Abstract
Background. This meta-analysis examined the effects of exercise training on length of hospital stay, postoperative complications, exercise capacity, 6-minute walking distance (6MWD), and health-related quality of life (HRQoL) in patients following resection of non–small cell lung cancer (NSCLC). Methods. This review searched PubMed, EMBASE, and the Cochrane Collaboration data base up to August 16, 2015. It includes 15 studies comparing exercise endurance and quality of life before versus after exercise training in patients undergoing lung resection for NSCLC. Results. This review identified 15 studies, 8 of which are randomized controlled trials including 350 patients. Preoperative exercise training shortened length of hospital stay; mean difference (MD): −4.98 days (95% CI = −6.22 to −3.74, P < .00001) and also decreased postoperative complications for which the odds ratio was 0.33 (95% CI = 0.15 to 0.74, P = .007). Four weeks of preoperative exercise training improved exercise capacity; 6MWD was increased to 39.95 m (95% CI = 5.31 to 74.6, P = .02) .While postoperative exercise training can also effectively improve exercise capacity, it required a longer training period; 6MWD was increased to 62.83 m (95% CI = 57.94 to 67.72) after 12 weeks of training (P < .00001). For HRQoL, on the EORTC-QLQ-30, there were no differences in patients’ global health after exercise, but dyspnea score was decreased −14.31 points (95% CI = −20.03 to −8.58, P < .00001). On the SF-36 score, physical health was better after exercise training (MD = 3 points, 95% CI = 0.81 to 5.2, P = .007) while there was no difference with regard to mental health. The I2 statistics of all statistically pooled data were lower than 30%. There was a low amount of heterogeneity among these studies. Conclusions. Evidence from this review suggests that preoperative exercise training may shorten length of hospital stay, decrease postoperative complications and increase 6MWD. Postoperative exercise training can also effectively improve both the 6MWD and quality of life in surgical patients with NSCLC, but requiring a longer training period.
Keywords: exercise training, non–small lung cell cancer, rehabilitation, surgical resection, exercise endurance, health-related quality of life
Introduction
Lung cancer has been the most common cancer worldwide for several decades. Its incidence is ever increasing1 and is associated with the highest mortality.2-4 Patients with lung cancer report poorer health-related quality of life (HRQoL) and a higher prevalence of psychological distress than patients with other types of cancer. The major components of lung cancer treatment are chemotherapy, radiotherapy and surgery.5
Non–small cell lung cancer (NSCLC) is common, with surgical resection being the treatment of choice for stage I to III cancers.6 Exercise training may decrease the length of hospital stay and postoperative complications in such patients.7 Several studies have already shown that exercise training improved exercise capacity and HRQoL in NSCLC patients who underwent surgery.8-14 But the research has not disseminated into clinical practice and exercise training following lung resection is not yet routine.15 Although research on exercise programs in people with surgery for NSCLC suggests exercise interventions are safe and likely to be effective, yet at the same time there is demand for more data from further randomized controlled trials.16 The National Institute of Health and Clinical Excellence guidelines on lung cancer identified the need for further work to examine rehabilitation programs before and after surgery, stating that outcomes should include mortality, pulmonary complications, pulmonary function, and HRQoL assessment.17 There are multiple published reports on the benefits of preoperative exercise training in lung cancer patients. It may shorten length of hospital stay, reduce postoperative complications. Postoperative exercise training can improve exercise capacity and quality of life as well. Although the topic is frequently studied it is unfortunate that the studies are varied due to small sample sizes and difference in their approaches. Data on the effects of exercise training prior to or after surgery in NSCLC patients are still limited.
Aims
Thus, this review performed a random effects meta-analysis of available past and current studies on exercise training in surgical NSCLC patients with the aim to ascertain the effectiveness of exercise rehabilitation prior to and after surgery.
Methods
The study was designed according to the standards set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.18
Data Sources and Search
This review searched PubMed, EMBASE, and the Cochrane Collaboration databases using the key words “exercise training” or “rehabilitation,” “physical training” or “physical exercise” and “non-small cell cancer.” The search was limited to English language articles published by August 16, 2015.
Interventions and Outcome Measures
This review includes 15 studies comparing exercise endurance and HRQoL before and after exercise training in patients undergoing lung resection for NSCLC. Study inclusion criteria were the following: length of hospital stay, postoperative complications, 6-minute walk distance (6MWD), the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC-QLQ-C30),19 and 36-Item Short Form Health Survey (SF-36).20
Patients managed in any setting, that is, hospital, community facility, or home were included if they received an exercise-based intervention that included at least an aerobic exercise training component performed by the lower limbs (bicycle, treadmill walking) lasting 1, 4, 12, or 20 weeks, either alone or as part of a comprehensive rehabilitation program defined as also including components of muscle training, breathing exercises, health education, and psychological treatment.
Reviews, editorials, letters, case reports, and conference abstracts were excluded. Studies were excluded if there was an overlap in patients with another study within the same analysis. Thus, if some patients could possibly have been included in both the controlled and uncontrolled study analyses, they were only included once in any given analysis. Therefore, there was no overlap in populations included in our meta-analyses.
Data Extraction and Assessment of Risk of Bias
Two reviewers (HJN, PY) independently extracted data from eligible studies. Disagreements were resolved by consensus. Data pertaining to baseline characteristics of study subjects (number of subjects, age, sex, type of patients), exercise training program, duration and follow up, 6MWD, length of hospital stay, post-operative complication, EORTC-QLQ-C30 score (global Health, dyspnea score) and SF-36 score (physical function and mental health) were extracted.
For randomized controlled trials (RCTs), risk of bias was assessed for the domains as suggested by the Cochrane Handbook of Systematic Reviews,21 specifically emphasizing on sequence generation, allocation concealment, blinding, outcomes assessment, and selective reporting for the 8 randomized control trials included. For each criterion, risk of bias was assessed as (1) low risk of bias (adequate fulfillment of the respective criterion), (2) unclear (insufficient information to judge about fulfillment or nonfulfillment of the respective criterion), and (3) high risk of bias (inadequate fulfillment or nonfulfillment of the respective criterion).21,22 Risk of publication bias was assessed for each meta-analysis that included at least 10 studies.21 So this review did not detect clear publication bias as the numbers of included studies were small. As for the 5 single group trials (SGTs) and 2 controlled trials (CTs) reviewed, we used the Ottawa Quality Assessment Scale Cohort Studies. In the Newcastle-Ottawa Scale, studies are assigned up to 4 stars for selection, 2 for comparability, and 3 for outcome. For uncontrolled studies, the maximum available stars in the Newcastle-Ottawa scale is 3 for selection, 0 for comparability, and 3 for outcome.
Outcomes
The main outcome measure for the present analysis was length of hospital stay and postoperative complication. The following additional secondary parameters which were reported explicitly and clearly in part of the studies were also assessed: 6MWD, EORTC-QLQ-C30 score, and SF-36 score.
Data Synthesis and Statistical Analysis
The difference in change of length of hospital stay, postoperative complications, 6MWD, and EORTC-QLQ-C30 score and SF-36 score after exercise training versus control was pooled, stratified and analyzed using random-effects meta-analysis models with inverse variance weighting. The magnitude of heterogeneity present was estimated using the I2 statistic, an estimate of the proportion of the total observed variance that is attributed to between study variance.
Pooled effects on hospital stay, postoperative complication, 6MWD, EORTC-QLQ-C30, score and SF-36 score were presented as weighted mean differences (MDs) or odds ratio (ORs) with corresponding 95% confidence intervals (CIs). This review considered P < .05 as significant. Throughout, values are presented as mean ± SD unless otherwise stated. Analyses were performed using the Cochrane Collaboration Review Manager (version 5.2, Cochrane Collaboration, Copenhagen, Denmark).
Results
Characteristics of the Studies
Of 351 articles identified initially, 27 were retrieved for more detailed evaluation, Subsequently, 15 studies (8 randomized controlled trials)23-37 that included 350 patients were finally included in the analyses (Figure 1). Table 1 summarizes the design and methods of the included studies. The studies included patients with stage I to IV NSCLC. All the patients were adults referred for resection by thoracotomy or video-assisted thoracoscopic surgery. Some of them had chronic obstructive pulmonary disease. Eight studies including 238 patients delivered exercise training after surgery. Seven studies including 112 patients delivered exercise training before surgery. The exercise training program was largely similar, including bicycle, walking, breathing, and so on. Mean duration of the exercise training program was 8 ± 7 weeks (1, 4, 12, or 20 weeks). The mean age of subjects across studies ranged from 54 to 70 years.
Figure 1.
Flowchart showing the progress through the stages of meta-analysis.
Table 1.
Participants in Reviewed Trials.
| Study/First Author Year | Preoperative/Postoperative | Type of Study | Control Patients (F), n | Training Patients (F), n | Type of Patients | Age (Years) |
Exercise Training | Frequency | Length of intervention | Duration and Follow up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Patients | Training Patients | ||||||||||
| Arbane 2011 | Post | RCT | 26 | 27 | NSCLC, stage I-IV,100% post-surgical(open thoracotomy or VATS) | 62.6 (32-47) | 65.4 (47-82) | Resistance (weights) + aerobic (walking, marching, and recumbent bike) | 2×/day | 12 weeks (+5 days) | Aerobic 5-10 min for each component |
| Granger 2013 | Post | RCT | 8 | 7 | suspected lung cancer (those confirmed ¼ 10(67%)), stage I-IV, surgery all patients | 72.4 ± 12.4 | 57 ± 16.2 | Aerobic (walking + cycling) + resistance (upper and lower) + stretching | 2×/day until discharge then twice weekly | Postsurgery to discharge + 8 weeks outpatient | 1 h |
| Stigt 2013 | Post | RCT | 26 | 23 | NSCLC resectable, thoracotomy 4 weeks post-discharge | 63.2 ± 10.3 | 63.6 ± 10.2 | Aerobic (cycling) + resistance | 2×/week | 12 weeks | 1 h |
| Edvardsen 2015 | Post | RCT | 31 | 30 | NSCLC, stage I-IV,open thoracotomy or VATS. Stratified for receiving chemotherapy and having COPD | 65.9 ± 8.5 | 64.4 ± 9.3 | Aerobic (walking on treadmill) + resistance + muscle training | 3×/week | 20 weeks | 1 h |
| Arbane 2014 | Post | RCT | 67 | 64 | NSCLC, stage I-IV, surgery resection by thoracotomy or VATS | 68 (11) | 67 (11) | strength training + walking | Once daily | 4 weeks | 30 min |
| Benzo 2011 | Pre | RCT | 9 | 10 | lung cancer resection by open thoracotomy or VATS and moderate-severe COPD | 72 (6.69) | 70.2 (8.61) | Aerobic (treadmill, step and arm ergometer) + resistance + breathing | 2×/day over 5 days | 1 week | 20 min lower extremity + upper, extremity + strength, exercises + breathing, 20 min + education |
| Pehlivan 2011 | Pre | RCT | 30 | 30 | NSCLC stageIA-IIIB, surgery (lobectomy/pneumonectomy) | 54.76 ± 8.45 | 54.1 ± 8.53 | Aerobic (walking on treadmill + walking around the center) + breathing | 3×/day walking + 2×/day chest physiotherapy | 1 week preop until discharge | According to patient’s tolerance |
| Morano 2013 | Pre | RCT | 12 | 12 | NCSLC stage I-IIIA with pulmonary disease and impaired spirometry, surgical resection by thoracotomy or VATS | 68.6 ± 7.3 | 64.8 ± 8 | Strength and endurance + breathing + flexibility | 5×/week | 4 weeks | 10 min increasing to 30 min + resistance training + 10-30 min of IMT |
| Sekine 2005 | Pre | CT | 60 | 22 | NSCLC stage I-IV with COPD, thoracotomy | 70.4 ± 4.6 | 69 ± 5.5 | Breathing (incentive spirometry, abdominal breathing, huffing, and coughing) + aerobic (walking) | Daily (breathing 5× per day) | 2 weeks | Breathing 15 min 5×/day + pulmonary exercises for 30 min and walking more than 5000 steps every day |
| Cesario 2007 | Post | CT | 186 | 25 | NSCLC, Surgical (lateral muscle sparing thoracotomy) | NR | NR | Aerobic (cycling, walking) + breathing | 5×/week | 4 weeks | 3 h |
| Coats 2013 | Pre | SGT | 13 | under investigation for NSCLC (stage I-IV) awaiting surgical resection | 59 ± 9 | Aerobic (walking + cycling) + resistance | 3-5×/week | 4 weeks | Aerobic 30 min + muscle strength exercises | ||
| Jones 2007 | Pre | SGT | 25 | suspected surgical lung cancer stage I-IIIA | 65 ± 10 | Aerobic (cycling) | 5×/week on consecutive days | Until surgical resection, mean of 30 sessions | 20-30 min + 5 min warm up and 5 min cool down | ||
| Peddle-Mclntyre 2012 | Post | SGT | 17 | 94% NSCLC stage I-IIIB and limited stage SCLC, on average 3 and a half years post-surgical | 66.7 (50-85) | Resistance + breathing + stretching | 3×/week nonconsecutive days | 10 weeks (28 sessions) | NR | ||
| Reisenberg and Lubbe 2010 | Post | SGT | 45 | NSCLC stage I-IIIB+SCLC (2 limited an 1 extensive), undergone treatment (88% surgical); time since last treatment no more than 14 days | 60.2 ± 8.0 | Aerobic (cycling) | Daily | 28 days | 30 min per day interval training (3-5 min) | ||
| Jones 2008 | Post | SGT | 20 | NSCLC stage I-IIIB, 80% surgery | 62 ± 11 | Aerobic (cycling) | 3×/week on consecutive days | 14 weeks | 15-45 min increasing over 14 weeks | ||
Abbreviations: CT, controlled trial; RCT, randomized controlled trial; SGT, single group trial; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer; COPD, chronic obstructive pulmonary disease; NR, not reported; IMT, inspiratory muscle training; VATS, video-assisted thoracoscopic surgery.
For RCTs, evaluation of risk of bias of each trial and assessment of risk of bias by individual trials are illustrated in Figures 2 and 3, respectively. Three trials23,24,28 had a high drop-out rate in the control group, but failed to address this incomplete outcome with intention-to-treat analysis. Four trials24,25,27,29 were open to bias with false-positive results because of failure to blind participants in relation to intervention delivery. The risk of bias was low in the other studies; a detailed assessment is available in Table 2.
Figure 2.

Overall risk of bias assessment using the Cochrane tool.
Figure 3.

Risk of bias assessment by individual trials.
Table 2.
Bias Assessment of Cohort and Uncontrolled Studiesa.
| Study | Selection | Comparability | Outcome |
|---|---|---|---|
| Sekine 2005 | ☆☆☆ | ☆☆☆ | ☆☆☆ |
| Cesario 2007 | ☆☆☆ | ☆☆ | ☆☆☆ |
| Coats 2013 | ☆☆☆ | ☆☆ | |
| Jones 2007 | ☆☆☆ | ☆☆ | |
| Peddle-Mclntyre 2012 | ☆☆☆ | ☆☆☆ | |
| Reisenberg and Lubbe 2010 | ☆☆☆ | ☆☆ | |
| Jones 2008 | ☆☆☆ | ☆☆ |
The Ottawa Quality Assessment Scale Cohort Studies. In the Newcastle-Ottawa Scale, studies are assigned up to 4 stars for selection, 2 for comparability, and 3 for outcome. For uncontrolled studies, the maximum available stars in the Newcastle-Ottawa scale is 3 for selection, 0 for comparability, and 3 for outcome.
Effect of Exercise Training on Length of Hospital Stay and Postoperative Complications
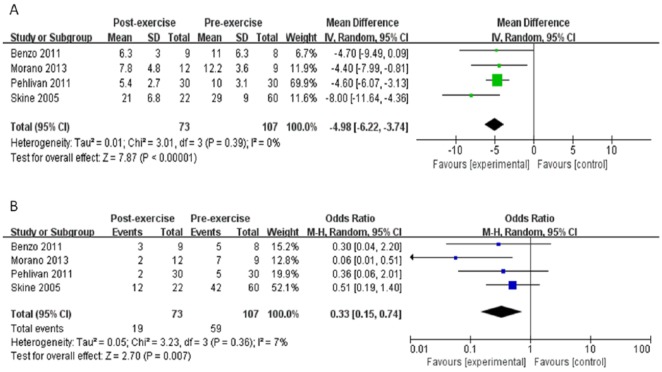
Preoperative exercise training shortened the length of hospital stay. In 4 studies,25-27,32 there was a marked decrease in length of stay of −4.98 days (95% CI = −6.22 to −3.74) after exercise training (P < .00001) (Figure 4A). No heterogeneity was apparent among studies (I2 = 0%). Exercise training in these 4 studies,25-27,32 also effectively decreased postoperative complications; the OR was 0.33 (95% CI = 0.15 to 0.74, P = .007) (Figure 4B). There was a low heterogeneity (I2 = 7%) among these studies.
Figure 4.
Meta-analysis of length of hospital stay and postoperative complication. (A) Changes of length of hospital stay after exercise training. (B) Changes of postoperative complication. CI, confidence interval(s); IV, inverse variance; SD, standard deviation; M-H, Mueller-Hinton.
Effect of Exercise Training on 6-Minute Walk Distance
Preoperative exercise training improved exercise capacity in 3 studies,26,33-34 6MWD was increased to 39.95 m (95% CI = 5.31 to 74.6) after 4 weeks training (P = .02); there was no heterogeneity (I2 = 0%) among these studies. While postoperative exercise training also effectively improved exercise capacity in the other 6 studies,23,28,29,31,36,37 6MWD was increased to 62.83 m (95% CI = 57.94 to 67.72) only after 12 weeks training (P < .00001). There was a low amount of heterogeneity (I2 = 7%) among these studies. (Figure 5A and B).
Figure 5.
Meta-analysis of 6-minute walk distance (6WMD). It shows the changes of 6MWD stratified by follow-up time after exercise training. A demonstrates shows the changes of 6WMD in preoperative exercise training patients. B shows the changes of 6WMD in postoperative exercise training patients. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Effect of Exercise Training on EORTC-QLQ-30 Score
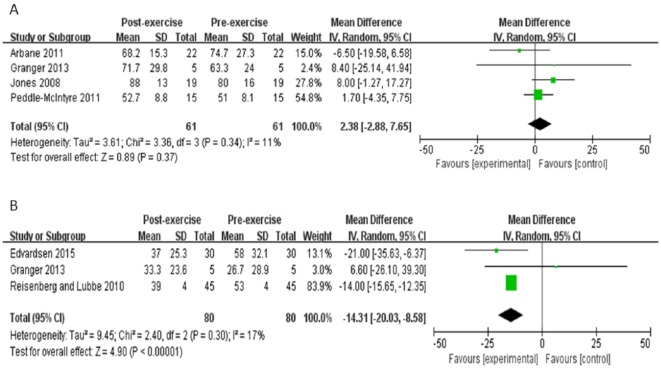
Four studies reported global health score.23,28,35,36 There was no alteration in patients’ global health after exercise training, cumulative MD 2.4 points (95% CI = −2.9 to 7.7, P = .37).
However, low heterogeneity was apparent among studies (I2 = 11%) (Figure 6A). Meanwhile, the other 3 studies reported the dyspnea score.28,30,37 Exercise training decreased the dyspnea score to −14.3 points (95% CI = −20 to −8.6, P < .00001). There was a low heterogeneity (I2 = 17%) between these studies (Figure 6B).
Figure 6.
Meta-analysis of Quality of Life Questionnaire. (A) Changes of EORTC-QLQ-30 in global health. (B) Changes of EORTC-QLQ-30 in dyspnea score. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Effect of Exercise Training on HRQoL Questionnaire SF-36 Score
Four studies reported HRQoL Questionnaire SF-36 score (physical health)28,30,35,36 and 3 reported the SF-36 score (mental health).28,35,36 Although the SF-36 score improved with exercise training (MD = 3 points, 95% CI = 0.81 to 5.2, P = .007), there was no alteration in mental health of patients (MD = 1.9 points, 95% CI = −0.5 to 4.4, P = .12) (Figure 7A and B). No heterogeneity was related to both physical health and mental health (I2 = 0%) (Figure 7A and B).
Figure 7.
Meta-analysis of Quality of Life Questionnaire. (A) Changes of SF-36 score in physical health. (B) Changes of SF-36 score in mental health. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Discussion
This review aims to evaluate the effects of exercise training on length of hospital stay, postoperative complications, exercise capacity (6MWD) and HRQoL in patients following resection of NSCLC. Preoperative exercise training may shorten length of hospital stay, decrease postoperative complications, and increase the 6MWD. Although postoperative exercise training also effectively improved the 6MWD and quality of life in surgical patients with NSCLC, the time taken for improvement was longer.
Data from 15 studies (8 RCTs) and 350 patients were included, which is still a small number for a meta-analysis. It comprehensively represents, however, most of the published experience of exercise training in patients with NSCLC prior to and after resection. In a meta-analysis, especially when the outcome is continuous, the number of included studies is more important than the number of patients included.
The outcome is clear as the studies show preoperative exercise training shortens the length of hospital stay and decreases postoperative complications. The results show that preoperative exercise training may shorten length of hospital stay by −4.98 days. Arbane et al23 reported that postoperative exercise training may shorten length of hospital stay by −2.1 days. Although more data are warranted, these results further strengthen the fact that preoperative exercise is more effective in shortening the length of hospital stay. Shortened hospital stay after exercise training may be associated with increased exercise capacity, increased muscle strength, reduced fatigue and improved pulmonary function.
The 6MWD was increased to 39.95 m after 4 weeks training (P = .02) in patients with preoperative exercise training, while postoperative exercise training also effectively improved the 6MWD to 62.83 m after 12 weeks training (P < .00001). This is consistent with the study of Cavalheri et al,38 which showed that postoperative exercise training improves the 6MWD. This meta-analysis demonstrates that both preoperative and postoperative exercise training can increase exercise capacity. These very positive effects on recovery of patients may relate to the improvement of their cardiopulmonary function, leading to an improved exercise tolerance in these patients. Furthermore, our meta-analyses shows that postoperative exercise training was more effective than preoperative exercise training. This may indicate that early postoperative exercise training is more likely to prompt recovery of pulmonary function and motor function in patients than preoperative exercise training.
This review also suggests that exercise training conferred an improved quality of life for patients following lung resection for NSCLC including the EORTC-QLQ-30 and the SF-36 scores. On the EORTC-QLQ-30, global health was no different after exercise, but the dyspnea score was lower (MD = −14.3 points, 95% CI = −20 to −8.6, P < .00001) after exercise. This demonstrates that exercise training improved dyspnea in postoperative patients. It is known that resistance training can increase peak oxygen uptake, especially in severely deconditioned adults.39 Hagerman et al40 showed that cancer patients’ regained muscle mass, improved their performance of daily life activities, reduced cancer-related fatigue and improved HRQoL after whole-body resistance training. One of the possible reasons perhaps is that exercise training decreased the dyspnea score. The SF-36 score showed an improved physical health but had no effective in mental health of patients undergoing lung resection after exercise training. This outcome is different from that of Cavalheri et al.38 They suggest that exercise training had little effect on HRQoL for people following lung resection for NSCLC. The article by Cavalheri et al38 reviewed 3 studies measuring the HRQoL. One used the EORTC-QLQ-C30 (Arbane et al23), one used the St George’s Respiratory Questionnaire (Stigt et al29), and one used the SF-36 (Brocki et al41). HRQoL in our article used the same measured parameter. The increased HRQol following exercise training may be related to increased muscle strength, reduced fatigue, and improved daily life activities. We recommend that future RCTs use equal measurement parameters in the relevant patient population.
Some of the included studies exposed some methodological flaws, thereby introducing high risk of biases into these trials, that is, some trials failed to blind research subjects, intervention delivery, and outcome assessors and some trials included insufficient sample sizes, which meant there was a potential risk of overestimating positive outcomes. Despite the difficulties, studies should blind the outcome assessors to minimize potential methodological biases. Therefore, the reliability of the evidence presented here is clearly limited.
Additionally, there were some other limitations to be considered when interpreting the results of this meta-analysis. First, there were not enough randomized controlled trials providing sufficient data on 6MWD and HRQoL. Second, inclusion was restricted to published studies and may therefore be affected by publication bias. Third, the follow-up rate was quite limited in many of the included studies. Most studies were short-term follow-ups of less than 3 months. Fourth, the exercise training programs was similar. However, the duration, intensity, frequency, and modality of exercise training varied between trials. The generalizability of our findings may therefore be limited. To improve generalizability, future exercise intervention trials should include larger, long-term, multicenter randomized controlled exercise training studies, which should include more data of quadriceps strength, forced expiratory volume in 1 second (FEV1), and so on. Few of the included studies reported the actual level of exercise training undertaken by participants.
Conclusion
Preoperative exercise training may shorten length of hospital stay, decrease postoperative complications, and increase the 6WMD while postoperative exercise training effectively improves 6MWD and improves HRQoL in surgical patients with NSCLC. Larger RCTs with long-term follow-up are needed to confirm the sustained efficacy and safety of exercise training in such a patient population.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Program of Shanghai Municipal Commission of Health and Family Planning (20144Y0196), Shanghai Natural Science Foundation (15ZR1434400), and Program of National Natural Science Foundation of China (81500040).
References
- 1. Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent non-small-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409-417. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [DOI] [PubMed] [Google Scholar]
- 3. Araujo LH, Lammers PE, Matthews-Smith V, et al. Somatic mutation spectrum of non–small cell lung cancer in African Americans: a pooled analysis. J Thorac Oncol. 2015;10:1430-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farley A, Aveyard P, Kerr A, Naidu B, Dowswell G. Surgical lung cancer patients’ views about smoking and support to quit after diagnosis: a qualitative study. J Cancer Surviv. 2016;10:312-319 [DOI] [PubMed] [Google Scholar]
- 5. Ferguson MK. Preoperative assessment of pulmonary risk. Chest. 1999;115:58-63. [DOI] [PubMed] [Google Scholar]
- 6. Sherwood JT, Brock MV. Lung cancer: new surgical approaches. Respirology. 2007;12:326-332. [DOI] [PubMed] [Google Scholar]
- 7. Takaoka ST. The value of preoperative pulmonary rehabilitation. Thorac Surg Clin. 2005;15:203-211. [DOI] [PubMed] [Google Scholar]
- 8. Reeve J, Stiller K, Nicol K, et al. A postoperative shoulder exercise program improves function and decreases pain following open thoracotomy: a randomised trial. J Physiother. 2010;56:245-252. [DOI] [PubMed] [Google Scholar]
- 9. Jones L, Eves N, Peterson B, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical non–small cell lung cancer patients: a pilot study. Cancer. 2008;113:3430-3439. [DOI] [PubMed] [Google Scholar]
- 10. Riesenberg H, Lubbe A. In-patient rehabilitation of lung cancer patients—a prospective study. Support Care Cancer. 2010;18:877-882. [DOI] [PubMed] [Google Scholar]
- 11. Spruit M, Janssen P, Willemsen S, Hochstenbag M, Wouters E. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer. 2006;52:257-260. [DOI] [PubMed] [Google Scholar]
- 12. Cesario A, Ferri L, Galetta D, et al. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer. 2007;57:175-180. [DOI] [PubMed] [Google Scholar]
- 13. Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;18:CD003793. [DOI] [PubMed] [Google Scholar]
- 15. Reeve J, Denehy L, Stiller K. The physiotherapy management of patients undergoing thoracic surgery: a survey of current practice in Australia and New Zealand. Physiother Res Int. 2007;12:59-71. [DOI] [PubMed] [Google Scholar]
- 16. Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health-related quality of life for patients with non–small cell lung cancer: a systematic review. Lung Cancer. 2011;72:139-153. [DOI] [PubMed] [Google Scholar]
- 17. National Institute for Health and Clinical Excellence. The Diagnosis and Treatment of Lung Cancer (Update). London, England: National Institute for Health and Clinical Excellence; 2013. http://guidance.nice.org.uk/CG121/Guidance. Accessed December 6, 2013. [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 20. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://handbook.cochrane.org/. Accessed June 20, 2015.
- 22. Zeng Y, Luo T, Finnegan-John J, Cheng AS. Meta-analysis of randomized controlled trials of acupuncture for cancer-related fatigue. Integr Cancer Ther. 2013;13:193-200. [DOI] [PubMed] [Google Scholar]
- 23. Arbane G, Tropman D, Jackson D, Garrod R. Evaluation of an early exercise intervention after thoracotomy for non–small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomized controlled trial. Lung Cancer. 2011;71:229-234. [DOI] [PubMed] [Google Scholar]
- 24. Arbane G, Douiri A, Hart N, et al. Effect of postoperative physical training on activity after curative surgery for non-small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy. 2014;100:100-107. [DOI] [PubMed] [Google Scholar]
- 25. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94:53-58. [DOI] [PubMed] [Google Scholar]
- 27. Pehlivan E, Turna A, Gurses A, Gurses HN. The effects of preoperative short term intense physical therapy in lung cancer patients: a randomized controlled trial. Ann Thorac Cardiovasc Surg. 2011;17:461-468. [DOI] [PubMed] [Google Scholar]
- 28. Granger CL, Chao C, McDonald CF, Berney S, Denehy L. Safety and feasibility of an exercise intervention for patients following lung resection: a pilot randomized controlled trial. Integr Cancer Ther. 2013;12:213-224. [DOI] [PubMed] [Google Scholar]
- 29. Stigt JA, Uil SM, van Riesen SJ, et al. A randomized controlled trial of post-thoracotomy pulmonary rehabilitation in patients with resectable lung cancer. J Thorac Oncol. 2013;8:214-221. [DOI] [PubMed] [Google Scholar]
- 30. Edvardsen E, Skjønsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax. 2015;70:244-250. [DOI] [PubMed] [Google Scholar]
- 31. Cesario A, Ferri L, Galetta D, et al. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer. 2007;57:175-180. [DOI] [PubMed] [Google Scholar]
- 32. Sekine Y, Chiyo M, Iwata T, et al. Perioperative rehabilitation and physiotherapy for lung cancer patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg. 2005;53:237-243. [DOI] [PubMed] [Google Scholar]
- 33. Coats V, Maltais F, Simard S, et al. Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Can Respir J. 2013;20:e10-e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones LW, Peddle CJ, Eves ND, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590-598. [DOI] [PubMed] [Google Scholar]
- 35. Jones LW, Eves ND, Peterson BL, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients. Cancer. 2008;113:3430-3439. [DOI] [PubMed] [Google Scholar]
- 36. Peddle-McIntyre CJ, Bell G, Fenton D, McCargar L, Courneya KS. Feasibility and preliminary efficacy of progressive resistance exercise training in lung cancer survivors. Lung Cancer. 2012;75:126-132. [DOI] [PubMed] [Google Scholar]
- 37. Riesenberg H, Lübbe AS. In-patient rehabilitation of lung cancer patients—a prospective study. Support Care Cancer. 2010;18:877-882. [DOI] [PubMed] [Google Scholar]
- 38. Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training for people following lung resection for non-small cell lung cancer—a Cochrane Systematic review. Cancer Treat Rev. 2014;40:585-594. [DOI] [PubMed] [Google Scholar]
- 39. Strasser B, Steindorf K, Wiskemann J, et al. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45:2080-2090. [DOI] [PubMed] [Google Scholar]
- 40. Hagerman FC, Walsh SJ, Staron RS, et al. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci. 2000;55:B336-B346. [DOI] [PubMed] [Google Scholar]
- 41. Brocki B, Rodkjær L, Nekrasas V, Due K, Dethlefsen C, Andreasen J. Rehabilitation after lung cancer operation; a randomised controlled study [abstract]. Ann ERS Annu Congress. 2010;331s. [Google Scholar]