Abstract
Aim of the study. To examine the antiproliferation and anti-invasion of Eupolyphaga sinensis Walker 70% ethanol extract (ESWE) on breast cancer and elucidate the underlying signaling mechanisms. Methods. MTT and colony formation assays were used to investigate the effect of ESWE on proliferation of breast cancer cells in vitro. The xenograft mouse tumor model was used to determine the effect of ESWE on breast cancer in vivo. To investigate the underlying molecular mechanisms, we used western blotting to analyze the expression of ERK1/2, CXCR4, matrix metalloproteinase 2 (MMP2), and MMP9 pretreated with ESWE. The stromal cell–derived factor (SDF)-1α-induced migration and invasion potential of breast cancer cells were examined by wound-healing assays and Matrigel invasion chamber assays. Results. ESWE effectively inhibited the proliferation of MDA-MB-435s and MDA-MB-231 cells and exhibited antitumor effects in an MDA-MB-231 xenograft mice model. Furthermore, ESWE suppressed the activity of ERK1/2, a key molecule of MAPK signaling. We also observed that ESWE treatment led to downregulation of CXCR4 expression as well as greatly reduced MMP2 and MMP9. ESWE affected CXCR4 expression partially through the modulation of autocrine vascular endothelial growth factor. However, suppression of CXCR4 expression was the result of downregulation of mRNA expression. Inhibition of CXCR4 expression by ESWE further correlated with the suppression of SDF-1α-induced migration and invasion in breast cancer cells. Conclusion. ESWE exerted its antiproliferation and antiinvasion by regulating MAPK signaling and related metastasis factorsand thus could be a useful therapeutic candidate for breast cancer intervention.
Keywords: Eupolyphaga sinensis Walker, breast cancer, ERK1/2, CXCR4, MMP2, MMP9
Introduction
Insects have proved to be very important as sources of drugs for modern medicine. In China, some insects are considered safe and used as traditional Chinese medicine. Eupolyphaga sinensis Walker (ESW) belonging to the family Corydiidae (Blattodea) is widely distributed in China, and it is an important insect used in Chinese traditional medicine.1 In Chinese folk and traditional medical practices, it is said to enhance immune response and promote blood circulation by removing blood stasis and is widely used as a natural health product to treat many diseases, including bone injury and immune-related diseases.2,3 Moreover, ESW is usually used to control pain.4 Some reports showed that ESW, one of the main components in Dahuang zhechong pill, can alleviate hepatic fibrosis by decreasing the secretion of tumor necrosis factor-α and interleukin-13 through downregulation of p38 and ERK phosphorylation.5 In recent years, researchers have discovered antitumor and immunomodulatory effects of extracts from ESW on lung cancer, hepatocarcinoma, and gastric adenocarcinoma.6,7 Although some studies have been reported, few studies on breast cancer have been done yet.
Stromal cell–derived factor-1α (SDF-1α, also known as CXCL12) and its receptor CXCR4 have been widely associated with metastasis of several epithelial and hematopoietic tumors, including breast, prostate, ovary, and lung cancers.8,9 Subsequent research has expanded the role of CXCR4 to regulate carcinogenesis and primary tumor growth. Whereas the expression of CXCR4 is very low or absent in normal breast tissue, CXCR4 expression is upregulated in cancer metastasis, leading to enhanced signaling.10,11 Because of its involvement in both metastasis and primary tumor growth, CXCR4 is an ideal target to investigate novel therapeutic interventions.
The matrix metalloproteinases (MMPs), a family of zinc-dependent proteinases involved in the degradation of the extracellular matrix (ECM), degrade the basement membrane and ECM, thus facilitating the invasion of malignant cells through connective tissues and blood vessel walls and resulting in the establishment of metastases.12,13 The gelatinases A (MMP2) and B (MMP9) are 2 members of the MMP family that are expressed in human cancer and play a critical role in tumor cell invasion and metastasis.
We previously demonstrated that ESWE could downregulate several key growth and metastasis factors in hepatocellular carcinoma cells using genome-wide microarray analysis.14 In the present study, to examine the effect of ESW on breast cancer, ESW 70% ethanol extract (ESWE) was tested for its antitumor effects and the underlying signaling mechanisms in vitro and in vivo.
Materials and Methods
Reagents
The raw material of ESW used in the study was commercially available as dry matter, which was derived from Jiang Su (China). Leibovitz’s L15, DMEM (Dulbecco’s Modified Eagle Medium), F12 medium, insulin, hydrocortisone, cholera toxin, MG132, and chloroquine were purchased from Sigma-Aldrich (St Louis, MO). Fetal bovine serum (FBS) and horse serum were obtained from Lanzhou national hyclone Bio-engineering Co, Ltd, China. Recombinant human SDF-1α and epidermal growth factor were purchased from PeproTech (Rocky Hill, USA). Antibodies against CXCR4 were obtained from Abcam (Burlingame, USA). MMP2 rabbit mAb and MMP9 rabbit mAb were obtained from Epitomics (USA). p44/42 MAPK (ERK1/2) rabbit mAb and p-p44/42 MAPK (p-ERK1/2) rabbit mAb were purchased from Cell Signaling (USA). Vascular endothelial growth factor (VEGF165) rabbit mAb and horseradish peroxidase (HRP)-conjugated GAPDH (glyceraldehyde 3-phosphate dehydrogenase) monoclonal antibody were from Proteintech Group (Chicago, IL). Total RNA extraction kit was from Fastagen (Fastagen, Shanghai, China). PrimeScript RT Master Mix Perfect Real Time Kit (DRR036A) and SYBR Premix Ex Taq II were from TaKaRa. Lipofectamine 2000 was from Invitrogen. Other reagents used were analytical grades.
Cell Culture
MDA-MB-435s and MDA-MB-231 breast cancer cell lines were obtained from Shanghai Institute of Cell Biology in the Chinese Academy of Sciences in 2012. A recent study presented the related evidences and suggested that the MDA-MB-435s cell line originated from breast tissue.15,16 They were maintained in Leibovitz’s L15 medium supplemented with 10% (v/v) FBS and incubated cultures at 37°C without CO2. MCF-10A breast cells were kindly provided by Dr Xiao Li (Xi’an Jiaotong University) and grown in a 5% CO2-humidified incubator at 37°C in medium composed of DMEM/F12 supplemented with 5% horse serum, 20 ng/mL epidermal growth factor, 10 µg/mL insulin, 0.5 µg/mL hydrocortisone, 100 ng/mL cholera toxin, 100 units/mL penicillin, and 100 units/mL streptomycin.
Preparation of ESWE
Extraction of ESWE was done using the method described previously.14
Cell Viability Assay
Exponentially growing cells were plated into a 96-well plate (Costar, USA); 24 hours after seeding, cells were incubated in the absence or presence of ESWE for 48 hours. The cell viability was evaluated using MTT assay, as described previously.17
Colony Formation Assay
MDA-MB-435s and MDA-MB-231 cells were plated in 6-well plates (100 cells per well). After incubating for 24 hours, the cells were treated with 0.1, 0.2 mg/mL ESWE for 10 to 15 days. Colonies with cell numbers of >50 cells per colony were photographed and counted after staining with 0.01% crystal violet solution. All the experiments were performed in triplicate wells in 3 independent experiments.
In Vivo Tumor Model
All animal experiments were performed according to the guidelines and approval of the Institutional Animal Care and Use Committee of Xi’an Jiaotong University. Four- to six-week-old immunodeficient female Balb/c mice were purchased from Shanghai Laboratory Animal center of the Chinese Academy of Sciences and housed under aseptic and ventilated conditions. The mice were inoculated by subcutaneous injection into the mammary fat pad with 0.2 mL MDA-MB-231 cells (1 × 107 cells/mL) resuspended in 5% saline. Tumor size was measured on alternate days using a vernier caliper and calculated as length × width2 × 0.5 (in cm3). Body weights were monitored weekly as an indicator of overall health. Treatment began when tumors were palpable and no less than 0.1 cm3 in volume. The mice were randomly assigned to control and treatment groups (n = 4 per group). Animals were administrated ESWE orally daily at doses of 200 and 400 mg/kg, respectively, whereas control animals received equivalent volumes of dissolvant. After a total of 14 days of treatment, mice were killed humanely, and tumor tissue was removed and weighed. The tumor growth inhibition was calculated using the following formula:
Western Blot Analysis
ESWE-treated whole-cell extracts were lysed in lysis buffer, and lysates were then spun at 12 000g for 10 minutes to remove insoluble material and resolved on a 10% SDS gel. After electrophoresis, the proteins were electrotransferred to a polyvinylidene fluoride membrane, blocked with 5% nonfat milk to minimize nonspecific binding and probed with primary antibodies overnight at 4°C. The blot was washed, exposed to HRP-conjugated secondary antibodies for 2 hours, and CXCR4/HER2 expression was detected by chemiluminescence emission (ECL, Millipore, USA).
RNA Extraction and Polymerase Chain Reaction (PCR) Analysis
Total RNA isolation was performed using the total RNA extracted kit (Fastagen, China) according to the manufacturer’s protocol. Real-time (RT)-PCR was performed using PrimeScript RT Master Mix Perfect Real Time kit (TaKaRa DRR036A, Japan). RT-PCR was performed using SYBR Premix Ex TaqTM II and a Thermal Cycle Dice Real time system (TaKaRa, Japan). The result was analyzed using the manufacturer’s program (Thermal Cycler Dice Real Time System). The primer sequences were as follows:
GAPDH forward primer: 5′-GCACCGTCAAGGCTGAGAAC-3′
GAPDH reverse primer: 5′-TGGTGAAGACGCCAGTGGA-3′
CXCR4 forward primer: 5′-CCTGCCTGGTATTGTCATCCTG-3′
CXCR4 reverse primer: 5′-ACTGTGGTCTTGAGGGCCTTG-3′
Melt curve analysis was performed at the end of each PCR to confirm the specificity of the PCR product. Threshold cycle (Ct) values of CXCR4 in each sample were normalized with the GAPDH expression.
Wound Healing Assay
MDA-MB-435s and MDA-MB-231 cells were planted into 6-well plates and allowed to grow to 70% confluency in complete medium. Cells were then serum starved for 24 hours, and cell monolayers were scratched with a pipette tip. Wounded monolayers were then washed several times with serum-free medium to remove floating cells and photographed in a microscope. Cells were incubated in medium in the absence or presence of ESWE for 48 hours. After incubation, the growth medium was then changed to basal medium with or without SDF-1α. Then, 24 hours later, cell migration into the wound surface and the average distance that the cells migrated were determined under an inverted microscope.
Invasion Assay
Cancer cells were suspended in medium and seeded into the Millicell chambers with polycarbonate membranes of 8-µm pore size coated with 100 µL 1 mg/mL Matrigel (Becton Dickinson, USA). After preincubation with or without ESWE for 48 hours, Millicell chambers were placed into 24-well plates in which was added the basal medium only or basal medium containing 100 ng/mL SDF-1α. After incubation, the upper surface of Millicell chambers was wiped off with a cotton swab, and invading cells were fixed with 100% methanol and then stained with 0.2% crystal violet (Beijing Chemical Works, China). The invading cell numbers were counted in 5 randomly selected microscope fields (200×).
Statistical Analysis
All values are expressed as means ± standard error of the mean. Statistics was determined with ANOVA. Results were considered statistically significant if the P value was <.05.
Results
ESWE Inhibited Proliferation and Colony Formation of Breast Cancer Cells
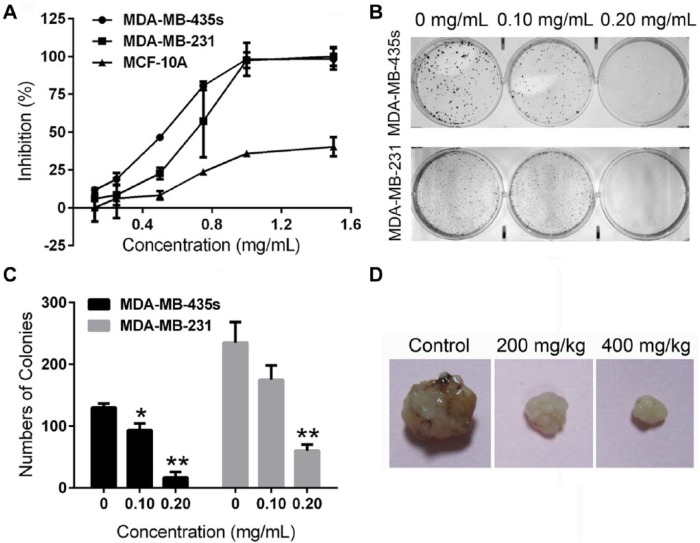
To assess the effects of ESWE on breast cancer cell growth, breast cancer cells MDA-MB-435s and MDA-MB-231 were treated with ESWE at indicated concentrations. MCF-10A was a normal breast cell line that was used as a comparison. Cell inhibition was measured using MTT, an antiproliferative assay. We observed that ESWE inhibited MDA-MB-435s and MDA-MB-231 in a concentration-dependent manner. The 50%-growth inhibitory concentrations (IC50s) of ESWE at 48 hours were 0.564 and 0.724 mg/mL, respectively, whereas it was considerably less active in MCF-10A cells (IC50 = 2.294 mg/mL; Figure 1A). In addition, the colony formation assay showed that ESWE generated a significantly lower number of colonies in comparison with the control (Figure 1B). These findings indicated that ESWE had potential antitumor properties in breast carcinoma in vitro.
Figure 1.
Eupolyphaga sinensis Walker 70% ethanol extract (ESWE) suppressed breast cancer cell proliferation and colony formation. A. Effect on proliferation of MDA-MB-435s, MDA-MB-231, and MCF-10A cells by ESWE. Cells were treated with ESWE at a series of increasing concentrations (0.125, 0.25, 0.50, 0.75, 1.00, 1.50 mg/mL) for 48 hours. ESWE inhibited MDA-MB-435s and MDA-MB-231 cell growth in a dose-dependent manner and was considerably less active on MCF-10A cells. B. Effect on colony formation of MDA-MB-435s and MDA-MB-231 cells by ESWE. C. Quantification of (B); data are represented as the means ± standard error of the mean from 3 repeated experiments. D. Photograph of the representative tumor in each group.
ESWE Suppressed Tumor Growth in Breast Tumor Mice Model
To determine the antitumor effect of ESWE in vivo, we also investigated the ability of ESWE to suppress tumor growth using a mice model. Figure 1D and Table 1 show that there was significant reduction of tumor in the mice treated with ESWE compared with the vehicle control tumor-bearing mice. After 14 days of treatment, a significant inhibition could be observed in the ESWE treatment groups compared with the control group; final tumor growth inhibitions were 56.60% for ESWE at a dose of 200 mg/kg and 62.26% at a dose of 400 mg/kg. No body weight loss or other abnormalities were observed in the ESWE-treated mice, indicating that there were no toxic effects in our treatment regimen.
Table 1.
In Vivo Inhibitory Effect of ESWE on MDA-MB-231 Cells.a
| Treatment | Control | ESWE | |
|---|---|---|---|
| Dose (mg/kg) | — | 200 | 400 |
| Initial tumor volume (mm3) | 101.22 ± 26.64 | 105.17 ± 32.88 | 107.12 ± 27.06 |
| Final tumor weight (g) | 0.53 ± 0.20 | 0.23 ± 0.18b | 0.20 ± 0.15b |
| Tumor growth inhibition, g/g (%) | — | 56.60 | 62.26 |
| Final body weight (g) | 22.15 ± 3.22 | 21.14 ± 4.29 | 21.26 ± 5.11 |
Abbreviation: ESWE, Eupolyphaga sinensis Walker 70% ethanol extract.
Values are mean ± standard error of the mean.
P < .01 versus control.
MAPK Signaling Pathway Involved the Inhibitory Effect of ESWE on Breast Cancer Proliferation
We first determined the effect of ESWE on the MAPK signaling pathway, which constitutes a large modular network that regulates a variety of physiological processes, such as cell growth, differentiation, and apoptotic cell death.18 Therefore, we investigated the effect of ESWE on ERK1/2 activity, a key molecule of this pathway. As shown in Figure 2, 48 hours incubation with ESWE reduced phosphorylated ERK1/2 protein expression in a dose-dependent manner in MDA-MB-435s and MDA-MB-231 cells, suggesting that the MAPK signaling pathway involved the ESWE’s antiproliferation effect.
Figure 2.
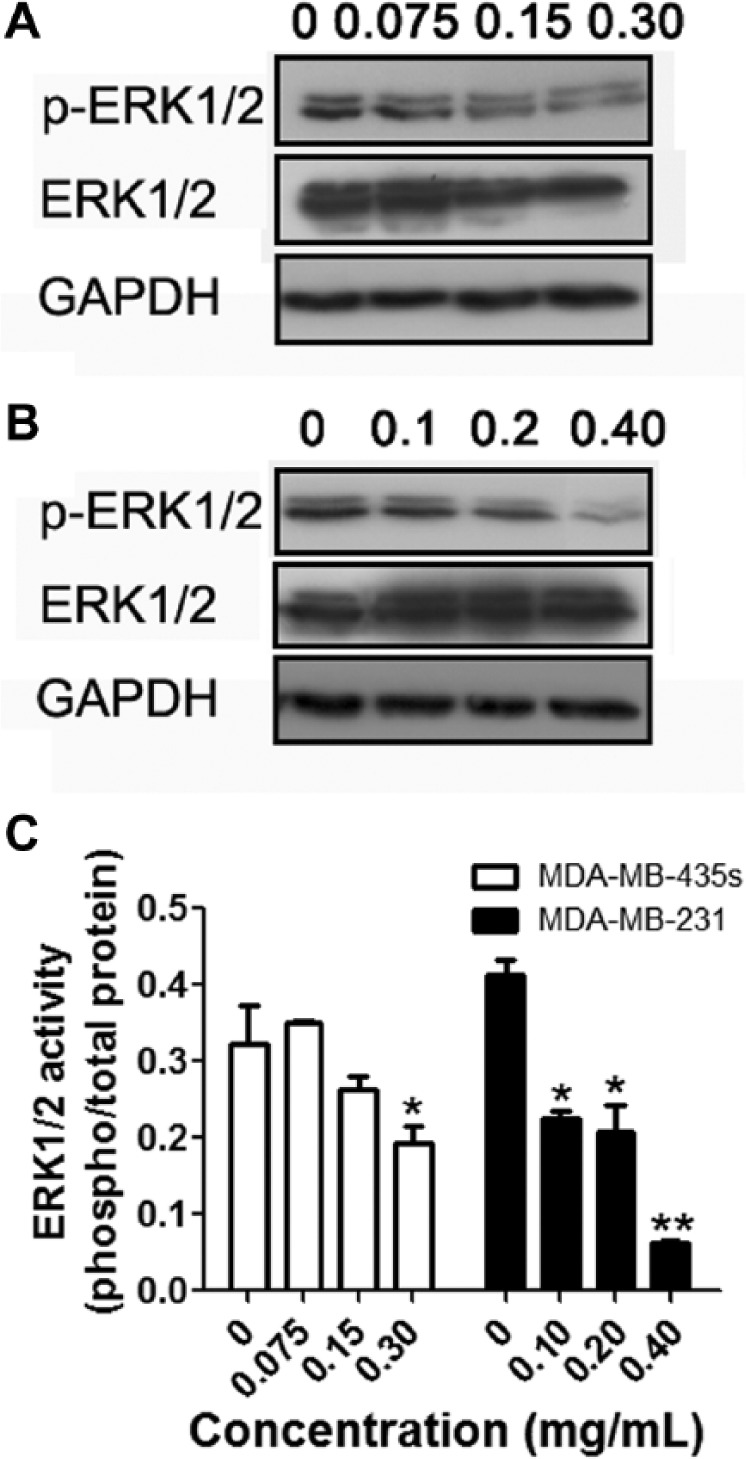
Eupolyphaga sinensis Walker 70% ethanol extract (ESWE) suppressed ERK1/2 activity in breast cancer cells. MDA-MB-435s (A) and MDA-MB-231 (B) cells were incubated with ESWE at different concentrations for 48 hours. Whole-cell extracts were prepared and analyzed by western blot analysis with antibodies against p-ERK1/2, ERK1/2, and GAPDH (glyceraldehyde 3-phosphate dehydrogenase). C. Quantification of (A) and (B). The results shown were representative of 3 independent experiments.a
a*P < .05, **P < .01 compared with untreated control cells.
ESWE Suppressed the Expression of CXCR4 Protein in Breast Cancer Cells
Previous microarray analysis of Agilent microarray-based gene expression profiling indicated that ESWE could downregulate CXCR4 expression in hepatocellular carcinoma SMMC-7721 cells. We hypothesize that ESWE treatment reduces CXCR4 signaling and, therefore, cell proliferation and migration. In this study, we first investigated the expression level of CXCR4 in MDA-MB-231 and MDA-MB-435s and found that CXCR4 protein expression in the 2 breast cancer cell lines was downregulated by the ESWE in a dose-dependent manner at the concentrations used in the experiments (Figure 3).
Figure 3.
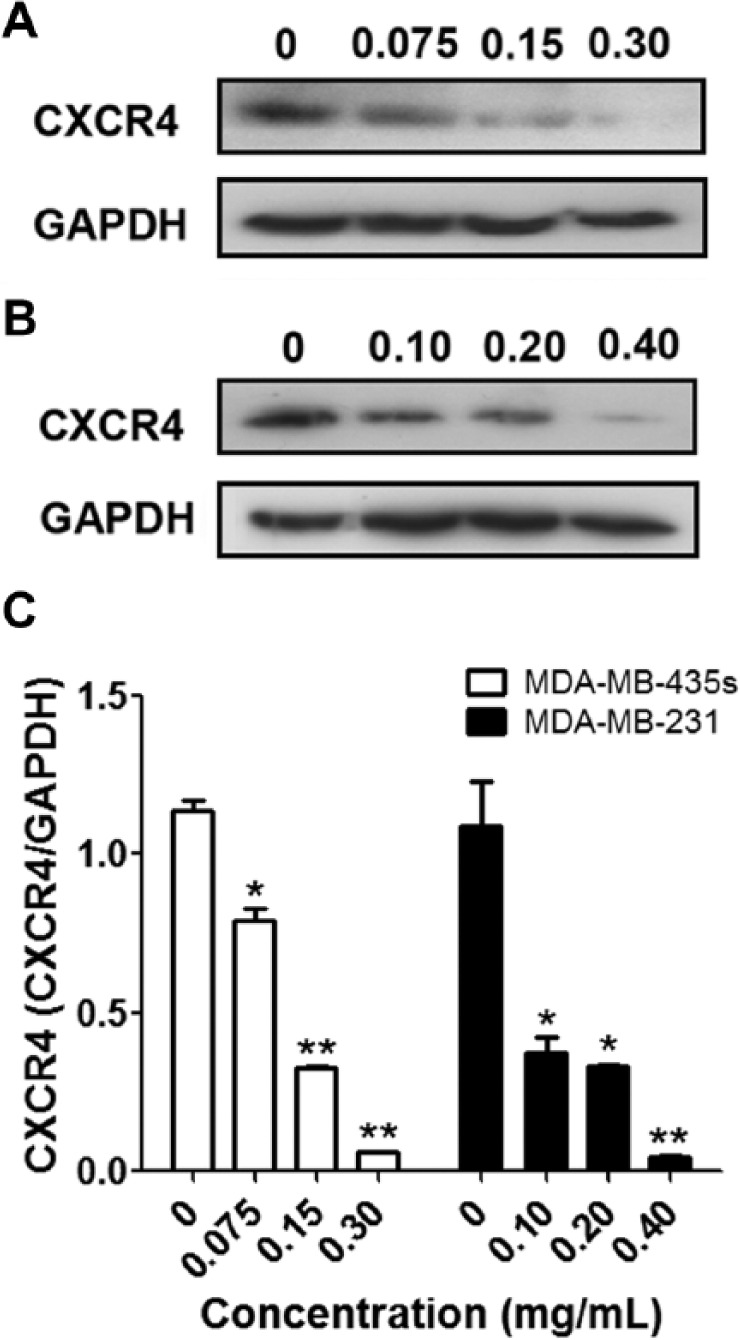
Eupolyphaga sinensis Walker 70% ethanol extract (ESWE) downregulated CXCR4 in breast cancer cells: MDA-MB-435s (A) and MDA-MB-231 (B) cells were incubated with ESWE at different concentrations for 48 hours. Whole-cell extracts were prepared and analyzed by western blot analysis with antibodies against CXCR4. The same blots were stripped and reprobed with GAPDH antibody to show equal protein loading. C. Quantification of (A) and (B). The results shown are representative of 3 independent experiments.a
a*P < .05, **P < .01 compared with untreated control cells.
ESWE Affected CXCR4 Expression Through the Modulation of Autocrine VEGF165
VEGF produced by breast carcinoma cells is critical for their invasion because CXCR4 has been reported to mediate migration of breast carcinoma cells toward SDF-1α, and this migration is dependent on autocrine VEGF.19 We also examined whether ESWE affected CXCR4 expression through the modulation of autocrine VEGF in breast cancer cells. MDA-MB-435s and MDA-MB-231 cells were incubated with different concentrations of ESWE for 48 hours and then examined for VEGF165 expression by western blot analysis using specific antibodies. We found that VEGF165 expression was partially affected after ESWE treatment (Figure 4), thus suggesting that downregulation of CXCR4 expression by ESWE might partially be a result of modulation of autocrine VEGF165.
Figure 4.
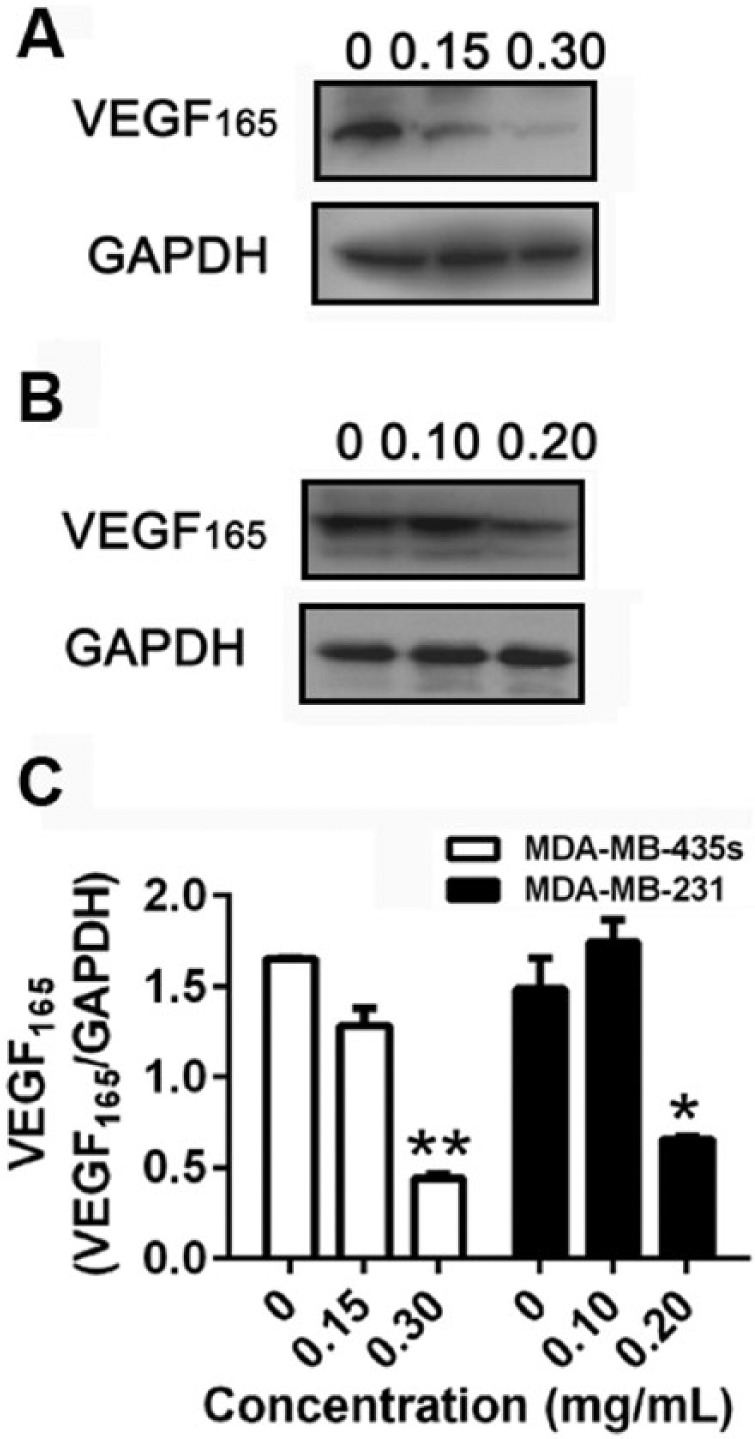
Effect of ESWE on VEGF expression in breast tumor cells: MDA-MB-435s (A) and MDA-MB-231 (B) cells were treated with the indicated concentrations of ESWE for 48 hours, after which western blotting for VEGF165 was done as described above. The same blots were stripped and reprobed with GAPDH antibody to show equal protein loading. C. Quantification of (A) and (B). The results shown are representative of 3 independent experiments.a
Abbreviation: ESWE, Eupolyphaga sinensis Walker 70% ethanol extract; VEGF, vascular endothelial growth factor.
a*P < .05, **P < .01 compared with untreated control cells.
Downregulation of CXCR4 by ESWE Was Partially Mediated Through Its Degradation
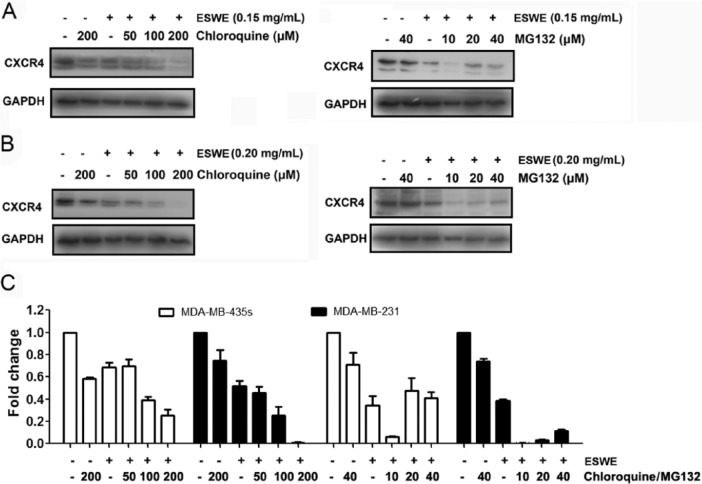
Because ESWE could downregulate CXCR4 expression, we examined the ability of chloroquine, a lysosomal inhibitor, to block ESWE-induced degradation of CXCR4 because CXCR4 has been shown to undergo ligand-dependent lysosomal degradation.20 The cells were pretreated with chloroquine for 1 hour before exposure to ESWE. Our results showed that chloroquine had no effect on ESWE-induced degradation of CXCR4 (Figure 5A), suggesting that this is an unlikely basis for the suppression of ESWE on CXCR4 expression.
Figure 5.
Eupolyphaga sinensis Walker 70% ethanol extract (ESWE) suppressed CXCR4 partial by proteasomal degradation: MDA-MB-435s (A) and MDA-MB-231 (B) cells were treated with indicated concentrations of chloroquine or MG132 for 1 hour, followed by treatment with ESWE for 48 hours. Whole-cell extracts were prepared and analyzed by western blot analysis using antibodies against CXCR4. The same blots were stripped and reprobed with GAPDH antibody to show equal protein loading. C. Quantification of (A) and (B). The results shown are representative of 3 independent experiments.
CXCR4 has also been shown to undergo ubiquitination at its lysine residue followed by degradation.20,21 We next investigated whether ESWE induces downregulation of CXCR4 through proteasomal degradation. To determine this, we examined the ability of MG132, a proteasome inhibitor, to block ESWE-induced degradation of CXCR4. MDA-MB-231 and MDA-MB-435s cells were pretreated with MG132 for 1 hour before being exposed to ESWE. As shown in Figure 5B, MG132 only slightly prevented ESWE-induced degradation of CXCR4, suggesting that this was arguably not the primary pathway for suppression of expression of CXCR4.
ESWE Downregulated CXCR4 mRNA Expression in Breast Cancer Cells
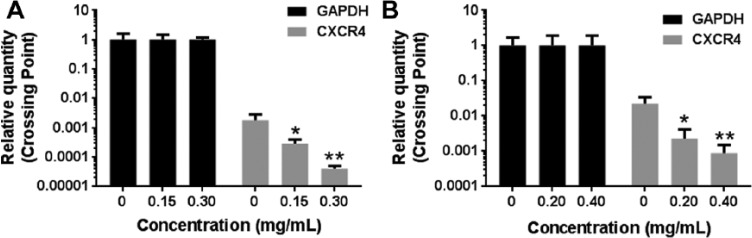
Because downregulation of CXCR4 expression by ESWE was not completely caused by enhancement of degradation, we investigated whether suppression occurred at the transcriptional level using quantitative PCR (RT-PCR). Cells were treated with ESWE at different concentrations and then examined for steady-state mRNA level of CXCR4. As shown in Figure 6, ESWE induced downregulation of CXCR4 mRNA expression in a concentration-dependent manner.
Figure 6.
Eupolyphaga sinensis Walker 70% ethanol extract (ESWE) suppressed CXCR4 mRNA level in breast tumor cells. MDA-MB-435s (A) and MDA-MB-231 (B) cells were treated with ESWE for the indicated concentrations for 48 hours, and then, real-time polymerase chain reaction was performed to measure the relative quantities of CXCR4 mRNA, with GAPDH as endogenous control for measurement of equal loading of RNA samples. The results shown are representative of 3 independent experiments.a
a*P < .05, **P < .01 compared with untreated control cells.
ESWE Inhibited SDF-1α-Induced Breast Cancer Cell Invasion
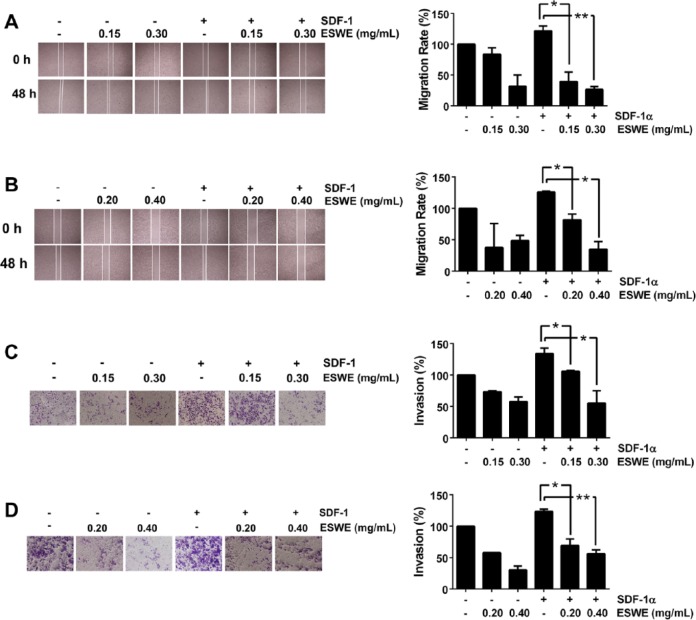
The expression of CXCR4/SDF-1α in breast tumors has been correlated with a poor prognosis, with increased metastasis.22 We found that both MDA-MB-231 and MDA-MB-435s cells migrated faster under the influence of SDF-1α, and this effect was abolished on treatment with ESWE (Figures 7A and 7B). To elucidate further the effect of ESWE on SDF-1α-induced cell invasion, we also found using an in vitro invasion assay that treatment with ESWE suppressed SDF-1α-induced invasion of both MDA-MB-231 and MDA-MB-435s cells (Figures 7C and 7D).
Figure 7.
ESWE inhibited migration and invasion of breast tumor cells: (A) and (B), Photographs of wounds of cells treated with ESWE. Wound-healing assay was performed for evaluating the inhibitory effect of ESWE on MDA-MB-435s (A) and MDA-MB-231 (B) cell migration. Confluent monolayers of cells were scarred and pretreated with ESWE for 48 hours before being exposed to 100 ng/mL SDF-1α for 24 hours. The average distance that the cells migrated into the wound surface was determined under an inverted microscope. The representative photographs show the same area at time 0 and after 48 hours of incubation. (C) and (D), Photographs of the cell invasion through the Matrigel-coated polycarbonate membrane stained by 0.2% crystal violet. MDA-MB-435s (C) and MDA-MB-231 (D) cells were seeded in the top chamber of the Matrigel. After pretreatment with or without ESWE for 48 hours, Millicell chambers were incubated with either the basal medium only or basal medium containing 100 ng/mL SDF-1α for 24 hours. After incubation, they were assessed for cell invasion as described in the Materials and Methods section. The representative photographs show the cell migration through the polycarbonate membrane stained by 0.2 % crystal violet.
Abbreviation: ESWE, Eupolyphaga sinensis Walker 70% ethanol extract; SDF, stromal cell–derived factor.
ESWE Downregulated MMP2 and MMP9 Protein Expression in Breast Cancer Cells
Besides CXCR4, MMP2 and MMP9 play a key role in cancer cell invasion and metastasis. The potential effects of ESWE pretreatment on MMP2 and MMP9 expression were determined by western blot analysis. As shown in Figure 8, ESWE exerted an inhibitive effect on the expression of MMP2 and MMP9 in the 2 breast cancer cell types.
Figure 8.
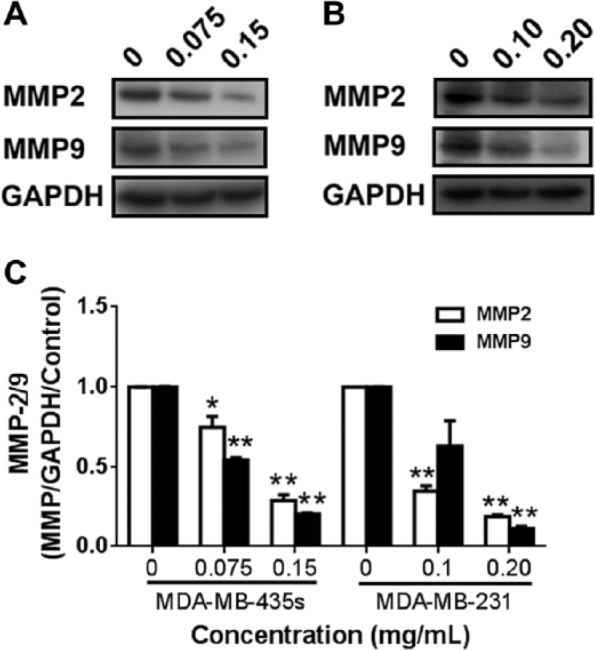
Effect of ESWE on expression of MMP-2 and MMP-9 in breast tumor cells: (A) and (B), western blot analysis of MMP-2 and MMP-9 protein expression in MDA-MB-435s cells (A) and MDA-MB-231 cells (B) after treatment with ESWE at indicated concentrations for 48 hours. C. Quantification of (A) and (B). The results shown are representative of 3 independent experiments. Data are expressed as mean ± standard error of the mean.a
Abbreviations: ESWE, Eupolyphaga sinensis Walker 70% ethanol extract; MMP, matrix metalloproteinase.
a*P < .05, **P < .01 compared with untreated control cells.
Discussion
ESW is a well-known edible and medicinal insect in China, which is used indigenously as traditional medicine in the treatment of bone injury, blood stasis, and immune-related diseases. Modern pharmacological studies indicate that ESWE shows obvious pharmacological activities and that it can inhibit growth of various tumors.23-25 Although the antitumor activity of ESW has been reported in some studies, few are related to the effect on breast cancer, and its antitumor mechanism is largely unknown. The current study focuses on its inhibition of breast cancer growth and invasion. In this study, we showed that ESWE had a significant inhibitory effect on breast cancer growth in vitro and in vivo. In cell experiments, ESWE obviously suppressed breast cancer cell proliferation and colony formation but was considerably less active in normal breast cells. In the mouse models, ESWE significantly inhibited primary tumor growth in nude mice xenograft models involving inoculation of MDA-MB-231 tumor cells into fat pads. The molecular mechanism by which ESWE affects cell proliferation and invasion involved the regulation of MAPK signaling as well as metastasis-related protein CXCR4, MMP2, and MMP9.
MAPK signaling plays a critical role in cell proliferation by regulating cell growth. Blockade of its key molecule is an effective method of controlling tumor cell proliferation. Western blot assay showed that the levels of phosphorylated ERK1/2 protein gradually increased with ESWE at the concentrations we tested. It provided an antiproliferative advantage for ESWE to breast cancer cells associated with this signaling pathway, for survival.
In previous studies, we found that ESWE also could inhibit CXCR4 using genome-wide microarray analysis.14 Here we determined whether it could suppress the expression and function of CXCR4, a chemokine receptor that has been closely linked with tumor cell proliferation, invasion, and metastasis in breast cancer. The CXCR4 chemokine receptor has been found to be overexpressed in breast cancer, and the involvement of CXCR4 in regulating breast cancer metastasis has been well established. Increased chemokine signaling through upregulated receptors on tumor cells is a hallmark for late-stage cancer patients.26-29 Studies point to genetic and microenvironmental factors, although it is still unclear what leads to the overexpression of CXCR4 in tumor cells.30
Our results clearly indicate that ESWE also suppressed CXCR4 expression in breast cancer cells, which was proved in our study using western blot analysis. Thereafter, we decided to investigate the various possible mechanisms by which ESWE could cause downregulation of CXCR4 expression in breast cancer cells.
VEGF, a major angiogenic factor, is also a requisite autocrine factor for breast carcinoma invasion in vitro. Previous findings indicate that a VEGF autocrine pathway induces CXCR4 expression in breast carcinoma cells, thus promoting their directed migration toward specific chemokines.19 Here, we found that ESWE modulated VEGF expression in breast cancer cells, suggesting that it might affect CXCR4 expression by affecting VEGF autocrine. We will further investigate the relevance of VEGF to ESWE on modulation of CXCR4 in subsequent work.
Many studies have documented the ligand-dependent downregulation of CXCR4 expression by lysosomal degradation, which involves atrophin-interacting protein 4-mediated ubiquitination and degradation.20 Our results, however, suggest that downregulation of CXCR4 by ESWE was not induced entirely through proteasomal degradation, therefore, we analyzed whether the inhibition of CXCR4 could possibly occur at the transcriptional level. Indeed, we found that ESWE downregulated the expression of CXCR4 mRNA in breast cells as observed by quantitative PCR analysis.
We hypothesized that ESWE treatment reduced CXCR4 signaling and, therefore, cell proliferation, migration, and invasion. Binding of SDF-1α to CXCR4 activates a variety of intracellular signal transduction pathways that regulate cell survival, migration, and adhesion. We further investigated the effect of ESWE on SDF-1α-induced migration and invasion of breast cancer cells. Inhibition of CXCR4 expression by ESWE significantly impaired SDF-1α-induced migration and invasion of both MDA-MB-435s and MDA-MB-231cells in a Matrigel invasion assay. This showed the critical role of the CXCR4 receptor in treatment with ESWE in downregulating SDF-1α-induced migration and invasion of breast cancer cells.
The expression and activity of MMPs against matrix macromolecules have been linked to the development of malignant phenotypes and the promotion of cell invasiveness and metastasis.31,32 The anti-invasive action of ESWE was reflected by its suppressive effects on the expression of MMP2 and MMP9, 2 major MMPs mediating the degradation of the ECM. In our study, ESWE treatment could reduce the protein expression of MMP2 and MMP9. These results suggest that the anti-invasive action of ESWE was also mediated, at least in part, by diminishing the ability of breast cancer cells to degrade the components of ECM by modulating MMP2 and MMP9 expression.
Conclusion
Taken together, our data showed that ESWE was able to significantly inhibit breast cancer growth, migration, and invasion. The mechanism underlying the above effects was attributed to attenuation of the activity of ERK1/2 and downregulation of the expression of CXCR4, MMP2, and MMP9.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Natural Science Foundation of China (Grant No. 81227802, 81302800, and 81370088), Natural Science Basic Research Plan in Shaanxi Province of China (Grant No.2015JQ8296), National Science Foundation for Postdoctoral Scientists of China (Grant No. 2013M532062), The Project of Shaanxi Star of Science and Technology (2012KJXX-06), and Supporting Plan of Education Ministry’s New Century Excellent Talents (NCET-13-0467).
References
- 1. Feng Y, Zhao M, He Z, Chen Z, Sun L. Research and utilization of medicinal insects in China. Entomol Res. 2009;39:313-316. [Google Scholar]
- 2. Ahn MY, Ryu KS, Lee YW, Kim YS. Cytotoxicity and L-amino acid oxidase activity of crude insect. Arch Pharm Res. 2000;23:5. [DOI] [PubMed] [Google Scholar]
- 3. Wu W, Ren Q, Li C, et al. Characterization and comparative profiling of MicroRNAs in a sexual dimorphism insect, Eupolyphaga sinensis Walker. PLoS ONE. 2013;8:e59016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu L, Lao XL, Ge A, Yu S, Li J, Mansky PJ. Chinese herbal medicine for cancer pain. Integr Cancer Ther. 2007;6:208-234. [DOI] [PubMed] [Google Scholar]
- 5. Cai HB, Sun XG, Liu ZF, et al. Effects of dahuangzhechong pills on cytokines and mitogen activated protein kinase activation in rats with hepatic fibrosis. J Ethnopharmacol. 2010;132:157-164. [DOI] [PubMed] [Google Scholar]
- 6. Ge GF, Yu CH, Yu B, Shen ZH, Zhang DL, Wu QF. Antitumor effects and chemical compositions of Eupolyphaga sinensis Walker ethanol extract. J Ethnopharmacol. 2012;141:178-182. [DOI] [PubMed] [Google Scholar]
- 7. Wang FX, Wu N, Wei JT, et al. A novel protein from Eupolyphaga sinensis inhibits adhesion, migration, and invasion of human lung cancer A549 cells. Biochem Cell Biol. 2013;91:244-251. [DOI] [PubMed] [Google Scholar]
- 8. Furusato B, Mohamed M, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497-505. [DOI] [PubMed] [Google Scholar]
- 9. Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238:30-41. [DOI] [PubMed] [Google Scholar]
- 10. Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17-32. [DOI] [PubMed] [Google Scholar]
- 11. Schmid BC, Rezniczek GA, Leodolter S, Zeillinger R. CXCR4 is expressed in ductal carcinoma in situ of the breast and in atypical ductalhyperplasia. Breast Cancer Res Treat. 2004;84:247-250. [DOI] [PubMed] [Google Scholar]
- 12. Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260-1270. [DOI] [PubMed] [Google Scholar]
- 13. Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178-193. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Zhan Y, Zhang D, et al. Eupolyphaga sinensis Walker displays inhibition on hepatocellular carcinoma through regulating cell growth and metastasis signaling. Sci Rep. 2014;4:e5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292-5293. [DOI] [PubMed] [Google Scholar]
- 16. Hollestelle A, Schutte M. Comment Re: MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:OF1. [DOI] [PubMed] [Google Scholar]
- 17. Zhan YZ, Zhang YM, Chen YN, Wang N, Zheng L, He LC. Activity of taspine isolated from Radix et Rhizoma Leonticis against estrogen-receptor-positive breast cancer. Fitoterapia. 2011;82:896-902. [DOI] [PubMed] [Google Scholar]
- 18. Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signalling pathways in the regulation of cell survival. FASEB J. 2008;22:954-965. [DOI] [PubMed] [Google Scholar]
- 19. Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203-7206. [PubMed] [Google Scholar]
- 20. Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282:36971-36979. [DOI] [PubMed] [Google Scholar]
- 21. Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509-45512. [DOI] [PubMed] [Google Scholar]
- 22. Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27:97-105. [DOI] [PubMed] [Google Scholar]
- 23. Ge GF, Yu CH, Wu QF. Anti-proliferative effects of ethanol extract from Eupolyphaga sinesis Walker in vitro. China J Tradit Chin Med Pharm. 2013;28:826-828. [Google Scholar]
- 24. Dai B, Zhan Y, Qi J, Zhang Y. Eupolyphaga sinensis Walker inhibits human chronic myeloid leukemia cell K562 growth by inducing G2-M phase cell cycle arrest and targeting EGFR signaling pathway and in S180 tumor-bearing mice. Environ Toxicol Pharmacol. 2014;37:1177-1185. [DOI] [PubMed] [Google Scholar]
- 25. Dai B, Qi J, Liu R, Zhang Y. Eupolyphaga sinensis Walker demonstrates angiogenic activity and inhibits A549 cell growth by targeting the KDR signaling pathway. Mol Med Rep. 2014;10:1590-1596. [DOI] [PubMed] [Google Scholar]
- 26. Cabioglu N, Yazici MS, Arun B, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686-5693. [DOI] [PubMed] [Google Scholar]
- 27. Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18:1240-1242. [DOI] [PubMed] [Google Scholar]
- 28. Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60:273-276. [DOI] [PubMed] [Google Scholar]
- 29. Helbig G, Christopherson KW, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631-21638. [DOI] [PubMed] [Google Scholar]
- 30. Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355-10362. [DOI] [PubMed] [Google Scholar]
- 31. Lapteva N, Yang AG, Sanders DE, Strube RW, Chen SY. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 2005;12:84-89. [DOI] [PubMed] [Google Scholar]
- 32. Smith MCP, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604-8612. [DOI] [PubMed] [Google Scholar]