Abstract
Background. The use of natural health products in prostate cancer (PrCa) is high despite a lack of evidence with respect to safety and efficacy. Fish-derived omega-3 fatty acids possess anti-inflammatory effects and preclinical data suggest a protective effect on PrCa incidence and progression; however, human studies have yielded conflicting results. Methods. A search of OVID MEDLINE, Pre-MEDLINE, Embase, and the Allied and Complementary Medicine Database (AMED) was completed for human interventional or observational data assessing the safety and efficacy of fish-derived omega-3 fatty acids in the incidence and progression of PrCa. Results. Of 1776 citations screened, 54 publications reporting on 44 studies were included for review and analysis: 4 reports of 3 randomized controlled trials, 1 nonrandomized clinical trial, 20 reports of 14 cohort studies, 26 reports of 23 case-control studies, and 3 case-cohort studies. The interventional studies using fish oil supplements in patients with PrCa showed no impact on prostate-specific antigen levels; however, 2 studies showed a decrease in inflammatory or other cancer markers. A small number of mild adverse events were reported and interactions with other interventions were not assessed. Cohort and case-control studies assessing the relationship between dietary fish intake and the risk of PrCa were equivocal. Cohort studies assessing the risk of PrCa mortality suggested an association between higher intake of fish and decreased risk of prostate cancer–related death. Conclusions. Current evidence is insufficient to suggest a relationship between fish-derived omega-3 fatty acid and risk of PrCa. An association between higher omega-3 intake and decreased PrCa mortality may be present but more research is needed. More intervention trials or observational studies with precisely measured exposure are needed to assess the impact of fish oil supplements and dietary fish-derived omega-3 fatty acid intake on safety, PrCa incidence, treatment, and progression.
Keywords: fish oil, omega-3, fish, prostate cancer, prostate carcinoma, PSA
Introduction
Prostate cancer (PrCa) accounts for almost one quarter of cancers diagnosed among men. In Canada, there are approximately 24 000 new cancer cases (24% of all new male cancer cases) expected in 2015.1 While 5-year survival rates have dramatically improved, PrCa is still the third leading cause of cancer death among men, with nearly 4000 deaths (10.1% of all male cancer deaths) expected in 2015 in Canada.1 However, PrCa incidence and mortality varies 60-fold globally, with a dramatic increase observed in immigrants moving from low- to high-risk countries, suggesting that dietary and lifestyle factors play a role in its etiology and pathogenesis.2 With Canadian men having approximately a 1 in 8 (12.8%) lifetime probability of being diagnosed with prostate cancer, there is strong interest in dietary and natural health product (NHP) interventions, which may be effective in either the prevention or treatment of PrCa.
There is widespread evidence that many patients with PrCa take NHPs, frequently without any clinical supervision. Surveys conducted in the United States, Britain, Australia, and Canada suggest that complementary and alternative medicine (CAM) is widely used among prostate cancer patients; with estimates of CAM prevalence between 25 to 90%3-7 and rates of disclosure to the patient’s physician or oncologist as low as 25%.8
Fish-derived omega-3 fatty acids have emerged as a topic of interest in the prevention and treatment of PrCa. The omega-3 fatty acids found in fish include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These fatty acids exhibit anti-inflammatory properties through their impact on prostaglandin synthesis.9 Populations with a high consumption of fish, such as those in Japan and Alaskan Inuit, have lower rates of PrCa than populations who consume the more typical Western diet, where intake of fish and the anti-inflammatory fish-derived omega-3 fatty acids is generally lower,2 and intake of pro-inflammatory omega-6 fatty acids is generally much higher.10 Additionally, a wide range of mechanisms by which omega-3 fatty acids affect cancer development have been elucidated11 and a large number of in vitro and animal studies show that EPA and DHA have inhibitory effects on PrCa growth and progression.12-17 Briefly, some of the chief mechanisms of action behind the cancer modulating effects of fish oil include the following: suppression of raft-associated signal transduction, promotion of BAD-dependent apoptosis via the PI3K/AKT survival pathway (phosphatidylinositol 3-kinase and serine/threonine protein kinase AKT), reduction of oxidative stress-induced endothelial Ca2+ influx via transient receptor potential channels (TSPs) and nuclear factor erythroid-2-related factor 2 (Nrf2) activation, and resolution of inflammation through the action of E-resolvins (RvE1 and RvE2), D-resolvins (RvD1 and RvD2), and protectin (PD1) on cyclooxygenase (COX) and lipoxygenase (LOX) pathways.18
The clinical potential of omega-3 fatty acids in the context of PrCa prevention and treatment remains controversial, and the results of numerous randomized controlled trials (RCTs), case-control studies, epidemiological reports, and systematic reviews assessing the role of fish-derived omega-3 fatty acids in the incidence and progression of PrCa have been largely inconsistent. The equivocal results are complicated by high variability in study methodology in terms of measurements of exposure (eg, food frequency questionnaires [FFQ]; plasma, serum or prostate tissue fatty acid levels); PrCa outcomes (eg, incidence, mortality, or progression) and biomarkers (eg, prostate-specific antigen [PSA] or inflammatory markers such as cyclooxygenase-2) used to assess fish-derived omega-3 fatty acid status and clinical outcomes.
To our knowledge, there are no evidence-based guidelines currently available for patients or clinicians indicating whether fish-derived omega-3 fatty acids are safe or effective in the context of PrCa treatment, progression, and prevention. In the absence of guidelines, men often self-prescribe based on limited information found on the Internet or obtained from family and friends.19
Patients and clinicians need to have access to reliable, credible, and evidence-based information about potential risks and benefits when making decisions about using NHPs in the context of PrCa. Because previous studies have reported mixed or conflicting result on the relationship between fish-derived omega-3 fatty acid and PrCa, thorough analysis of the entire body of literature is warranted. Therefore, we performed a systematic, evidence-based review of the available literature regarding fish-derived omega-3 fatty acids for the treatment and prevention of PrCa, with the purpose of developing comprehensive knowledge translation tools for oncology health professionals and, ultimately, patients with PrCa.
Methods
Methods of the analysis and inclusion criteria were specified in advance and documented in a registered protocol (PROSPERO 2014:CRD42014013014).20
Search Strategy
Electronic search strategies were developed by an experienced medical information specialist in consultation with the review team (Appendix A). Using the OVID platform, we searched OVID MEDLINE, Pre-MEDLINE, Embase, and the Allied and Complementary Medicine Database (AMED). We also searched the Cochrane Library on Wiley and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) on EBSCO and 2 Chinese-language databases (Wanfang and China National Knowledge Infrastructure databases). All database searches were performed on July 21, 2014 and updated June 21, 2015. Searches included controlled vocabulary terms (eg, “Prostatic Neoplasms,” “Fatty Acids, Omega-3,” “Docosahexaenoic Acids”) and key words (eg, prostate cancer, Omega 3, PUFAs). There were no language or date restrictions on any of the searches. ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP) were searched for current and completed clinical trials.
Inclusion Criteria
Eligible studies assessed male patients of any age for primary or secondary prevention or progression of PrCa. The studies assessed, used, or measured dietary and/or supplemental intake of fish-derived omega-3 fatty acids with or without other supplements or measured the omega-3 content in biological samples obtained from participants. Studies assessing non–fish-derived omega-3 fatty acids such as alpha-linoleic acid or flaxseed oil were excluded. Studies using placebos, comparator groups consisting of natural or pharmacological agents, or no control were eligible. Eligible study designs included RCTs, non–randomized controlled trials (non-RCTs), and observational studies (case-control, cohort) but not preclinical or cross-sectional studies.
Record Screening and Selection
All citations identified by the systematic search were downloaded into a reference database. Two investigators independently reviewed all identified titles and abstracts for eligibility. Disagreement regarding inclusion of records was determined by consensus and third party arbitration by another member of the research team, when appropriate. Duplicate reports of studies were included.
Data Extraction
Data were extracted independently by 2 investigators using data abstraction sheets that had been developed and piloted among the investigative team. Outcome measures extracted included relative risk (RR), hazard ratio (HR), and odds ratio (OR). Outcome measures that were adjusted for known PrCa risk factors such as age, ethnicity, family history, smoking status, and body mass index (BMI) were extracted when available. When not reported, unadjusted outcomes were extracted. Study authors were contacted to clarify results reported in 2 publications21,22; however, no new information was obtained.
Outcomes
The primary outcome of this review is primary prevention of PrCa. Secondary outcomes include PSA level, PSA doubling time, Gleason score, recurrence, tumor response rates, survival, immune function (clinical or surrogate parameters), quality of life (QOL), other cancer symptoms or chemotherapy-related side effects, adverse events/toxicities, the Eastern Cooperative Oncology Group (ECOG) and/or Karnofsky performance scores, Edmonton Symptom Assessment Scale (ESAS), prognostic scores (Glasgow PS), or other cancer markers or relevant surrogates. Additionally, we assessed for interactions, defined as a pharmacological or clinical responses to the administration or co-exposure of a treatment and another substance that modifies either the effectiveness or safety of the treatment.23,24
Exposure
Data on the dose of fish-derived omega-3 fatty acid intake were extracted from the studies. When these data were not reported as mg of EPA + DHA per day, a conversion was performed to create a standardized dose by weight. To facilitate conversion when intake was reported in servings of fish, a standard of 0.798 g EPA + DHA per servings of fish was used. This was calculated by finding the average EPA + DHA content among the different fish and seafood types listed in the Dietitians of Canada online reference.25 When intake of fatty acids was reported as a percentage of daily caloric intake, a standard of 2350 calories per day was utilized based on the Health Canada Food Guide recommendations for adult men with low activity level. The percentage was multiplied by 2350 calories and divided by 9 cal/g to approximate the grams of fish oil taken in per day.
Risk of Bias Assessment
The quality of clinical trials was assessed using the Cochrane Risk of Bias tool.26 The quality of cohort and case-control studies was assessed using the Newcastle-Ottawa Scale.27 All studies were assessed for their source of funding (industry vs nonindustry). Assessment was completed independently by 2 investigators and disagreement was resolved by consensus or third party arbitration, when appropriate. For nonrandomized trials, we assessed quality according to reporting of blinding of patients and assessors, a priori sample size estimation and low loss to follow-up (<20%).
Data Analysis
Analysis was completed separately for each type of study design. A random-effects model was used to pool results from the studies, including the following measures of effect and 95% confidence intervals (CIs): RR, HR, and OR. When available, adjusted measures of effect were used. Forest plots were created to display the results for different types of studies that were pooled. When available, the effect of total fish-derived omega-3 fatty acids on total incidence of cancer was utilized. When these statistics were not available, other statistics were reported, such as the effect of EPA only and DHA only on PrCa risk or the effects of omega-3 fatty acids on advanced and nonadvanced PrCa. Additional forest plots were created to display the effects of fish-derived omega-3 fatty acid son PrCa mortality, to compare analyses of EPA alone to DHA alone and to compare studies utilizing different methods of assessing fish-derived omega-3 fatty acid exposure.
Planned sensitivity analyses included an assessment of the effect of methodological quality of included trials, withdrawals/losses to follow-up, and funding source, when feasible. Homogeneity was assessed using the I-squared statistic and the Zalen test. If some degree of homogeneity existed (I2 < 75%), a meta-regression analysis (using STATA) was conducted to determine the extent to which factors contributed to heterogeneity. Variable coefficients are displayed in the beta-coefficient with 95% CIs and appropriate ORs with P values. A funnel plot test, using Eggers test for publication bias, was used to assess the likelihood of publication bias. Both quantitative (meta-analysis) and qualitative narrative synthesis of studies (based on study design) were planned.
Results
Of 1776 records screened, 54 publications, reporting on 44 studies, were included for review and analysis. Figure 1 shows a flowchart of the literature search and study selection. Search of the Chinese databases yielded 17 records, none of which met criteria for inclusion. Data extraction by 2 reviewers was found to have a high degree of agreement and consensus was reached on all articles. Meta-analysis was not completed due to the significant heterogeneity in the observational data and limited amount of interventional data. The results reported in the studies found were predominantly focused on cancer prevention. Eight publications reported on disease management and progression.28-35 Many of the secondary outcomes that were planned in this review were not assessed by any of the included studies.
Figure 1.
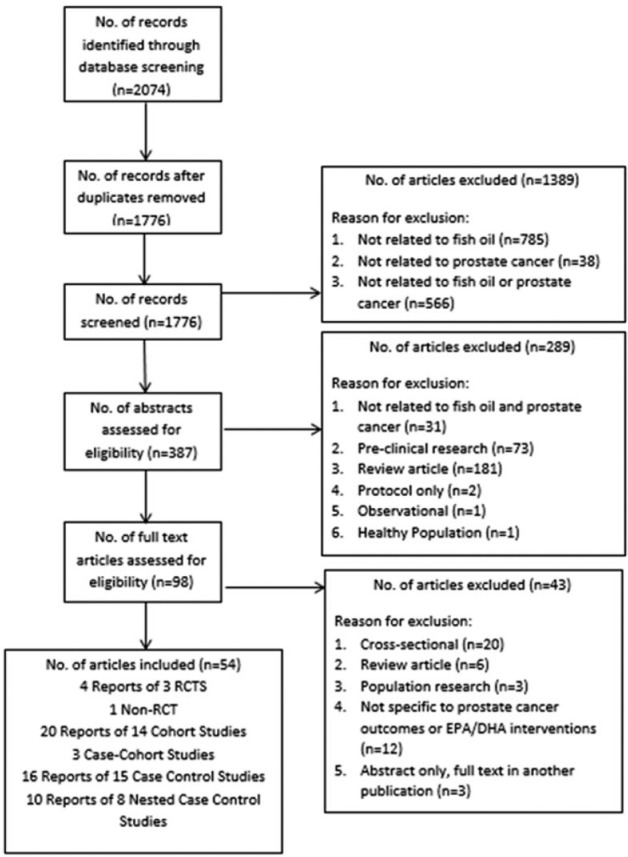
Literature flowchart.
Interventional Studies
Five publications28-32 reported on the results of 4 clinical trials, including 1 non-RCT (Table 1). Of the 4 trials, 1 was in patients with localized PrCA preprostatectomy,29 1 in patients undergoing active surveillance,31 1 in postprostatectomy patients,28 and 1 in patients with untreated localized or regional PrCA.32 Interventions included a low-fat diet plus fish oil supplementation (2400-5500 mg per day of fish oil providing 1600 to 2400 mg of EPA + DHA). PSA levels remained unchanged in all four trials (Table 1). Many reported a lack of significant effect on inflammatory markers; however, there were significant reductions in malignant epithelial cell proliferation (Ki-67) in one of the trials (decrease of 32.2% P < .05)29 and decreased cell-cycle progression score, pro-inflammatory fatty acids 15-S-hydroxyeicosatetraenoic acid and leukotriene B4 in another study30 (Table 1). The intervention studies analyzed were of short duration. Three of the 4 trials were three months or shorter in duration29,31,32 and the remaining study was 2 years.28
Table 1.
Characteristics of Human Trials Investigating Supplemental Fish-Derived Omega-3 Fatty Acids in Patients With Prostate Cancer.
| Ref | n | Random | Control | Blind | PrCa Status; Other Treatments | Intervention | Duration (Months) | Effect of Intervention on PSA | Effect on Inflammatory Markers | Effect on Additional Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized clinical trials | ||||||||||
| Higashihara et al (2010)28 | 62 | Yes | No Tx | NR | PrCa PSA < 0.2 ng/mL 3 months postprostatectomy; None | 2400 mg/d EPA ethyl ester | 48 | Equal PSA failure rate (4 in EPA group, 8 in placebo; Kaplan-Meier P = .16) | ||
| Aronson et al (2011)29 | 48 | Yes | Western diet (15:1 n-6:n-3 ratio), no placebo | Single | Localized PrCa preprostatectomy; scheduled for surgery in ≥4 weeks | Low-fat diet + 5.5 g/d FO (1835 mg DHA, 1000 mg EPA); 2:1 n-6:n-3 ratio | 1-1.5 | ↔ PSA (change of 0.08 ± 0.4 mg/mL vs −0.09 ± 0.3 P = .53) | ↔ serum IGF-1 (P = .25) ↔ serum IGFBP-1 (P = .84) ↔ serum IGFBP-3 (P = .14) ↔ urine PGEM (P = .36) ↔ Pr tissue PGE2 ↔ COX-2 |
↓ malignant epithelial cell proliferation (Ki67) by 32.2% (P < .05) ↓22RV1 cell proliferation (−5.0% ± 1.8% vs 0.6% ± 1.9% P = .039) ↔ angiogenesis, apoptosis immunostaining |
| Galet et al (2014)30; post hoc analysis of Aronson et al (2011)29 | As Aronson 2011 | ↓ 15(S)-HETE (−7.2 ± 6.6 vs 24.7 ± 11.4 in placebo P = .02) ↓ LTB4 post-LFFO vs pre-LFFO, but ↔ relative to control (14.9 ± 5.6 vs −9.7 ± 7.4 in placebo) |
↓ cell-cycle progression score (P = .03) | |||||||
| Chan et al (2011)31 | 69 | Yes | Placebo | Double | Low-grade PrCa; active surveillance | 3 g/d FO (1098 mg EPA, 549 mg DHA) | 3 | ↔ PSA (change of 0.20 ng/mL vs −0.46 P = .39) | ↔ in COX-2 expression (change of 0.39 ± 1.98 vs 0.40 ± 2.19 in placebo) | |
| Nonrandomized clinical trial | ||||||||||
| Aronson et al (2001)32 | 9 | No | N/A | N/A | Untreated localized or regional PrCa; none | Low-fat diet + 3 g/d FO (1800 mg EPA, 1200 mg DHA) + 800 IU vit E | 3 | ↔ PSA (baseline: 11.15 ± 2.9 ng/mL), final: 13.12 ± 4.0 | ↓ COX-2 in 4 of 7 patients compared with baseline (not statistically powered to detect) | |
Abbreviations: PrCa, prostate cancer; n-3, omega-3 fatty acid; n-6, omega-6 fatty acid; N/A, not applicable; NR, not reported; FO, fish oil; LFFO, low-fat diet + fish oil; Tx, treatment; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; PSA, prostate-specific antigen; IGF-1, insulin-like growth factor 1; IGFBP-1, insulin-like growth factor binding protein 1; IGFBP-3, insulin-like growth factor binding protein 3; PGEM, prostaglandin E2 metabolite; PGE2, prostaglandin E2; COX-2, cyclooxygenase-2; Pr, protein; CHO, carbohydrate; vit, vitamin; FA, fatty acid; ↔, no change; ↑, increase; ↓, decrease.
Observational Evidence
Prospective Cohort Studies of Primary Prevention
Seventeen publications reported on 11 cohort studies investigating primary prevention of PrCA (Table 2). These studies assessed dietary fish-derived omega-3 fatty acids and supplementary fish oil intake (13 assessed diet alone, 1 assessed supplement intake alone, and 3 assessed both diet and supplement intake) and assessed incidence and mortality of PrCa over an average of 12.2 years (range 4-23.5 years). One additional study discussed in the nested case-control section of this review contained a prospective cohort component36 that assessed the relationship between dietary intake and supplemental fish oil and the relationship with prostate cancer. In the studies assessing dietary intake, the highest quartile or quintile of intake was compared with the lowest, which included individuals who consumed very little or no dietary fish. Sixteen publications reported on the association of fish-derived omega-3 fatty acid intake with PrCa incidence. Of these, 3 studies10,21,36 reported on advanced and nonadvanced prostate cancer separately resulting in a total of 19 analyses. The publications reported RR or HR and 12 publications reported outcomes that had been adjusted for other factors.
Table 2.
Prospective Cohort Studies of Dietary and Supplemental Fish-Derived Omega-3 Fatty Acids and Risk of Primary Prostate Cancer.
| Reference | Cohort/Study Name | Cohort n | Cases n | Geographic Area | PrCa Status at Baseline; Other Tx | Exposure Assessment | Years f/u | Highest group (quartile, quintile, etc.); Dose | Effect of exposure on outcome (highest vs. lowest exposure) |
|---|---|---|---|---|---|---|---|---|---|
| Allen et al (2004)49 | Life Span Study | 18 115 | 196 | Hiroshima or Nagasaki, Japan | No evidence of disease | FFQ | 16.9 | Eating fish almost daily or more often; 684 mg/d |
↑ risk of PrCa RR 1.77 (1.01-3.11) P = .07 |
| Augustsson et al (2003)45 | Health Professionals Follow-Up Study | 47 882 | 2482 | USA | Never | FFQ | 12 | Eating fish more than 3×/wk; 342 mg/d |
↓ risk total PrCa mvRR 0.93 (0.80-1.08), advanced PrCa mvRR 0.83 (0.61-1.13), metastatic PrCa mvRR 0.56 (0.37-0.86) |
| FO supp use | ↔ risk of PrCa | ||||||||
| Leitzmann et al (2004)46 | Health Professionals Follow-Up Study | 47 866 | 2965 | USA | Never | FFQ and supplement inquiry | 14 | Dietary EPA + DHA >0.214% energy; 558 mg/d |
↓ risk of PrCa RR 0.89 (0.77-1.04) P = .002 ↔ fatal PrCa 0.68 (0.40-1.17) P trend = .12 |
| FO supp >2.5 g/d | ↔ risk of PrCa mvRR 0.89 (0.62-1.30) P = .91 | ||||||||
| Giovannucci et al (1993)37 | Health Professionals Follow-Up Study | 47 885 | 300 | USA | Never | FFQ | 4 | Median of 0.55 g/day n-3 fat (from fish); 550 mg/d |
↔ risk of PrCa RR 0.90 (0.51-1.61) P = .30 |
| Bonner et al (2012)47 | New York State Angler Cohort | 17 110 | 58 | USA | Never | Self-administered food questionnaire | 17 | Ever eaten fish from Lake Ontario (vs never) | ↓ risk of PrCa RR 0.5 (0.3-0.8) |
| Brasky et al (2011)50 | VITamins And Lifestyle (VITAL) Cohort | 35 239 | 1602 | USA | Never | Questionnaire of supplement use | 6.1 | User of fish oil supp (>1 d/wk for >1 year) | ↔ risk of PrCa mvHR 0.98 (0.82-1.17) P = .61 |
| Daniel et al (2011)39 | NIH-AARP Diet and Health Study | 293 466 | 23 453 | USA | Never | FFQ | 9.1 | 21.4 g fish/1000 kcal; 535 mg/d |
↔ risk of PrCa HR 1.02 (0.98-1.06) P = .67 |
| Pelser et al (2013)10 | NIH-AARP Diet and Health Study | 288 268 | 23 281 | USA | Never | FFQ | 9 | EPA 0.036% energy | ↔ risk of advanced/nonadvanced PrCa mvHR 0.93 (0.82-1.04) P = .15; mvHR 1.05 (1.00-1.10) P = .69 ↓ risk of fatal PrCa mvHR 0.82 (0.64-1.04) Ptrend = .02 |
| EPA + DHA 0.103% energy; 269 mg/d |
↔ risk of advanced (mvHR 0.97 (0.86-1.09) P = .31); nonadvanced (mvHR 1.04 (1.00-1.10) P = .45); or fatal PrCa (mvHR 0.87 (0.68-1.10) P = .10) | ||||||||
| Bosire et al (2013)51 | NIH-AARP Diet and Health Study | 293 464 | 23 453 | USA | Never | FFQ | 8.9 | >0.66 ounce/d of fish; 199 mg/d |
↓ risk of fatal PrCa mvHR 0.79 (0.65-0.96) |
| ≥250 mg/d marine n-3; 250 mg/d |
↓ risk of fatal PrCa mvHR 0.94 (0.90-0.98) | ||||||||
| Terry et al (2001)48 | N/A | 6274 | 466 | Sweden | Never | Self-administered food questionnaire | 21.4 | Fish accounted for “large part of diet” | ↓ risk of PrCa mvRR 0.43 (0.22-0.83) P < .05; ↓ risk of PrCa death mvRR 0.30 (0.17-0.56) P < .01 |
| Crowe et al (2008)40 | EPIC | 142 520 | 2727 | 10 European countries | Never | FFQ | 8.7 | Not defined | ↔ risk of PrCa Total PrCa risk mvHR per 1% increase in energy from fish fat: 1.00 (0.93-1.07) P = .977 ↔ risk of localized, advanced, high-grade, or low-grade PrCa |
| Kristal et al (2010)21 | Prostate Cancer Prevention Trial | 9559 | 1703 | USA and Canada | Never; Finasteride or placebo | FFQ and supplement questionnaire | 7 | Total EPA + DHA >0.28 mg/d; 0.28 mg/d |
↔ risk of PrCa For GS 2-7 OR 1.11 (0.94-1.31) P = .230 For GS 9-10 OR 1.46 (0.86-2.50) P = .193 |
| Pham et al (2009)52 | Miyako Study | 5589 | 21 deaths | Japan | Never | Self-administered questionnaire | 13.4 | Fish consumed at least 2-4×/wk; 342 mg/d |
↓ risk PrCa death mvHR 0.12 (0.05-0.32) |
| Chavarro et al (2008)41 | Physician’s Health Study | 20 167 | 2162 cases, 230 deaths | USA | Never; aspirin and beta-carotene | FFQ | 19 | Fifth quintile of seafood n-3 FA intake | ↔ risk of PrCa mvRR 1.09 (0.95-1.25) P = .55 ↓ risk of PrCa death mvRR 0.65 (0.42-0.99) P = .02 |
| Sato et al (2008)42 | Osaki National Health Insurance Subscribers Cohort Study | 24 895 | 95 | Japan | Never | FFQ | 7 | Fish intake >100 g/d; 1064 mg/d |
↔ risk of PrCa; mvHR 0.72 (0.40-1.33) P = .23 Among >70-year-olds mvHR 0.44 (0.18-1.11) P = 0.08 |
| Wallstorm et al (2007)43 | Malmo Diet and Cancer Cohort | 10 564 | 817 | Sweden | Never | Questionnaire (including Supplement) and Menu record | 11 | 1.30 g/d of EPA + DHA supplement; 1300 mg/d |
↔ risk of PrCa mvRR 1.26 (1.00-1.59) P = .056 |
| 0.47 g/d EPA | ↑ risk of PrCa mvRR 1.30 (1.03-1.64) P = .043 | ||||||||
| 0.88 g/d DHA | ↔ risk of PrCa mvRR 1.26 (1.00-1.59) P = .062 |
||||||||
| Chavarro et al (2010)44; full text not published | Physician’s Health Study | 488 | 94 Pr CA deaths | USA | Never | Blood FA levels at baseline | 23.5 | Quartiles of serum FAs | ↔ risk of PrCa death (data not provided in abstract) |
| Torfadottir et al (2013)36 | AGES-Reykjavik Cohort Study | 133 | 1944 | Iceland | Never | FFQ | 7 | Once a week or more intake of salted or smoked fish | ↑ risk advanced PrCa intake later life OR 2.28 (95% CI: 1.04, 5.00) ↓ risk of localized PrCa intake later life AOR 0.63 (0.40-1.00) |
| Fish oil use daily | ↓ risk of advance PrCa HR 0.43 (0.19-0.95) with use in later life |
Abbreviations: PrCa, prostate cancer; mvRR, multivariate relative risk; mvHR, multivariate hazard ratio; AOR, adjusted odds ratio; FFQ, Food Frequency Questionnaire; Tx, treatment; N/A, not applicable; ×/wk, times per week; f/u, follow-up; Bl, baseline; ↓, decrease; ↑, increase; ↔ no effect; GS, Gleason score; FO fish oil; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; n-3, omega-3; NR, not reported; Supp, supplement.
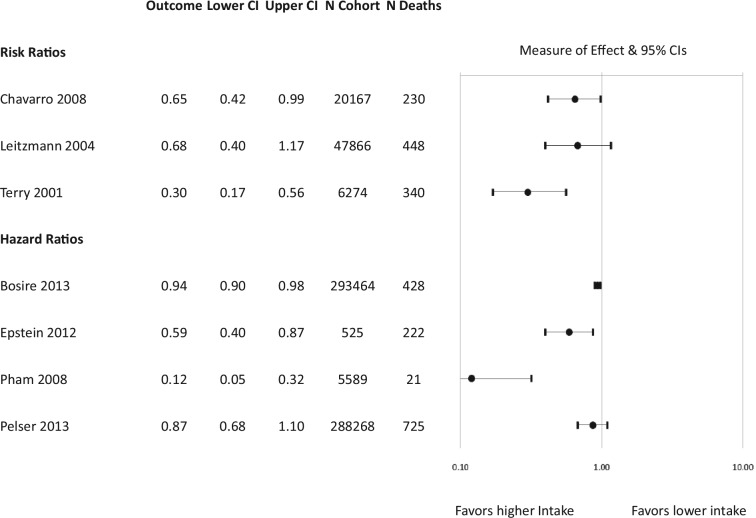
Of the 19 analyses assessing PrCa incidence, 12 did not show a statistically significant association.10,21,37-44 Five analyses showed a significant association between increased intake of fish-derived omega-3 fatty acids and decreased PrCa incidence.36,45-48 In 2 of these studies, the authors concluded that statistical significance was achieved based on the P value despite nonsignificant 95%Cis.45,46 Two analyses reported a significant association between increased intake and increased PrCa risk.36,49 In a subanalysis, another study demonstrated a positive association (ie, increased PrCa) with increased EPA intake but not with increased intake of DHA or EPA + DHA.43 The results of the primary cohort studies are presented in a forest plot (Figure 2); with the exception of 1 study where the data were not available.44
Figure 2.
Risk of prostate cancer (PrCa) incidence with fish-derived omega-3 fatty acid intake: Prospective cohort studies. When the effect of fish-derived omega-3 fatty acid intake on total PrCa risk was not available, subanalyses reporting on different stages of cancer (eg, advanced cancer and nonadvanced cancer) are reported. *Study authors concluded that the results achieved statistical significance based on P value and confidence interval.
Five prospective cohort analyses assessed the impact of fish oil supplements (or the combination of diet and fish oil supplements in 1 study); 4 demonstrated no statistically significant association with the risk of PrCa incidence.43,45,46,50 One demonstrated a relationship between daily supplemental fish oil intake in later life and a decrease in advanced PrCa incidence.36
Among the 7 cohort study reports assessing the risk of death related to PrCa, 5 studies reported a significant association between higher intake of fish-derived fatty acids and decreased risk of death.35,41,48,51,52 Of the 2 remaining studies, one showed a significant association between decreased risk of death and higher EPA intake and a nonsignificant association with higher total EPA + DHA.10 The remaining study showed an association between decreased risk of death and higher total EPA + DHA intake that approached significance.46
Prospective Cohort Studies of Secondary Prevention
Three publications reported on cohort studies assessing progression or risk of death among patients with PrCa (Table 3).31,34,35 In these 3 studies, patients underwent conventional individualized treatment for their PrCa. One study measured the time to PrCa-related death35 while one defined progression as either PrCa death, bone metastases from PrCa, biochemical recurrence, or initiation of secondary treatment.34 The third study asked the patient’s treating physician to whether or not the patient’s prostate cancer had recurred or progressed since the initial treatment33 and when not available defined progression as 2 or more successive rises in PSA, initiation of second therapy or positive scans for metastasis. Two studies utilized an FFQ to assess dietary fish intake after diagnosis of PrCa and analyzed risk of disease progression; no impact from high fish intake was found33,34. The third study assessed risk of PrCa death and found an association between lower risk and higher fish-derived omega-3 fatty acid intake in the year prior to diagnosis.35
Table 3.
Prospective Cohort Studies of Dietary Fish-Derived Omega-3 Fatty Acids and Risk of Prostate Cancer Progression or Death.
| Reference | Cohort/Study Name | Cohort n | Cases n | Geographic Area | PrCa Status at Baseline | Study Objective | Exposure Assessment | Years f/u | Highest Exposure Group (Quartile, Quintile, etc); Dose | Effect of Exposure on Outcome (Highest vs Lowest Exposure) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chan et al (2006)31 | Health Professionals Follow-up Study | 1202 | 392 cases progression | USA | Present | Prevent progression | FFQ of intake postdiagnosis | 10 | Not reported | ↔ Risk of PrCa progression HR 0.73 (0.52-1.02) |
| Richman et al (2010)34 | Cancer of the Prostate Strategic Urologic Research Endeavour (CaPSURE) | 1294 | 127 cases progression | USA | Present (excluding advanced or metastatic) | Prevent progression | FFQ of intake postdiagnosis | 2 | Median 4.3 servings/wk; 490 mg/d |
↔ Risk of PrCa progression mvHR 1.13 (0.70-1.84) |
| Epstein et al (2012)35 | N/A | 525 | 222 cases of PrCa death | Sweden | Present | Prevent death due to PrCa | FFQ of intake 1 year prediagnosis | 20 | 0.8 g marine FAs/day; 800 mg/d |
↓ risk PrCa death HR 0.59 (0.4-0.87) P = .04 |
Abbreviations: PrCa, prostate cancer; mvRR, multivariate relative risk; mvHR, multivariate hazard ratio; AOR, adjusted odds ratio; FFQ, Food Frequency Questionnaire; ×/wk times per week; f/u follow-up; Bl, baseline; ↓, decrease; ↑, increase; ↔ no change; RBC, red blood cells; FA, fatty acids.
The results of the primary and secondary cohort studies assessing risk of death are presented together in a forest plot (Figure 3).
Figure 3.
Risk of prostate cancer (PrCa) mortality with fish-derived omega-3 fatty acid intake: Prospective cohort studies case-cohort, case-control and nested case-control studies.
Dose of Fish-Derived Omega-3 Fatty Acids
Among the cohort studies, the amount of fish-derived omega-3 fatty acid intake in the highest quartile of dietary intake varied widely, ranging from approximately 200 to 1300 mg/d of EPA + DHA (Table 2). No relationship between the dose of the exposure and study outcome was apparent. Individual studies reporting findings of increased risk of PrCA incidence, decreased risk of PrCA incidence, and no effect on risk of PrCA incidence had the following average intake of fish-derived omega-3 fatty acids in the highest quartile: 577, 373, and 666 mg, respectively.
Three case-cohort studies assessed the impact of fish intake on PrCa incidence (Table 4). Two studies showed no association between fish intake and PrCa.53,54 One study showed no association between EPA intake and PrCa and a relationship between higher dietary intake of DHA and higher incidence of PrCa.55
Table 4.
Case-Cohort Studies of Fish-derived omega-3 fatty acids and Prostate Cancer Incidence.
| Reference | Cohort/Study Name | Cases n | Controls n | Geographic Area | Exposure Assessment | Highest Group (Quartile, Quintile, etc) | Effect of Exposure on Outcome (Highest vs Lowest Exposure) |
|---|---|---|---|---|---|---|---|
| Assessing blood levels of fatty acids | |||||||
| Bassett et al (2013)53 | Melbourne Collaborative Cohort Study | 464 | 1717 | Australia | PPL FAs and FFQ at baseline | Quintiles %PPL EPA and DHA (not defined) | ↔ risk of PrCa EPA mvHR 1.05 (0.93-1.18) P = .42; DHA mvHR 0.99 (0.88-1.11) P = .86 |
| Dietary intake quintiles (not defined) | ↔ risk of PrCa EPA mvHR 0.79 (0.56-1.12) P = .53; DHA mvHR 0.878 (0.61-1.25) P = .52 |
||||||
| Brasky et al (2013)55 | SELECT Trial | 834 | 1393 | USA, Canada, Puerto Rico | Serum PPL at baseline | EPA >0.82% total FAs | ↔ risk of total, low-grade or high-grade PrCa |
| DHA >3.62% total FAs | ↑ Total PrCa and low-grade PrCa DHA mvHR 1.39 (1.06-1.82) P = .009; mvHR 1.42 (1.06-1.89) P = .08 ↔ risk of high-grade PrCa with DHA |
||||||
| Assessing dietary intake of fatty acids | |||||||
| Schuurman et al (1999)54 | The Netherlands Cohort Study | 642 | 1525 | Netherlands | FFQ at baseline of cohort study | EPA intake 0.10 g/d DHA intake 0.18 g/d |
↔ PrCa risk for EPA RR 1.00 (0.73-1.35) P = .10 and DHA RR 1.03 (0.75-1.40) P = .19 |
Abbreviations: PPL, plasma phospholipid; FA, fatty acids; PrCA, prostate cancer; mvRR, multivariate relative risk; mvHR, multivariate hazard ratio; FFQ, Food Frequency Questionnaire; ↓ decrease, ↑ increase, ↔ no effect; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Sixteen publications reported on 15 case-control studies and 10 publications reported on 8 nested case-control studies. These results are presented in Tables 5 and 6 and displayed in Figures 4 and 5. There was considerable heterogeneity in the results of the different studies as well as the methodology, analyses and reporting in individual studies. The studies varied in their method of assessing exposure, the time of exposure, the type of fish eaten and the marine fatty acids measured (EPA, DHA, EPA + DHA). Of the case-cohort, case-control, and nested case-control studies, 10 utilized whole blood, plasma, serum or RBC fatty acid analysis to assess fish-derived omega-3 fatty acid exposure, whereas 21 assessed intake through participant recall using a FFQ.
Table 5.
Case Control Studies of Fish-derived omega-3 fatty acids and Prostate Cancer Incidence.
| Reference | Cohort/Study Name | Cases n | Controls n | Geographic Area | Exposure Assessment | Highest Group (Quartile, Quintile, etc) | Effect of Exposure on Outcome (Highest vs Lowest Exposure) |
|---|---|---|---|---|---|---|---|
| Assessing blood levels of fatty acids | |||||||
| Ukoli et al (2010)64 | N/A | 48 African American, 66 Nigerian | 125 African American, 274 Nigerian | Nigeria/USA | FFQ and plasma FA | Quartiles of plasma FA (not defined) | In African Americans: ↑ PrCa risk, Q3 vs Q1 DHA OR 6.63 (2.02-21.77) Ptrend < .004 ↔ PrCa risk Q4 vs Q1 DHA OR 1.35 (0.40-4.61) |
| Cheng (2013)81 | Carotene and Retinol Efficacy Trial (CARET) | 458 | 1369 | USA | Serum PPL FA at baseline | Quartiles of serum EPA + DHA (not defined) | ↔ risk of nonaggressive PrCa EPA + DHA mvOR 1.14 (0.80-1.63) P = .45 ↔ risk of aggressive PrCa mvOR 1.05 (0.71-1.55) P = .53 |
| Assessing dietary intake of fatty acids | |||||||
| Vlajinac et al (2010)62 | N/A | 101 | 202 | Serbia | 150-item FFQ | Tertiles and terciles of average daily intake (not defined) | ↑ PrCa risk OR 2.60 (1.45-4.65) p<0.01 (mostly canned/processed) |
| Williams (2011)82 | N/A | 79 | 187 | Virginia | Harvard 12-month FFQ | EPA 0.079-0.83% and DHA 0.10% to 0.64% (of total energy) | ↔ PrCa risk EPA OR 1.13 (0.56-2.24) P = .73 DHA 0.82 (0.40-1.68) P = .6 |
| Mina et al (2008)60 | National Enhanced Cancer Surveillance System (NECSS) | 1534 | 1607 | Canada | 60-item FFQ | Fresh/canned fish ≥2 servings /wk | ↔ PrCa risk AOR 1.10 (0.84-1.42) |
| Preserved (smoked/dried/salted fish) ≥1 serving /wk | ↓ PrCa risk 1-3 servings/mo preserved fish (vs 0/mo) AOR 0.78 (0.64-0.95) | ||||||
| Hu et al (2008)69 | National Enhanced Cancer Surveillance System (NECSS) | 1799 | 5039 | Canada | Self-administered 69-item FFQ for previous 2 years | ≥5 ounce fish/wk | ↔ PrCa risk OR 0.8 (0.7-1.0) P = .08 |
| Hedelin et al (2007)61 | Cancer Prostate in Sweden (CAPS) Study | 1499 (diet only), 1378 with blood samples | 1130 (diet only), 782 with blood samples | Sweden | Self-administered 261-item 12-month FFQ | ≥1 serving/wk | Salmon-type: ↓ PrCa risk OR 0.57 (0.43-0.76) Cod/saithe/fish fingers: ↑ PrCa risk OR 1.45 (1.12-1.88) Shellfish:↑ PrCa risk OR 0.81 (1.28-2.56) |
| 0.11 g EPA + DHA/day-MJ | ↓ PrCa risk OR 0.70 (0.51-0.97) | ||||||
| Chen (2005)83 | N/A | 237 | 481 | Taiwan | Interviewed FFQ for previous 10 years | Intake of “more” compared with others | ↔ PrCa risk AOR 1.12 (0.80-1.56) |
| Sonoda et al (2004)56 | N/A | 140 | 140 | Japan | Interviewed 102-item FFQ for previous 5 years | ≥130.7 g/d fish intake | ↓ PrCa risk OR 0.45 (0.20-1.02) P = .04 |
| Pawlega et al (1996)57 | N/A | 76 | 152 | Cracow, Poland | Self-administered 44-item FFQ for previous 20 years | Fish consumption ≥once/wk vs <rarely | ↓ PrCa risk smoked fish OR 0.5 (0.2-0.8) P < .05 ↓ PrCa risk fried fish 0.5 (0.2-0.9) P < .05 |
| Talamini et al (1992)68 | N/A | 271 | 685 | Northern Italy | Interviewed 14-item FFQ for preceding year | Fish intake ≥2 servings/wk | ↔ PrCa risk OR 0.79 (0.53-1.17) P = .36 |
| Deneo-Pellegrini (2012)84 | N/A | 326 | 1488 | Uruguay | Interviewed 64-item FFQ for previous 5 years | Tertiles of fish intake (continuous servings per year, not defined) | ↔ PrCa risk OR 1.34 (0.95-1.89) P = .09 |
| Kristal et al (2002)66 | Seattle-Puget Sound Surveillance Epidemiology and End Results Registry | 605 | 592 | Seattle, WA | Self-administered FFQ for previous 3-5 years | >0.24 g/d EPA + DHA intake (food and supplements) | ↔ local PrCa risk AOR 1.05 (0.68-1.63) P = .51 ↔ regional/distant PrCa risk AOR 0.84 (0.44-1.58) P = .81 |
| Joshi et al (2012)63 | California Collaborative Prostate Cancer Study | 717 localized, 1140 advanced | 1096 | California | Interviewed FFQ for previous 12-month intake | Tertiles fish intake White fish: >12.41-167 g/1000 kcal/d |
↔ PrCa risk T3 intake tuna, dark fish or deep-fried fish ↑ risk advanced PrCa white fish AOR 1.3 (1.0-1.7) Ptrend = .014 |
| Raimondi et al (2010)58 | N/A | 197 | 197 | Canada | FFQ for 1 year prior to diagnosis | Finfish/shellfish intake >30.4 g/d | ↓ PrCa risk OR 0.54 (0.30-0.97) P = .05 |
| Fradet et al (2009)59 | N/A | 466 | 478 | USA | FFQ reflecting the period before diagnosis | EPA intake 0.167 g/d DHA intake 0.368 g/d |
↓ PrCa risk EPA AOR 0.35 (0.24-0.52) P < .0001; DHA AOR 0.36 (0.25-0.53) P < .0001 |
| >1 serving per week dark fish | ↓ PrCa risk AOR 0.43 (0.29-0.63) Ptrend < .0001 | ||||||
| >1 serving per week white fish | ↓ PrCa risk AOR 0.66 (0.45-0.96) Ptrend = .32 | ||||||
| >1 serving per week shellfish | ↓ PrCa risk AOR 0.51 (0.35-0.74) Ptrend < .0001 | ||||||
| >1 serving per week tuna | ↓ PrCa risk AOR 0.75 (0.51-1.09) P = .04 | ||||||
| >1 serving per week fried fish | ↓ PrCa risk AOR 0.56 (0.37-0.86) P = .03 | ||||||
| Assessing blood levels and dietary intake of fatty acids | |||||||
| Norrish et al (1999)22 | Auckland Prostate Study | 317 | 480 | Auckland, New Zealand | Self-administered 107-item FFQ and RBC EPA and DHA | Quartiles EPA and DHA intake (not defined) | ↔ PrCa risk EPA mvRR 0.96 (0.63-1.48) DHA mvRR 1.10 (0.71-1.70) |
| RBC EPA >0.83 mol% and RBC DHA >1.70 mol% | ↓ PrCa risk EPA RR 0.59 (0.37-0.95) DHA RR 0.62 (0.39-0.98) | ||||||
Abbreviations: PrCa, prostate cancer; mvRR, multivariate relative risk; mvHR, multivariate hazard ratio; AOR, adjusted odds ratio; FFQ, Food Frequency Questionnaire; ×/wk, times per week; f/u follow-up; Bl, baseline; ↓, decrease; ↑, increase; ↔, no change; FA, fatty acids; PPL, plasma phospholipids; RBC, red blood cell; n-3, omega-3; PUFA, polyunsaturated fatty acids; sICAM-1, soluble intercellular adhesion molecule-1; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Table 6.
Nested Case-Control Studies of Fish-Derived Omega-3 Fatty Acids and Prostate Cancer Incidence.
| Reference | Cohort/Study Name | Cases n | Controls n | Geographic Area | Exposure Assessment | Highest Group (Quartile, Quintile, etc) | Effect of Exposure on Outcome (Highest vs Lowest Exposure) |
|---|---|---|---|---|---|---|---|
| Assessing blood levels of fatty acids | |||||||
| Dahm et al (2012)65 | European Prospective Investigation into Cancer and Nutrition (EPIC) cohort | 962 | 1061 | 10 European countries | Plasma FAs at baseline | Quintiles of plasma marine n-3 PUFA (not defined) | ↑ risk of PrCa based on treelet transform analysis OR 1.36 (0.99, 1.86) Ptrend = .041 |
| Crowe et al (2008)40 | European Prospective Investigation into Cancer and Nutrition (EPIC) | 926 | 926 | 10 European countries | Plasma PPL FAs | EPA 1.95-9.49 mol% | ↔ risk of total PrCa mvRR 1.31 (0.96-1.81) P = .09 ↑ risk of high-grade PrCa mvRR 2.00 (1.07-3.76) Ptrend = 0.031 ↔ risk of localized, advanced or low-grade PrCa |
| DHA 5.34-10.37 mol% | ↔ risk of total PrCa DHA mvRR 1.39 (1.02-1.90) P = .158 ↔ risk of localized, advanced, high-grade or low-grade PrCa |
||||||
| Brasky et al (2011)38 | Prostate Cancer Prevention Trial | 1658 | 1803 | USA | Serum PPL FA at baseline | EPA >0.74% | ↔ risk of low-grade or high-grade PrCa |
| DHA >3.30% | ↑ risk high-grade PrCa OR 2.50 (1.34, 4.65) P = .04 ↔ risk of low-grade PrCa |
||||||
| EPA + DHA >4.02% | ↔ risk of low-grade ↔ risk of high-grade PrCa AOR 1.99 (1.08-3.68) P = .08 |
||||||
| Park (2009)85 | The Multiethnic Cohort Study | 376 | 729 | USA | RBC FA at baseline | EPA >0.77% DHA >8.00% |
↔ risk PrCa |
| Chavarro et al (2008)41 | Physician’s Health Study | 476 | 476 | USA | Whole blood FAs | EPA >2.36% | ↓ risk of localized PrCa mvRR 0.57 (0.36-0.92) P = .02 ↔ risk of advanced, aggressive or nonaggressive PrCa |
| DHA >3.37% | ↓ risk of localized PrCa mvRR 0.60 (0.39-0.93) P = .07 ↔ risk of advanced, aggressive or nonaggressive PrCa |
||||||
| Harvei et al (1997)67 | N/A | 141 | 141 | Norway | Serum PPL FAs | EPA 2.00% DHA 5.67% |
↔ risk of PrCa EPA OR 1.2 (0.6-1.2) P = .1; DHA OR 1.0 (0.5-1.8) P = .08 |
| Assessing dietary intake of fatty acids | |||||||
| Touvier (2012)86 | SUVIMAX (Supplementation en Vitamines et Mineraux AntioXydants) Cohort Study | 129 | 760 | France | 24-hour dietary records every 2 mo for first 2 years of study; baseline plasma sICAM-1 | 1.2 g/d n-3 fatty acid intake in women, 1.6 g/d in men | Relation between sICAM-1and PrCa modulated by n-3 PUFA intake; sICAM-1 associated with ↑ risk PrCa in patients with n-3 intakes below the median OR 6.1; (1.1-34.5) Ptrend = .03; no association in patients with intakes above median OR 0.3 (0.1-1.6) Ptrend = .2 |
| Torfadottir et al (2013)36 | AGES-Reykjavik Cohort Study | 343 | 1914 | Iceland | FFQ assessing early, mid- and late-life fish intake | >4 servings total fish/wk | ↔ PrCa risk with intake early- and midlife AOR 0.87 (95% CI: 0.66, 1.13), 1.05 (95% CI: 0.71, 1.57) |
| Once a week or more intake of salted or smoked fish | ↑ risk advanced PrCa intake early life OR 1.98 (95% CI: 1.08, 3.62); ↔ risk with intake in midlife ↔ risk of total and localized PrCa with intake in early life, midlife |
||||||
| Fish oil use daily | ↔ risk of total, localized, or advanced PrCa with supplementation in early life or midlife | ||||||
| Assessing blood levels and dietary intake of fatty acids | |||||||
| Gann (1994)87 | Physician’s Health Study | 120 | 120 | USA | Plasma FAs and FFQ at baseline | Quartiles of plasma FAs (not defined) | ↔ risk of PrCa EPA RR 0.87 (0.41-1.82) P = .81 |
Abbreviations: PrCa, prostate cancer; mvRR, multivariate relative risk; mvHR, multivariate hazard ratio; AOR, adjusted odds ratio; FFQ, Food Frequency Questionnaire; ×/wk, times per week; f/u follow-up; Bl, baseline; ↓, decrease; ↑, increase; ↔, no change; FA, fatty acids; PPL, plasma phospholipids; RBC, red blood cell; n-3, omega-3; PUFA, polyunsaturated fatty acids; sICAM-1, soluble intercellular adhesion molecule-1; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
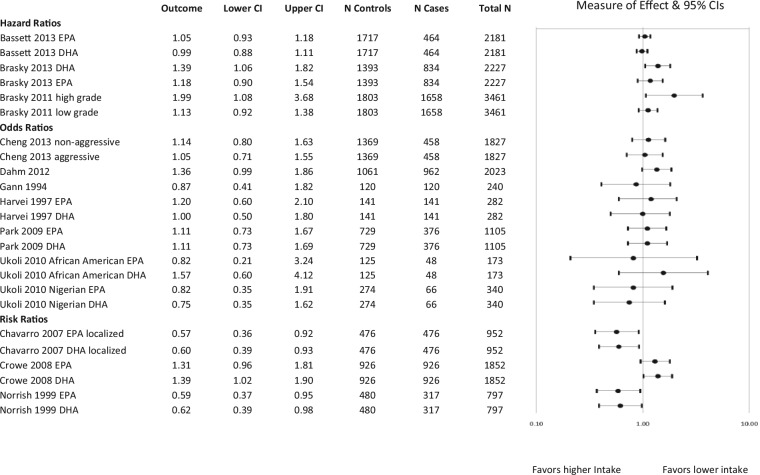
Figure 4.
Risk of prostate cancer (PrCa) incidence with fish-derived omega-3 fatty acid intake: Case-cohort, case-control, and nested case-control studies using blood assessment of fatty acids. When the effect of total fish-derived omega-3 fatty acid intake on PrCa risk was not available, sub-analyses reporting on individual fatty acids or different stages of cancer are reported.
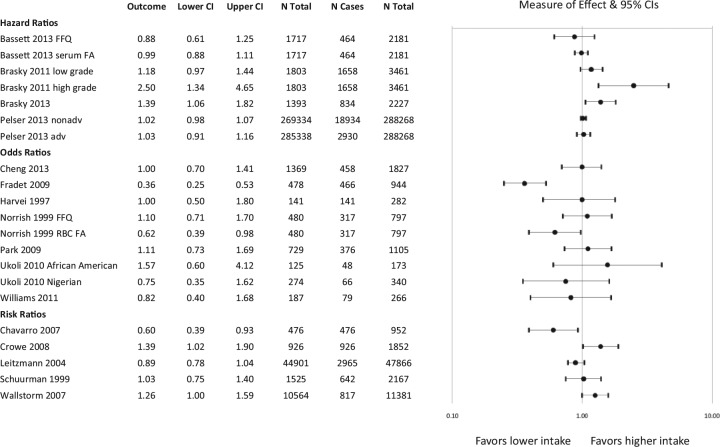
Figure 5.
Risk of prostate cancer (PrCa) incidence with fish-derived omega-3 fatty acid intake: Case-cohort, case-control, and nested case-control studies using Food Frequency Questionnaire (FFQ) assessment of fatty acid exposure. When the effect of total fish-derived omega-3 fatty acid intake on PrCa risk was not available, subanalyses reporting on individual fatty acids, different stages of cancer, and different time points of fish exposure are reported.
Among those that assessed fish-derived omega-3 fatty acid intake through recall with significant findings, 8 analyses showed an association between higher intake and lower risk of PrCa incidence56-61 while 4 analyses showed an association with higher risk of incidence.36,61-63 Among the studies that assessed blood levels, 3 analyses reported an association between higher intake and lower risk22,41 and 5 reported an association with higher risk of incidence.40,50,55,64,65 Among the studies reporting an association between higher fish intake on recall and higher risk, the type of fish intake assessed was related to the outcome. One study showed an association with increased risk with higher intake of cod, saithe, shellfish, and “fish fingers” but an association with decreased risk with higher intake of salmon-type fish while adjusting for intake of the other fish types.61 It also showed that when overall intake of EPA + DHA was assessed, the effect was protective. Another study showed an association with increased risk with higher white fish intake (unadjusted for intake of other fish) and no effect from higher intake of tuna, dark fish, or deep fried fish.63 Another study that found an association with increased risk reported mixed results including an increased risk of advanced PrCa with higher intake of salted or smoked fish in early life, a lower risk of localized PrCa with intake later in life and no effect on other stages of PrCa and other intake time points.36
No case-cohort, case-control, or nested case-control studies reported on the risk of death.
One case-control study assessed supplemental fish oil intake in addition to dietary fish intake; however, the intake amounts were combined in analysis to reflect overall fish-derived omega-3 fatty acid intake.66 One nested case-control study assessed supplemental fish oil intake and found that daily use in early or midlife was not associated with and PrCa risk.36
When the observational results were organized by the type of fish-derived fatty acid analyzed (EPA and DHA), no association was observed (Figures 6 and 7).
Figure 6.
Risk of prostate cancer (PrCa) incidence with eicosapentaenoic acid (EPA) intake: Observational data. When the effect of total fish-derived omega-3 fatty acid intake on PrCa risk was not available, subanalyses reporting on individual fatty acids or different stages of cancer are reported.
Figure 7.
Risk of prostate cancer (PrCa) incidence with docosahexaenoic acid (DHA) intake: Observational data. When the effect of total fish-derived omega-3 fatty acid intake on PrCa risk was not available, sub-analyses reporting on individual fatty acids or different stages of cancer are reported.
Adverse Events and Interactions
In the RCTs and non-RCT, adverse events ranged from none to mild. In 1 study, 2 of 32 subjects experienced nausea and withdrew.28 One study reported increased flatulence (5 subjects compared with 1 in the placebo group), self-limiting diarrhea (2 participants compared with 1 in the placebo group), and eructation (1 participant).29 Two studies stated that no adverse events were reported.31,32 No studies reported a statistically significant difference in adverse reaction rates between fish oil and control/placebo arm.
Among the studies that used fish oil supplement interventions in patients with PrCa, no interactions with conventional care were reported; however, the studies excluded many participants receiving conventional cancer therapies. Of the 3 RCTs and 1 non-RCT, 3 excluded men receiving antihormonal therapies, 2 excluded men receiving 5-alpha-reductase inhibitors, and 3 excluded men taking anti-inflammatory medication. None of the studies included men receiving chemotherapy or radiation; 2 studies included men who were untreated,31,32 1 included men postprostatectomy,28 and 1 preprostatectomy.29 No clinical studies specifically reported an active assessment of interactions with other therapies, surgical procedures, or medications.
Risk of Bias
Among the RCTs, all studies had a high risk of bias when evaluated with the Cochrane Risk of Bias Assessment; the results are displayed in Figure 8. Risk was identified in most studies due to incomplete outcome data and in some studies due to lack of blinding for participants and in outcome assessment. Allocation concealment was unclear in all studies. Among the 3 RCTs, 1 was industry funded,28 1 was non–industry funded,29 and 1 was funded by a combination.31 The 1 nonrandomized clinical trial did not use blinding, had no loss to follow-up; and a priori sample size estimation and funding source were unclear.32
Figure 8.
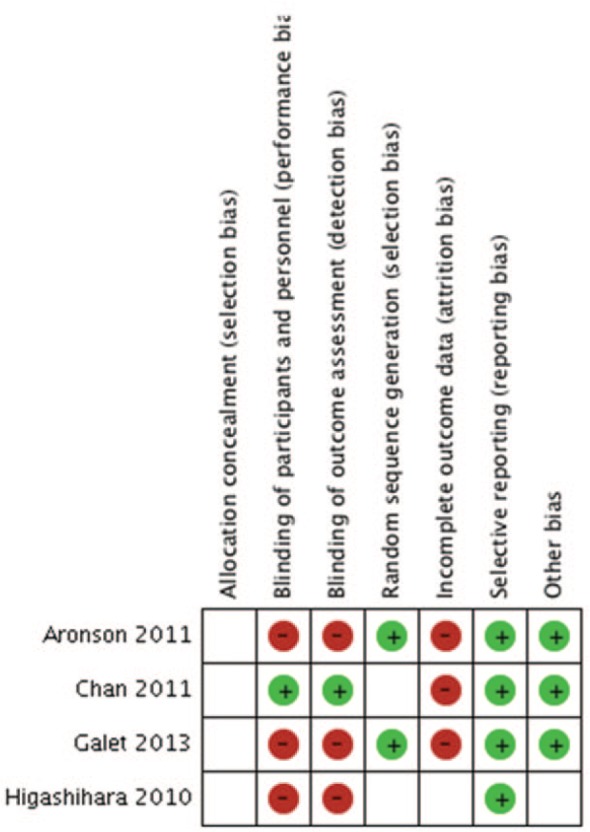
Cochrane Risk of Bias Assessment of randomized controlled trials (RCTs). (+), low risk of bias; (−), high risk of bias; neither symbol, unclear risk of bias.
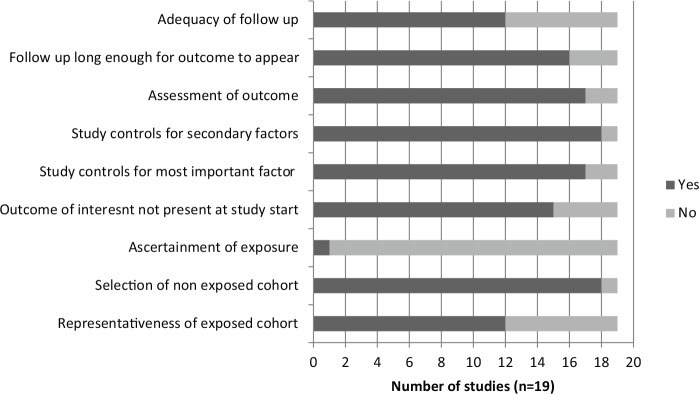
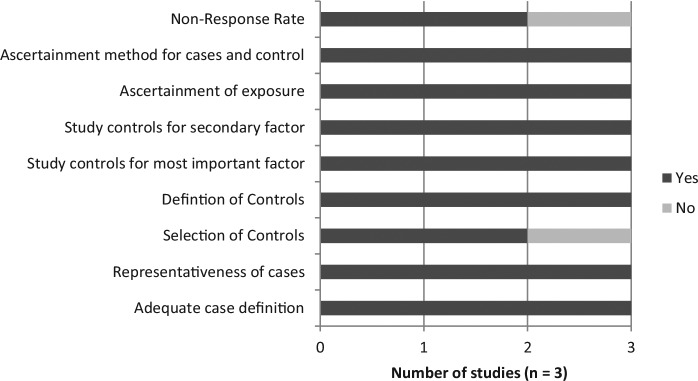
Among the observational studies, the cohort studies, case-control studies and case-cohort studies had an average score of 7.06, 7.27, and 8.33 out of 9, respectively, on the Newcastle-Ottawa Quality Assessment Scale. Two cohort studies were not assessed because full text publication in English was not available.42,44 The results are displayed in Figures 9, 10, and 11. The most notable deficiency in both the cohort and case-control studies was for the ascertainment of fish-derived omega-3 fatty acid exposure as most studies relied on written, self-report of dietary information rather than a structured interview. Additionally, many of the case-control studies failed to report the difference in nonresponse rate between cases and controls. Among the observational studies, 40 were funded by nonindustry sources, 2 by a combination of industry and nonindustry sources,34,36 and 8 were unclear in their reporting of funding.43,47,56-58,67-69
Figure 9.
Newcastle-Ottawa Quality Assessment Scale: Cohort studies.
Figure 10.
Newcastle-Ottawa Quality Assessment Scale: Case-control studies.
Figure 11.
Newcastle-Ottawa Quality Assessment Scale: Case-cohort studies.
Discussion
Summary of Findings
The interventional studies of fish-derived omega-3 in patients with PrCa showed no impact on PSA levels; however, some studies showed a decrease in inflammatory markers. A small number of mild adverse events were reported and interactions with other interventions were not assessed. Cohort, case-cohort and case-control studies assessing the risk of PrCa incidence were equivocal. Cohort studies assessing the risk of PrCa mortality suggested an association between higher intake and decreased risk. The results of this review are consistent with other systematic reviews conducted,70,71 which also assessed prevention and secondary PrCa outcomes using diet and supplement exposure and found no association.
Study Weaknesses: Method of Exposure Ascertainment in Observational Studies
While all of the cohort studies assessed patient-reported fish-derived omega-3 fatty acid intake to ascertain exposure, case-control and nested case-control studies utilized either patient questionnaires or blood levels to assess fish-derived omega-3 fatty acid exposure; however, both have limitations. The method of assessing fish-derived omega-3 fatty acid exposure may be of importance as more of the studies utilizing blood levels reported an association with increased risk of PrCa while more of the studies assessing self-reported intake reported an association with deceased risk of PrCa. A recent study compared the different methods of fish intake assessment.72 They followed a group of men with low-risk PrCa undergoing active surveillance to first repeat biopsy and assessed relationships between the risk of progression and the different methods of fatty acid assessment—dietary recall, RBC fatty acids, and prostate tissue fatty acids. While dietary intake and RBC fatty acids were not correlated with risk of progression, men with the highest tertile of prostate EPA had a significantly lower risk of progression (OR = 0.08, 95% CI 0.01-0.72, P = .02).
Blood assessment is limited by the challenge of differentiating endogenously produced fats from those consumed and the impact of individually varying rates of metabolism and deposition in tissues as a result of genetic, dietary and lifestyle differences.73 Additionally an individual fatty acid is reported as a percentage of total fatty acids, not as an absolute amount; as a result, larger intake of one fatty acid could affect the relative proportion of others. Lastly, disease can affect fatty acid levels; for example, tumors selectively take up high amounts of polyunsaturated fatty acids.73 Among the studies included in this review that used blood samples, 9 of 12 assessed plasma or serum fatty acids, 2 assessed RBC fatty acids and 1 assessed whole blood. Plasma fatty acids are known to reflect short-term or recent fat intake while RBCs reflect the previous 3 weeks’ to 3 months’ intake due to their longer life span.74 Considering the length of time required for the pathogenesis of cancerous lesions, these fatty acid evaluations that reflect relatively recent intake may be of limited utility in assessing a role in causation, particularly if the participant has made changes to their diet over time.
Diet recall also presents challenges, including intentionally or unintentionally inaccurate recall and low reproducibility. Studies assessing the reproducibility of FFQs for polyunsaturated fatty acids have found a Spearman correlation coefficient from 0.38 to 0.59.75 With cancer known to develop over a long period of time, some of the shorter times between observation of exposure and outcome of interest may neither be sufficient to allow for detection, progression, or substantive change, nor represent the time when carcinogenesis or early cancer development occurred. Conversely, when studies asked participants to recall dietary patterns from 10 years ago, there could be concern about the patients’ ability to do so accurately.
Possible Mechanisms of Protective Effect
There have been a number of proposed mechanisms by which fish-derived omega-3 fatty acids may influence PrCa risk or progression, largely related to their effect on decreasing inflammation through inhibition of the cyclooxygenase enzymes76 and affecting the immune system. Inflammation is suspected to trigger PrCa progression77 and the use of nonsteroidal anti-inflammatory drugs (NSAIDS) results in a decreased risk of PrCa.61 In vitro studies assessing the impact of fish oil on PrCa cells demonstrate increased cancer cell death,12 enhanced cytotoxic effects of docetaxel,13 prolongation of the androgen-dependent state,14 and inhibition of cell adhesion, invasion and migration, which effect metastasis.15,16 A mouse study comparing diets based on fish, olive, corn, or animal fat showed slowed tumor growth and increased survival in the fish oil group.17
Alternatively, another factor in the fish may be exerting an anticancer effect. Dietary fish is a source of vitamin D78 and while results are varied, vitamin D is suspected to have a possible protective effect in prostate cancer.79 None of the studies reviewed assessed vitamin D levels; however, one study acknowledged that the protective effect of fish oil supplementation may have been related to the vitamin D content found in fish liver oil, the most common form of fish oil supplementation used in the study population.36
Possible Mechanisms of Harmful Effect
Some of the studies reviewed found associations between higher fish-derived omega-3 fatty acid exposure and PrCa incidence risk. No biological mechanism has been proposed to explain these results. The studies suggesting harm were more likely to be retrospective studies than prospective studies and many showed a combination of harmful, protective or null effect, resulting in unclear conclusions. Although they were of short duration, the intervention trials did not produce results suggesting a risk in the form of side effects, or negative effects on clinical outcomes such as laboratory markers or mortality.
One study suggested that the omega-3:omega-6 ratio might be of more importance than the absolute amount of marine omega-3 intake because of their competitive metabolism and antagonistic effects on inflammation.61 The study conducted by Joshi et al63 explored the relationship between different types of fish, different methods of preparation, different levels of “done-ness” (ie, preconsumption cooking and/or preparation) and the risk of PrCa. Fish that was pan-fried or cooked to “well done” was associated with increased risk while fish cooked “just until done,” “well done at low temperatures,” and “just done at high temperatures” was not. The authors concluded that mutagenic heterocyclic amines produced in cooking might be responsible for the association.
Other studies hypothesized that environmental toxins, such as polychlorinated biphenyls found in the fish that was ingested,43 or involved in processing and packaging,62 could be the biologically active constituent responsible for the association with cancer rather than the fatty acids EPA + DHA.
While not part of this systematic review, a brief search of preclinical data yielded only 1 study showing procancer effects of EPA at low concentrations in cancer cell lines. The remaining 50 in vitro/in vivo studies reported anticancer effects or mechanisms of omega-3 fatty acids (Appendix B). Unlike the observational studies that posed very large challenges in assessing exposure, the cell culture and animal studies involved doses of fish oil exposure that were was precisely known.
Alternatively, the relationship may be related to a behavioral factor rather than a biological one. PrCa risk is increased with increasing education, which may be associated with more health conscious behaviors such as consuming dietary fish or fish oil supplements.80
Systematic Review Strengths and Limitations
Strengths of our review include a comprehensive search including all interventional and observational studies conducted in humans. Our methodology, including duplication of screening and data extraction demonstrates reliability of the synthesis.
One significant limitation of this review relates to primary research available and specifically the lack of well designed, long duration studies examining the effects of fish oil interventions in patients with or without PrCa. Of the 4 interventional studies reported on, 3 studies were 3 months or shorter in duration, which may not be an adequate length of time to observe a response to treatment, progression, recurrence of PrCa or to monitor long-term adverse events. Because of the relatively small number of studies assessing the role of fish oil in patients with PrCa, the majority of the evidence included in this review assessed primary prevention of PrCa. While this information may be useful in understanding a potential anticancer or procancer effect of these constituents, it is a significant limitation in answering the original question of whether or not fish oil supplementation is indicated in patients with PrCa. Because the majority of the studies were observational, the results provide limited information on causality and may reflect correlations or other associations. Additionally, the methods used to assess exposure possessed limitations. Although we included a large number of studies, it was not possible to pool the data due to heterogeneity.
Conclusions
Taken together, there are inadequate data to determine if fish-derived omega-3 fatty acids are associated with PrCa incidence and progression and how to advise PrCa patients who are considering fish oil supplementation. Preliminary research suggests that an association between higher omega-3 intake and decrease PrCa mortality may be present but more research is needed. Because of the challenges related to assessing exposure, more intervention trials or observational studies with precisely measured exposure and longer duration are needed to assess the impact of supplemental or dietary fish-derived omega-3 fatty acid intake on PrCa incidence, treatment and progression.
Appendix A
Prostate Cancer—Omega 3
Final Search Strategy
2014 Jul 21
OVID Searches
Database: AMED (Allied and Complementary Medicine) <1985 to July 2014>, Embase Classic+Embase <1947 to 2014 July 18>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present> Search Strategy:
——————————————————————————–
1 exp Prostatic Neoplasms/ (253131)
2 ((prostate or prostatic) adj3 (cancer* or carcinoid* or carcinoma* or carcinogen* or adenocarcinoma* or adeno-carcinoma* or malignan* or neoplasia* or neoplasm* or sarcoma* or tumour* or tumor*)).tw. (230129)
3 ((prostate or prostatic) adj3 (anticancer* or anti-cancer* or anticarcinogen* or anti-carcinogen* or antineoplas* or anti-neoplas* or chemoprevent* or chemo-prevent* or (tumo?r adj2 suppress*))).tw. (2285)
4 Prostate/de (3838)
5 Prostatic Hyperplasia/ (47434)
6 ((prostate or prostatic) adj3 (hyperplasia* or adenoma* or hypertroph* or enlarg*)).tw. (40106)
7 BPH.tw. (19805)
8 Prostate-Specific Antigen/ (55668)
9 (PSA or prostate-specific antigen* or prostatic-specific antigen* or gamma-seminoprotein* or (hK3 adj1 Kallikrein) or semenogelase or seminin).tw. (76299)
10 or/1-9 (349314)
11 Fatty Acids, Omega-3/ (25712)
12 Eicosapentaenoic Acid/ (15147)
13 (eicosapentanoic acid* or icosapentaenoic acid* or icosapentaenoate or omega-3-eicosapentaenoic acid* or timnodonic acid* or eicosapen).tw. (600)
14 (“5,8,11,14,17-Eicosapentaenoic Acid” or “5,8,11,14,17-Icosapentaenoic Acid”).tw. (143)
15 EPA.tw. (24393)
16 Eicosapentaenoic Acid.rn. (4358)
17 exp Docosahexaenoic Acids/ (18855)
18 (docosahexaenoic acid* or docosahexaenoate or dhasco).tw. (18092)
19 dha.tw. (20493)
20 25167-62-8.rn. (17617)
21 (“omega 3” or omega3 or n-3 fatty acid* or n3 fatty acid* or n-3 polyunsaturated fatty acid* or n-3 poly-unsaturated fatty acid* or n3 polyunsaturated fatty acid* or n3 poly-unsaturated fatty acid* or n-3 PUFA or n3 PUFA or PUFAs).tw. (35392)
22 (Maxepa or Omacor).tw. (998)
23 or/11-22 (80037)
24 10 and 23 (816)
25 exp Fishes/ (309824)
26 exp Food/ (1803802)
27 exp Diet/ (439211)
28 25 and (26 or 27) (34591)
29 (fish$2 or fishoil* or shellfish$2 or shell fish$2 or seafood* or sea food* or marine*).tw. (403998)
30 28 or 29 (415408)
31 10 and 30 (2226)
32 24 or 31 (2842)
33 exp Animals/ not (exp Animals/ and Humans/) (8758181)
34 32 not 33 (2574)
35 (comment or editorial or interview or letter or news).pt. (2839640)
36 34 not 35 (2521)
37 36 use prmz (860)
38 exp prostate cancer/ (228398)
39 ((prostate or prostatic) adj3 (cancer* or carcinoid* or carcinoma* or carcinogen* or adenocarcinoma* or adeno-carcinoma* or malignan* or neoplasia* or neoplasm* or sarcoma* or tumour* or tumor*)).tw. (230129)
40 ((prostate or prostatic) adj3 (anticancer* or anti-cancer* or anticarcinogen* or anti-carcinogen* or antineoplas* or anti-neoplas* or chemoprevent* or chemo-prevent* or (tumo?r adj2 suppress*))).tw. (2285)
41 prostate hypertrophy/ (29272)
42 ((prostate or prostatic) adj3 (hyperplasia* or adenoma* or hypertroph* or enlarg*)).tw. (40106)
43 BPH.tw. (19805)
44 prostate specific antigen/ (55668)
45 (PSA or prostate-specific antigen* or prostatic-specific antigen* or gamma-seminoprotein* or (hK3 adj1 Kallikrein) or semenogelase or seminin).tw. (76299)
46 or/38-45 (337349)
47 omega 3 fatty acid/ (28576)
48 icosapentaenoic acid/ (10789)
49 (eicosapentanoic acid* or icosapentaenoic acid* or icosapentaenoate or omega-3-eicosapentaenoic acid* or timnodonic acid* or eicosapen).tw. (600)
50 (“5,8,11,14,17-Eicosapentaenoic Acid” or “5,8,11,14,17-Icosapentaenoic Acid”).tw. (143)
51 EPA.tw. (24393)
52 1553-41-9.rn. (164)
53 icosapentaenoic acid.rn. (9979)
54 docosahexaenoic acid/ (13096)
55 (docosahexaenoic acid* or docosahexaenoate or dhasco).tw. (18092)
56 dha.tw. (20493)
57 25167-62-8.rn. (17617)
58 docosahexaenoic acid.rn. (11891)
59 (“omega 3” or omega3 or n-3 fatty acid* or n3 fatty acid* or n-3 polyunsaturated fatty acid* or n-3 poly-unsaturated fatty acid* or n3 polyunsaturated fatty acid* or n3 poly-unsaturated fatty acid* or n-3 PUFA or n3 PUFA or PUFAs).tw. (35392)
60 (Maxepa or Omacor).tw. (998)
61 or/47-60 (80408)
62 46 and 61 (839)
63 exp fish/ (173462)
64 exp food/ (1803802)
65 exp diet/ (439211)
66 63 and (64 or 65) (19916)
67 (fish$2 or fishoil* or shellfish$2 or shell fish$2 or seafood* or sea food* or marine*).tw. (403998)
68 66 or 67 (410094)
69 46 and 68 (2203)
70 62 or 69 (2836)
71 exp animal experimentation/ or exp models animal/ or exp animal experiment/ or nonhuman/ or exp vertebrate/ (37835447)
72 exp humans/ or exp human experimentation/ or exp human experiment/ (28781117)
73 71 not 72 (9055973)
74 70 not 73 (2577)
75 (editorial or letter).pt. (2524137)
76 74 not 75 (2527)
77 76 use emczd (1648)
78 prostatic neoplasms/ (119512)
79 ((prostate or prostatic) adj3 (cancer* or carcinoid* or carcinoma* or carcinogen* or adenocarcinoma* or adeno-carcinoma* or malignan* or neoplasia* or neoplasm* or sarcoma* or tumour* or tumor*)).tw. (230129)
80 ((prostate or prostatic) adj3 (anticancer* or anti-cancer* or anticarcinogen* or anti-carcinogen* or antineoplas* or anti-neoplas* or chemoprevent* or chemo-prevent* or (tumo?r adj2 suppress*))).tw. (2285)
81 exp prostatic hypertrophy/ (47487)
82 ((prostate or prostatic) adj3 (hyperplasia* or adenoma* or hypertroph* or enlarg*)).tw. (40106)
83 BPH.tw. (19805)
84 (PSA or prostate-specific antigen* or prostatic-specific antigen* or gamma-seminoprotein* or (hK3 adj1 Kallikrein) or semenogelase or seminin).tw. (76299)
85 or/78-84 (315938)
86 fatty acids/ (172045)
87 (eicosapentanoic acid* or icosapentaenoic acid* or icosapentaenoate or omega-3-eicosapentaenoic acid* or timnodonic acid* or eicosapen).tw. (600)
88 (“5,8,11,14,17-Eicosapentaenoic Acid” or “5,8,11,14,17-Icosapentaenoic Acid”).tw. (143)
89 EPA.tw. (24393)
90 (docosahexaenoic acid* or docosahexaenoate or dhasco).tw. (18092)
91 dha.tw. (20493)
92 (“omega 3” or omega3 or n-3 fatty acid* or n3 fatty acid* or n-3 polyunsaturated fatty acid* or n-3 poly-unsaturated fatty acid* or n3 polyunsaturated fatty acid* or n3 poly-unsaturated fatty acid* or n-3 PUFA or n3 PUFA or PUFAs).tw. (35392)
93 (Maxepa or Omacor).tw. (998)
94 or/86-93 (230884)
95 85 and 94 (851)
96 fishes/ (141926)
97 exp food/ (1803802)
98 exp diet/ (439211)
99 96 and (97 or 98) (21782)
100 (fish$2 or fishoil* or shellfish$2 or shell fish$2 or seafood* or sea food* or marine*).tw. (403998)
101 99 or 100 (409563)
102 85 and 101 (2072)
103 95 or 102 (2769)
104 exp Animals/ not (exp Animals/ and Humans/) (8758181)
105 103 not 104 (2474)
106 (comment or editorial or interview or letter or news).pt. (2839640)
107 105 not 106 (2450)
108 107 use amed (7)
109 37 or 77 or 108 (2515)
110 remove duplicates from 109 (1778) [UNIQUE RECORDS]
111 110 use prmz (821) [MEDLINE RECORDS]
112 110 use emczd (956) [EMBASE RECORDS]
113 110 use amed (1) [AMED RECORD]
***************************
Cochrane Library
Search Name: Prostate Cancer - Omega 3
Date Run: 21/07/14 13:43:26.496
Description: Final 2014 Jul 21
ID Search Hits
#1 [mh “Prostatic Neoplasms”] 3374
#2 ((prostate or prostatic) near/3 (cancer* or carcinoid* or carcinoma* or carcinogen* or adenocarcinoma* or adeno-carcinoma* or malignan* or neoplasia* or neoplasm* or sarcoma* or tumour* or tumor*)):ti,ab,kw 5130
#3 ((prostate or prostatic) near/3 (anticancer* or anti-cancer* or anticarcinogen* or anti-carcinogen* or antineoplas* or anti-neoplas* or chemoprevent* or chemo-prevent* or (tumo*r* near/2 suppress*))):ti,ab,kw 48
#4 [mh Prostate/de] 103
#5 [mh “Prostatic Hyperplasia”] 1361
#6 ((prostate or prostatic) near/3 (hyperplasia* or adenoma* or hypertroph* or enlarg*)):ti,ab,kw 1980
#7 BPH:ti,ab,kw 856
#8 [mh “Prostate-Specific Antigen”] 998
#9 (PSA or prostate-specific antigen* or prostatic-specific antigen* or gamma-seminoprotein* or (hK3 near/1 Kallikrein) or semenogelase or seminin):ti,ab,kw 2239
#10 {or #1-#9} 7311
#11 [mh ^”Fatty Acids, Omega-3”] 1258
#12 [mh “Eicosapentaenoic Acid”] 647
#13 (eicosapentanoic next acid*) or (icosapentaenoic next acid*) or icosapentaenoate or (“omega-3-eicosapentaenoic” next acid*) or (timnodonic next acid*) or eicosapen:ti,ab,kw 260
#14 (“5,8,11,14,17-Eicosapentaenoic Acid” or “5,8,11,14,17-Icosapentaenoic Acid”):ti,ab,kw 1
#15 EPA:ti,ab,kw 830
#16 [mh “Docosahexaenoic Acids”] 694
#17 (docosahexaenoic next acid*) or docosahexaenoate or dhasco:ti,ab,kw 1399
#18 dha:ti,ab,kw 995
#19 “omega 3” or omega3 or ((“n-3 fatty” or “n3 fatty” or “n-3 polyunsaturated fatty” or “n-3 poly-unsaturated fatty” or “n3 polyunsaturated fatty” or “n3 poly-unsaturated fatty”) next acid*) or “n-3 PUFA” or “n3 PUFA” or PUFAs:ti,ab,kw 2804
#20 Maxepa or Omacor:ti,ab,kw 106
#21 {or #11-#20} 3714
#22 #10 and #21 21
#23 [mh Fishes] 218
#24 [mh Food] or [mh Diet] 29205
#25 #23 and #24 155
#26 fish or fishes or fishoil* or shellfish* or (shell next fish*) or seafood* or (sea next food*) or marine*:ti,ab,kw 3060
#27 #25 or #26 3064
#28 #10 and #27 17
#29 #22 or #28 33
DSR - 3
DARE - 2
CENTRAL - 27
Cochrane Groups - 1 (did not download)
CINAHL.
2014 Jul 21
| # | Query | Limiters/Expanders | Results |
|---|---|---|---|
| S38 | S35 NOT S36 | Limiters: Exclude MEDLINE records Expanders: Apply related words Search modes: Boolean/Phrase |
57 |
| S37 | S35 NOT S36 | Expanders: Apply related words Search modes: Boolean/Phrase |
113 |
| S36 | PT comment or editorial or interview or letter or news | Expanders: Apply related words Search modes: Boolean/Phrase |
306 747 |
| S35 | S31 NOT S34 | Expanders: Apply related words Search modes: Boolean/Phrase |
127 |
| S34 | S32 NOT (S32 AND S33) | Expanders: Apply related words Search modes: Boolean/Phrase |
26 845 |
| S33 | (MH “Human”) | Expanders: Apply related words Search modes: Boolean/Phrase |
848 532 |
| S32 | (MH “Animals+”) | Expanders: Apply related words Search modes: Boolean/Phrase |
29 062 |
| S31 | S22 OR S30 | Expanders: Apply related words Search modes: Boolean/Phrase |
130 |
| S30 | S10 AND S29 | Expanders: Apply related words Search modes: Boolean/Phrase |
55 |
| S29 | S23 OR S27 OR S28 | Expanders: Apply related words Search modes: Boolean/Phrase |
5992 |
| S28 | TI ( fish or fishes or fishoil* or shellfish* or (shell n1 fish*) or seafood* or (sea n1 food*) or marine* ) OR AB ( fish or fishes or fishoil* or shellfish* or (shell n1 fish*) or seafood* or (sea n1 food*) or marine* ) | Expanders: Apply related words Search modes: Boolean/Phrase |
4355 |
| S27 | S24 AND (S25 OR S26) | Expanders: Apply related words Search modes: Boolean/Phrase |
2413 |
| S26 | (MH “Diet+”) | Expanders: Apply related words Search modes: Boolean/Phrase |
48 485 |
| S25 | (MH “Food+”) | Expanders: Apply related words Search modes: Boolean/Phrase |
66 225 |
| S24 | (MH “Fish”) | Expanders: Apply related words Search modes: Boolean/Phrase |
2413 |
| S23 | (MH “Seafood+”) | Expanders: Apply related words Search modes: Boolean/Phrase |
2998 |
| S22 | S10 AND S21 | Expanders: Apply related words Search modes: Boolean/Phrase |
96 |
| S21 | S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | Expanders: Apply related words Search modes: Boolean/Phrase |
5246 |
| S20 | TI ( Maxepa or Omacor ) OR AB ( Maxepa or Omacor ) | Expanders: Apply related words Search modes: Boolean/Phrase |
22 |
| S19 | TI ( “omega 3” or omega3 or ((“n-3 fatty” or “n3 fatty” or “n-3 polyunsaturated fatty” or “n-3 poly-unsaturated fatty” or “n3 polyunsaturated fatty” or “n3 poly-unsaturated fatty”) n1 acid*) or “n-3 PUFA” or “n3 PUFA” or PUFAs ) OR AB ( “omega 3” or omega3 or ((“n-3 fatty” or “n3 fatty” or “n-3 polyunsaturated fatty” or “n-3 poly-unsaturated fatty” or “n3 polyunsaturated fatty” or “n3 poly-unsaturated fatty”) n1 acid*) or “n-3 PUFA” or “n3 PUFA” or PUFAs ) | Expanders: Apply related words Search modes: Boolean/Phrase |
2309 |
| S18 | TI dha OR AB dha | Expanders: Apply related words Search modes: Boolean/Phrase |
633 |
| S17 | TI ( (docosahexaenoic n1 acid*) or docosahexaenoate or dhasco ) OR AB ( (docosahexaenoic n1 acid*) or docosahexaenoate or dhasco ) | Expanders: Apply related words Search modes: Boolean/Phrase |
684 |
| S16 | (MH “Docosahexaenoic Acids”) | Expanders: Apply related words Search modes: Boolean/Phrase |
975 |
| S15 | TI EPA OR AB EPA | Expanders: Apply related words Search modes: Boolean/Phrase |
801 |
| S14 | TI ( “5,8,11,14,17-Eicosapentaenoic Acid” or “5,8,11,14,17-Icosapentaenoic Acid” ) OR AB ( “5,8,11,14,17-Eicosapentaenoic Acid” or “5,8,11,14,17-Icosapentaenoic Acid” ) | Expanders: Apply related words Search modes: Boolean/Phrase |
1 |
| S13 | TI ( (eicosapentanoic n1 acid*) or (icosapentaenoic n1 acid*) or icosapentaenoate or (“omega-3-eicosapentaenoic” n1 acid*) or (timnodonic n1 acid*) or eicosapen ) OR AB ( (eicosapentanoic n1 acid*) or (icosapentaenoic n1 acid*) or icosapentaenoate or (“omega-3-eicosapentaenoic” n1 acid*) or (timnodonic n1 acid*) or eicosapen ) | Expanders: Apply related words Search modes: Boolean/Phrase |
13 |
| S12 | (MH “Eicosapentaenoic Acid”) | Expanders: Apply related words Search modes: Boolean/Phrase |
678 |
| S11 | (MH “Fatty Acids, Omega-3”) | Expanders: Apply related words Search modes: Boolean/Phrase |
3404 |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 | Expanders: Apply related words Search modes: Boolean/Phrase |
13 411 |
| S9 | TI ( (PSA or prostate-specific antigen* or prostatic-specific antigen* or gamma-seminoprotein* or (hK3 N1 Kallikrein) or semenogelase or seminin) ) OR AB ( (PSA or prostate-specific antigen* or prostatic-specific antigen* or gamma-seminoprotein* or (hK3 N1 Kallikrein) or semenogelase or seminin) ) | Expanders: Apply related words Search modes: Boolean/Phrase |
2125 |
| S8 | (MH “Prostate-Specific Antigen”) | Expanders: Apply related words Search modes: Boolean/Phrase |
2517 |
| S7 | TI BPH OR AB BPH | Expanders: Apply related words Search modes: Boolean/Phrase |
401 |
| S6 | TI ( ((prostate or prostatic) N3 (hyperplasia* or adenoma* or hypertroph* or enlarg*)) ) OR AB ( ((prostate or prostatic) N3 (hyperplasia* or adenoma* or hypertroph* or enlarg*)) ) | Expanders: Apply related words Search modes: Boolean/Phrase |
713 |
| S5 | (MH “Prostatic Hypertrophy”) | Expanders: Apply related words Search modes: Boolean/Phrase |
1332 |
| S4 | (MH “Prostate/DE”) | Expanders: Apply related words Search modes: Boolean/Phrase |
42 |
| S3 | TI ( ((prostate or prostatic) N3 (anticancer* or anti-cancer* or anticarcinogen* or anti-carcinogen* or antineoplas* or anti-neoplas* or chemoprevent* or chemo-prevent* or (tumo#r N2 suppress*))) ) OR AB ( ((prostate or prostatic) N3 (anticancer* or anti-cancer* or anticarcinogen* or anti-carcinogen* or antineoplas* or anti-neoplas* or chemoprevent* or chemo-prevent* or (tumo#r N2 suppress*))) ) | Expanders: Apply related words Search modes: Boolean/Phrase |
59 |
| S2 | TI ( ((prostate or prostatic) N3 (cancer* or carcinoid* or carcinoma* or carcinogen* or adenocarcinoma* or adeno-carcinoma* or malignan* or neoplasia* or neoplasm* or sarcoma* or tumour* or tumor*)) ) OR AB ( ((prostate or prostatic) N3 (cancer* or carcinoid* or carcinoma* or carcinogen* or adenocarcinoma* or adeno-carcinoma* or malignan* or neoplasia* or neoplasm* or sarcoma* or tumour* or tumor*)) ) | Expanders: Apply related words Search modes: Boolean/Phrase |
7,608 |
| S1 | (MH “Prostatic Neoplasms”) | Expanders: Apply related words Search modes: Boolean/Phrase |
10,199 |
Appendix B
Preclinical Studies Reference List
- 1. Apte S, Friedrichs W, Hursting S, et al. Delayed progression to hormone-independent prostate cancer through modulation of mTOR by omega-3 fatty acids. Cancer Res 2010;70(8 suppl 1). Paper presented at:101st Annual Meeting of the American Association for Cancer Research; April 17-21, 2010; Washington, DC. [Google Scholar]
- 2. Apte SA, Cavazos DA, Whelan KA, Degraffenried LA. A low dietary ratio of omega-6 to omega-3 fatty acids may delay progression of prostate cancer. Nutr Cancer. 2013;65:556-562. [DOI] [PubMed] [Google Scholar]
- 3. Apte SA, Cavazos W, Hursting S, Nogureira L, Degraffenried LA. mTOR is a target for omega-3 fatty acid modulation of prostate cancer progression. Cancer Res 2011:70(8 suppl 1). Paper presented at: 102nd Annual Meeting of the American Association for Cancer Research; April 2-6, 2011; Orlando, FL. [Google Scholar]
- 4. Begin ME, Ells G, Das UN, Horrobin DF. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986;77:1053-1062. [PubMed] [Google Scholar]
- 5. Begin ME, Das UN, Ells G, Horrobin DF. Selective killing of human cancer cells by polyunsaturated fatty acids. Prostaglandins Leukot Med. 1985;19:177-186. [DOI] [PubMed] [Google Scholar]
- 6. Begin ME, Ells G, Das UN. Selected fatty acids as possible intermediates for selective cytotoxic activity of anticancer agents involving oxygen radicals. Anticancer Res. 1986;6:291-295. [PubMed] [Google Scholar]
- 7. Bianchini F, Giannoni E, Serni S, Chiarugi P, Calorini L. 22: 6n-3 DHA inhibits differentiation of prostate fibroblasts into myofibroblasts and tumorigenesis. Br J Nutr. 2012;108:2129-2137. [DOI] [PubMed] [Google Scholar]
- 8. Brown I, Wahle K, Cascio M, Pertwee S, Heys S. Omega-3 fatty acids can exert their anticancer and chemotherapy enhancing effects through the endocannabinoid system. Eur J Surg Oncol. 2016;42:591-563. Paper presented at: BASO—The Association for Cancer Surgery Scientific Conference; November 7-8, 2011; London, United Kingdom.27005885 [Google Scholar]
- 9. Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by omega 6 and its inhibition by omega 3 PUFAs. Br J Cancer. 2006;94:842-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bureyko T, Hurdle H, Metcalfe JB, Clandinin MT, Mazurak VC. Reduced growth and integrin expression of prostate cells cultured with lycopene, vitamin E and fish oil in vitro. Br J Nutr. 2009;101:990-997. [DOI] [PubMed] [Google Scholar]
- 11. Cavazos DA, deGraffenried LA. Impact of omega-3 fatty acids on obesity-induced DNA oxidation and prostate cancer progression. Cancer Res. 2010;70(8 suppl 1). Paper presented at: 101st Annual Meeting of the American Association for Cancer Research; April 17-21, 2010; Washington, DC. [Google Scholar]
- 12. Cavazos DA, deGraffenried LA. DHA enhances genotoxic stress in prostate cancer cells through suppression of NF-B. Cancer Res 2011;71(8 suppl 1). Paper presented at: 102nd Annual Meeting of the American Association for Cancer Research; April 2-6, 2011; Orlando, FL. [Google Scholar]
- 13. Cavazos DA, Price RS, Apte SS, deGraffenried LA. Docosahexaenoic acid selectively induces human prostate cancer cell sensitivity to oxidative stress through modulation of NF-B. Prostate. 2011;71:1420-1428. [DOI] [PubMed] [Google Scholar]
- 14. Chung BH, Mitchell SH, Zhang JS, Young CY. Effects of docosahexaenoic acid and eicosapentaenoic acid on androgen-mediated cell growth and gene expression in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:1201-1206. [DOI] [PubMed] [Google Scholar]
- 15. du Toit PJ, van Aswegen CH, du Plessis DJ. The effect of essential fatty acids on growth and urokinase-type plasminogen activator production in human prostate DU-145 cells. Prostaglandins Leukot Essent Fatty Acids. 1996;55:173-177. [DOI] [PubMed] [Google Scholar]
- 16. Duregger AD, Ramoner R, Pante J, Helmut K. Differential metabolic effects of medium-chain triglycerides and omega-3 fatty acids in benign and malignant prostate cells-evidence for a ketogenic diet as adjuvant therapy for prostate cancer. Eur Urol 2014;13(1):e305 Paper presented at: 29th Annual Congress of the European Association of Urology, April 11-15, 2014; Stockholm, Sweden. [Google Scholar]
- 17. Edwards IJ, Sun H, Berguin IM, et al. In vivo and in vitro regulation of syndecan 1 in prostate cells by n-3 polyunsaturated fatty acids. J Biol Chem. 2008;283:18441-18449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eser PO, Vanden Heuvel JP, Araujo J, Thompson JT. Marine- and plant-derived omega-3 fatty acids differentially regulate prostate cancer cell proliferation. Mol Clin Oncol. 2013;1:444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fradet V, Neveu B, Morel X, et al. Anti-inflammatory effects of fish oil (omega-3 fatty acids) on normal human prostate epithelium. Urology. 2012;78(3 suppl 1);S340-S341. Paper presented at: 31st Congress of the Societe Internationale d’Urologie, SIU 2011; October 16-20, 2011; Berlin, Germany. [Google Scholar]
- 20. Friedrichs W, Ruparel SB, Marciniak RA, deGraffenried L. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr Cancer. 2011;63:771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu Z, Wu J, Wang S, et al. Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis. 2013;34:1968-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hopkins M., Liu Z., Meier K. Effects of eicosapentaenoic acid and the GPR120 agonist TUG-891 on lysophosphatidic acid signaling in human prostate cancer cells. FASEB J. 2014;28(1 suppl 1). Paper presented at: Experimental Biology; April 26-30, 2014; San Diego, CA. [Google Scholar]
- 23. Hu Y, Sun H, O’Flaherty JT, Edwards IJ. 15-Lipoxygenase-1-mediated metabolism of docosahexaenoic acid is required for syndecan-1 signaling and apoptosis in prostate cancer cells. Carcinogenesis. 2013;34:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu Y., Sun H, Owens RT, et al. Syndecan-1-dependent suppression of PDK1/Akt/bad signaling by docosahexaenoic acid induces apoptosis in prostate cancer. Neoplasia. 2010;12:826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes-Fulford M, Chen Y, Tjandrawinata RR. Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis. 2001;22:701-707. [DOI] [PubMed] [Google Scholar]
- 26. Istfan NW, Person KS, Holick MF, Chen TC. 1α,25-Dihydroxyvitamin D and fish oil synergistically inhibit G1/S-phase transition in prostate cancer cells. J Steroid Biochem Mol Biol. 2007;103(3-5):726-730. [DOI] [PubMed] [Google Scholar]
- 27. Karmali RA. Eicosanoids in neoplasia. Prev Med. 1987;16:493-502. [DOI] [PubMed] [Google Scholar]
- 28. Karmali RA, Reichal P, Cohen LA, et al. The effects of dietary omega-3 fatty acids on the DU-145 transplantable human prostatic tumor. Anticancer Res. 1987;7:1173-1179. [PubMed] [Google Scholar]
- 29. Kelavkar UP, Hutzley J, Dhir R, Kim P, Allen KG, McHugh K. Prostate tumor growth and recurrence can be modulated by the omega-6: omega-3 ratio in diet: athymic mouse xenograft model simulating radical prostatectomy. Neoplasia. 2006;8:112-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelavkar UP, Hutzley J, McHugh K, Allen KG, Parwani A. Prostate tumor growth can be modulated by dietarily targeting the 15-lipoxygenase-1 and cyclooxygenase-2 enzymes. Neoplasia. 2009;11:692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kobayashi N, Barnard RJ, Henning SM, et al. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006;12:4662-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li CC, Hou YC, Yeh CL, Yeh SL. Effects of eicosapentaenoic acid and docosahexaenoic acid on prostate cancer cell migration and invasion induced by tumor-associated macrophages. PLoS One. 2014;9:e99630. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Li CC, Hou YC, Pai MH, Yeh SL. Effects of eicosapentaenoic acid and docosahexaenoic acid on prostate cancer cell migration induced by tumor-associated macrophage. Ann Nutr Metab 2013;63(suppl 1):1215 Paper presented at: 20th International Congress of Nutrition; September 15-20, 2013; Granada, Spain. [Google Scholar]
- 34. Lim K, Shin K, Jing N, et al. Docosahexaenoic acid-induced autophagy is related to inhibition of mtor by reactive oxygen species in p53 mutant cancer cells. Euro J Cancer 2012;48:S33-S34. Paper presented at: 22nd Biennial Congress of the European Association for Cancer Research; July 7-10, 2012; Barcelona, Spain. [Google Scholar]
- 35. Lloyd J, Masko EM, Antonelli Does type of dietary fat matter? Prostate cancer xenograft progression in a SCID mouse model with varying dietary fat sources. J Urol 2010;183(4 suppl 1):e40 Paper presented at: 2010 Annual Meeting of the American Urological Association; May 29-June 3, 2010; San Francisco, CA. [Google Scholar]
- 36. Lloyd JC, Masko EM, Wu C, et al. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013;16:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McEntee MF, Ziegler C, Reel D, et al. Dietary n-3 polyunsaturated fatty acids enhance hormone ablation therapy in androgen-dependent prostate cancer. Am J Patho. 2008;173:229-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Motaung E, Prinsloo SE, van Aswegen CH, du Toit PJ, Becker PJ, du Plessis DJ. Cytotoxicity of combined essential fatty acids on a human prostate cancer cell line. Prostaglandins Leukot Essent Fatty Acids. 1999;61:331-337. [DOI] [PubMed] [Google Scholar]
- 39. Nakajima T, Kubota N, Tsutsumi T, et al. , Eicosapentaenoic acid inhibits voltage-gated sodium channels and invasiveness in prostate cancer cells. Br J Pharmacol. 2009;156:420-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Narayanan NK, Narayanan BA, Reddy BS, A combination of docosahexaenoic acid and celecoxib prevents prostate cancer cell growth in vitro and is associated with modulation of nuclear factor-κB, and steroid hormone receptors. Int J Oncol. 2005;26:785-792. [PubMed] [Google Scholar]
- 41. O’Flaherty JT, Hu Y, Wooten RE, et al. 15-Lipoxygenase metabolites of docosahexaenoic acid inhibit prostate cancer cell proliferation and survival. PLoS One. 2012;7:e45480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pandalai PK, Pilat MJ, Yamazaki K, Naik H, Pienta KJ. The effects of omega-3 and omega-6 fatty acids on in vitro prostate cancer growth. Anticancer Res. 1996;16:815-820. [PubMed] [Google Scholar]
- 43. Rose DP, Connolly JM. Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate. 1991;18:243-254. [DOI] [PubMed] [Google Scholar]
- 44. Shaikh IA, Brown I, Schofield AC, Wahle KW, Heys SD. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: the role of genes associated with the NF-kappaB pathway. Prostate. 2008;68:1635-1646. [DOI] [PubMed] [Google Scholar]
- 45. Shin S, Jimg K, Kim N, et al. Docosahexaenoic acid induces autophagy through reactive oxygen species /mTOR pathway in p53 mutant cancer cells. Cancer Res 2012;72(8 suppl 1). Paper presented at: 103rd Annual Meeting of the American Association for Cancer Research; March 31-April 4 2012, Chicago, IL. [Google Scholar]
- 46. Shin S, Jing K, Jeong S, et al. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. Biomed Res Int. 2013;2013:568671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song KS, Shin S, Jing Omega-3 polyunsaturated fatty acids induce apoptosis cell death and inhibit LPS-induced invasion in PC3 cells. Cancer Res. 2010;70(8 suppl 1). Paper presented at: 101st Annual Meeting of the American Association for Cancer Research; April 17-20, 2010; Washington, DC. [Google Scholar]
- 48. Vang K, Ziboh VA. 15-lipoxygenase metabolites of gamma-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: possible implications of dietary fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2005;72:363-372. [DOI] [PubMed] [Google Scholar]
- 49. Wang S, Wu J, Suburu J, et al. Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis. 2012;33:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Whelan KA, Cavazos, DeGraffenried LA. Defining the role of NF-kB as a target for omega-3 fatty acids in prostate cancer. Cancer Res 2011:71(8 suppl 1). Paper presented at: 102nd Annual Meeting of the American Association for Cancer Research; April 2-6, 2011; Orlando, FL [Google Scholar]
- 51. Yi L, Zhang QY, Mi MT. Role of Rho GTPase in inhibiting metastatic ability of human prostate cancer cell line PC-3 by omega-3 polyunsaturated fatty acid [in Chinese]. Ai Zheng. 2007;26:1281-1286. [PubMed] [Google Scholar]
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a CIHR Knowledge Synthesis Grant (KRS 316843).
References
- 1. Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2015. Toronto, Ontario, Canada: Canadian Cancer Society; 2015. [Google Scholar]
- 2. Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr. 2010;92:1223-1233. [DOI] [PubMed] [Google Scholar]
- 3. Klempner SJ, Bubley G. Complementary and alternative medicines in prostate cancer: from bench to bedside? Oncologist. 2012;17:830-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klafke N, Eliott JA, Wittert GA, Olver IN. Prevalence and predictors of complementary and alternative medicine (CAM) use by men in Australian cancer outpatient services. Ann Oncol. 2012;23:1571-1578. [DOI] [PubMed] [Google Scholar]
- 5. McDermott CL, Blough DK, Fedorenko CR, et al. Complementary and alternative medicine use among newly diagnosed prostate cancer patients. Support Care Cancer. 2012;20:65-73. [DOI] [PubMed] [Google Scholar]
- 6. Bishop FL, Rea A, Lewith H, et al. Complementary medicine use by men with prostate cancer: a systematic review of prevalence studies. Prostate Cancer Prostatic Dis. 2011;14:1-13. [DOI] [PubMed] [Google Scholar]
- 7. Eng J, Ramsum D, Verhoef M, Guns E, Davison J, Gallagher R. A population-based survey of complementary and alternative medicine use in men recently diagnosed with prostate cancer. Integr Cancer Ther. 2003;2:212-216. [DOI] [PubMed] [Google Scholar]
- 8. Nam RK, Fleshner N, Rakovitch E, et al. Prevalence and patterns of the use of complementary therapies among prostate cancer patients: an epidemiological analysis. J Urol. 1999;161:1521-1524. [PubMed] [Google Scholar]
- 9. Poorani R, Bhatt AN, Dwarakanath BS, Das UN. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance [published online September 1 2015]. Eur J Pharmacol. doi: 10.1016/j.ejphar.2015.08.049 [DOI] [PubMed] [Google Scholar]
- 10. Pelser C, Mondul AM, Hollenbeck AR, Park Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2013;22:697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu Z, Suburu J, Chen H, Chen YQ. Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. Biomed Res Int. 2013;2013:824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shin S, Jing K, Jeong S, et al. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. Biomed Res Int. 2013;2013:568671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaikh IA, Brown I, Schofield AC, Wahle KW, Heys SD. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: the role of genes associated with the NF-kappaB pathway. Prostate. 2008;68:1635-1646. [DOI] [PubMed] [Google Scholar]
- 14. Friedrichs W, Ruparel SB, Marciniak RA, deGraffenried L. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr Cancer. 2011;63:771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi L, Zhang QY, Mi MT. Role of rho GTPase in inhibiting metastatic ability of human prostate cancer cell line PC-3 by omega-3 polyunsaturated fatty acid [in Chinese]. Ai Zheng. 2007;26:1281-1286. [PubMed] [Google Scholar]
- 16. Li CC, Hou YC, Yeh CL, Yeh SL. Effects of eicosapentaenoic acid and docosahexaenoic acid on prostate cancer cell migration and invasion induced by tumor-associated macrophages. PLoS One. 2014;9:e99630, 92014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Lloyd JC, Masko EM, Wu C, et al. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013;16:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gu Z, Suburu J, Chen H, Chen YQ. Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. Biomed Res Int. 2013;2013:824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walsh MC, Trentham-Dietz A, Schroepfer TA, et al. Cancer information sources used by patients to inform and influence treatment decisions. J Health Commun. 2010;15:445-463. [DOI] [PubMed] [Google Scholar]
- 20. Fritz H, Balneaves L, Breau RH, Cooley Fish-derived omega-3 fatty acids and prostate cancer: a systematic review of the literature. PROSPERO 2014:CRD42014013014 http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013014. Accessed June 10, 2016. [DOI] [PMC free article] [PubMed]
- 21. Kristal AR, Arnold KB, Neuhouser ML, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010;172:566-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuhlmann J, Muck W. Clinical-pharmacological strategies to assess drug interaction potential during drug development. Drug Safety. 2001;24:715-725. [DOI] [PubMed] [Google Scholar]
- 24. Seely D, Oneschuk D. Interactions of natural health products with biomedical cancer treatments. Curr Oncol. 2008;15(suppl 2):s109 es181-s110 es106. [PMC free article] [PubMed] [Google Scholar]
- 25. Dietitians of Canada. Information about omega-3 fats. http://www.dietitians.ca/Your-Health/Nutrition-A-Z/Fat/Food-Sources-of-Omega-3-Fats.aspx. Accessed June 10, 2016.
- 26. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed June 10, 2016
- 28. Higashihara E, Itomura M, Terachi T, et al. Effects of eicosapentaenoic acid on biochemical failure after radical prostatectomy for prostate cancer. In Vivo. 2010;24:561-565. [PubMed] [Google Scholar]
- 29. Aronson WJ, Kobayashi N, Barnard RJ, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila). 2011;4:2062-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galet C, Gollapudi K, Stepanian S, et al. Effect of a low-fat fish oil diet on proinflammatory eicosanoids and cell-cycle progression score in men undergoing radical prostatectomy. Cancer Prev Res (Phila). 2014;7:97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan JM, Weinberg V, Magbanua MJ, et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control. 2011;22:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aronson WJ, Glaspy JA, Reddy ST, Reese D, Heber D, Bagga D. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology. 2001;58:283-288. [DOI] [PubMed] [Google Scholar]
- 33. Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control. 2006;17:199-208. [DOI] [PubMed] [Google Scholar]
- 34. Richman EL, Stampfer MJ, Paciorek A, Broering JM, Carroll PR, Chan JM. Intakes of meat, fish, poultry, and eggs and risk of prostate cancer progression. Am J Clin Nutr. 2010;91:712-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Epstein MM, Kasperzyk JL, Mucci LA, et al. Dietary fatty acid intake and prostate cancer survival in Orebro County, Sweden. Am J Epidemiol. 2012;176:240-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torfadottir JE, Valdimarsdottir UA, Mucci LA, et al. Consumption of fish products across the lifespan and prostate cancer risk. PLoS One. 2013;8(4):e59799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giovannucci E, Rimm EB, Colditz GA, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571-1579. [DOI] [PubMed] [Google Scholar]
- 38. Brasky TM, Till C, White E, et al. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173:1429-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res. 2011;4:1903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crowe FL, Key TJ, Appleby PN, et al. Dietary fat intake and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Am J Clin Nutr. 2008;87:1405-1413. [DOI] [PubMed] [Google Scholar]
- 41. Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008;88:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato F, Shimazu T, Kuriyama S, et al. Fish intake and the risk of prostate cancer in Japan: a prospective cohort study [in Japanese]. Nippon Hinyokika Gakkai Zasshi. 2008;99:14-21. [DOI] [PubMed] [Google Scholar]
- 43. Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective study on dietary fat and incidence of prostate cancer (Malmo, Sweden). Cancer Causes Control. 2007;18:1107-1121. [DOI] [PubMed] [Google Scholar]
- 44. Chavarro JE, Stampfer MJ, Campos H, Sesso HD, Ma J. Pre-diagnostic blood fatty acid levels and prostate cancer mortality. Cancer Res. 70(8 suppl 1). Paper presented at: 101st Annual Meeting of the American Association for Cancer Research; April 17, 2010; Washington, DC. [Google Scholar]
- 45. Augustsson K, Michaud DS, Rimm EB, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64-67. [PubMed] [Google Scholar]
- 46. Leitzmann MF, Stampfer MJ, Michaud DS, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204-216. [DOI] [PubMed] [Google Scholar]
- 47. Bonner M, Swanson M, Joseph G, Vena J. Lake Ontario fish consumption and the incidence of colorectal, lung, and prostate cancer in the New York State angler cohort. Epidemiology. 2012; 5(suppl 1):S301 Paper presented at: 24th Annual Conference of the International Society for Environmental Epidemiology; August 26-30, 2012; Columbia, SC. [Google Scholar]
- 48. Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764-1766. [DOI] [PubMed] [Google Scholar]
- 49. Allen NE, Sauvaget C, Roddam AW, et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Control. 2004;15:911-920. [DOI] [PubMed] [Google Scholar]
- 50. Brasky TM, Kristal AR, Navarro SL, et al. Specialty supplements and prostate cancer risk in the VITamins and Lifestyle (VITAL) cohort. Nutr Cancer. 2011;63:573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bosire C, Stampfer MJ, Subar AF, et al. Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. Am J Epidemiol. 2013;177:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pham TM, Fujino Y, Kubo T, et al. Fish intake and the risk of fatal prostate cancer: findings from a cohort study in Japan. Public Health Nutr. 2009;12:609-613. [DOI] [PubMed] [Google Scholar]
- 53. Bassett JK, Severi G, Hodge AM, et al. Plasma phospholipid fatty acids, dietary fatty acids and prostate cancer risk. Int J Cancer. 2013;133:1882-1891. [DOI] [PubMed] [Google Scholar]
- 54. Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019-1027. [PubMed] [Google Scholar]
- 55. Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105:1132-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sonoda T, Nagata Y, Mori M, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95:238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pawlega J, Rachtan J, Dyba T. Dietary factors and risk of prostate cancer in Poland. Results of case-control study. Neoplasma. 1996;43:61-63. [PubMed] [Google Scholar]
- 58. Raimondi S, Mabrouk JB, Shatenstein B, Maisonneuve P, Ghadirian P. Diet and prostate cancer risk with specific focus on dairy products and dietary calcium: a case-control study. Prostate. 2010;70:1054-1065. [DOI] [PubMed] [Google Scholar]
- 59. Fradet V, Cheng I, Casey G, Witte JS. Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clin Cancer Res. 2009;15:2559-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mina K, Fritschi L, Johnson KC, Canadian Cancer Registries Epidemiology Research Group. An inverse association between preserved fish and prostate cancer: results from a population-based case-control study in Canada. Nutr Cancer. 2008;60:222-226. [DOI] [PubMed] [Google Scholar]
- 61. Hedelin M, Chang ET, Wiklund F, et al. Association of frequent consumption of fatty fish with prostate cancer risk is modified by COX-2 polymorphism. Int J Cancer. 2007;120:398-405. [DOI] [PubMed] [Google Scholar]
- 62. Vlajinac H, Ilic M, Marinkovic J, Sipetic S. Nutrition and prostate cancer. J Balk Union Oncol. 2010;15:698-703. [PubMed] [Google Scholar]
- 63. Joshi AD, John EM, Koo J, Ingles SA, Stern MC. Fish intake, cooking practices, and risk of prostate cancer: results from a multi-ethnic case-control study. Cancer Causes Control. 2012;23:405-420. [DOI] [PubMed] [Google Scholar]
- 64. Ukoli FA, Fowke JH, Akumabor P, et al. The association of plasma fatty acids with prostate cancer risk in African Americans and Africans. J Health Care Poor Underserved. 2010;21(1 suppl):127-147. [DOI] [PubMed] [Google Scholar]
- 65. Dahm CC, Gorst-Rasmussen A, Crowe FL, et al. Fatty acid patterns and risk of prostate cancer in a case-control study nested within the European prospective investigation into cancer and nutrition. Am J Clin Nutr. 2012;96:1354-1361. [DOI] [PubMed] [Google Scholar]
- 66. Kristal AR, Cohen JH, Qu P, Stanford JL. Associations of energy, fat, calcium, and vitamin D with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:719-725. [PubMed] [Google Scholar]
- 67. Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545-551. [DOI] [PubMed] [Google Scholar]
- 68. Talamini R, Franceschi S, La VC, Serraino D, Barra S, Negri E. Diet and prostatic cancer: a case-control study in northern Italy. Nutr Cancer. 1992;18:277-286. [DOI] [PubMed] [Google Scholar]
- 69. Hu J, La VC, DesMeules M, Negri E, Mery L, Canadian Cancer Registries Epidemiology Research Group. Meat and fish consumption and cancer in Canada. Nutr Cancer. 2008;60:313-324. [DOI] [PubMed] [Google Scholar]
- 70. Alexander DD, Bassett JK, Weed DL, Barrett EC, Watson H, Harris W. Meta-analysis of long-chain omega-3 polyunsaturated fatty acids (LComega-3PUFA) and prostate cancer. Nutr Cancer. 2015;67:543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lovegrove C, Ahmed K, Challacombe B, Khan MS, Popert R, Dasgupta P. Systematic review of prostate cancer risk and association with consumption of fish and fish-oils: analysis of 495,321 participants. Int J Clin Pract. 2015;69:87-105. [DOI] [PubMed] [Google Scholar]
- 72. Moreel X, Allaire J, Leger C, et al. Prostatic and dietary omega-3 fatty acids and prostate cancer progression during active surveillance. Cancer Prev Res (Phila). 2014;7:766-776. [DOI] [PubMed] [Google Scholar]
- 73. Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(suppl 3):925S-932S. [DOI] [PubMed] [Google Scholar]
- 74. Rise P, Eligini S, Ghezzi S, Colli S, Galli C. Fatty acid composition of plasma, blood cells and whole blood: relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot Essent Fatty Acids. 2007;76:363-369. [DOI] [PubMed] [Google Scholar]
- 75. Kohlmeier L. Future of dietary exposure assessment. Am J Clin Nutr. 1995;61(3 suppl):702S-709S. [DOI] [PubMed] [Google Scholar]
- 76. Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8:3046-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hamid AR, Umbas R, Mochtar CA. Recent role of inflammation in prostate diseases: chemoprevention development opportunity. Acta Medica Indonesiana. 2011;43(1):59-65. [PubMed] [Google Scholar]
- 78. Lu Z, Chen TC, Zhang A, et al. An evaluation of the vitamin D3 content in fish: is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol. 2007;103:642-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Deschasaux M, Souberbielle JC, Latino-Martel P, et al. A prospective study of plasma 25-hydroxyvitamin D concentration and prostate cancer risk. Br J Nutr. 2016;115:305-314. [DOI] [PubMed] [Google Scholar]
- 80. Pudrovska T, Anishkin A. Clarifying the positive association between education and prostate cancer: a Monte Carlo simulation approach. J Appl Gerontol. 2015;34:293-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cheng TY, King IB, Barnett MJ, et al. Serum phospholipid fatty acids, genetic variation in myeloperoxidase, and prostate cancer risk in heavy smokers: a gene-nutrient interaction in the carotene and retinol efficacy trial. Am J Epidemiol. 2013;177(10):1106-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Williams CD, Whitley BM, Hoyo C, et al. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res. 2011;31(1):1-8. [DOI] [PubMed] [Google Scholar]
- 83. Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes and Control. 2006;17(2):199-208. [DOI] [PubMed] [Google Scholar]
- 84. Deneo-Pellegrini H, Ronco AL, De SE, et al. Food groups and risk of prostate cancer: A case-control study in Uruguay. Cancer Causes and Control.23 (7) (pp 1031-1038), 2012. Date of Publication: July 2012. 2012(7): 1031-1038. [DOI] [PubMed] [Google Scholar]
- 85. Park SY, Wilkens LR, Henning SM, et al. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes and Control. 2009;20(2):211-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Touvier M, Kesse-Guyot E, Andreeva VA, et al. Modulation of the association between plasma intercellular adhesion molecule-1 and cancer risk by n-3 PUFA intake: a nested case-control study. Am J Clin Nutr. 2012;95(4):944-950. [DOI] [PubMed] [Google Scholar]
- 87. Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86(4):281-286. [DOI] [PubMed] [Google Scholar]