Abstract
Hypotheses. Sleep disorders are associated with an increased risk of cancer, including breast cancer (BC). Physical activity (PA) can produce beneficial effects on sleep. Study design. We designed a randomized controlled trial to test the effect of 3 months of physical activity on sleep and circadian rhythm activity level evaluated by actigraphy. Methods. 40 BC women, aged 35-70 years, were randomized into an intervention (IG) and a control group (CG). IG performed a 3 month of aerobic exercise. At baseline and after 3 months, the following parameters were evaluated both for IG and CG: anthropometric and body composition measurements, energy expenditure and motion level; sleep parameters (Actual Sleep Time-AST, Actual Wake Time-AWT, Sleep Efficiency-SE, Sleep Latency-SL, Mean Activity Score-MAS, Movement and Fragmentation Index-MFI and Immobility Time-IT) and activity level circadian rhythm using the Actigraph Actiwatch. Results. The CG showed a deterioration of sleep, whereas the IG showed a stable pattern. In the CG the SE, AST and IT decreased and the AWT, SL, MAS and MFI increased. In the IG, the SE, IT, AWT, SL, and MAS showed no changes and AST and MFI showed a less pronounced change in the IG than in the CG. The rhythmometric analysis revealed a significant circadian rhythm in two groups. After 3 months of PA, IG showed reduced fat mass %, while CG had improved weight and BMI. Conclusion. Physical activity may be beneficial against sleep disruption. Indeed, PA prevented sleep worsening in IG. PA can represent an integrative intervention therapy able to modify sleep behaviour.
Keywords: breast cancer, physical activity, sleep, sleep quality, actigraphy, anthropometry
Introduction
Sleep is a state resulting from the interaction between physiological and behavioral factors. Quantity and quality of sleep represent important factors for the quality of life, which can have positive or negative influence on individual health.1-3 The assessment of the risks and benefits associated with a particular life style is a precondition to modifying habits that are responsible for the development of pathological processes.
Evidence exists that sleep disorders are associated with an increased risk of cancer. For instance, shift workers show an increased risk of numerous cancers.4-8 On the other hand, the diagnosis of cancer and/or cancer therapies are often associated with fatigue and depression that can exacerbate a preexisting sleep disorder or contribute to initiating one. Breast cancer (BC) is the most common malignancy in women, and it is the main cause of death from women’s cancer. BC survivors are constantly increasing, and research investments to identify modifiable factors associated with BC recurrences are increasing too. Elucidation of the relationships between sleep quality and cancer is thus of interest both in prevention of the primary pathology as well as in the prevention of potential recurrences.
Physical activity (PA) is one of the lifestyle-related factors that are decisive for the quality of sleep.9-13 The relationship between sleep and PA has been actively investigated in the past, and several studies have shown that engaging in regular physical exercise can produce beneficial effects on nighttime sleep.9,10,14 Certainly, PA reduces body weight, insulin resistance,15 and inflammation,13,16,17 which are all important factors related to sleep. Extensive research has been conducted to evaluate the positive impact of exercise after BC diagnosis. These studies provided support for reduced mortality from this disease among women who engage in moderate PA.18-25 However, few studies26,27 have evaluated the effect of a structured program of aerobic PA on nocturnal sleep by using actigraphy. Mustian et al26 demonstrated that 4 weeks of yoga is a useful therapy for posttreatment cancer survivors with impaired sleep, improving sleep quality; significant improvements have been demonstrated with respect to global sleep quality, sleep latency, sleep duration, sleep efficiency. Payne et al27 suggest that a prescribed home-based walking exercise intervention may improve sleep in older women receiving hormonal treatment for BC. It stands to reason that the practice of a regular PA in women with BC can help regularize the sleep-wake cycle and improve nocturnal sleep.
The aim of this randomized controlled trial was to evaluate the effect of an aerobic PA program on sleep behavior in BC survivors. If PA is able to act positively on sleep, this finding would strengthen the importance of exercise in the integrated treatment of cancer. Actigraphy, which offers one of the best-known alternatives to polysomnography, was used to derive sleep parameters.28-32
Materials and Methods
Subjects
The women included in this study belong to the Diet and Androgen-5 study (DIANA-5),33,34 an ongoing multi-institutional randomized controlled trial aiming to test the hypothesis that a dietary change based on the Mediterranean diet and macrobiotic principles, together with moderate PA, can reduce the incidence of additional BC-related events in women with BC. Inclusion criteria in DIANA-5 study were as follows:
mastectomy or conservative surgery for primary invasive BC, any type, within the previous 5 years
no history or objective evidence of recurrent disease or metachronous ipsilateral or contralateral BC
35 to 70 years of age at recruitment
not scheduled for and not currently undergoing chemotherapy
no other cancer diagnosis within the last 10 years
no comorbidity requiring a specific diet or contraindicating a high-fiber diet, moderate calorie restriction, or physical exercise
no medical or social condition that would interfere with participation in a 5-year diet and physical exercise study
able to fill-in self-administered questionnaires
In February 2014, 42 women with BC belonging to the intervention arm of the DIANA-5 study agreed to participate in the present study (see the Statistical Analysis section for a description of the power analysis carried out to choose the sample size). In addition to the inclusion criteria mentioned above, the women had no family or working impediment that could preclude participation in the PA program. All women had participated in DIANA-5 for at least 2 years and received the same dietary recommendations and activities.
Women were randomized in the intervention group (IG) or the control group (CG). Since 2 women of IG dropped out because of medical problems, the IG was composed of 19 subjects while the CG had 21. The dropouts in the IG occurred before the beginning of the PA program.
The mean age (± SD) at study entry was similar for both groups with no statistical differences: 55.2 (± 6.8) years for the IG and 58.2 (± 6.4) years for the CG.
Women in the CG received only the WCRF/AICR recommendation to be physically active for at least 30 minutes every day. Women in the IG received the same recommendations, but were also requested to participate in a 3-month active PA program that included 2 sessions of 1-hour brisk walking per week. During the 3 months of the trial program all women, both IG and CG, continued to participate in the DIANA-5 study activities including cooking classes and nutritional advice. An informed consent document was obtained from each participant before the beginning of the intervention, and all risks and benefits of the study were explained. The study was approved by the Ethical Review Board of the National Cancer Institute of Milan (Italy).
Physical Activity intervention
Women included in the IG attended a program of aerobic PA over a period of 3 months, from the first week of March to the last week of May 2014. The PA program was conducted by 2 sport therapy experts and included 2 sessions of 1-hour brisk walking per week, on Wednesday and Friday. Each training session also included 10 minutes of cool-down of static stretching at the end of the walking. Data about the attendance to the training were collected each day before the training started. At the beginning of the physical tasks, each subject wore the heart rate monitor (Polar s810, Polar Electro Oy, Kempele, Finland), set to record the beat-to-beat interval of every heartbeat. The heart rate data collected during the walking sessions were downloaded to the Polar Precision Performance software 3.0. The mean heart rate (HRmean) of each participant was studied through descriptive analysis. The IG performed an aerobic PA since the range of the HRmean of the group was 58% to 73% with a mean group of 68% of the maximum heart rate. The adherence to PA was 86.8 ± 4.9% (expressed as percentage of the total numbers of training sessions). The IG PA sessions were carried out on fixed days and times with the same trainer during 3 months.
At baseline (last week of February/PRE) and at the end (first week of June/POST) of the 3-month PA program, both groups underwent an anthropometric and body composition evaluation, an actigraph-based monitoring of sleep and activity-level parameters, and an armband-based monitoring of motion-level parameters.
Anthropometric and Body Composition Evaluation
Anthropometric and body composition measurements (percentages of fat mass and lean mass) were taken using a body segmental impedance balance, Tanita BC-418 (Body Composition Analyzer BC-41 AM, Tanita Corporation, Tokyo, Japan). Waist circumference was measured with a measuring tape in the midpoint between the lowest rib and the iliac crest during exhale.
Actigraphic Monitoring of Sleep Patterns
The subjects wore the Actigraph Actiwatch (Cambridge Neurotecnology, Cambridge, UK) for 1 week (from Monday to Sunday) on the nondominant hand for the evaluation of sleep parameters, both at baseline and after 3 months of PA. We used a low actigraphic sensitivity threshold (80 counts per epoch). Together with the actigraph, each subject received a diary to record the information about bed time, wake up time, hours of naps, hours without wearing the actigraph, and number of nocturnal awakenings. The data recorded by the actigraph were analyzed by the Actiwatch Sleep Analysis Software with the aim of deriving parameters capable to quantify the quantity and quality of sleep. Data analysis started with the onset of nocturnal rest (bedtime) and ended with the onset of daytime activity (wake time). Exact bedtimes and waking times were set for each subject using information from the rest-activity pattern and diary. The software yielded the following sleep parameters.
Actual Sleep Time (AST)
The amount of time between Sleep Start and Sleep End. This is determined by the summation of the number of epochs that do not exceed the sensitivity threshold and multiplying that value by the epoch length in min.
Actual Wake Time (AWT)
The total time spent awake according to the epoch-by-epoch wake/sleep categorization.
Sleep Efficiency (SE)
The percentage of time in bed actually spent sleeping.
Sleep Latency (SL)
The period of time required for sleep onset after retiring to bed. SL is the period between Bed Time and Sleep Start. This is automatically calculated by an algorithm, based on lack of movement following Bed Time.
Immobility Time (IT)
The total time, expressed in percentage, spent without recording any movement within the period of Sleep Start and Sleep End.
Mean Activity Score (MAS)
It represents the average activity score recorded between Sleep Start and Sleep End.
Movement and Fragmentation Index (MFI)
The addition of the Movement Index (percentage time spent moving) and the Fragmentation Index (percentage of immobile phases of one minute). MFI is used as an index of restlessness.
Actigraphic Monitoring of Activity Level and Circadian Rhythm
The data recorded by the actigraph were analyzed using Actiwatch Activity Analysis software to obtain the activity data for all women at baseline and after 3 months. These data were used to evaluate the activity-level circadian rhythm for each subject. A parametric portrait of the circadian rhythm was obtained consisting of 3 parameters:
MESOR (M). This represents the mean activity for a 24-hour period.
Amplitude (A). This measures one half the extent of the rhythmic variation in a cycle.
Acrophase (φ). This measures the time interval within which the highest values of activity are expected.
Armband-Based Monitoring of Energy Expenditure
The SenseWear Pro 3 Armband (Body-Media, Pittsburgh, USA) was used to collect data on energy expenditure (EE) and motion level.35-37 All women wore the device for 1 week on the right upper arm over the triceps muscle. All patients were instructed to continuously wear the Armband for an average of 7 consecutive days except during water-based activities. Algorithms provided by the manufacturer combine the sensor data with age, body weight, height, gender, smoking status, and handedness to produce minute-by-minute estimates of Total Energy Expenditure and to quantify, with selectable thresholds of Metabolic Equivalents, daily motion level.
The armband provided the following metabolic and motion level parameters:
Total Energy Expenditure (TEE)
Numbers of steps per day
Metabolic Equivalent (MET = 1 kcal/kg/h)
Physical Activity Level (PAL = TEE/REE), where REE is the Rest Energy Expenditure
Daily targets PAL ≥ 1.7 were used to classify subjects as physically active or not. The SenseWear software estimates Physical Activity Duration (PAD) spent for a specific period above a set threshold in METs. Usually, the threshold value of 3.0 METs is an acceptable value of the minimum activity (by sedentary lifestyle), which is the equivalent of a brisk walk.
Statistical Analysis
Unless otherwise stated, the statistical analyses were conducted using SPSS version 21 software (IBM Corporation, Armonk, NY), and a P value less than or equal to .05 was considered statistically significant. Below we describe the power analysis carried out to choose the sample size of the study, as well as the statistical analyses of sleep, rhythmometric, anthropometric, and motion-level parameters.
Power Analysis for Determination of the Sample Size
In order to arrive at a reasonable sample size for each of the 2 groups participating in the study, we carried out a power analysis that focused on the ability of the study to detect changes in sleep efficiency, which is perhaps the most clinically meaningful actigraphy-based sleep parameter. To perform the power analysis, we needed an estimation of the variability of Sleep Efficiency. In a previous investigation,3 we had found that Sleep Efficiency in normal subjects was about 84 ± 5% (mean ± SD), and thus we set SD = 5%. We decided that the magnitude of the clinical difference of interest in Sleep Efficiency between the PRE and POST conditions (ie, the smallest effect that we wanted to be able to detect) was in the range 3% to 4%. By setting the power (1 − β) to .80 and the significance level α to .05 (2-tailed), we found that the sample size of each group had to be between 14 and 24 subjects (calculations were carried using the sample size calculator developed by Russel Lenth and available at http://www.stat.uiowa.edu/~rlenth/Power). We finally set the sample size to 21 subjects per group.
Analysis of Sleep Parameters
Sleep behavior was described by 7 actigraphy-based sleep parameters (AST, AWT, SE, SL, IT, MAS, and MFI). Each parameter was evaluated twice (pretest and posttest) in both groups (intervention and control). The parameter results were expressed as mean ± SD. The distribution of each parameter in the pretest and posttest conditions was tested for normality by graphical methods and the Shapiro-Wilk’s test. All the parameters were normally distributed with the exception of SL and IT. In order to test the null hypothesis for no difference between the pretest scores of the control and intervention groups, SL and IT were subjected to the nonparametric Mann-Whitney rank test, whereas AST, AWT, SE, MAS, and MFI were subjected to the unpaired Student’s t test. SL and IT were then subjected to the Wilcoxon paired-sample test to test the null hypothesis for no difference between the pretest and posttest scores, whereas the 5 sleep parameters displaying normal distribution (AST, AWT, SE, MAS, and MFI) were subjected to repeated-measures multivariate analysis of variance (RM-MANOVA) to test whether there was an overall change of sleep behavior over time between the intervention and control groups. Before running RM-MANOVA, a correlation-matrix analysis was carried out to ascertain the degree of correlation between each pair of dependent variables. We found that AWT and SE were highly and negatively correlated (Pearson’s r = −0.82 in both the pretest and posttest conditions) and AWT and MAS were highly and positively correlated (r = 0.81 and r = 0.85 in the pretest and posttest conditions, respectively). In order to avoid multicollinearity, the offending variable AWT was removed from RM-MANOVA and was subjected to a mixed ANOVA characterized by one within-subjects factor (time, with 2 levels: pretest and posttest) and one between-subjects factor (group, with 2 levels: intervention and control). The purpose of the 2-way repeated-measures ANOVA was to establish whether AWT differed between the 2 groups over time and whether there was an interaction between group and time. The set of the other 4 dependent variables (AST, SE, MAS, and MFI) was subjected to RM-MANOVA (within-subjects factor: time; between-subjects factor: group). RM-MANOVA provided both a multivariate effect and a set of univariate effects. The multivariate effect quantified the overall impact that group, time, and their interaction had on sleep behavior (described by that linear combination of the dependent variables providing the best separation between the groups and the time points). The univariate effects quantified how much the mean value of each dependent variable differed across groups and time points. Within each univariate effect, the interaction between group and time was also evaluated. The presence of a significant interaction indicated that the modality of change of the sleep parameter over time (from the pretest to the posttest) depended on the group. When the univariate analysis showed a significant interaction, the mean of the dependent variable was compared across time points by performing 2 paired Student’s t tests, one for the control group and one for the intervention group. In each case, cutoff levels for statistical significance were adjusted according to Bonferroni’s correction to account for multiple comparisons (cutoff = 0.05/2 = 0.025).
Rhythmometric Analysis of Activity
To evaluate the activity-level circadian rhythm, we analyzed the activity data using single cosinor method.38,39 Hinging on the least-squares method, the single cosinor method identifies and evaluates the cosine mathematical function that best fits the data as a function of time. The function, f(t) = M + A cos(ωt + φ), defines 3 parameters characteristic of each statistically significant rhythm: M is the MESOR; A is the amplitude; φ is the acrophase. MESOR (Midline Estimating Statistic of Rhythm) is a rhythm-adjusted mean that approximates the arithmetical mean of the data for a 24-hour period, and amplitude is the measure of one half of extent of the rhythmic variation in a cycle. Acrophase indicates, with its 95% confidence limits (CL), the time interval within which the highest values of activity are expected. The 3 parameters are usually indicated with the relevant 95% confidence intervals. Each parameter was evaluated twice (pretest and posttest) in both groups (intervention and control). The rhythmometric parameters of activity levels (MESOR, Amplitude, and Acrophase) were then processed with the average of population mean cosinor. This method, applied to the rhythmometric parameters of each subject’s circadian variables, evaluates the rhythmometric characteristics of the activity levels of the population and provides a significant P value (P < .05) when a circadian rhythm is present.39 The rhythmometric analysis was carried out with Time Series Analysis-Seriel Cosinor 6.0 by Expert Soft Technologie.
The statistical analysis of the rhythmometric parameters (MESOR, Amplitude, and Acrophase) followed the same rationale outlined above for the sleep parameters. The distribution of each parameter in the pretest and posttest conditions was tested for normality by graphical methods and the Shapiro-Wilk’s test. No marked deviations from normality were detected, and the parameters were expressed as mean ± SD. In order to test the null hypothesis for no difference between the pretest scores of the control and intervention groups, the parameters were subjected to the unpaired Student’s t test. Then, the 3 parameters were subjected to RM-MANOVA to test whether there was an overall change of circadian rhythmicity over time between the intervention and control groups. Before running RM-MANOVA, a correlation-matrix analysis was carried out to ascertain the degree of correlation between each pair of dependent variables. We found that MESOR and amplitude were highly and positively correlated (r = −0.93 in both the pretest and posttest conditions). In order to avoid multicollinearity, MESOR was removed from RM-MANOVA and was subjected to a mixed ANOVA. Finally, RM-MANOVA was carried out on a set of 2 dependent variables (Amplitude and Acrophase). RM-MANOVA provided both a multivariate effect and a set of univariate effects that were evaluated using the same approach as discussed above for the sleep parameters.
Analysis of Anthropometric and Motion-Level Parameters
The anthropometric parameters (weight, body mass index [BMI], waist circumference, fat mass, lean mass) and the motion-level parameters (TEE, number of steps, METs average, and PAL) were analyzed as follows. Each parameter was evaluated twice (pretest and posttest) in both groups (intervention and control). The distribution of each parameter in the pretest and posttest conditions was tested for normality by graphical methods and the Shapiro-Wilk’s test. All the parameters were normally distributed and were expressed as mean ± SD. In order to test the null hypothesis for no difference between the pretest scores of the control and intervention groups, the parameters were subjected to the unpaired Student’s t test. To test the null hypothesis for no difference between the pretest and posttest scores for no difference across time points, 2 paired Student’s t tests were performed, one for the control group and one for the intervention group. In each case, cutoff levels for statistical significance were adjusted according to Bonferroni’s correction to account for multiple comparisons (cutoff = 0.05/2 = 0.025).
We investigated the correlation between anthropometric, PA data, and sleep parameters with the Pearson correlation coefficient (r).
Results
Sleep Parameters
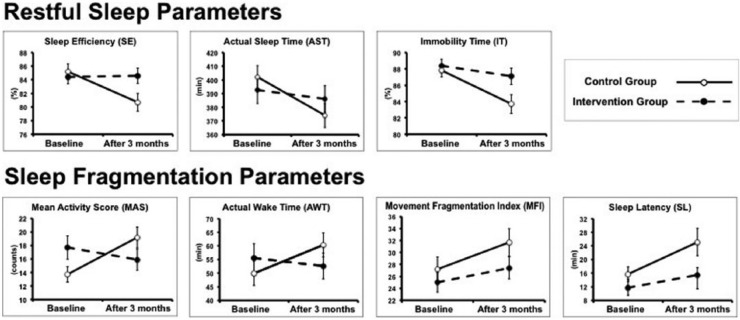
Table 1 shows the sleep parameters measured at baseline (PRE) and after 3-months (POST) in the intervention group (IG) and in the control group (CG). Figure 1 allows a visual appreciation of the trajectories of the sleep parameters in the 2 groups from PRE to POST. Three out of 7 parameters (ie, SE, AST, IT) are representative for restful sleep (the higher the parameter, the better). The other 4 parameters (ie, MAS, AWT, MFI, SL) are representative for fragmented sleep (the lower the parameter, the better). The presence of an interaction between group and time is visualized as 2 nonparallel lines (one line per group) connecting the levels of the sleep parameter measured at the 2 time points (PRE and POST).
Table 1.
Sleep Parameters at Baseline (PRE) and After 3 Months (POST) for Intervention Group (IG) Versus Control Group (CG)a.
| Parameters | IG |
CG |
||||
|---|---|---|---|---|---|---|
| PRE | POST | Effect of Time | PRE | POST | Effect of Time | |
| SE (%) | 84.4 ± 4.3 | 84.6 ± 4.9 | ns | 85.2 ± 5.2 | 80.7 ± 6.0 | P < .001b |
| AST (minutes) | 392.6 ± 42.4 | 386.1 ± 42.2 | P = .03c | 402.1 ± 37.5 | 374.1 ± 40.3 | P = .03c |
| IT (%) | 88.4 ± 3.5 | 87.1 ± 4.4 | ns | 87.8 ± 3.6 | 83.7 ± 5.3 | P < .001d |
| MAS (counts) | 17.7 ± 7.6 | 15.9 ± 6.8 | ns | 13.7 ± 5.2 | 19.2 ± 7.1 | P < .001b |
| AWT (minutes) | 55.6 ± 22.5 | 52.6 ± 20.5 | ns | 49.9 ± 20.4 | 60.3 ± 20.4 | P = .002e |
| MFI | 25.0 ± 7.1 | 27.4 ± 8.0 | P = .02c | 27.2 ± 9.4 | 31.7 ± 10.6 | P = .02c |
| SL (minutes) | 11.7 ± 11.1 | 15.4 ± 9.5 | ns | 15.7 ± 10.0 | 25.1 ± 18.5 | P = .046d |
Abbreviations: SE, sleep efficiency; AST, actual sleep time; IT, immobility time; MAS, mean activity score; AWT, actual wake time; MFI, Movement & Fragmentation Index; SL, sleep latency; ANOVA, analysis of variance; RM-MANOVA, repeated-measures multivariate analysis of variance.
Values presented as mean ± SD. No significant differences were observed in the sleep parameters between IG and CG in the PRE condition. See the main text for details about the statistical analyses.
RM-MANOVA followed by univariate analyses that found a significant interaction between group and time. The effect of time was assessed by using paired Student’s t tests with Bonferroni’s correction.
RM-MANOVA followed by univariate analyses that found no interaction between group, but a significant effect of time.
Wilcoxon test.
Mixed ANOVA that found a significant interaction between group and time. The effect of time was assessed by using paired Student’s t tests with Bonferroni’s correction.
Figure 1.
Visual appreciation of the trajectories of the sleep parameters in the IG and CG from PRE (baseline conditions) to POST (after 3 months). Three out of 7 parameters (ie, SE, AST, IT) are representative of Restful Sleep (the higher the parameter, the better). The other 4 parameters (ie, MAS, AWT, MFI, SL) are representative of Fragmented Sleep (the lower the parameter, the better). The presence of an interaction between group and time is visualized as 2 nonparallel lines (one line per group) connecting the levels of the sleep parameter measured at the 2 time points (PRE and POST). For the statistical analysis, refer to the text.
Prior to the beginning of the experimental protocol, IG and CG were comparable for all the sleep parameters (no significant differences were observed in the sleep parameters of the 2 groups in the PRE condition).
The Wilcoxon test showed that differences between the PRE and POST levels of parameters SL and IT were not statistically significant in the IG, but were statistically significant in the CG (P = .046 for SL and P < .001 for IT). The mixed ANOVA performed on parameter AWT showed a statistically significant interaction between time and group (F[1, 38] = 6.7, P = .014), indicating that the changes displayed by this parameter differed between the 2 groups from PRE to POST. The source of such interaction was examined by performing 2 paired Student’s t tests with Bonferroni’s correction of the significance level (α = 0.05/2 = 0.025). We found that AWT increased significantly in the CG (P = .002) from PRE to POST, but remained unchanged in the IG.
The multivariate outcome of RM-MANOVA showed the presence of a significant interaction between time and group (Pillai’s trace = 2.33, F[4, 35] = 2.81, P = .04), indicating that the 2 groups exhibited a different pattern of change of sleep behavior across time. The univariate analyses showed a significant interaction between time and group for SE (F[1, 38] = 7.8, P = .008) and MAS (F[1, 38] = 9.2, P = .004), while AST came close to but did not achieve the statistical significance (P = .06). Both SE and MAS were then subjected to 2 paired Student’s t tests with Bonferroni’s correction of the significance level (α = 0.05/2 = 0.025) to determine the source of the interaction. We found that SE decreased significantly in the CG (P < .001) from PRE to POST, but remained unchanged in the IG, whereas MAS increased significantly in the CG (P < .001) from PRE to POST, but remained unchanged in the IG. The 2 parameters that did not achieve a significant interaction between time and group (ie, AST [P = .06] and MFI [P = .4]), nevertheless, displayed a significant main effect of time. AST showed a significant decrease from PRE to POST (F[1, 38], P = .03), while MFI showed a significant increase from PRE to POST (F[1, 38], P = .02). All these results indicate that the subjects of the CG showed a generalized deterioration of their sleep behavior, whereas the subjects of the IG maintained their sleep behavior more stable.
Activity-Level Circadian Rhythm
Table 2 reports the rhythmometric parameters measured in the IG and CG. The single cosinor method revealed the presence of a statistically significant activity level circadian rhythm (P < .001) in each one of the 40 women. The population mean cosinor applied to the IG and CG, at baseline (PRE) and after 3 months (POST), revealed the presence of a significant circadian rhythm with in the 2 groups (P < .001). MESOR, Amplitude, and Acrophase were not different in the 2 groups in the pretest condition. The mixed ANOVA carried out on MESOR showed no statistically significant main effect for time and group, neither a statistically significant interaction between time and group, indicating that MESOR was virtually the same in the 2 groups and remained stable across time. RM-MANOVA showed a statistically significant effect of time (Pillai’s trace = 0.17, F[2, 37] = 3.6, P = .04), but no interaction between time and group, indicating that the circadian rhythm (as portrayed by the linear combination of Amplitude and Acrophase) changed at virtually the same rate in the 2 groups between PRE and POST. The univariate analyses established that the only effect achieving statistical significance was the main effect of time on Amplitude, that decreased significantly between PRE and POST (F[1, 38] = 6.4, P = .02).
Table 2.
Rhythmometric Analysis (Population Mean Cosinor) for Intervention Group (IG) and Control Group (CG) at Baseline (PRE) and After 3 Months (POST)a.
| Groups | MESOR (Mean ± SE) | Amplitude (Mean and 95% CL) | Acrophase (h:min) (Mean and 95% CL) | |
|---|---|---|---|---|
| IG (N = 19) | PRE | 232.9 ± 18.1 | 197.8 (167.4-228.1) | 14:52 (12:30-17:14) |
| POST | 221.9 ± 10.8 | 177.9 (164.8-191) | 15:00 (12:45-17:15) | |
| CG (N = 21) | PRE | 223.7 ± 14.5 | 197.2 (177.5-216.8) | 14:31 (12:23-16:39) |
| POST | 215.8 ± 9.5 | 179.6 (166.1-193.1) | 14:47 (12:32-17:02) |
Abbreviation: CL, confidence limit.
The table reports results of the actigraphy-based analysis of the activity-level circadian rhythmicity in the IG and CG at baseline (PRE) and after 3 months (POST). The population mean cosinor applied to IG and CG at PRE and POST revealed the presence of a significant circadian rhythm in the 2 groups (P < .001). MESOR, Amplitude, and Acrophase were not different in the 2 groups in pretest conditions. Amplitude decreased significantly between PRE and POST (F[1, 38] = 6.4, P = .02). MESOR and Acrophase remained unchanged.
Anthropometric and Motion-Level Parameters
Table 3 shows the anthropometric and motion-level parameters measured at baseline (PRE) and after 3 months (POST) in the IG and CG. We ascertained that, prior to the beginning of the experimental protocol, IG and CG were comparable for all the anthropometric and motion-level parameters (no significant differences were found in anthropometric and motion-level parameters of the 2 groups in the PRE condition). Of note, prior to the beginning of the PA program, both groups presented an inactive lifestyle (PAL < 1.7 METs). Table 3 also reports the statistical assessment of the comparison between the PRE versus POST anthropometric and motion-level parameters in the 2 groups. After 3 months of PA, CG had significantly improved weight and BMI (P = .01). Only in the IG we observed a significant reduction in fat mass % (P = .02). No changes in the indices of motion level were found in IG and CG.
Table 3.
Anthropometric and Motion-Level Data at Baseline (PRE) and After 3 Months (POST) for Intervention Group (IG) Versus Control Group (CG)a.
| Parameters | IG (N = 19) |
CG (N = 21) |
||||
|---|---|---|---|---|---|---|
| PRE | POST | P | PRE | POST | P | |
| Weight (kg) | 67.1 ± 11.9 | 66.6 ± 11.8 | .03 | 64.5 ± 9.6 | 63.7 ± 9.5 | .01 |
| BMI (kg/m2) | 25.4 ± 3.8 | 25.2 ± 3.7 | .04 | 24.6 ± 3.4 | 24.3 ± 3.4 | .01 |
| Waist circumference (cm) | 80.5 ± 11.0 | 79.8 ± 10.0 | .04 | 80.3 ± 8.7 | 80.1 ± 9.1 | ns |
| Fat mass (%) | 33.7 ± 5.5 | 31.7 ± 6.6 | .02 | 33.2 ± 6.2 | 32.7 ± 5.5 | ns |
| Lean mass (%) | 66.4 ± 5.4 | 68.2 ± 6.2 | .04 | 66.8 ± 6.2 | 67.9 ± 5.5 | ns |
| TEE (kcal) | 2058.0 ± 180.9 | 2111.7 ± 222.2 | ns | 2123.0 ± 222.3 | 2113.7 ± 213.3 | ns |
| Number of steps | 9790.9 ± 2817.2 | 11071.1 ± 3520.9 | .04 | 11001.6 ± 3702.2 | 11672 ± 3981.2 | ns |
| METs average | 1.32 ± 0.14 | 1.42 ± 0.17 | .04 | 1.41 ± 0.18 | 1.43 ± 0.17 | ns |
| PAL | 1.49 ± 0.10 | 1.55 ± 0.12 | ns | 1.56 ± 0.14 | 1.57 ± 0.13 | ns |
Abbreviations: BMI, body mass index; TEE, total energy expenditure; PAL, physical activity level.
Values presented as mean ± SD. The comparisons between IG and CG, both PRE and POST, did not show any statistical difference. Statistical significance is fixed at P < .025 (Bonferroni correction). The anthropometric and motion-level parameters’ comparison between IG and CG in baseline condition and after 3 months showed no significant difference.
Relationship Between Sleep Parameters and Anthropometric and Motion-Level Parameters
At basal condition, in all 40 women, correlation analysis showed that SE was inversely and significantly correlated with fat mass percentage (r = −0.38, P < .05) and was directly correlated with lean mass percentage (r = +0.39, P < .05). SL was significantly and directly correlated with BMI (r = +0.34, P < .05) and body fat mass percentage (r = +0.35, P < .05) and inversely correlated with lean mass percentage (r = −0.33, P < .05). MFI was significantly and inversely correlated with the number of steps (r = −0.32, P < .05).
Discussion
The major finding of this study was that a 3-month program of aerobic PA, performed twice a week, exerted a protective effect on the sleep behavior of women with BC. While in most of the previous studies sleep disturbances in women with BC were measured by using questionnaires, in the present study we have used actigraphic monitoring to evaluate sleep patterns at baseline and at the end of 3 months of PA program. The actigraph-based monitoring is certainly a more objective method in order to evaluate the effect of the exercise intervention on sleep scores.
Under baseline conditions, IG and CG patients showed virtually superimposable sleep behaviors with comparable actigraphy-based sleep parameters. After 3 months, IG and CG patients showed differential changes in sleep behavior, as documented by the results of the RM-MANOVA (applied to parameters AST, SE, MAS, MFI) and by the results of the parametric univariate analysis (applied to parameter AWT) and nonparametric univariate analysis (applied to parameters SL and IT). While CG patients showed a generalized deterioration of sleep behavior, IG patients showed a more stable pattern. In the CG group, all of the 7 sleep parameters displayed significant changes from PRE to POST that could be interpreted as a deterioration of sleep quality, since it was seen that the 3 Restful Sleep Parameters (SE, AST, IT) decreased and the 4 Sleep Fragmentation Parameters (AWT, SL, MAS, MFI) increased. In contrast, in the IG group, 5 out of 7 sleep parameters (SE, IT, AWT, SL, MAS) measured at the end of the PA program showed no changes with respect to the baseline assessment. Of note is that the 2 parameters (AST and MFI) whose patterns of change were not significantly different in the 2 groups across the 2 time points showed a less pronounced change in the IG than in the CG (see Table 1 and Figure 1).
Payne et al,27 who evaluated the efficacy of a prescribed home-based walking intervention on sleep disturbances by actigraphy, found significant differences between an exercise group and usual care group in Actual Wake Time and Actual Sleep Time. Less movement during sleep also was noted in the exercise group, suggesting that a walking exercise intervention improves sleep in women receiving hormonal treatment for BC, although the authors did not find significant modification of SE, perhaps due to the small sample size and subsequent limited power. Instead, in our study the sample is twice as big compared to the study of Payne and colleagues.
In this study we decided not to include subjective sleep evaluations because the questionnaires usually refer to a longer period of nocturnal rest (eg, the Pittsburgh Sleep Quality Index is a 19-item self-report scale that measures sleep quality over the past month),40 and we preferred to analyze only 1 week with the actigraphs to be more accurate and objective, especially when evaluating the effects of the aerobic PA in the IG. The marked worsening in sleep parameters of CG patients was unexpected and the reasons for this outcome are unclear. It stands to reason that this may be due to seasonal environmental modifications, possibly interacting with the underlying disease of these women. However, since a healthy control group of the same sex and age was not included in our study, the individual contribution of the above-mentioned factors cannot be elucidated from our data.
No clinical or pathological modification occurred in CG that could justify the sleep worsening during the trial, since all CG women maintained good health without pathological BC complications. In the following, we will limit ourselves to speculate about the likely environmental factors that, based on the existing literature, might have affected the sleep of the 2 groups. Sleep modifications could be related to the day length, seasonal adaptation, and the Daylight Saving Time (DST) transition. It is well known that seasonal changes in morning light and especially sunrise times41,42 can affect sleep behavior. Specifically, in a study carried out in the European population, Kantermann et al42 found seasonal fluctuations in sleep duration, with about 20 minutes less sleep in summer than in winter. In our study, we carried out the first sleep monitoring (the baseline condition) in the last week of February and the second monitoring (the final condition) in the first week of June, that is, close to the transition from spring to summer. Interestingly, from February to June, the AST in the CG decreased of about 28 minutes, a figure consistent with the findings of Kantermann and coworkers.
It is also well documented that seasonal variations in ambient temperature can influence the circadian rhythm and sleep quality. For instance, Haskell et al43 suggested that the wakefulness and the worsening of sleep are typical effects of heat exposure conditions. Some other studies43-45 have pointed out that poor sleep and fatigue are associated with the increase of ambient temperature in hot seasons. In our study, the mean ambient temperature in Milan changed from 7.1°C in February to 22.6°C in June 2014 (source: Meteorological Weather Forecast, Italian Military Aviation). Such a marked increase in ambient temperature might have contributed to the sleep deterioration observed in the CG.
The seasonal and DST transition are additional factors that might have played a role in the sleep deterioration observed in the CG. The DST transition occurred on the last Sunday of March and produced an abrupt increase in the available daylight in the evening. Disrupted night-sleep is one of the main DST consequences. Several studies41,46,47 have shown that the spring transition can compromise sleep by reducing both sleep duration and sleep efficiency. For instance, Kantermann et al42 reported that the spring transition induces, in healthy subjects, a reduction of sleep duration for 8 consecutive weeks following the transition. In our study, the second sleep monitoring was carried out in the first week of June, and thus slightly beyond the interval investigated by Kantermann and colleagues.42 Although it is not unreasonable to hypothesize that the second sleep monitoring was influenced by the seasonal change and the DST transition, this only remains a matter of speculation. Another consideration is that sleep behavior3 and DST adaptation48 are chronotype specific. Allebrandt et al48 showed that the evening-types have a shorter sleep during the DST transition. It would have been interesting to evaluate the CG sleep worsening also in relation to the chronotypes, but we did not assess the circadian typology of the subjects.
All in all, we purport that the CG group experienced a difficult adjustment to the above-mentioned environmental changes, leading to a deterioration of sleep parameters. Since the IG patients were exposed to the same disruption as the CG patients, one reasonable explanation for the substantial stability of their sleep behavior is that the PA program counteracted the worsening effect of the environmental and seasonal factors.
It is worth making some specific comments about the changes of parameter SE since it is probably the most widely used parameter to summarize the sleep quality of an individual. In CG patients, SE decreased from 84.6% to 80.7%. In contrast, in IG patients SE moved from 84.4% to 86.4%. Since SE = 85% is considered the clinical cutoff for clinically significant sleep disruption,49 these results seem to suggest that IG patients were able to maintain their SE just above the cutoff, whereas CG patients exhibited almost a 5% drop of SE below the cutoff. It is worth emphasizing that BC women having SE > 85% have been shown to have lower mortality50 and that sleep quality is positively related to quality of life in cancer patients.8 If, however, we make a comparison with sleep parameters proposed by Natale and colleagues51 who fixed the cutoff value for sleep efficiency (low sensitivity) at 87%, neither the IG nor the CG achieve a good sleep quality.
Thus, the ability of PA to preserve SE warrants further investigation because this ability may have some potential in prolonging life and improving well-being of BC survivors.52 Referring to activity-level circadian rhythm, we could observe a substantially stability in the circadian rhythm parameters; MESOR and Acrophase were the same in the 2 groups and remained unchanged across time.
Taking into consideration the anthropometric and body composition parameters, we observed changes in both the CG and IG. At baseline, all women had similar parameters, so they started to join the project with the same physical characteristics. After 3 months IG decreased significantly the percentage of fat mass and tended to increase the lean mass but not significantly. Therefore, the improvement of these parameters confirms, as reported in the literature,18,20 that PA program is able to modify risk factors related to BC prognosis.
These results in the IG are due to the PA program, and we hypothesized that the improvements for the CG in BMI and body weight is partly due to the diet that the women are following. In fact, women included in this study were extracted from the group of a dietary intervention trial (DIANA-5), so some positive changes could be influenced by the effect of diet and by the baseline recommendation to be physically active; this clearly appears in CG for the positive changes of anthropometric parameters. After 3 months neither IG nor CG modified any motion-level parameter.
Conclusion
The results of the present study suggest that aerobic PA has a protective effect against factors promoting sleep disruption. PA is an integrative intervention therapy that has the potential to render sleep behavior less sensitive to detrimental environmental factors.
Acknowledgments
We would like to thank all the women involved in this study for their great participation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav. 2007;90:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Castro Toledo Guimaraes LH, de Carvalho LBC, Yanaguibashi G, do Prado GF. Physically active elderly women sleep more and better than sedentary woman. Sleep Med. 2008;9:488-493. [DOI] [PubMed] [Google Scholar]
- 3. Vitale JA, Roveda E, Montaruli A, Galasso L, Weydahl A, Caumo A. Chronotype influences activity circadian rhythm and sleep: difference in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32:405-415. [DOI] [PubMed] [Google Scholar]
- 4. Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65:9595-9600. [DOI] [PubMed] [Google Scholar]
- 5. Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:1502-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66:5521-5525. [DOI] [PubMed] [Google Scholar]
- 7. Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117:841-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tatrow K, Montgomery GH, Avellino M, Bovbjerg DH. Activity and sleep contribute to levels of anticipatory distress in breast surgery patients. Behav Med. 2004;30:85-91. [DOI] [PubMed] [Google Scholar]
- 9. Youngstedt SD. Exercise effects on sleep. Clin Sports Med. 2005;24:355-365. [DOI] [PubMed] [Google Scholar]
- 10. Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4:387-402. [DOI] [PubMed] [Google Scholar]
- 11. Mansikkamäki K, Raitanen J, Nygård CH, et al. Sleep quality and aerobic training among menopausal women: a randomized controlled trial. Maturitas. 2012;72:339-345. [DOI] [PubMed] [Google Scholar]
- 12. Roveda E, Sciolla C, Montaruli A, Calogiuri G, Angeli A, Carandente F. Effects of endurance and strength acute exercise on night sleep quality. Int Sportmed J. 2011;12:113-124. [Google Scholar]
- 13. Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12:323-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sherrill DL, Kotchou K, Quan SF. Association of physical activity and human sleep disorders. Arch Intern Med. 1998;158:1894-1898. [DOI] [PubMed] [Google Scholar]
- 15. Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907-912. [DOI] [PubMed] [Google Scholar]
- 16. Autenrieth C, Schneider A, Doring A, et al. Association between different domains of physical activity and markers of inflammation. Med Sci Sports Exerc. 2009;41:1706-1713. [DOI] [PubMed] [Google Scholar]
- 17. Friedenreich CM, Neilson HK, Woolcott CG, et al. Inflammatory marker changes in a yearlong randomized exercise intervention trial among postmenopausal women. Cancer Prev Res. 2012;5:98-108. [DOI] [PubMed] [Google Scholar]
- 18. Friedenreich CM. The role of physical activity in breast cancer etiology. Semin Oncol. 2010;37:297-302. [DOI] [PubMed] [Google Scholar]
- 19. Friedenreich CM, Woolcott CG, McTiernan A, et al. Alberta Physical Activity and Breast Cancer Prevention Trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28:1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Courneya KS, Mackey JR, Jones LW. Coping with cancer: can exercise help? Phys Sports Med. 2000;28:49-51. [DOI] [PubMed] [Google Scholar]
- 21. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomised controlled trial of exercise training in post-menopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660-1668. [DOI] [PubMed] [Google Scholar]
- 22. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. Cancer J Clin. 2012;62:242-274. [DOI] [PubMed] [Google Scholar]
- 23. Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479-2486. [DOI] [PubMed] [Google Scholar]
- 25. Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379-386. [DOI] [PubMed] [Google Scholar]
- 26. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Payne JK, Held JH, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35:635-642. [DOI] [PubMed] [Google Scholar]
- 28. Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288-302. [DOI] [PubMed] [Google Scholar]
- 29. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342-392. [DOI] [PubMed] [Google Scholar]
- 30. Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy sleep. Sleep. 2007;30:1362-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehnkering H, Strauss A, Wegner B, Siegmund R. Actigraphic investigations on the activity-rest behavior of right- and left-handed students. Chronobiol Int. 2006;23:593-605. [DOI] [PubMed] [Google Scholar]
- 32. Lehnkering H, Siegmund R. Influence of chronotype, season and sex of subject on sleep behavior of young adults. Chronobiol Int. 2007;24:875-888. [DOI] [PubMed] [Google Scholar]
- 33. Pasanisi P, Villarini A, Bruno E, Raimondi M, Gargano G, Berrino F. Nutritional advice to breast cancer survivors. Support Care Cancer. 2010;18:29-33. [DOI] [PubMed] [Google Scholar]
- 34. Villarini A, Pasanisi P, Traina A, et al. Lifestyle and breast cancer recurrences: the DIANA-5 trial. Tumori. 2011;97:693-710. [DOI] [PubMed] [Google Scholar]
- 35. Jakicic J, Marcus M, Gallagher K, et al. Evaluation of the SenseWear Pro Armband™ to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36:897-904. [DOI] [PubMed] [Google Scholar]
- 36. Scheers T, Philippaerts R, Lefevre J. Variability in physical activity patterns as measured by the SenseWear Armband: how many days are needed? Eur J Appl Physiol. 2012;112:1653-1662. [DOI] [PubMed] [Google Scholar]
- 37. St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85:742-749. [DOI] [PubMed] [Google Scholar]
- 38. Halberg F, Carandente F, Cornelissen G, Katinas GS. Glossary of chronobiology. Chronobiologia. 1977;4:1-189. [PubMed] [Google Scholar]
- 39. Nelson W, Tong LY, Lee JK, Halberg F. Methods of cosinor-rhythmometry. Chronobioogial. 1979;6:305-323. [PubMed] [Google Scholar]
- 40. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 41. Harrison Y. The impact of daylight saving time on sleep and related behaviours. Sleep Med Rev. 2013;17:285-292. [DOI] [PubMed] [Google Scholar]
- 42. Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Current Biol. 2007;17:1996-2000. [DOI] [PubMed] [Google Scholar]
- 43. Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. The effects of high and low ambient temperatures on human sleep stages. Electroencephalogr Clin Neurophysiol. 1981;51:494-501. [DOI] [PubMed] [Google Scholar]
- 44. Okamoto-Mizuno K, Tsuzuki K. Effects of season on sleep and skin temperature. Int J Biometeorol. 2010;54:401-409. [DOI] [PubMed] [Google Scholar]
- 45. O’Connel SE, Griffiths PL, Clemes SA. Seasonal variation in physical activity, sedentary behavior and sleep in sample of UK adults. Ann Hum Biol. 2014;41:1-8. [DOI] [PubMed] [Google Scholar]
- 46. Tonetti L, Erbacci A, Fabbri M, Martoni M, Natale V. Effects of transitions into and out of daylight saving time on the quality of the sleep/wake cycle: an actigraphic study in healthy university students. Chronobiol Int. 2013;30:1218-1222. [DOI] [PubMed] [Google Scholar]
- 47. Lahti T, Leppamaki S, Lonnqvist J, Partonen T. Transition to daylight saving time reduces sleep duration plus sleep efficiency of the deprived sleep. Neurosci Lett. 2006;406:174-177. [DOI] [PubMed] [Google Scholar]
- 48. Allebrandt KV, Teder-Laving M, Kantermann T, et al. Chronotype and sleep duration: the influence of season of assessment. Chronobiol Int. 2014;31:731-740. [DOI] [PubMed] [Google Scholar]
- 49. Shutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504. [PMC free article] [PubMed] [Google Scholar]
- 50. Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Natale V, Leger D, Martoni M, Bayon V, Erbacci A. The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med. 2014;15:111-115. [DOI] [PubMed] [Google Scholar]
- 52. Spahn G, Choi KE, Kennemann C, et al. Can a multimodal mind-body program enhance the treatment effects of physical activity in breast cancer survivors with chronic tumor-associated fatigue? A randomized controlled trial. Integr Cancer Ther. 2013;12:291-300. [DOI] [PubMed] [Google Scholar]