Abstract
Oyster has gained much attention recently for its anticancer activity but it is unclear whether calcium, the major antitumor ingredient in oyster shell, is responsible for the anticarcinogenic role of the oyster. To address this issue, C57BL/6 mice were fed with the carcinogen 4-nitroquinoline-1-oxide (4NQO, 50 µg/mL) and normal diet or a diet containing oyster powder, oyster calcium, or calcium depleted oyster powder. The tongue tissue specimens isolated from these mice were histologically evaluated for hyperplasia, dysplasia, and papillary lesions, and then analyzed for proliferation and differentiation markers by immunohistochemistry. The results showed that mice on the diet containing oyster calcium significantly reduced rates of tumors in the tongue and proliferation and enhanced differentiation in the oral epithelium compared with the diet containing calcium depleted oyster powder. These results suggest that calcium in oyster plays a critical role in suppressing formation of oral squamous cell carcinoma and proliferation and promoting differentiation of the oral epithelium.
Keywords: oyster, calcium, oral carcinogenesis, proliferation, differentiation
Introduction
Oral cancer is a malignant neoplasm with high mortality and morbidity. Approximately 90% of oral cancer is squamous cell carcinoma, which most commonly involves the tongue. The occurrence oral squamous cell carcinoma is strongly associated with smoking, alcohol, betel nut chewing, and human papillomavirus infection.1 Oral cancer has been ranked as the sixth most common cancer with more than 650 000 new cases and 50% associated deaths each year by the World Health Organization.2,3 The 5-year survival rate is as low as 50%.4
Oyster meat is one of the popular seafood in many coastal countries, and oyster shell is customarily discarded. However, the oyster shell has been commonly used in traditional Chinese medicine as an antitumor formula in clinical practice as illustrated in ancient medicine books and Chinese Pharmacopoeia. Oyster shell could nourish yin and subdue the overflowing of yang and was used to induce sedation, soften hard masses, eliminate nodulation, and arrest discharges.5 It is the shell of Ostrea gigas Thunberg, Ostrea talienwhanensis Crosse, or Ostrea rivularis Gould (Fam Ostreidae) and is naturally rich in many essential vitamins and minerals, including protein, iron, calcium, zinc, and vitamin C.5 Owing to the long-term application in traditional Chinese medicine, oyster shell has been included in the first batch of resources those allowing utilization in drug and supplement food by the Ministry of Health of China in 2002.6 Oyster shells, composed primarily (90% to 95%) of calcium carbonate, have been consumed as a very good source of calcium supplement and multiple oyster products in the form of chewable tablets, granules, and capsules.7
Oyster has been reported to have antitumor activity in the recent years.8-10 There are lines of evidence from epidemiological studies showing optimal reduction of risk of cancers, including colorectal, breast, and renal epithelial cancer in individuals with a high intake of calcium.11-13 Thus, calcium in the oyster shell might be an important component against oral carcinogenesis. In addition to calcium, oyster shells contain trace elements, including iron, copper, and zinc, as well as amino acids, including glutamic acid and aspartic acid.14 However, it remains uncertain whether calcium in the oyster shell possesses antitumor activity of the oyster shell. To determine whether calcium in the oyster plays a role in preventing oral carcinogenesis, we compared effects of oyster powder (OPow), oyster extract (OEx), and oyster calcium (OCa) on the development of oral tumors induced by 4-nitroquinoline-1-oxide (4NQO) in C57BL mice.
Methods
Materials and Preparation of the Diet
Oyster shell, shell of Ostrea gigas Thunberg, was purchased from Chinese Materia Medica Co. (Beijing, China). Dried oyster shells (10 000 g, OPow) were pulverized and extracted twice with 80% ethanol, and the residue was oyster calcium (OCa). The 80% ethanol extract was evaporated in vacuum to yield a green yellow residue (47.2 g), namely oyster extract (OEx). The mice were fed with a normal diet or a diet containing OPow, OCa, or OEx, which were made by Keaoxieli Inc. (Beijing, China). The calcium content in the diet was determined by permanganate titration. 4-NQO was purchased from Meryer Chemical Technology Shanghai Co. (Shanghai, China). Drinking water containing 4-NQO was freshly prepared every week in deionized water and was administered to mice in light-shielded water bottles.
Animals and Treatment
Male and female wild-type C57BL/6 mice were supplied by Beijing Vital Laboratory Animal Technology (Beijing, China). Fifteen animals per cage were housed in our animal facility in a 12-hour light-dark cycle. The animal protocol was approved by the Animal Ethics Committee at the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences. A total of 126 weaned mice (C57BL/6, 4 weeks old, 60 males and 60 females) were randomly divided into the normal diet (n = 30), OPow (n = 34), OEx (n = 33), and OCa (n = 29) groups. Mice in these groups were fed with the regular diet containing calcium hydrogen phosphate (1.3% calcium), OPow (2.0% calcium), OEx (1.3% calcium), or OCa (2.0% calcium) as shown in Figure 1A. Following 16 weeks of 4NQO (50 µg/mL in drinking water) exposure, mice were then fed with normal drinking water for 12 weeks. The mice in each group were evaluated for body weight at appropriate intervals. On termination of the study at 28 weeks, these mice were euthanized and tongue tissues were collected. The number of tumors (larger than 1 or 2.5 mm in diameter) on the tongue was counted, rates of tumors were determined and their tongues and blood samples were collected for the subsequent experiments.
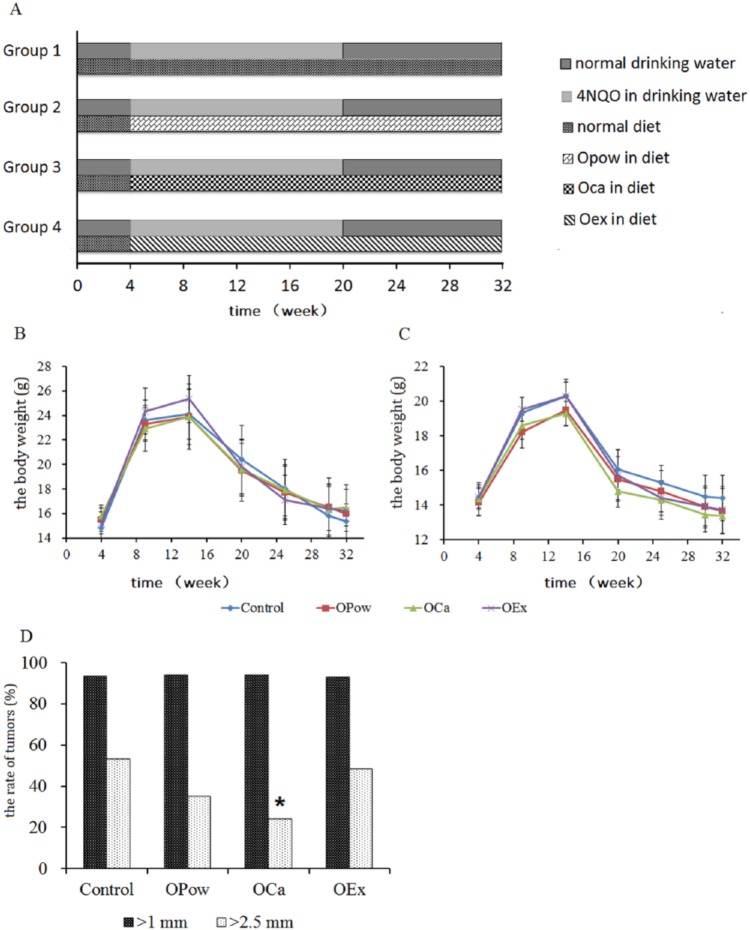
Figure 1.
(A) The experimental timelines and effects of the diet containing oyster powder (OPow), oyster extract (OEx), or oyster calcium (OCa) on the tumor rates in mice treated by 4NQO. Mice were randomly divided into 4 groups including the group on the normal diet (the control group), the group on OPow diet, the group on OCa diet, and the group on OEx diet. All the groups were given 50 mg/mL of 4NQO in drinking water for 16 consecutive weeks. The diets include the normal diet containing 1.3% calcium, OPow containing 2.0% calcium, OEx containing 1.3% calcium, and OCa containing 2.0% calcium. The concentration of calcium in each diet was determined as described in Materials and Methods. The body weight in male mice (B) and female mice (C) were recorded and plotted. At the end of study, the mice were sacrificed and examined for oral tumors. The number of tumors is shown the bar graph (D). The data are expressed as mean ± SD. *P < .05 (compared with the control group). The results showed that the mice on OPow, OCa, or OEx did not display any difference in body weight when compared with mice on the normal diet. The rates of oral tumors larger than 1.0 mm in diameter were almost 93% in all the groups and there is no difference between each group. The rate of tumors larger than 2.5 mm in the OCa group is significantly lower than that in the normal diet group. However, there was no significant difference in the rate of tumors larger than 2.5 mm between the OEx group or the OPow group and the normal diet group.
Histological and Immunohistochemical Assessment
The tongue tissues were fixed in buffered formalin. The paraffin-embedded 4 mm-thick tongue tissue sections from different groups were processed for H&E staining. Briefly, tongue tissues were collected from all the animals in each group (n=30) and serial sectioning was performed. The levels of proliferating cell nuclear antigen (PCNA), keratin 1, involucrin, filaggrin, and loricrin using immunohistochemistry were analyzed by immunohistochemistry as previously described15 and quantitated by counting number of cells stained and total number of cells in 5 representative regions of the sections. The percentages of positively stained cells were calculated. For routine histological analysis, the results were examined under a light microscope and reviewed by 2 certified pathologists at the Second Xiangya Hospital. All data are presented as mean ± standard deviation.
Statistical Analysis
The statistical significance among the groups was tested by chi-square test using SPSS software and P < .05 was considered significant.
Results and Discussion
Mice on Oyster Calcium Displayed Reduced Rates of Oral Tumors
To determine whether oyster calcium has any effect on the development of tongue tumors, we fed mice with the normal diet or the diet containing OPow, OCa, or OEx and 4NQO and examined rates of tumors in the tongue. Starting 14 weeks, all mice showed a significant decrease in body weight in all groups of mice. Mice on OPow, OCa, or OEx did not display any difference in body weight when compared with mice on the normal diet (Figure 1B and C). The rates of oral tumors larger than 1.0 mm in diameter were almost 93% in all the groups and there was no difference between each group. However, the rates of oral tumors larger than 2.5 mm for each group were as follows: 53.3% (16 out of 30) in the normal diet group, 48.3% (14 out of 29) in the OEx group, 35.3% (12 out of 34) in the OPow group, and 24.2% (8 out of 33) in the OCa group (Figure 1D). The rate of tumors larger than 2.5 mm in the OCa group was significantly lower than that in the normal diet group. However, there was no significant difference in the rate of tumors larger than 2.5 mm between the OEx group or the OPow group and the normal diet group. All the tumors were sectioned and examined by 3 certified pathologists in the Second Xiangya Hospital. The hematoxylin and eosin staining showed oral squamous cell carcinoma in all tumors isolated. These data indicate that calcium in the oyster plays a critical role in suppressing development of oral tumors.
Oyster Calcium Promoted Differentiation of the Normal Oral Epithelium
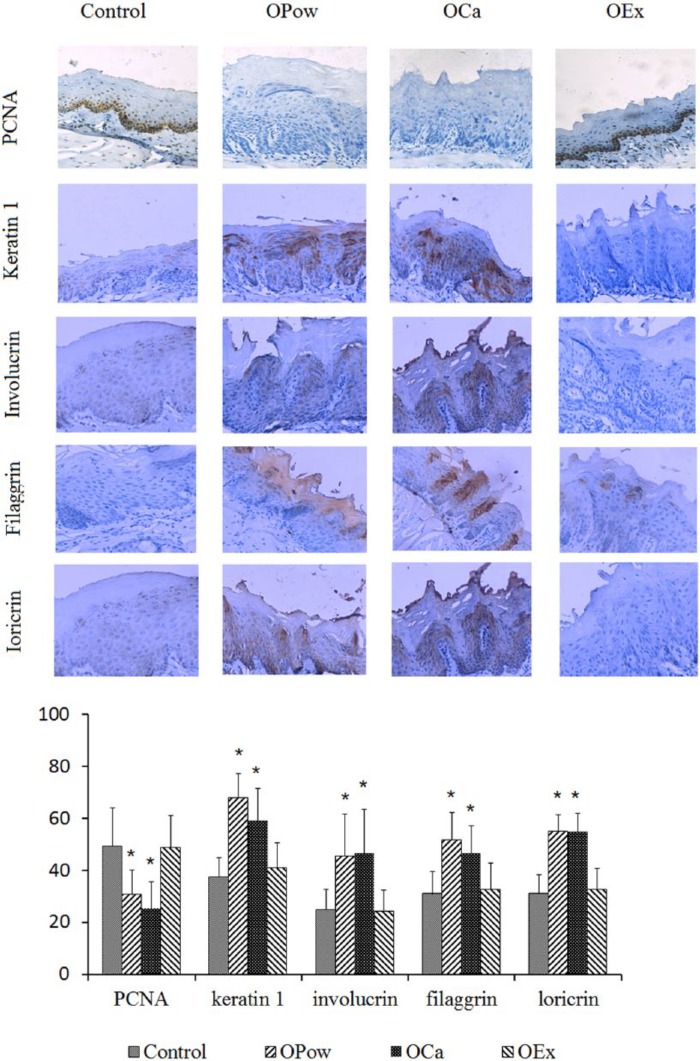
To determine whether oyster calcium affects proliferation and differentiation of oral epithelium, the expression levels of PCNA, keratin 1, involucrin, filaggrin, or loricrin in the normal oral epithelium of mice were examined by immunohistochemistry. The results showed that levels of PCNA in the normal oral epithelium was significantly lower and levels of keratin 1, involucrin, filaggrin, and loricrin in mice on OPow or OCa were higher compared to that in mice on OEx or on the normal diet (P < .05, Figure 2). These data indicate that calcium in the oyster suppresses proliferation and promotes differentiation in the oral epithelium.
Figure 2.
Effects of OPow, OCa, or OEx diet on the proliferation and differentiation of oral epithelia. (A) The tongue was removed from the mouse on the normal diet (the control group) or the diet containing OPow, OCa, or OEx and the tongue tissue was fixed in formalin solution and embedded in paraffin blocks for pathological analysis and immunohistochemistry using the antibodies against the proliferating cell nuclear antigen (PCNA), keratin 1, involucrin, filaggrin, or loricrin. Positive expression is shown in brown and the counterstaining is shown in blue. (B) The bar graph shows quantitation of the levels of differentiation markers in the cells. The quantitation for each section was obtained by counting the number of positive cells and total number of cells in the corresponding region in 5 representative regions in each section. The data are expressed as mean ± SD, *P < .05 (compared with the control group). The results showed that levels of PCNA in the normal oral epithelium was significantly lower and levels of keratin 1, involucrin, filaggrin, and loricrin in mice on OPow or OCa were higher compared with that in mice on OEx or on the normal diet (P < .05).
Discussion
Continuous exposure of a synthetic chemical carcinogen 4NQO to mice to induce oral tumorigenesis has been used to bring about the carcinomatous changes that are considered to occur in the same way as they do in humans.16 In the present study, the 4NQO-induced oral carcinogenesis and histopathological alterations in the tongue tissue in mice on the oyster diet with or without calcium was examined to investigate whether calcium in the oyster suppresses oral carcinogenesis. The results showed that mice on OCa had a reduced rate compared with that in mice on regular diet, suggesting that oyster calcium suppresses oral carcinogenesis. In the present study, however, the oyster extract did not show any anticancer activity. This is discrepant from previous studies, which have shown that the oyster shell extract has cytotoxic effects on the human hepatoma cell line BEL-7402, the cervical cancer cell line Hela and the murine leukemic cell line P388.17 The discrepancy between the present studies and previous studies is unclear. One of the possible reasons might be the differences in the method for preparation or the dosage used between the present studies and previous studies.
As increased cell proliferation and decreased differentiation are prominent features during carcinogenesis,18 the effect of oyster calcium on proliferation of noncancerous oral epithelium was examined by PCNA staining in the present study. The results showed that the oral epithelium in OPow and OCa groups displayed decreased levels of PCNA and increased levels of differentiation markers including keratin 1, involucrin, filaggrin, and loricrin than that in the control and OEx groups. These data suggest that oyster calcium plays a critical role in suppressing proliferation and inducing differentiation in the oral epithelium. Given that cancer cells are usually less differentiated and more proliferated, compared with normal cells, increased proliferation and decreased differentiation might be among the mechanisms by which dietary calcium suppresses oral carcinogenesis.
Many types of marine shells containing biogenic calcium carbonate, such as pearl shell (Zhen-Zhu-Mu in Chinese), abalone shell (Shi-Jue-Ming in Chinese), oyster shell (Mu-Li in Chinese), and cuttlebone (Hai-Piao-Qiao in Chinese), are common crude drugs in traditional Chinese medicine.19 In the present study, biogenic calcium carbonate has been demonstrated to be the major effective ingredient preventing tongue carcinogenesis in oyster shell. Therefore, oyster calcium as a diet supplement suppresses the tumorigenesis and may be used as a potential marine drug.
Conclusion
In conclusion, dietary oyster calcium suppresses oral carcinogenesis and proliferation and promotes differentiation in the oral epithelium. Therefore, oyster calcium as a diet supplement suppresses the tumorigenesis and has potential for use as a marine drug.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by National Natural Science Foundation of China (81072219, 81272973, and 81471055).
References
- 1. Macfarlane GJ, Zheng T, Marshall JR, et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer B Oral Oncol. 1995;31B:181-187. [DOI] [PubMed] [Google Scholar]
- 2. Otoh EC, Johnson NW, Olasoji HO, Danfillo IS, Adeleke OA. Intra-oral carcinomas in Maiduguri, north-eastern Nigeria. Oral Dis. 2005;11:379-385. [DOI] [PubMed] [Google Scholar]
- 3. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309-316. [DOI] [PubMed] [Google Scholar]
- 4. Kujan O, Sloan P. Dilemmas of oral cancer screening: an update. Asian Pac J Cancer Prev. 2013;14:3369-3373. [DOI] [PubMed] [Google Scholar]
- 5. China Pharmacopoeia Committee. Chinese Pharmacopoeia (I). Beijing, China: China Medical Science Press;2010:161-162. [Google Scholar]
- 6. The list of resources those allowing utilized in drug and supplement food. March 4, 2002. http://wsb.moh.gov.cn/mohwsjdj/s3593/200810/38175.shtml. Accessed March 4, 2002.
- 7. Yang X, Zhou SL, Ma AC, Xu HT, Guan HS, Liu HB. Chemical profiles and identification of key compound caffeine in marine-derived traditional Chinese medicine ostreae concha. Mar Drugs. 2012;10:1180-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang YK, He HL, Wang GF, et al. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immune-stimulating effects in BALB/c mice. Mar Drugs. 2010,8:255-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li P, Li QF, Shi SL, Liang Y. Regulation of bioactive peptides of oyster (BPO) on the cell cycle and gene expression of human gastric adenocarcinoma celllineBGC-823. Chin J Mar Drugs. 2007;26:1-8. [Google Scholar]
- 10. Zhong JW, Chen JW, Li X, Cai BC, Hou KL. Influence of oyster on the hypnotic effect of pentobarbital sodium in mice. Chin Arch Tradit Chin Med. 2009;27:499-501. [Google Scholar]
- 11. Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer. 2014;135:1940-1948. doi: 10.1002/ijc.28840. [DOI] [PubMed] [Google Scholar]
- 12. Hong Z, Tian C, Zhang X. Dietary calcium intake, vitamin D levels, and breast cancer risk: a dose-response analysis of observational studies. Breast Cancer Res Treat. 2012;136:309-312. [DOI] [PubMed] [Google Scholar]
- 13. Hu J, Mao Y, White K; Canadian Cancer Registries Epidemiology Research Group. Diet and vitamin or mineral supplements and risk of renal cell carcinoma in Canada. Cancer Causes Control. 2003;14:705-714. [DOI] [PubMed] [Google Scholar]
- 14. Zhang H, Zhang L, Liu Y. Studies on chemical components and pharmacological activities of Osdraconis (Longgu) and Ostreae concha. Chin J Chin Mater Med. 2011;36:1839-1840. [PubMed] [Google Scholar]
- 15. Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161-171. [DOI] [PubMed] [Google Scholar]
- 16. Vered M, Yarom N, Dayan D. 4NQO oral carcinogenesis: animal models, molecular markers and future expectations. Oral Oncol. 2005;41:337-339. [DOI] [PubMed] [Google Scholar]
- 17. Guan HS, Liu HB, Yang X, inventors. A method of preparation and application of the Ostreae concha extract. China patent. 2010102803618. January 26, 2011. [Google Scholar]
- 18. Cao Z, Yu D, Fu S, et al. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol Lett. 2013;218:174-185. [DOI] [PubMed] [Google Scholar]
- 19. Wu QG, Liang YY, Liu SJ, Jiang QE. Research progress in testacean of traditional Chinese medicine. J Guangdong Pharm Univ. 2012;28:212-217. [Google Scholar]