
- 82. Sala E, Warren R, Duffy S, Welch A, Luben R, Day N. High risk mammographic parenchymal patterns and diet: a case-control study. Br J Cancer. 2000;83:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Knight JA, Martin LJ, Greenberg CV, et al. Macronutrient intake and change in mammographic density at menopause: results from a randomized trial. Cancer Epidemiol Biomarkers Prev. 1999;8:123-128. [PubMed] [Google Scholar]
- 84. Tseng M, Byrne C, Evers KA, Daly MB. Dietary intake and breast density in high-risk women: a cross-sectional study. Breast Cancer Res. 2007;9:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bertone-Johnson ER, McTiernan A, Thomson CA, et al. Vitamin D and calcium supplementation and one-year change in mammographic density in the women’s health initiative calcium and vitamin D trial. Cancer Epidemiol Biomarkers Prev. 2012;21:462-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Atkinson C, Warren RM, Sala E, et al. Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 2004;6:R170-R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maskarinec G, Verheus M, Steinberg FM, et al. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr. 2009;139:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev. 2003;12:165-169. [DOI] [PubMed] [Google Scholar]
- 89. Verheus M, van Gils CH, Kreijkamp-Kaspers S, et al. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2632-2638. [DOI] [PubMed] [Google Scholar]
- 90. Masala G, Assedi M, Bendinelli B, et al. Glycemic index, glycemic load and mammographic breast density: the EPIC Florence longitudinal study. PLoS One. 2013;8:e70943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tseng M, Byrne C, Kurzer MS, Fang CY. Equol-producing status, isoflavone intake, and breast density in a sample of U.S. Chinese women. Cancer Epidemiol Biomarkers Prev. 2013;22:1975-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Voevodina O, Billich C, Arand B, Nagel G. Association of Mediterranean diet, dietary supplements and alcohol consumption with breast density among women in South Germany: a cross-sectional study. BMC Public Health. 2013;13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vachon CM, Sellers TA, Janney CA, et al. Alcohol intake in adolescence and mammographic density. Int J Cancer. 2005;117:837-841. [DOI] [PubMed] [Google Scholar]
- 94. Mishra G, McCormack V, Kuh D, Hardy R, Stephen A, dos Santos Silva I. Dietary calcium and vitamin D intakes in childhood and throughout adulthood and mammographic density in a British birth cohort. Br J Cancer. 2008;99:1539-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mishra GD, dos Santos Silva I, McNaughton SA, Stephen A, Kuh D. Energy intake and dietary patterns in childhood and throughout adulthood and mammographic density: results from a British prospective cohort. Cancer Causes Control. 2011;22:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Haars G, van Gils CH, Elias SG, Lokate M, van Noord PA, Peeters PH. The influence of a period of caloric restriction due to the Dutch famine on breast density. Int J Cancer. 2010;126:2211-2215. [DOI] [PubMed] [Google Scholar]
- 97. Nuttall ME, Stroup GB, Fisher PW, Nadeau DP, Gowen M, Suva LJ. Distinct mechanisms of action of selective estrogen receptor modulators in breast and osteoblastic cells. Am J Physiol Cell Physiol. 2000;279:C1550-C1557. [DOI] [PubMed] [Google Scholar]
- 98. Boyd N, Martin L, Stone J, Little L, Minkin S, Yaffe M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11:1048-1053. [PubMed] [Google Scholar]
- 99. Kim J, Han W, Moon HG, et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2012;14:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sørensen TI, Lynge E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res. 2014;16:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Narvaez CJ, Matthews D, LaPorta E, Simmons KM, Beaudin S, Welsh J. The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Front Physiol. 2014;5:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906-913. [DOI] [PubMed] [Google Scholar]
- 103. Diorio C, Pollak M, Byrne C, et al. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14:1065-1073. [DOI] [PubMed] [Google Scholar]
- 104. Li Z, Hu CY, Mo BQ, Xu JD, Zhao Y. Effect of beta-carotene on gene expression of breast cancer cells (in Chinese). AiZheng. 2003;22:380-384. [PubMed] [Google Scholar]
- 105. Bowman GL, Shannon J, Ho E, et al. Reliability and validity of food frequency questionnaire and nutrient biomarkers in elders with and without mild cognitive impairment. Alzheimer Dis Assoc Disord. 2011;25:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Ann Transl Med. 2014;2:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Work ME, Reimers LL, Quante AS, Crew KD, Whiffen A, Terry MB. Changes in mammographic density over time in breast cancer cases and women at high risk for breast cancer. Int J Cancer. 2014;135:1740-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. van Gils CH, Hendriks JH, Holland R, et al. Changes in mammographic breast density and concomitant changes in breast cancer risk. Eur J Cancer Prev. 1999;8:509-515. [DOI] [PubMed] [Google Scholar]
- 109. Kerlikowske K, Ichikawa L, Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99:386-395. [DOI] [PubMed] [Google Scholar]
- 110. Li J, Humphreys K, Eriksson L, Edgren G, Czene K, Hall P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J Clin Oncol. 2013;31:2249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]