Abstract
Purpose: To define the use of complementary and alternative medicine (CAM) in individuals presenting for care at a comprehensive cancer center. Patients and Methods: A total of 17 639 individuals presenting to an NCI-designated Comprehensive Cancer Center (and consortium sites) completed a questionnaire regarding CAM use. Data were analyzed using the univariate χ2 test to assess CAM use associated with a number of variables, including cancer status, age, gender, marital status, ethnicity, race, employment, and education level. Results: Eighty-seven percent of individuals who completed the CAM survey acknowledged CAM therapy use within the previous 12 months. Of the 5 broad categories of CAM, the most commonly used were biologically based approaches (14 759/17 639 [83.67%]), mind-body interventions (4624/17 485 [26.45%]), manipulative and body-based therapies (3957/17 537 [22.56%]), alternative medical systems (429/15 952 [2.69%]), and energy therapies (270/15 872 [1.7%]). CAM use was more prevalent among women, non-Hispanics, Caucasians, patients 60 to 69 years of age, and those who are married, have a higher level of education, and are employed (P < .005). Conclusions: This is the largest report of CAM use in individuals presenting for care at a comprehensive cancer center. Our analysis revealed that a very high percentage of patients utilize CAM. Because many of these CAM interventions are not studied in oncology patients, additional research on safety, efficacy, and mechanisms of action are essential. Furthermore, it is important that oncologists understand CAM modalities and counsel their patients about their use.
Keywords: cancer diagnosis, comprehensive cancer center, cancer survivor, questionnaire, suspicion of cancer
Introduction
Complementary and alternative medicine (CAM) is defined as “a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine.”1 Complementary medicine can be defined as that used in conjunction with conventional medicine, whereas alternative medicine is that used instead of conventional medicine. Integrative medicine is defined as an approach to medicine that “combines conventional and CAM treatments for which there is evidence of safety and effectiveness.”1
According to the 2007 National Health Interview Survey, which assessed 32 810 US civilian, noninstitutionalized individuals, 38.3% of adults used CAM during the previous 12 months, with the most common being non-vitamin, non-mineral natural products. Since their last survey in 2002, deep-breathing exercises, meditation, massage therapy, and yoga have increased in use. CAM was more prevalent among 30- to 69-year-old women and adults with higher levels of education, higher economic status, those living in the West, former smokers, and those hospitalized during the past year.2,3
Despite scarce data on CAM’s safety and effectiveness, use of these interventions is common among cancer patients worldwide. In 1998, a systematic review that included 26 surveys from 13 countries showed a 31% CAM use among adults with cancer (range = 7% to 64%).4 Recent studies have suggested that these numbers are higher, with rates up to 83% in the United States5 and 98% in China.6
A population-based study from the National Health Interview Survey showed that cancer survivors were more likely to use CAM therapies than individuals without cancer, with 65% reporting CAM use in their lifetime and 43% having used these therapies in the previous 12 months, compared with 52% and 37%, respectively, among noncancer individuals (P < .001).7 In general, cancer patients use CAM for disease-related symptoms, treatment-related adverse effects not addressed by conventional treatment, improving quality of life, its presumed antineoplastic or cancer preventive properties, its presumed pro-immune activity, and more control and responsibility of their own care.7-13
In 2003, Moffitt Cancer Center embarked on a long-term initiative to realize personalized care for individuals with cancer, termed Total Cancer Care (TCC). Patients prospectively provide written, informed consent to be included as part of this institutional review board–approved protocol. All new patients at Moffitt and our consortium sites (currently 17 hospitals in 10 different states) are offered consent to be included, which includes collection of detailed demographic and medical information and tumor and liquid specimens. Patients also consent to be followed for life and to be recontacted for future studies. At enrollment, patients complete a detailed questionnaire consisting of 179 questions that include demographics such as gender, age, race, marital, and socioeconomic status; exposure to medications, recreational drugs, tobacco products, infectious agents, carcinogens, and solar radiation; and their use of integrative services and products. Questions about integrative services and products are included within the cancer risk assessment section.
Here, we aimed to assess CAM use, and factors associated with CAM use, in patients with a cancer diagnosis or conditions that portend to cancer, those having procedures to rule out a cancer diagnosis, or those with a high suspicion of cancer, presenting to a comprehensive cancer center. We reviewed clinical, demographic, and CAM use data for all patients who had enrolled in the TCC and also completed the integrative medicine portion of their questionnaire.
Methods
Approximately 84 000 individuals have enrolled in the TCC from January 2003 to January 2014. The TCC questionnaire assesses sociodemographic characteristics; medical, surgical, family, and cancer histories; use of CAM during the past 12 months; and quality of life. An analysis of prospectively collected data, including all individuals enrolled at Moffitt Cancer Center, was conducted.
The CAM component of the TCC questionnaire consists of 40 multiple-choice questions incorporating the 5 broad categories of CAM (listed in Table 1). Patients were classified as CAM users if they used at least one therapy in any of the 5 categories over the past 12 months.
Table 1.
Complementary and Alternative Medicine Categories.
| Category | Intervention |
|---|---|
| 1. Biologically Based Approaches | Vitamins, minerals, non-mineral non-vitamin natural products, diet-based therapies, chelation therapy, diets, herbs, tea |
| 2. Mind-Body Interventions | Yoga, spirituality, relaxation, art and music therapy, biofeedback, meditation, aromatherapy, deep breathing exercises, hypnosis, Tai chi, progressive relaxation, guided imagery |
| 3. Energy Therapies | Reiki, magnets, Qigong, healing touch |
| 4. Manipulative and Body-Based Therapies | Massage, chiropractic care, osteopathy, reflexology, acupuncture, acupressure |
| 5. Alternative Medical Systems | Homeopathy, naturopathy, folk medicine, Ayurveda |
The univariate χ2 test was used to assess CAM use with respect to cancer status and demographic characteristics, including age, gender, marital status, ethnicity, race, employment status, and education level. P < .05 was considered statistically significant.
Results
Among 84 000 individuals enrolled in the TCC, 17 639 patients (20.9%) completed the CAM portion of the questionnaire, comprising the individuals in our analysis (CAM patient group).
We found that 15 388 patients (87.2%) in the CAM patient group acknowledged some form of CAM use in the past 12 months. In the CAM patient group, 6810 (38.6%) had a diagnosis of invasive cancer at the time of their initial visit; the remaining patients had pre-invasive cancer or were being evaluated for suspicion of cancer.
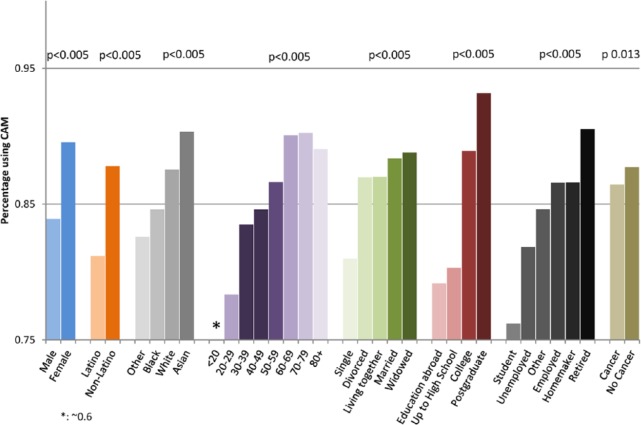
Table 2 and Figure 1 summarize patient demographics. Totals are based only on individuals who answered the question. CAM users tended to be female, non-Hispanic/Latino, age 60 to 69 years old, married, have a high education level, and employed or retired. Overall, patients with a cancer diagnosis were less likely to use CAM therapy than those without any known cancer at the time of the questionnaire (86.4% vs 87.7%, P = .01).
Table 2.
Demographics of Individuals Using Complementary and Alternative Medicine.
| n | % | |
|---|---|---|
| Gender | ||
| Female | 10 366 | 58.90 |
| Male | 7232 | 41.10 |
| Total | 17 598 | 100.00 |
| Ethnicity | ||
| Non-Hispanic/Latino | 16 084 | 91.42 |
| Hispanic/Latino | 1510 | 8.58 |
| Total | 17 594 | 100.00 |
| Race | ||
| Asian | 269 | 1.56 |
| Black or African American | 981 | 5.68 |
| Other | 529 | 3.06 |
| White | 15 492 | 89.70 |
| Total | 17 271 | 100.00 |
| Age, years | ||
| <20 | 177 | 1.00 |
| 20-29 | 702 | 3.99 |
| 30-39 | 1274 | 7.23 |
| 40-49 | 2605 | 14.79 |
| 50-59 | 3979 | 22.59 |
| 60-69 | 4736 | 26.89 |
| 70-79 | 3134 | 17.79 |
| 80+ | 1005 | 5.71 |
| Total | 17 612 | 100.00 |
| Marital status | ||
| Cohabiting/living together | 693 | 3.96 |
| Divorced/separated | 1805 | 10.32 |
| Married | 11 514 | 65.83 |
| Single | 2236 | 12.78 |
| Widowed | 1242 | 7.10 |
| Total | 17 490 | 100.00 |
| Education | ||
| Attended school in another country | 192 | 1.09 |
| College | 9750 | 55.41 |
| Postgraduate or professional school | 2959 | 16.82 |
| Up to high school | 4695 | 26.68 |
| Total | 17 596 | 100.00 |
| Employment | ||
| Employed | 6995 | 39.76% |
| Homemaker | 918 | 5.22% |
| Other | 676 | 3.84% |
| Retired | 6589 | 37.45% |
| Student | 311 | 1.77% |
| Unemployed | 2105 | 11.96% |
| Total | 17 594 | 100.00% |
| Cancer diagnosis at presentation | ||
| No | 10 829 | 61.39 |
| Yes | 6810 | 38.61 |
| Total | 17 639 | 100.00 |
Figure 1.
Characteristics of individuals using any CAM.
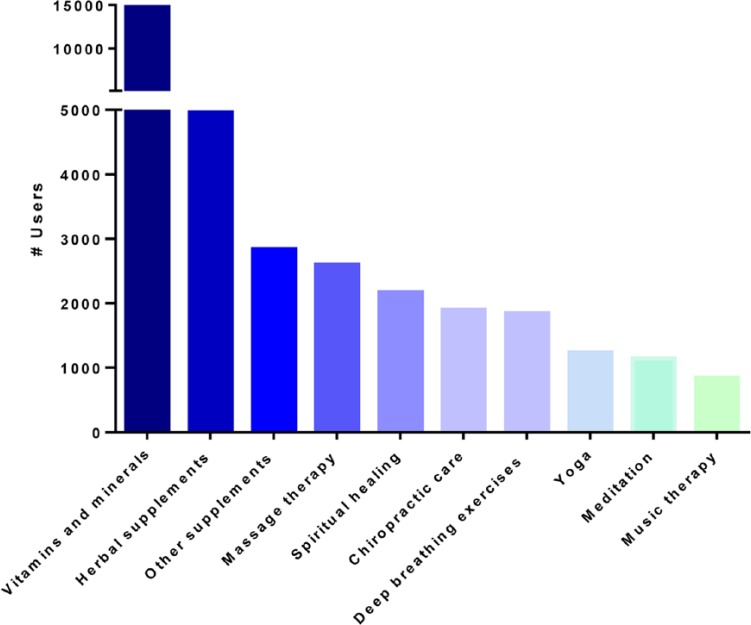
Of the 15 388 CAM users, 14 514 (94.3%) used vitamins and minerals, 4959 (32.2%) used herbal supplements, 2844 (18.5%) used other supplements (nonspecified supplements, probiotics, or herbal/botanical products), 2600 (16.9%) used massage therapy, and 2171 (14.1%) used spiritual healing/prayer (Figure 2). Excluding vitamins, minerals, and herbal supplements, massage therapy was the most frequently used CAM therapy in those <60 years old, whereas spiritual healing was most common among patients >60 years old.
Figure 2.
The 10 most commonly used complementary and alternative therapies.
Regarding use results distributed by the 5 CAM categories, biologically based were most common (83.67% of 17 639 respondents), followed by mind-body interventions (26.56%; 4624/17 485), manipulative and body-based therapies (22.56%; 3957/17 537), alternative medical systems (2.69%; 429/15 952), and energy therapies (1.7%; 270/15 872). In addition, biologically based approaches (see Table 1) were most commonly used in patients 60 to 79 years old, those of Asian race, those who were highly educated, and in retired individuals (Supplementary Figure S1. Mind-body interventions (see Table 1) were more commonly used among young adults 30 to 39 years old (P < .005; Supplementary Figure S2). Energy therapies (Supplementary Figure S3) and alternative medical systems (Supplementary Figure S4) were mostly used by races other than white, black, or Asian (P = .0014). Manipulative and body-based therapies (Supplementary Figure S5) were more commonly used by white (P < .005), young adults 30 to 39 years old (P < .005), and those with high socioeconomic status (P < .005).
The most commonly used biologic approaches were vitamins/minerals, herbs, and other supplements (94.3%, 32.2%, and 18.5% of all CAM users, respectively), whereas the most popular mind-body interventions were spiritual healing and prayer, deep-breathing relaxation, and yoga (14%, 12%, and 8%, respectively). We found that 17% used massage therapy and 12.3% used chiropractic care. Figures 3, 4A, and 4B illustrate the most common modalities in each CAM category among all CAM users, the most commonly used vitamins and minerals, and the most commonly used herbs.
Figure 3.
Most commonly used modality in each category.
Figure 4.
(A) Most commonly used vitamin and mineral supplements. (B) Most commonly used herbal supplements.
Discussion
CAM use is common, but variable, worldwide, with use ranging between 25% and 70%.11-13 In those with cancer, prevalence has been reported as 31.4% (range = 7% to 64%).4
Our study demonstrates an abundant use of CAM among individuals presenting to a comprehensive cancer center, with 87.2% of patients who responded to the CAM component of our questionnaire reporting CAM use over the past 12 months. To our knowledge, this is the largest study to date worldwide to describe CAM practices among individuals presenting for cancer care.
Our findings are consistent with a previous survey of 453 patients with cancer, which reported 83.3% of patients having used at least one CAM approach,5 higher than CAM use in the general population (~38% as estimated by the latest NCCAM survey1), and higher than use among cancer patients in Europe (35.9%).14
High CAM use among patients presenting to a comprehensive cancer center may be explained by the anxiety and the stress associated with cancer or a possible cancer diagnosis. Multiple studies have shown an association between increased CAM use and anxiety, emotional instability, fatigue, and poorer health status in the general population.11,13,15 A British survey of 600 patients who had been recently diagnosed with cancer showed that those using complementary therapies were more anxious, as rated by the hospital anxiety and depression scale, than those receiving conventional treatment only.16 In another study of 480 patients with newly diagnosed early-stage breast cancer, new use of alternative medicine was a marker of greater psychosocial distress and worse quality of life, as these patients reported more depression, worse general mental health, and greater fear of cancer recurrence.17 Individuals with cancer also seek alternative therapies hoping that these will cure their illness, with some stating that CAM helps them have better control over their disease and disease-related symptoms.5,14,16,18,19
Our study was unique in that it compared CAM use between patients with and without a definitive cancer diagnosis. Although one would expect CAM use to be more prevalent in patients with diagnosed cancer for the reasons mentioned above, this was not demonstrated in our present analysis, with use more common among patients without any known cancer at their initial visit. This could be related to fear associated with a referral to a specialized cancer center for a possible diagnosis of cancer and the perception that CAM may support health and reduce anxiety. Nonetheless, CAM use was highly prevalent in both groups (86.4% vs 87.7%).
These numbers underline the difference in CAM practice between the United States and European countries, where the reported use is 35.9%.14 This could be a reflection of cultural variability and the influence of multiple ethnic groups in the United States, a lack of national policies and regulations associated with CAM practices in Europe, greater access to CAM therapies by those with higher socioeconomic status, or limited CAM research overall.14,20,21
CAM use was correlated with age, gender, race, marital status, employment status, and education. This is in accordance with previous studies that showed higher use with high socioeconomic status, higher education level, and female gender.1,5,17,22-29 However, as compared to other studies, our CAM users were older. This could be related to the fact that more than 70% of the individuals enrolled in TCC were >50 years old, which may have biased our results.
Biologically based approaches, massage, chiropractic care, and mind-body interventions were the most commonly used CAM therapies, confirming earlier reports.1,5,20,30 Vitamins/minerals and herbs were the most prevalent, with 82.3% and 28.1% use among all participants. Individuals often consider these products “natural,” making them an attractive option; however, data on adverse effects of these products and their interactions with antineoplastic drugs are scarce. Some of these products have been shown to induce allergic reactions and various organ toxicities.31-35 Other products can interfere with the pharmacokinetics and metabolism of certain cytotoxic agents, leading to subtherapeutic levels or increased toxicity.12-16,36-43 Furthermore, intake duration of certain supplements may have variable effects on drug metabolism. Until further research is accomplished, physicians should discuss CAM use with their patients and provide them with the available information.
Conclusions
Limitations of this study are inherent to a prospective, questionnaire trial. Only 20.9% completed the CAM portion of the questionnaire, which may be because of age (57% age >60 years), length of questionnaire, and not completing this section as they did not participate in CAM use. Despite this relatively low participation rate, this is the largest study worldwide to assess CAM use in a single institution (>17 000 participants). Our population was predominantly white, elderly, and highly educated, which may not be representative of the US population as a whole.
Because many CAM interventions have not been studied in oncology patients, additional research on safety, efficacy, and mechanisms of action are essential. Furthermore, it is important that oncologists understand CAM modalities and counsel their patients about their use. Health care institutions should offer patients safe and effective CAM therapies for improved symptom control and quality of life.
Supplementary Material
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance. Total Cancer Care® is enabled, in part, by the generous support of the DeBartolo Family, and we thank the many patients who so graciously provided data and tissue to the Total Cancer Care Consortium.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplementary Material: The supplementary material is available at http://journals.sagepub.com/doi/suppl/10.1177/1534735416660384
References
- 1. National Center for Complementary and Alternative Medicine. The use of complementary and alternative medicine in the United States. http://nccam.nih.gov/sites/nccam.nih.gov/files/camuse.pdf. Accessed October 20, 2014.
- 2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23. [PubMed] [Google Scholar]
- 3. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;(343):1-19. [PubMed] [Google Scholar]
- 4. Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer. 1998;83:777-782. [DOI] [PubMed] [Google Scholar]
- 5. Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505-2514. [DOI] [PubMed] [Google Scholar]
- 6. Cui Y, Shu XO, Gao Y, et al. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Res Treat. 2004;85:263-270. [DOI] [PubMed] [Google Scholar]
- 7. Mao JJ, Palmer CS, Healy KE, Desai K, Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv. 2011;5:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vickers AJ, Cassileth BR. Unconventional therapies for cancer and cancer-related symptoms. Lancet Oncol. 2001;2:226-232. [DOI] [PubMed] [Google Scholar]
- 9. Kosty MP. PC-SPES: hope or hype? J Clin Oncol. 2004;22:3657-3659. [DOI] [PubMed] [Google Scholar]
- 10. Straus SE. Herbal medicines—what’s in the bottle? N Engl J Med. 2002;347:1997-1998. [DOI] [PubMed] [Google Scholar]
- 11. Fisher P, Ward A. Complementary medicine in Europe. BMJ. 1994;309:107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569-1575. [DOI] [PubMed] [Google Scholar]
- 13. Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279:1548-1553. [DOI] [PubMed] [Google Scholar]
- 14. Molassiotis A, Fernandez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655-663. [DOI] [PubMed] [Google Scholar]
- 15. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-252. [DOI] [PubMed] [Google Scholar]
- 16. Downer SM, Cody MM, McCluskey P, et al. Pursuit and practice of complementary therapies by cancer patients receiving conventional treatment. BMJ. 1994;309:86-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. Use of alternative medicine by women with early-stage breast cancer. JAMA. 1999;340:1773-1739. [DOI] [PubMed] [Google Scholar]
- 18. Lerner IJ, Kennedy BJ. The prevalence of questionable methods of cancer treatment in the United States. CA Cancer J Clin. 1992;42:181-191. [DOI] [PubMed] [Google Scholar]
- 19. Oneschuk D, Fennell L, Hanson J, Bruera E. The use of complementary medications by cancer patients attending an outpatient pain and symptom clinic. J Palliat Care. 1998;14:21-26. [PubMed] [Google Scholar]
- 20. Risberg T, Lund E, Wist E, et al. The use of non-proven therapy among patients treated in Norwegian oncological departments: a cross-sectional national multicenter study. Eur J Cancer. 1995;31A:1785-1789. [DOI] [PubMed] [Google Scholar]
- 21. Boon H, Stewart M, Kennard MA, et al. The use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18:2515-2521. [DOI] [PubMed] [Google Scholar]
- 22. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12:45-56. [PMC free article] [PubMed] [Google Scholar]
- 23. Helyer LK, Chin S, Chui BK, et al. The use of complementary and alternative medicines among patients with locally advanced breast cancer—a descriptive study. BMC Cancer. 2006;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rees RW, Feigel I, Vickers A, Zollman C, McGurk R, Smith C. Prevalence of complementary therapy use by women with breast cancer: a population-based study. Eur J Cancer. 2000;36:1359-1364. [DOI] [PubMed] [Google Scholar]
- 25. Navo MA, Phan J, Vaughan C, et al. An assessment of the utilization of complementary and alternative medication in women with gynecologic or breast malignancies. J Clin Oncol. 2004;22:671-677. [DOI] [PubMed] [Google Scholar]
- 26. Moschen R, Kemmler G, Schweigkofler H, et al. Use of alternative/complementary therapy in breast cancer patients a psychological perspective. Support Care Cancer. 2001;9:267-274. [DOI] [PubMed] [Google Scholar]
- 27. Lee MM, Lin SS, Wrensch MR, Adler SR, Eisenberg D. Alternative therapies used by women with breast cancer in four ethnic populations. J Natl Cancer Inst. 2000;92:42-47. [DOI] [PubMed] [Google Scholar]
- 28. Bernstein BJ, Grasso T. Prevalence of complementary and alternative medicine use in cancer patients. Oncology. 2001;15:1267-1272. [PubMed] [Google Scholar]
- 29. Sparber A, Bauer L, Curt G, et al. Use of complementary medicine by adult patients participating in cancer clinical trials. Oncol Nurs Forum. 2000;27:623-630. [PubMed] [Google Scholar]
- 30. Weis J, Bartsch H, Hennies F, et al. Complementary medicine in cancer patients: Demand, patients’ attitude, and psychological belief. Onkologie. 1998;21:144-149. [Google Scholar]
- 31. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther. 2004;76:1-17. [DOI] [PubMed] [Google Scholar]
- 32. Benninger J, Schneider HT, Schuppan D, Kirchner T, Hahn EG. Acute hepatitis induced by greater celandine (Chelidonium majus). Gastroenterology. 1999;117:1234-1237. [DOI] [PubMed] [Google Scholar]
- 33. Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387-391. [DOI] [PubMed] [Google Scholar]
- 34. Lord GM, Cook T, Arlt VM, Schmeiser HH, Williams G, Pusey CD. Urothelial malignant disease and Chinese herbal nephropathy. Lancet. 2001;358:1515-1516. [DOI] [PubMed] [Google Scholar]
- 35. Lampert N, Xu Y. Chinese herbal nephropathy. Lancet. 2002;359:796-797. [DOI] [PubMed] [Google Scholar]
- 36. Yeung KS, Gubili J, Cassileth B. Herb-drug interactions in oncology. ASCO Post. 2013;4(16). [Google Scholar]
- 37. Wang Z, Hamman MA, Huang SM, Lesko LJ, Hall SD. Effect of St John’s wort on the pharmacokinetics of fexofenadine. Clin Pharmacol Ther. 2002;71:414-420. [DOI] [PubMed] [Google Scholar]
- 38. D’Andrea GM. Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA Cancer J Clin. 2005;55:319-321. [DOI] [PubMed] [Google Scholar]
- 39. Bairati I, Meyer F, Gelinas M, et al. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J Clin Oncol. 2005;23:5805-5813. [DOI] [PubMed] [Google Scholar]
- 40. Lesperance ML, Olivotto IA, Forde N, et al. Mega-dose vitamins and minerals in the treatment of non-metastatic breast cancer: an historical cohort study. Breast Cancer Res Treat. 2002;76:137-143. [DOI] [PubMed] [Google Scholar]
- 41. Bairati I, Meyer F, Jobin E, et al. Antioxidant vitamins supplementation and mortality: a randomized trial in head and neck cancer patients. Int J Cancer. 2006;119:2221-2224. [DOI] [PubMed] [Google Scholar]
- 42. Weiger WA, Smith M, Boon H, Richardson MA, Kaptchuk TJ, Eisenberg DM. Advising patients who seek complementary and alternative medical therapies for cancer. Ann Intern Med. 2002;137:889-903. [DOI] [PubMed] [Google Scholar]
- 43. Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773-783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.