Abstract
Oxidative stress is a key component in carcinogenesis. Although radiation produces reactive oxygen species, some anticancer agents such as alkylating agents, platinum and antitumor antibiotics exert cytotoxicity by generating free radicals. Nonenzymatic exogenous antioxidants such as vitamins, minerals, and polyphenols can quench ROS activity. However, whether antioxidants alter antitumor effects during radiotherapy and some types of chemotherapy remains unclear. In the present study, we reviewed antioxidants as an adjuvant therapy for cancer patients during chemotherapy or radiotherapy. Electronic literature searches were performed to select all randomized controlled clinical trials (RCTs) in which antioxidants were administered to cancer patients along with chemotherapy or radiotherapy. Articles or abstracts written in English were included. In total, 399 reports received primary screening. Duplicated articles and those meeting the exclusion criteria (not RCT, not human, and no oral administration) were excluded. Finally, 49 reports matching the inclusion criteria were included. It was difficult to determine whether antioxidants affect treatment outcomes or whether antioxidants ameliorate adverse effects induced by chemotherapy and radiotherapy. It is desirable to use an evidence-based method to select supplements best suited to cancer patients. Although there are many opinions about risks or benefits of antioxidant supplementation, we could mostly conclude that the harm caused by antioxidant supplementation remains unclear for patients during cancer therapy, except for smokers undergoing radiotherapy.
Keywords: cancer, antioxidant, chemotherapy, radiotherapy, supplement, vitamin
Introduction
Cancer is the leading cause of death worldwide. Although outcomes of cancer therapy have improved, cancer becomes a systemic disease beyond a particular point. Because complete recovery of cancer patients following a single treatment is quite difficult, a multidisciplinary approach combined with surgery, chemotherapy, radiotherapy, and immunotherapy is usually utilized.1
Other approaches using complementary and alternative medicine (CAM) modalities are an important choice among cancer patients. Hyodo et al2 reported that 44.6% of cancer patients use CAM treatments in Japan. Patients undergoing chemotherapy tend to prefer CAM, particularly dietary supplements. However, it is not common for clinicians to use CAM as a general therapy in Japan because of the uncertainty regarding the safety and effects of CAM therapies.
Oxidative stress is a key component in the carcinogenesis process.3 Stimulated by endogenous and exogenous factors, reactive oxygen species (ROS) induce cellular damage.3 Although radiation certainly produces ROS, some anticancer agents such as alkylating agents and platinum and antitumor antibiotics exert cytotoxicity by generating free radicals. Some endogenous antioxidant defense mechanisms, such as superoxide dismutase, glutathione peroxidise, and catalase, can counterbalance oxidative microenvironments. Nonenzymatic exogenous antioxidants such as vitamins, minerals, and polyphenols also have the ability to quench ROS activity.3,4 Therefore, antioxidant therapies may alleviate the adverse effects of chemotherapy and/or radiotherapy but may antagonize antitumor effects by reducing oxidative damage. They prevent cellular damage of normal organs and tissues by reacting with oxidizing free radicals.5 However, whether antioxidants can antagonize antitumor effects of radiotherapy and some types of chemotherapy remains controversial. It is necessary to clarify whether or not these supplements interact with cancer therapy using radiation and chemotherapy.6 It is important to note that their effects on prognosis, such as survival rate and tumor progression, should be determined and the results disseminated widely. In this study, we reviewed antioxidants as an adjuvant therapy for cancer patients during chemotherapy or radiotherapy. As our end point, we aimed to provide information about their effectiveness and safety for survival, tumor development, and relief of adverse effects during chemotherapy or radiotherapy for patients with cancer.
Materials and Methods
Electronic literature searches were performed, and relevant articles from 1982 to July 1, 2014, were obtained. Published human clinical trials in English that used randomized controlled clinical trial (RCT) designs involving administration of antioxidant supplements to cancer patients during chemotherapy or radiotherapy were selected. Additional articles found in review articles that suited our inclusion criteria were added. The chosen results were evaluated by the authors independently according to the inclusion and exclusion criteria.
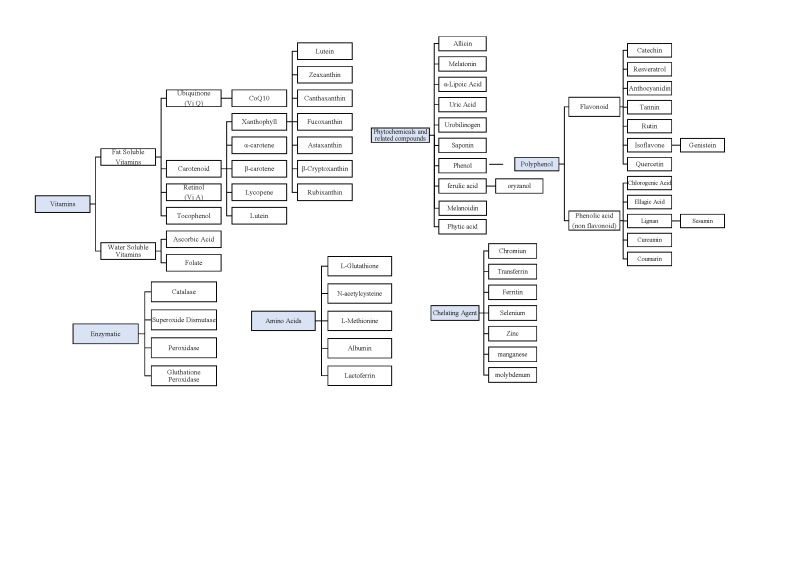
Studies that targeted cancer patients undergoing chemotherapy or radiotherapy were included. All cancer types and all chemotherapy or radiotherapy regimen types were included. Studies investigating chemopreventive effects using antioxidant supplements were excluded. Studies in which the antioxidant was administered by injection were excluded. Only oral supplements were included. Foods and Chinese medicine and herbs were excluded because it is unclear what their effective components might be. Names of antioxidants used in searches were researched and are listed in Supplementary Figure 1 (available at http://ict.sagepub.com/supplemental).7-10
Results
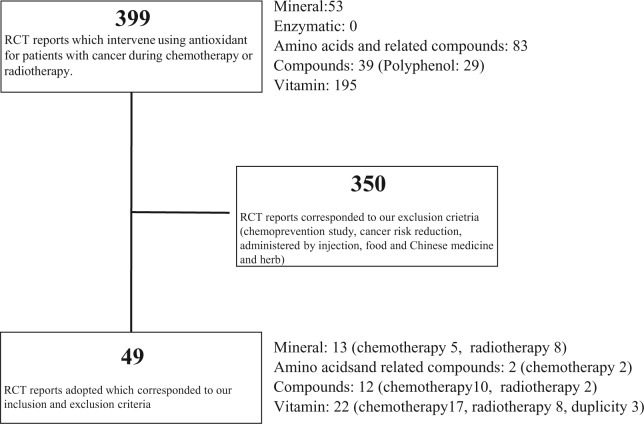
In total, 399 reports received primary screening. Thereafter, duplicated articles and those that met the exclusion criteria were excluded. Finally, 49 reports that met our inclusion criteria were included: 22 vitamin reports (17 chemotherapy, 8 radiotherapy, including 3 duplicates), 12 reports on phytochemicals and related compounds (10 chemotherapy, 2 radiotherapy), 2 amino acid and related component reports (both chemotherapy), and 13 mineral reports (5 chemotherapy, 8 radiotherapy). Detailed descriptions of the search flow are shown in Figure 1. In Tables 1 to 4, for each category, antioxidant effects on mitigation or aggravation of the adverse effects of cancer therapy are indicated in the representative results. Similarly, antioxidant interference with or enhancement of survival rate and/or antitumor efficacy of cancer therapy are also indicated in the tables.
Figure 1.
In total, 399 reports were primarily screened. Thereafter, duplicated articles and those that met the exclusion criteria were excluded. Finally, 49 reports that met our inclusion criteria were included: 22 vitamin reports (17 chemotherapy, 8 radiotherapy, including 3 duplicates), 12 phytochemicals and related compounds reports (10 chemotherapy, 2 radiotherapy), 2 amino acid and related substances reports (both chemotherapy) and 13 mineral reports (5 chemotherapy, 8 radiotherapy).
Abbreviation: RCT, randomized controlled trial.
Table 1.
List of Trials Using Antioxidant Vitamins.a
| Results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Reference | Design | Cancer | Participants (n) | Antioxidants | Chemotherapy | Radiation | End point | Results (Representative) | Survival/Tumor size |
| 1 | Takimoto et al (1982)26 | RCT | Lung, breast or thyroid | 40 | CoQ10 | Second day: fluorouracil, doxorubicin, cyclophosphamide (every 3 weeks) | First day: radiation 5 Gy (500 rads; cobalt60) | Prevention of cardiotoxicity | ● CTR ↑ in control group ● P < .01 vs CoQ10 group |
ND |
| 2 | Akihama et al (1983)24 | Double-blind and placebo-controlled trial | Acute/myeloid leukemia; malignant lymphoma | 19 | CoQ10 | Doxorubicin; cyclophosphamide; vincristine; prednisolone (6 pulse 21-day interval) | Hair loss; change in liver enzyme | ● Hair loss, NS ● AST/ALT ↑, P < .01 vs 0 week only in placebo group |
ND | |
| 3 | Okuma et al (1984)25 | RCT | Lung, malignant lymphoma or others | 80 | CoQ10 | Chemotherapy with adriamycin about 34.2 to 48 mg/body | Prevention of cardiotoxicity | ● QRS voltage: lower in control group ● P < .01 vs CoQ10 group |
||
| 4 | Iarussi et al (1994)27 | RCT | Acute lymphoblastic leukemia or non-Hodgkin lymphoma | 10 | CoQ10 | Adryblastin 120 mg + daunorubicin 120 mg | Prevention of cardiotoxicity | ● Group I: %LVFS ↓, P < .05 vs pretreatment ● Group II: %LVFS ↓, P < .002, %SWT ↓, P < .01 vs pretreatment |
ND | |
| 5 | Rusciani et al (2007)23 | RCT | Melanoma stage I to II | 81 | CoQ10 | γIFNα-2b | Metastases | P = .006 vs IFN group | ||
| 6 | Lesser (2013)28 | Double-blind and placebo-controlled trial | Breast cancer | 236 | ● Coenzyme Q10 + vitamin E ● Placebo + vitamin E |
Anthracycline, no anthracycline | Radiation | Fatigue | NS | ND |
| 7 | Meyskens et al (1995)36 | RCT | Chronic myelogenous leukemia | 153 | Vitamin A | Busulfan, 8 mg/m2/d, for 4 days every 4 weeks | Overall survival; progression-free survival | Grade 2 toxicities higher in Vitamin A supplementation group, P = .002 | ● Overall surviva l: P = .01 ● Progression-free survival: P = .023 |
|
| 8 | Dagdemir et al (2004)35 | RCT | Leukemia and lymphoma | 35 | Vitamin A | High-dose methotrexate (HDMTX), 3000 or 5000 mg/m2 every 24 hours, with leucovorin rescue | Intestinal absorption | Progression-free survival | ND | |
| 9 | Wadleigh (1992)51 | Double-blind and placebo-controlled trial | Head and neck cancer, esophageal cancer, hepatocellular cancer, and acute myelogenous leukemia | 18 | Vitamin E | ● Head and neck, esophageal: 5-FU + cisplatin ● Hepatocellular: doxorubicin ● Myelogenous leukemia: cytosine arabinoside, and doxorubicin |
Mucosal lesions healing | The number of patients who have complete resolution of lesions higher in vitamin E therapy; P = .025 | ND | |
| 10 | Pace et al (2003)46 | RCT | Solid malignancies (lung, ovarian, rhinopharynx, uretheral, gastric, testicular, esophageal, ethmoidal, and tongue cancer) | 27 | Vitamin E | Cisplatin cumulative dose >300 mg/m2 (administrated in combination regimens on the basis of tumor size) | Neuroprotective effect | Neurotoxicity scores, group 1 vs group 2; significantly higher in group 2; P < .01 | NS (clinical response) | |
| 11 | Ferreira (2004)45 | Double-blind and placebo-controlled trial | Cancer of the oral cavity and oropharynx | 54 | Vitamin E (oral rinse) | 2 Gy/section (Co60 unit), up to a cumulative dose of 44 Gy/4.5 weeks | Mucositis incidence and symptoms | ● Incidence density, P = .038 ● Questionnaire, P = .0001 |
NS between groups | |
| 12 | Argyriou et al (2006)48 | RCT | Solid or nonmyeloid malignancies (lung, breast, and ovarian cancer) | 32 | Vitamin E | Paclitaxel based (6 courses) | Efficacy and safety | ● Incidence PIPN significantly lower in group 1, P = .03 ● Adverse event, NS |
No statistical data | |
| 13 | Argyriou et al (2006)49 | RCT | Solid or nonmyeloid malignancies (lung, testicular, cervix, gastric, and head-and-neck cancer) | 30 | Vitamin E | ● Lung: cisplatin + etoposide+ irinotecan ● Testicular: cisplatin + etoposide + ifosfamide ● Gastric: cisplatin + docetaxel ● Head and neck: cisplatin + 5-FU |
Efficacy and safety | Incidence of neurotoxicity significantly higher in group 2, P = .026 | No statistical data | |
| 14 | Chitra and Shyamala Devi (2008)52 | RCT | Oral cavity cancer | 89 | Vitamin E | Telecobalt beam ≥6000 cGy | Salivary flow rate and the level of each component | ● Salivary flow rate, P < .001 2s vs 3a, P < 2b vs 3b ● Potassium level, P < .01 2s vs 3a, P < .001 2b vs 3b ● Ph, P < .01 2s vs 3a, P < .001 2b vs 3b ● Activity of amylase, P < .01 3a vs 3b ● Protein, P < .05 3a vs 3b ● Sodium, P < .05 2a vs 3a, P < .05 2b vs 3b |
ND | |
| 15 | Pace et al (2010)50 | Double-blind and placebo-controlled trial | Cancer patients receiving cisplatin-based chemotherapy | 41 | Vitamin E | Cisplatin (cumulative dose > 300 mg/m2) | Evaluate the neuroprotective effect | ● Incidence of neurotoxicity significantly lower in group 1, P < .01 ● Total neuropathy score significantly lower in group 1, P < .01 |
ND | |
| 16 | Kottschadeet al (2011)47 | Double-blind and placebo-controlled trial | Cancer patients receiving either taxanes or platinum-based chemotherapy | 189 | Vitamin E (dl-α-tocopherol) | Taxane, cisplatin, carboplatin, and/or oxaliplatin | Prevention effects of peripheral neuropathy | NS | ||
| 17 | Halperin et al (1993)54 | Double-blind and placebo-controlled trial | Primary or metastatic brain tumors | 65 | Vitamin C (l-ascorbic acid) | 14 to 70.3 Gy | Skin reaction | NS | ND | |
| 18 | Bairati et al (2005)40 | Double-blind and placebo-controlled trial | Squamous cell carcinoma of the head and neck area (stage I to II) | 540 | α-Tocopherol + β-carotene | Radiation | Adverse effect, symptoms | ● Adverse event (larynx) of α-tocopherol + β-carotene ↓ during radiotherapy (OR = 0.38: 95% CI = 0.21 to 0.71) ● QLQ-C30:diarrhea and sleep disturbance (P = .002) vs placebo ● Survival rate, ↓ (P = .12) |
NS; supplement ↓ vs placebo (P = .12) | |
| 19 | Pathak et al (2005)41 | RCT | Non–small-cell lung cancer (stage IIIb to IV) | 136 | Ascorbic acid + α-tocopherol + β-carotene | Paclitaxel and carboplatin | Response rate in chemotherapy/survival rate | NS | NS but slightly lower in supplementation group | |
| 20 | Meyer et al (2008)42 | Double-blind and placebo-controlled trial | Head and neck cancer (stage I to II) | 540 | ● α-Tocopherol + β-carotene ● α-Tocopherol |
Radiation | Recurrence and mortality | Incidence ↑in supplementation group ● Recurrence initial cancer: P = .03 ● Mortality from all cause: P = .02 ● Mortality from initial cancer: P = .04, during radiation therapy cigarette smokers vs nonsmokers |
||
| 21 | Fuchs-Tarlovsky et al (2011)43 | Double-blind and placebo-controlled trial | Cervical cancer (stage Ib1 to IIIb) | 103 | β-Carotene + vitamin C + vitamin E + selenium | Cisplatin | Radiation + cisplatin or radiation only, 50 Gy | QOL | ● Carbonylated protein ↓, P = .003 vs placebo ● QOL, P < .025 vs placebo |
ND |
| 22 | Suhail et al (2012)44 | RCT | Breast carcinoma (stage II) | 80 | Vitamin C, E | ● 5-FU 500 mg/m2
● Doxorubicin 50 mg/m2 ● Cyclophosphamide 500 mg/m2 Every 3 weeks for 6 cycles |
● Antioxidant enzymes ● DNA damage |
● MDA ↓, P < .01 vs chemotherapy alone ● GSH ↑, P < .01 vs chemotherapy alone ● SOD ↑, P < .01 vs chemotherapy alone ● CAT ↑, P < .01 vs chemotherapy alone GST ↑ ● P < .01 vs chemotherapy alone ● GR ↑, P < .01 vs chemotherapy alone ● DNA damage ↓, P < .01 vs chemotherapy alone |
ND | |
Abbreviations: CI, confidence interval; CTR, cardiothoracic ratio; IFN, interferon; NS, not significantly different between groups; OR, odds ratio; PIPN, peripheral neuropathy; RCT, randomized controlled clinical trial; LVFS, left ventricular fractional shortening; SWT, septal wall thickness; FU, fluorouracil; MDA, malondialdehyde; GSH, reduced glutathione; SOD, superoxide dismutase; QOL, quality of life; QLQ, quality of life questionnaire C30; CAT, catalase; GST, glutathione-S-transferase; GR, glutathione reductase ; ND, no data.
Table indicates the details of each RCT: design, cancer, participants, antioxidants, chemotherapy, radiation, end point, and results (survival/tumor size)
Table 4.
List of Trials Using Antioxidant Minerals.a
| Results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Reference | Design | Cancer | Participants (n) | Antioxidants | Chemotherapy | Radiation | End point | Results (Representative) | Survival/tumor size |
| 37 | Hu, YJ. (1997) 104 | Cross over RCT | cancer (lung, epidermoid carcinoma, adenocarcinoma,SCLC, breast, gastric carcinoma, primary liver cancer, esophagus carcinoma and colon carcinoma) |
41 | selenium | cisplatin 60 to 80 mg/m2 |
toxicity suppression effect of cisplatin (bone marrow suppression and nephrotoxicity) | ● urine NAG ↓in supplementation group p < 0.05 (24th h), <0.01 (48th h) vs. control group ● γGT ↓in supplementation group p < 0.05 (2nd hour), <0.001 (24th h), <0.02 (48th h) vs. control group ● urine AAP ↓in supplementation group p < 0.001 (2nd h), <0.01 (24th h), <0.02 (48th h) vs. control group leukopaenia ↓(bone marrow suppression) |
N.D | |
| 38 | Sieja, K. (2004) 105 | Double-blind and placebo controlled trial | ovarian cancer | 62 | selenium | doxorubicin cyclophosphamide vincristine prednisolone (6 pulse 21-day interval) |
side effect (● change in blood parameters ● serum and hair concentration) |
nausea (p<0.0000), vomiting (p<0.0000), stomatitis (p= 0.029), hair loss (p<0.05), flatulence (p< 0.0000), abdominal pain (p= 0.02), weakness (p< 0.0000), ill-being (p< 0.0000), loss of appetite (p< 0.0000)↓ in studied group vs. control group | N.D | |
| 39 | Buntzel, J. (2010) 103 | RCT | squamous cell carcinoma of the head and neck region | 39 | sodium selenite | 1.8 to 2.0 Gy (cumulative doses 60 to 72) | Toxicity suppression of radiotherapy | dysphagia↓in group 1 p = 0.05 vs. group 2 |
N.D | |
| 40 | Muecke, R. (2013) 101 | RCT | cervical and endometrial cancer (after surgical treatment) selenium concentration: less than 84 µg/ℓ |
81 | selenium | 3D conformal radiotherapy (6 to 18 MV linear accelerator) |
● Toxicity of radiotherapy ● clinical response |
● PTV (n.s) ● CTC score 2 (diarrhoea↓, p = 0.04 vs. CG) ● PTV > 1302 ml: CTC diarrhoea of score 2↓, p = 0.046 vs.CG |
N.D | |
| 41 | Elsendoorn, TJ. (2001) 106 | Double-blind and placebo controlled trial | cancer treated with cisplatin (testicular, bladder, sarcoma, gastric cancer, head-neck cancer and cervical cancer) | 27 | Vitamin C, E and selenium | testicular: cisplatin, etoposide, bleomycin bladder: cisplatin, methotrexate sarcoma: cisplatin doxorubicin gastric cancer: cisplatin, epidoxorubicin, 5-FU head and neck cancer: cisplatin, 5-FU, epi-adriamycin cervical cancer: cisplatin |
bone marrow, genotoxic and organ damage caused by chemotherapy | N.S. | N.D | |
| 42 | Weijl, NI.(2004) 107 | Double-blind and placebo controlled trial | malignant tumors (testicular cancer, sarcoma, gastrointestinal cancer, urogenital cancer, head and neck cancer, melanoma and so on.) |
48 | milky beverage include vitamin C and E and selenium ingredients: protein 3.42, carbohydrate 7.92, fat 0.05 |
cisplatin (total dose) supplementation group: 371 ± 139 placebo group: 339 ± 125 (mean ± SD) |
tumor response organ toxicity (plasma concentration) |
N.S | tumor response N.S |
|
| 43 | Ripamonti, C. (1998) 110 | placebo-controlled RCT | head and neck cancer | 18 | zinc sulphate | external beam radiotherapy 180 to 200 cGy (5 to 9 weeks) total dose 45 to 70 Gy |
toxicity prevention effect of radiation therapy | N.S | N.D | |
| 44 | Ertekin, MV. (2003) 113 | placebo-controlled RCT | head and neck cancer | 27 | zinc sulphate | 2-Gy fraction and 5 fractions/week (total 4000 to 7000 cGY) |
prevention of opportunistic bacterial and fungal infection |
Candida species much more in placebo vs. zinc group p = 0.000 Coagulase-negative staphylococci/Coagulase-positive staphylococci much more in placebo vs. zinc group p = 0.017 vs.0.031 |
N.D | |
| 45 | Ertekin, MV. (2004) 112 | placebo-controlled RCT | head and neck cancer | 27 | zinc sulphate | 2-Gy fraction and 5 fractions/week (total 4000 to 7000 cGY) |
preventive effect of radiation-induced oropharyngeal mucositis | mucositis↓in zinc supplementation p < 0.01 | N.D | |
| 46 | Ertekin, MV. (2004) 114 | placebo-controlled RCT | head and neck cancer | 27 | zinc sulphate | 2-Gy fraction and 5 fractions/week (total 4000 to 7000 cGY) |
antioxidant enzyme activities | SOD↓ in zinc group p < 0.03 vs. placebo (at first day after radiation) |
N.D | |
| 47 | Halyard, MY. (2007) 115 | Double-blind and placebo controlled trial | head and neck cancer | 169 | zinc | plan to receive ≥2000 cGy of external beam radiotherapy | prevention effect for radiation toxicity of change in taste | ● incidence of severe dysphagia p = 0.02 ● rate of patients maintained their weight p = 0.04 ● vs. placebo ● taste alteration N.S |
N.D | |
| 48 | Lyckholm, L. (2012) 111 | Double-blind and placebo controlled trial | cancer (acute leukaemia, bladder, breast, cervix, colon, lung, melanoma, non Hodgkin’s lymphoma, pancreas, prostate, sarcoma) |
41 | zinc | chemotherapy (carboplatin, docetaxel, 5-FU, gemcitabine, etoposide, paclitaxel, vincristine and so on) |
improvement in altered taste and smell | N.S | N.D | |
| 49 | Sangthawan, D. (2013) 109 | Double-blind and placebo controlled trial | head and neck cancer | 144 | zinc sulphate | 1.8 to 2.0Gy (total dose 50 to 70 Gy) |
benefit of relieving radiation-induced oral mucositis and pharyngitis | N.S | N.D | |
Abbreviations: CTC, common toxicity criteria system version 2a; PTV, planning target volume; RCT, randomized controlled clinical trial; NAG N-acetyl-β-D-glucosaminidase; γGT, γ-glutamyl transpeptidase; AAP, alanine aminopeptidase; SOD, superoxide dismutase.
Table indicates the details of each RCT: design, cancer, participants, antioxidants, chemotherapy, radiation, end point, and results (survival/tumor size).
Vitamins
The antioxidant effect of vitamins suggests that an adequate intake of these micronutrients would contribute to a lower risk of neoplastic diseases.11 Vitamins have also been used for reducing oxidative stress during chemotherapy and radiotherapy. The vitamins with antioxidant capacity researched in this study were ubiquinone, carotenoids, retinol, tocopherol, ascorbic acid, and folate.12,13 Multivitamins and combinations of multiple vitamins were also included. The results and details of clinical studies are summarized in Table 1 and Supplementary Table 1 (available at http://ict.sagepub.com/supplemental).
Ubiquinone (Vitamin Q)
Ubiquinone, also known as coenzyme Q10 (CoQ10), is a fat-soluble quinone. It has properties similar to those of other vitamins and is essential for the synthesis of adenosine 5-triphosphate (ATP). It plays an important role in the electron transport chain,14 functions as an antioxidant, and prevents lipid peroxidation.15,16 It is involved in optimal energy production for cell growth and maintenance within human cells.17 Adverse effects of CoQ10 may include insomnia, elevated liver enzymes, rash, nausea, epigastric pain, dizziness, photophobia, irritability, headache, and heartburn.18 Coenzyme Q10 is a lipid-soluble antioxidant that may protect against mitochondrial ROS,19 is an essential component of the electron transport system, and as a potent intracellular antioxidant, appears to prevent damage to the mitochondria of the heart.20 CoQ10 deficiency is significantly higher in cancer patients than in healthy populations.21 CoQ10 is widely promoted for enhancing or modulating the immune system.22
We found 5 clinical trials using CoQ10 alone23-27 and 1 trial28 using a combination of CoQ10 and other vitamins. These results indicated some effectiveness of CoQ10 as an adjuvant therapy in cancer treatment. Among 6 trials, 2 reported tumor development and survival rates. Rusciani et al23 indicated that the administration of γ interferon α-2b (γIFNα-2b) in combination with CoQ10 for 3 years significantly decreased melanoma metastases rates in patients who were followed up for 5 years compared with those in their control group.23 Although the mechanism by which coenzyme Q10 affected metastasis is not clear, it is unlikely that antioxidant effects interfered with IFN treatments because IFN appears to work through an immunological rather than a free-radical mechanism. CoQ10 also has an effect on the production of ATP required to compensate for the energy loss during chemotherapy. Chemotherapeutic agents could have deleterious effects on mitochondrial respiratory chains by interfering with CoQ10 and leading to calcium overload, causing myocardial cell necrosis.29 However, CoQ10 supplementation may be effective in protecting myocardial function from chemotherapeutic cardiotoxicity.25-27,30 Takimoto et al,26 Okuma et al,25 and Iarussi et al27 investigated whether CoQ10 supplementation prevents cardiotoxicity during chemotherapy with anthracycline antibiotics. In all 3 studies, it was reported that supplementation was effective in preventing cardiotoxicity caused by anthracycline antibiotics. However, there is some doubt about the evidence quality of these older studies, which have small sample sizes27 and other study design issues.25,26
These results indicate that CoQ10 may provide some protection against toxicity and deterioration associated with chemotherapy or radiotherapy. No adverse effects caused by CoQ10 supplementation were reported in any trial.
Retinol (Vitamin A)
Retinoids are reported to inhibit tumor growth on both exocrine and endocrine human pancreatic cell lines.31 Retinoids and IFNs act synergistically in inhibiting the growth of several cell lines.32 Furthermore, it is reported that retinol has a protective effect on the mucosa of the gastrointestinal system.33 However, very few RCTs were performed that combined cancer therapy and retinol supplementation. Regarding tumor development and survival rate, a report on retinol supplementation during cancer therapy was found. However, we excluded this study because it did not meet our inclusion criteria (article in French).34
Dagdemir et al35 reported that high-dose retinol supplementation with methotrexate reduces adverse effects of intestinal malabsorption during chemotherapy in children with leukemia and lymphoma. Although methotrexate is classified as an antimetabolite, it is not reported to increase oxidative stress. On the other hand, Meyskens et al36 reported that the control group in a vitamin A supplementation study had a significantly increased risk of disease progression and death, although toxicities greater than grade 2 were higher in the vitamin A supplementation group.
Tocopherol (Vitamin E)
The main constituent of vitamin E is α-tocopherol, a lipid-soluble vitamin. It is the most important natural antioxidant, scavenging ROS and boosting cellular antioxidative capacity to reduce oxidative damage.37 Radiation-induced oxygen free radicals have been implicated as mediators of radiation-induced mucosal cell injury.38,39
We found 3 clinical trials using α-tocopherol or vitamin E alone and another 6 trials using a combination of β-carotene, ascorbic acid, selenium, and CoQ10.16,28 Among these, 6 trials28,40-44 used α-tocopherol in combination with other vitamins. These were included in the “multiple combination with vitamins” category.
Regarding tumor development and survival rates, Ferreira et al45 conducted a double-blind and placebo-controlled RCT and concluded that mouthwashes (used to thoroughly rinse the oral cavity for 5 minutes and then swallowed immediately) containing vitamin E did not affect the survival rate of patients with cancer of the oral cavity and oropharynx. In addition, they reported that the supplementation reduced the incidence of mucosal adverse effects during radiotherapy. They reported no adverse effects caused by vitamin E supplementation. Two groups, Pace et al46 and Kottschade et al47 performed clinical trials using vitamin E for cancer patients treated with taxane or platinum. They reported that no significant difference in peripheral neuropathy was observed between the supplementation group and control group. Argyriou et al48,49 and Pace et al50 reported that vitamin E supplementation significantly protects against chemotherapy (cisplatin/paclitaxel)-induced neurotoxicity. Similarly, Wadleigh et al51 reported that application of vitamin E oil to the oral lesions may be effective for chemotherapy-induced mucositis. Chitra and Shyamala Devi52 performed a clinical trial for patients with oral cavity cancer. They concluded that α-tocopherol supplementation improves the salivary flow rate, thereby maintaining salivary parameters such as pH, activity of amylase, protein, and sodium during radiotherapy. These results suggest that α-tocopherol supplementation may have a preventive effect against radiation-induced oxygen free-radical toxicity.
Ascorbic Acid (Vitamin C)
Ascorbic acid, vitamin C, is a water-soluble antioxidant. It is thought to counteract free radicals and prevents organ and tissue damage caused by adverse effects of chemotherapy and radiotherapy.53 In this review, there were no reports found on tumor development and survival rates using ascorbic acid during cancer therapy.
Regarding radiotoxicity, Halperin et al54 investigated protective effects of a vitamin C solution on the skin. It was applied to the radiation site before radiotherapy in patients with brain tumors. However, the skin radiotoxicity score and diagnosis results indicated no significant effect. They reported a rusty discoloration on the skin caused by the application of ascorbic acid solution.
Carotenoid and Multiple Vitamin Combinations
More than 40 carotenoids have been identified in human blood samples; of these, α-carotene, β-carotene, lutein, β-cryptoxanthin, lycopene, and zeaxanthin are found at higher levels.55 It appears that carotenoids have various chemopreventive actions.56 Because of its cancer prevention and regression effects, lycopene has been adopted for use in various cancers types. It is also the most potent quencher of free radicals and is an immunomodulator.57,58
We found 4 trials using carotenoids in combination with other antioxidants. Among them, 3 trials investigated survival rates and reported both positive and negative data. Bairati et al40 indicated that β-carotene treatment in combination with α-tocopherol has the potential to decrease the occurrence and severity of adverse effects of radiations in patients with squamous cell carcinoma of the head and neck. In contrast, antioxidant vitamins may interfere with treatment efficacy, although this negative aspect was not found to be significant (P = .12). Although there was no statistical significance, the survival rate of the supplementation group was slightly lower than that of the placebo group. Although the studies of Bairati et al40 and Meyer et al42 were the same trial, we have included both of them because outcome variables presented in each article were different. Meyer et al42 performed a subgroup analysis that verified that α-tocopherol and β-carotene supplementation had a significant negative effect on cigarette smokers undergoing radiotherapy, particularly with respect to recurrence (P = .03), all-cause mortality (P = .02), and initial cancer (P = .04) of head and neck cancer (HNC). For patients who smoked during radiation therapy compared with those who did not smoke during radiation (not shown in Table 1), they reported high adjusted hazard ratios (HRs) for recurrence (HR = 2.41, smokers; HR = 1.07, nonsmokers), all-cause death (HR = 2.26, smokers; HR = 1.14, nonsmokers), and death from the initial cancer (HR = 3.38, smokers; HR = 1.06, nonsmokers). Furthermore, increased HRs were reported in patients who smoked even prior to and after radiation therapy. On the other hand, Pathak et al41 conducted an RCT for non–small-cell lung cancer (NSCLC) patients and researched the chemotherapy response and survival rate with and without supplementation using multiple antioxidants. They concluded that the data indicated a slightly higher response and survival rate in the supplementation group, but there was no significant difference between groups.
Regarding antioxidant effects of supplementation, a trial with antioxidant supplementation for patients with cervical cancer was performed by Fuchs-Tarlovsky et al.43,59 They indicated that β-carotene, vitamin C, vitamin E, and selenium supplementation lowered the level of carbonylated proteins and maintained higher QOL scores in global and cognitive ability (using quality of life 30(QOL-30) and quality of life questionnaire CX24 (QLQ-CX24)) compared with placebo, which may have decreased active oxygen induced by chemotherapy with cisplatin and/or radiotherapy. In addition, using combinations of vitamins, Suhail et al44 performed an RCT for healthy individuals and for breast cancer patients during chemotherapy. They concluded that using vitamin C and E therapy significantly prevented chemotherapy-induced DNA damage assessed in the peripheral lymphocytes. They did not indicate the survival rate and tumor response.
Phytochemicals and Related Compounds
In addition to minerals, amino acids, and vitamins, certain phytochemicals and related compounds have an antioxidant capacity. Here, the phytochemicals and related compounds with antioxidant efficacy that we studied included polyphenols, allicin, melatonin, α-lipoic acid, uric acid, urobilinogen, ferulic acid, melanoidin, phytic acid, and saponin. The results of the research and the details of the clinical trials are shown in Table 2 and Supplementary Table 1.
Table 2.
List of Trials Using Antioxidant Phytochemicals and Related Compounds.a
| Results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Reference | Design | Cancer | Participants (n) | Antioxidants | Chemotherapy | Radiation | End point | Results (Representative) | Survival/tumor size |
| 23 | Lissoni, P. (1997) 65 | RCT | metastatic solid tumor (lung cancer, breast cancer and gastrointestinal tract) | 80 | melatonin | Lung: cisplatin + etoposide Breast: mitoxantrone Gastro: 5-FU + folate |
toxicities of chemotherapy | toxicities of chemo vs. chemo + melatonin myleosuppression p = 0.006 asthaenia p = 0.0006 |
tumor regression: N.S but tends to be higher in melatonin group survival (1 year): higher in melatonin group vs. control p < 0.05 |
|
| 24 | Lissoni, P. (1997) 69 | RCT | consecutive NSCLC (patients who were unable to tolerate the most aggressive polychemotherapies with high dose cisplatin, anthracyclines, taxol, taxotere and genicitabine) | 70 | melatonin | doxorubicin cyclophosphamide vincristine prednisolone (6 pulse 21-day interval) |
● toxicities of chemotherapy ● survival rate ● clinical response |
toxicities of chemo vs. chemo + melatonin myleosuppression p < 0.05 neuropathy p < 0.05 asthaenia p < 0.01 10% of weight loss p < 0.001 |
survival rate: >p < 0.05 tumor regression: N.S but tends to be higher in melatonin group |
|
| 25 | Ghielmini, M. (1999) 61 | Double-blind and placebo controlled trial | NSCLC or small-cell lung cancer (not amenable to surgery or radiotherapy/no previous chemo and radiotherapy) | 20 | melatonin | carboplatin etoposide |
haematological toxicity | N.S | ||
| 26 | Lissoni, P. (2003) 64 | RCT | consecutive untreated metastatic NSCLC | 100 | melatonin | cisplatin 20 mg/m/day etoposide 100 mg/m/day |
● toxicities of chemotherapy ● survival rate ● clinical response |
toxicities of melatonin (vs. chemo alone) neurotoxicity p < 0.01 thrombocytopaenia p < 0.01 weight loss prevention p < 0.001 asthaenia p < 0.005 |
Survival from Kaplan–Meier method: higher in melatonin group vs. control p < 0.001 tumor regression: higher in melatonin group p < 0.05 Progressive disease: higher in control vs. melatonin group p < 0.01 |
|
| 27 | Cerea, G. (2003) 67 | RCT | metastatic colorectal cancer (previous chemotherapeutic contain 5-FU) | 30 | melatonin | CPT-11 125/mg/m2/week |
patients achieving disease control toxicity | patients of disease control archive↑in CPT-11 + melatonin vs. CPT-11 alone p < 0.05 | tumor response: N.S but slightly ↑ in melatonin group vs. control | |
| 28 | Lissoni, P. (2007) 68 | RCT | NSCLC | 100 | melatonin | cisplatin 20 mg/m2/day etoposide 100 mg/m2/day (3 consecutive days every 28 days) |
● toxicities of chemotherapy ● clinical response |
toxicities of chemo with melatonin and 5-MTT (vs. chemo alone) thrombocytopaenia p < 0.01 neurotoxicity p < 0.05 asthaenia p < 0.01 anorexia p < 0.05 |
clinical response (complete response): ↑in melatonin p < 0.05 | |
| 29 | Lissoni, P. (2007) 63 | RCT | consecutive cancer patients (NSCLC, colorectal, gastric) |
370 | melatonin | depends on tumor histotype cisplatin, etoposide, gemcitabine, oxaliplatin, 5-FU choice and combination of chemotherapy were established on the basis of tumor histotype |
● toxicities of chemotherapy ● survival rate ● clinical response |
toxicities of chemo with melatonin (vs. chemo alone) thrombocytopaenia p < 0.01 neurotoxicity p < 0.05 asthaenia p < 0.01 neoplastic cachexia p < 0.005 |
survival curve higher in melatonin group vs. control p < 0.05 clinical response↑in melatonin p < 0.01 disease control ↑in melatonin p < 0.01 |
|
| 30 | Lissoni, P. (1999) 66 | RCT | metastatic solid tumor (lung cancer, breast cancer, gastrointestinal tract neoplasms, head and neck cancers) |
250 | melatonin | NSCLC: cisplatin, etoposide, gemcitabine breast: doxorubicin, mitoxantrone, paclitaxel gastrointestinal tract: 5-FU + folic acid neck: cisplatin + 5-FU |
● toxicities of chemotherapy ● clinical response |
toxicities of CT + MLT (vs. CT) asthaenia p < 0.001 myelosuppression p < 0.001 and so on. |
survival (1 year) ↑in melatonin: all p < 0.05 Cisplatin + etoposide (NSCLC) : p < 0.001 5-FU + folic (gastro) : p < 0.05 doxorubicin (breast) : p < 0.05 regression rate melatonin group ↑: Cisplatin + etoposide (NSCLC): p < 0.001 5-FU + folic (gastro) : p < 0.05 doxorubicin (breast) : p < 0.05 Progression: longer in melatonin group : p < 0.05 |
|
| 31 | Guo, Y. (2014) 72 | Double-blind and placebo controlled trial | Cancer patients (gastrointestinal, lung, genitourinary, other) |
243 (70 completed) |
α-lipoic acid | Platinum based regimen (cisplatin, oxaliplatine) |
toxicity of neuropathy ADL pain ameliorating rate |
N.S between each group |
N.D | |
| 32 | Falsaperla, M (2005) 79 | RCT | hormone refractory prostate cancer | 48 | ellagic acid (extracted from Punica granatum seeds) | vinorelbine 25 mg/mq/week × 6 week estramustine 280 mg thrice daily × 42 days (28-day cycle) |
chemotoxicity | toxicities of group A (vs. group B) neutropaenia ↓ p < 0.05 |
N.S for survival (p = 0.2) N.S for clinical response between group |
|
| 33 | Grotz, KA. (2001) 84 | Double-blind and placebo controlled trial | head and neck cancer | 23 | coumarin + troxerutin | Total dose 60 Gy |
radiation toxicity (salivary glands, mucosa, pharynx, larynx and cutis) |
RTOG score: experimental ↓vs. placebo U3 : p = 0.015 U4 : p = 0.016 U5 : p = 0.007 U6 : p = 0.027 |
N.D | |
| 34 | Ryan, JL. (2013) 87 | Double-blind and placebo controlled trial | breast cancer | 30 | curcumin | 42.6 to 50.4 Gy (16 to 33 sessions) |
radiation toxicity for skin | Severity of radiation dermatitis p = 0.008 moist desquamation↓ p = 0.002 splitting ↓ p ≤ 0.021 |
||
Abbreviations: 5-MTT, 5-methoxytryptamine; CPT-11, irinotecan; NS, not significant; NSCLC, non–small-cell lung cancer; RCT, randomized controlled clinical trial; RTOG, Radiation Therapy Oncology Group; 5-FU, 5-fluorouracil; ADL activities of daily living; ND, no data.
Table indicates the details of each RCT: design, cancer, participants, antioxidants, chemotherapy, radiation, end point, and results (survival/tumor size).
Melatonin
Melatonin (5-methoxytryptamine) is a neurohormone secreted from the pineal body. Myeloprotective and immunoenhancing effects of melatonin have been demonstrated in in vitro and in vivo experimental models.60,61 Melatonin, a potent antioxidant, plays various roles in regulating circadian rhythms, sleep, tumor growth, and ageing. For many years, it has been known that it plays an important anticancer role.62 As an antioxidant agent, melatonin enhances the prevention of free-radical production and potentially protects against chemotherapy-induced toxicity.63
In this study, all the included articles used melatonin combined with chemotherapy. Many of them reported positive data regarding tumor development and survival rates63-66 when melatonin was combined with chemotherapy. The survival rate of patients with NSCLC and other cancers was significantly higher with melatonin supplementation when combined with chemotherapy using, for example, cisplatin and etoposide. Similarly, tumor regression rates were significantly higher with melatonin supplementation.66 The study of Cerea et al67 and many other studies63-66,68,69 reported positive results regarding toxicity reduction and clinical response. However, Ghielmini et al61 performed a double-blind and placebo RCT for NSCLC patients whose chemotherapy regimen included cisplatin and etoposide. They reported that there was no significant difference in regard to hematological toxicity in their trial.
In almost all the trials, melatonin supplements were administered in the evening. This was based on the idea that the synthesis of melatonin is strictly controlled by lighting conditions and shows a clear circadian rhythm, with lower levels during the daytime and significantly higher levels at night.70 There were no severe adverse effects of melatonin supplementation.
α-Lipoic Acid
The administration of α-lipoic acid has been shown to be effective in the treatment of diabetic distal sensorimotor neuropathy.71 Similarly, the effect of α-lipoic acid on chemotherapy-induced peripheral neuropathy has been investigated.72 The neuroprotective mechanism of α-lipoic acid is related to the reduction of oxidative stress from free-radical formation. It also has a protective effect during chemotherapy via the regulation of proinflammatory cytokines.73
Guo et al72 conducted a double-blind, placebo-controlled trial for patients undergoing platinum-based chemotherapy. Although only 28% of patients in the supplementation group and 30% in the placebo group completed this trial, they reported no significant effects in regard to the tumor reduction rate, activities of daily living, or chemotherapy-induced toxicity such as neurotoxicity and pain. No severe adverse effects caused by supplementation were found. This was the only RCT using α-lipoic acid; therefore, further RCTs using α-lipoic acid are required.
Polyphenols
Polyphenol74 compounds have been widely studied for their antioxidant properties. They are found in many foods such as chocolate, tea, red wine, and pomegranate juice, which are an integral part of the human diet.75 The polyphenols researched in this study were ellagic acid, coumarin, curcumin, catechin, resveratrol, anthocyanidin, tannin, rutin, isoflavone, quercetin, chlorogenic acid, and lignan.
Ellagic acid
Ellagic acid is an antioxidant substance that can be found in certain plants.76,77 It is an effective antimutagen and anticarcinogen phytotherapeutic agent that prevents carcinogens from binding to DNA. It may keep cancer cells from spreading and inhibits cancer onset and tumor proliferation during radiotherapy and chemotherapy in laboratory studies.78
Falsaperla et al79 conducted RCT using ellagic acid during chemotherapy for patients with hormone-refractory prostate cancer. They investigated clinical responses and survival rates. They indicated that the administration of alkaloid antitumor agents in combination with ellagic acid did not significantly affect the survival rate. However, ellagic acid supplementation significantly reduced chemotherapeutic neutropenia. A trend in serum prostate specific antigen reduction (>75%) was observed in the supplementation group, but there was no significant difference between each group. No significant adverse effects caused by ellagic acid supplementation were found. It is possible that ellagic acid reduces the effect of chemotherapy-induced toxicities; however, further studies are required.
Coumarin
Coumarins are benzopyrones that have antioxidant and anti-inflammatory effects.80 It is suggested that antioxidants exert their protective effect against cancer by inhibiting the formation of carcinogenic metabolites.81 However, some studies have reported that coumarin supplements cause liver toxicity.82,83 In this study, we found 1 RCT using coumarin supplementation, which did not present results about the survival rate or clinical response.
Grotz et al84 performed a double-blind and placebo RCT and reported that the combination of coumarin and troxerutin supplementation reduced the toxicity of radiation in patients with HNC. They reported no severe adverse effects caused by coumarin supplementation. However, there was only a single RCT using coumarin, and further RCTs are required to determine its safety and efficacy as a supplement.
Curcumin
Curcumin, a polyphenol contained in turmeric, has potent antioxidant, anticancer,85 and anti-inflammatory effects.86,87 Some clinical trials have revealed that curcumin lowered the toxicity of pancreatic cancer treatment, although diarrhea was an adverse effect in some.88
In this study, we found 1 RCT using curcumin supplementation, with no results concerning the survival rate or clinical response. Ryan et al87 performed a double-blind and placebo-controlled RCT for breast cancer patients. They reported that curcumin relieved radiation skin toxicities, without causing any severe adverse effects. Other trials88-90 were not categorized as RCTs.
Amino Acids and Related Substances
Recently, the antioxidant efficacy of certain amino acids and related substances—namely glutathione, N-acetylcysteine, methionine, albumin, lactoferrin, and arginine—has attracted attention. The results of the research and trial details are shown in Table 3 and Supplementary Table 1.
Table 3.
List of Trials Using Antioxidant Amino Acids and Related Compounds.a
| Results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Reference | Design | Cancer | Participants (n) | Antioxidants | Chemotherapy | Radiation | End point | Results (Representative) | Survival/tumor size |
| 35 | Lin, PC. (2006) 92 | Placebo-controlled RCT | stage III colorectal cancer patients with N2 disease | 14 | N-acetylcysteine | oxaliplatin (85 mg/m2) biweekly 5-FU (425 mg/m2) weekly leucovorin (20 mg/m2) |
chemotherapy-induced neurotoxicity | ● NCI-CTC at 8 cycles : p = 0.038 at 12 cycles : p = 0.01 vs. placebo ● Electrophysiologic evaluations N.S between each group |
N.D | |
| 36 | Heys, SD. (1998) 95 | Double-blind and placebo controlled trial | primary breast cancers | 96 | L-arginine | doxorubicin cyclophosphamide vincristine prednisolone (6 pulse 21-day interval) |
clinical and pathological response | pathological response ↑ in L-arginine group (tumors less than 6 cm in initial diameter) p = 0.04 vs. placebo | ||
Abbreviations: CTC, common toxicity criteria; NCI, National Cancer Institute; RCT, randomized controlled clinical trial.
Table indicates the details of each RCT: design, cancer, participants, antioxidants, chemotherapy, radiation, end point, and results (survival/tumor size).
N-Acetylcysteine
N-Acetylcysteine supplementation can increase whole blood concentrations of glutathione,91 which is a tripeptide associated with reduced chemotherapy-induced neurotoxicity.92 N-acetylcysteine has not been as well studied as other amino acids. Lin et al92 performed a placebo-controlled RCT for colorectal cancer patients. They did not indicate the results of survival rates or clinical response. They investigated the efficacy of reducing chemotherapy-induced toxicity. Their results showed that N-acetylcysteine significantly reduced chemotherapy-induced neurotoxicity. They reported no severe adverse effects caused by N-acetylcysteine supplementation. However, there was only 1 RCT using N-acetylcysteine, and further RCTs are required to determine its safety and efficacy as a supplement.
L-Arginine
l-Arginine is the biological precursor of endogenous nitric oxide, which is a potent vasodilator and key for immunological functions.93 It also regulates antioxidant-related signaling molecule expression.94
Heys et al95 conducted a double-blind, placebo-controlled RCT for patients with primary breast cancer. They indicated that l-arginine supplementation significantly increased the pathological response to chemotherapy using doxorubicin, cyclophosphamide, vincristine, and prednisolone in the supplementation group. However, they did not investigate survival rates or clinical response data. They reported no severe adverse effects caused by l-arginine supplementation. However, there was only 1 RCT using l-arginine, and further RCTs are required to determine its safety and efficacy as a supplement.
Minerals
Some minerals chelate substances, thereby exerting an antioxidant effect. These minerals were reported to be susceptible to oxidation-reduction reactions.7-10,96-100 In this study, chromium, transferrin (ferritin), selenium, manganese, molybdenum, and zinc met our inclusion criteria. The results of the research and trial details are shown in Table 4 and Supplementary Table 1.
Selenium
Selenium has been shown to possess cancer-preventive and cytoprotective qualities.100 It affects a wide range of biological processes, including energy metabolism and membrane integrity (by acting as an antioxidant), as well as protecting against DNA damage, exerting anti-inflammatory effects, and regulating the production of active thyroid hormone.100,101
Based on these capabilities, some clinical trials were performed with cancer patients during chemotherapy or radiotherapy. We found 6 results (4 chemotherapy, 2 radiotherapy) for selenium use during cancer therapy. No significant adverse effects of selenium supplementation were found. None of the selected trials reported survival rates or clinical response data, but a follow-up study assessed survival 6 years after 1 trial.101,102 The study reported that there was no significant difference in 6-year survival between groups.102
Buntzel et al103 and Muecke et al101 performed RCTs for patients undergoing radiotherapy for HNC, and cervical and endometrial cancer. They concluded that selenium supplements reduced the number of episodes and the severity of radiotoxicities, such as dysphagia and diarrhea.
Hu et al104 and Sieja and Talerczyk105 performed RCTs using placebo controls for patients with many cancer types undergoing chemotherapy using cisplatin. They concluded that selenium supplements reduced the nephrotoxicity and neurotoxicity of cisplatin. Elsendoorn et al106 and Weijl et al107 conducted a double-blind and placebo RCT for patients with many cancer types undergoing chemotherapy with cisplatin. They used a combined supplement of selenium, vitamin C, and vitamin E. In contrast to the result of Hu et al104 and Sieja and Talerczyk,105 they found no significant difference between trial groups regarding chemotherapy-induced organ toxicity.
Zinc
Zinc, a major dietary antioxidant, protects the airway epithelium against oxyradicals and other noxious substances. Zinc, therefore, has important implications for asthma and other inflammatory diseases, where the physical barrier is vulnerable and compromised.108 Some studies used zinc supplements to study its effect on maintaining and priming the immune system and tissue repair. There were 7 RCTs in this study, none of which provided data regarding survival rates or clinical responses. Most of them reported no severe adverse effects caused by zinc supplementation.
Sangthawan et al109 and Ripamonti et al110 performed RCTs using placebo controls for patients undergoing radiotherapy for HNC. They concluded that compared with placebo controls, zinc supplementation was not significantly effective in relieving radiotoxicities such as taste alterations, weight loss, nausea, and vomiting.
Similarly, Lyckholm et al111 found that zinc supplementation did not significantly improve chemotoxicity in terms of altered taste and smell. In addition, Ertekin et al112-114 and Halyard et al115 conducted an RCT for patients undergoing radiotherapy for HNC. They reported that zinc supplementation was effective in preventing opportunistic bacterial infections and radiotherapy-induced oropharyngeal mucositis and promoting antioxidant enzyme activities. Although the 3 articles of Ertekin et al112-114 reported on the same trial, we included all of them because the outcome variables presented in each article were different.
Discussion
Survival/Clinical Response
In 17 articles23,36,40-42,45,46,63-69,79,95,107 of 49 (approximately 35%) included in this study, effects on patient survival or clinical response during chemotherapy and/or radiotherapy were described. In 7 of 17 RCTs using melatonin supplementation, 4 reported a significantly increased survival rate and 4 reported a significantly increased the tumor regression rate. In addition, 1 trial each using vitamin A36 and multiple vitamins42 reported a significantly increased survival rate. On the other hand, 2 trials using multiple vitamins40,41 reported that there were no significant differences between supplementation and control groups, although the survival rates of the supplementation groups were slightly lower than those of the control groups. Two trials using vitamin E45,46 reported that there was no significant difference in clinical response between groups. Also, the RCT using ellagic acid reported that there were no significant differences between groups in survival rate and clinical response.79 Similarly, a trial using l-arginine supplementation reported a significant increase in the pathological response.95 On the other hand, a trial using combination supplement (vitamins C and E and selenium)107 reported that there was no significant difference in clinical response. In addition to our 49 results, Muecke et al102 performed a follow-up study of their 2013 report.101 They reported that there were no differences between groups in 6-year survival rate using selenium supplementation during radiation.
We list anticancer agents by class and whether they produce ROS in Supplementary Table 2 (available at http://ict.sagepub.com/supplemental).116 From the results of this review, there might be a possibility that potent antioxidant supplementation could reduce therapeutic effects of radiotherapy or chemotherapy using alkylating agents, platinum compounds, or anthracycline. These agents exert a therapeutic effect by generating active oxygen. Compared with regular doses (6-8 mg/d) as sleep aid, high-dose melatonin supplementation (20-40 mg/d dose) was administered in all trials in this review. In the case of melatonin and also for vitamin C, higher dose or stronger antioxidants might protect not only normal cells from ROS-generating therapies, but might also protect cancer cells themselves by helping them proliferate. On the other hand, it is also known that a higher dose of antioxidant can function as a pro-oxidant in cancer cells,99,117 suggesting that high-dose antioxidants might augment effects of ROS-generating therapies. Lower-dose antioxidant supplementation may protect normal cells and reduce the toxicity of radiation and chemotherapy.42 However, it is difficult to demonstrate either positive or negative effects of antioxidant supplementation on patient survival and growth inhibition of cancer cells in the studies reviewed in this article.
However, a negative effect was demonstrated by Meyer et al,42 studying HNC patients who were undergoing radiotherapy and who also smoked. Their data indicated that α-tocopherol and β-carotene supplementation with radiotherapy significantly increased recurrence and mortality in patients who smoked during radiation therapy, although not in nonsmoking patients. In evaluating the negative results of that study, 2 factors were suggested to interact with each other. The first was that antioxidant supplementation scavenged free radicals and reduced the damage caused by ionizing radiations. The second was that the carbon monoxide in cigarette smoke increased the blood carboxyhemoglobin,118 and oxygen transport abilities were adversely affected. This might have led to the proliferation of cancer cells that were resistant to hypoxia. It is known that carbon monoxide has 200 to 300 times higher affinity for hemoglobin than oxygen. It is difficult to generate ROS in a state of oxygen deficiency. Furthermore, cancer cells can adapt to hypoxia,119 which results in invasion, metastasis, and angiogenesis.120 Cancer cells stop growing during hypoxic cell cycle arrest, making any treatment less effective. It is, therefore, suggested that a combination of smoking with the consumption of a strong antioxidant during radiotherapy may create a favorable condition for cancer growth, resulting in lower survival rates.
Reduction of Adverse Effects: Chemotherapy
Among the 49 studies, 46 examined the reduction of adverse effects by antioxidant supplementation. In 34 trials, possible reductions in chemotoxicities or radiotoxicities using antioxidant supplementation were reported. On the other hand, only 1 RCT, using vitamin A, reported that supplementation possibly increased chemoinduced toxicities. The remaining 11 studies displayed no difference in toxicities between control and supplementation groups.
Chemotherapy and radiotherapy cause various adverse effects, which may in part be caused by free radicals and ROS.121 ROS generation causes various tissue or organ injuries122: doxorubicin and other anthracycline antibiotics are known to lead to cardiotoxicity123; cisplatin and other platinums lead to nephrotoxicity, ototoxicity, and peripheral neuropathy124,125; bleomycin leads to lung injury126; and alkylating agents cause DNA damage of drug-treated cells.8,127 Carcinogenesis may also occur as the result of tissue or organ injuries.122
In 18 RCTs in which platinum was used as the therapeutic agent, the effects of melatonin,61,63-65,67-69 selenium,104-107 and vitamin41,43,46,47,49-51 supplementation on chemotoxicities were reported. Among 7 trials using melatonin, 6 reported that melatonin supplementation significantly improved myelosuppression, weight loss, and neurotoxicity. Selenium supplementation was used in 4 trials. In 2 trials selenium supplementation was reported to be significantly effective for nephrotoxicity and QOL. Vitamins were used in 7 RCTs, showing a significant improvement of QOL in 1 trial and a significant decrease of various chemoinduced toxicities in 5 trials. Similarly, we found 7 RCTs studying the effects of melatonin63-66,68,69 or vitamin E48 on relieving toxicity of plant alkaloid–based chemotherapy, although these regimens were not considered to exert cytotoxicity by generating free radicals. All of them reported that melatonin or vitamin E supplementation was significantly effective in reducing toxicities. Furthermore, we found 5 studies26,36,69,105 that researched the interaction between antioxidants and alkylating chemotherapy (cyclophosphamide). In 3 trials26,69,105 out of 5 using cyclophosphamide regimens, there was a significant effect of antioxidants on chemoinduced toxicity. One trial36 using busulfan reported improvement in chemoinduced toxicities for more patients in the vitamin A supplementation group than in controls.
As described above, a significant relief in chemotherapy-induced toxicities was reported in many trials using various antioxidant supplements. However, among trials in which the same combination of chemotherapeutic agent and antioxidant was used, some reported effective outcomes, whereas others did not. This could be considered to be dependent on the dosage and/or patient’s background rather than on a scale difference of the trials.
Reduction of Adverse Effects: Radiation
In total, 19 radiotoxicity prevention trials were investigated, which specifically aimed to reduce toxicities affecting the mucosa, skin, salivary glands, and taste. Four of 19 trials reported no significant differences in toxicity between groups. Antioxidant supplements such as vitamin E,45,52 multivitamin combination,40,42,43 polyphenol,84,87 and zinc109-115 were effective in preventing radiation-induced toxicities in the skin, mucosa, and salivary glands.
Clinical Use of Antioxidant Supplements
Regarding the conflicting issues related to antioxidant use in cancer chemotherapy or radiotherapy, although a large, well-designed review of the relationship between mitigating effects of antioxidants and oxidative stress caused by anticancer agents is warranted, various aspects of the relationship between cancer and antioxidants have already been investigated. Using animal and in vitro experiments, Chandel and Tuveson128 demonstrated that antioxidants do not prevent cancer and may accelerate tumor development by targeting ROS in the cell. They caution that antioxidant supplementation should be carefully utilized in cancer patients undergoing concurrent cancer therapy. It may be highly arguable whether cancer patients during cancer therapy should freely use antioxidants.
Finally, the efficacy of each combination of anticancer agent with antioxidant supplement requires adequate verification. In the present research, there were no investigations in which the study drug proliferated the growth of cancer and increased mortality. However, if we want to use antioxidant supplements as CAM for cancer patients, further investigations are required for each and every combination of cancer, dietary supplement, and therapy. Unsupervised use of supplements should be avoided.129
One limitation of our review is that we included some trials with low Jadad scores. In most systematic reviews, trials scoring more than 3 are included. In our selected 49 studies, there were 23 trials that scored more than 3. However, we felt that Jadad scores themselves had limitations in describing study quality. Some studies met our inclusion criteria even though they had low Jadad scores. We have, therefore, included all studies but described them in detail in Tables 1 to 4 to allow the reader to judge study quality. With regard to statistical data integration, it is difficult to perform a meta-analysis with this set of studies because of variability of the data available for each antioxidant. Thus, data were compiled without statistical analysis.
To examine the viability and safety of antioxidants in pathological conditions and cancer therapy, trials should be performed with a single regimen, single type of cancer, and single antioxidant. Only such investigations would adequately describe the safety and effectiveness of antioxidant use by cancer patients during therapy. However, in our research, only 2 trials that met those conditions reported survival rate or tumor development. Similarly, only 5 trials that met those conditions reported effects on chemotoxicities or radiotoxicities. Therefore, we are unable to judge the effectiveness and safety of antioxidants definitively.
In conclusion, it was difficult to determine whether antioxidants may have an impact on treatment outcomes or whether they may ameliorate adverse effects of chemotherapy and radiotherapy. Discussion of antioxidant use has sometimes distinguished palliative versus curative regimens. For curative regimens, it is important not to inhibit therapy in any way, and patients are usually in better overall condition to tolerate side effects. In palliative or recurrent settings, however, patients are less able to tolerate side effects, and cytotoxic efficacy may be less of a concern than maintaining the patient in treatment. Basically, stable disease is acceptable in this situation if side effects can be managed. Thus, it is important that clinicians make an integrated decision, taking into account the following: (1) the antioxidant dosage and types, (2) the background and state of the patient, and (3) type of cancer and antitumor therapy.130 It is desirable to use an evidence-based method to select supplements best suited to cancer patients. Although there are many opinions about the risks or benefits of antioxidant supplementation, the only supportable conclusions based on the present research are that it is difficult to demonstrate definitively that antioxidants ameliorate therapeutic toxicities and that there is no evidence of antioxidant supplementation causing harm alongside cancer therapy, except for smokers undergoing radiotherapy.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ito T, Urushima H, Sakaue M, et al. Reduction of adverse effects by a mushroom product, active hexose correlated compound (AHCC) in patients with advanced cancer during chemotherapy: the significance of the levels of HHV-6 DNA in saliva as a surrogate biomarker during chemotherapy. Nutr Cancer. 2014;66:377-382. [DOI] [PubMed] [Google Scholar]
- 2. Hyodo I, Amano N, Eguchi K, et al. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol. 2005;23:2645-2654. [DOI] [PubMed] [Google Scholar]
- 3. Ziech D, Franco R, Georgakilas AG, et al. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem Biol Interact. 2010;188:334-339. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs-Tarlovsky V. Role of antioxidants in cancer therapy. Nutrition. 2013;29(1):15-21. [DOI] [PubMed] [Google Scholar]
- 5. Lamson DW, Brignall MS. Antioxidants in cancer therapy: their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304-329. [PubMed] [Google Scholar]
- 6. Labriola D, Livingston R. Possible interactions between dietary antioxidants and chemotherapy. Oncology (Williston Park). 1999;13:1003-1008, discussion 1008, 1011-1002. [PubMed] [Google Scholar]
- 7. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842-857. [DOI] [PubMed] [Google Scholar]
- 8. Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007;33:407-418. [DOI] [PubMed] [Google Scholar]
- 9. National Cancer Institute. Antioxidants and Cancer Prevention: Fact Sheet. Bethesda, MD: National Cancer Institute; Accessed September 28, 2015 Website. http://www.cancer.gov/about-cancer/causes-prevention/risk/diet/antioxidants-fact-sheet [Google Scholar]
- 10. Abrams DI, Weil A. Integrative Oncology. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- 11. Weisburger JH. Nutritional approach to cancer prevention with emphasis on vitamins, antioxidants, and carotenoids. Am J Clin Nutr. 1991;53(1, suppl):S226-S237. [DOI] [PubMed] [Google Scholar]
- 12. Houston MC. Treatment of hypertension with nutraceuticals, vitamins, antioxidants and minerals. Expert Rev Cardiovasc Ther. 2007;5:681-691. [DOI] [PubMed] [Google Scholar]
- 13. Mangge H, Becker K, Fuchs D, Gostner JM. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crane FL, Sun IL, Sun EE. The essential functions of coenzyme Q. Clin Investig. 1993;71(8, suppl):S55-S59. [DOI] [PubMed] [Google Scholar]
- 15. Nohl H, Gille L, Kozlov AV. Critical aspects of the antioxidant function of coenzyme Q in biomembranes. Biofactors. 1999;9:155-161. [DOI] [PubMed] [Google Scholar]
- 16. Premkumar VG, Yuvaraj S, Sathish S, Shanthi P, Sachdanandam P. Anti-angiogenic potential of coenzyme Q10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vascul Pharmacol. 2008;48:191-201. [DOI] [PubMed] [Google Scholar]
- 17. Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53:764-770. [DOI] [PubMed] [Google Scholar]
- 18. Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure: CoQ10 Drug Surveillance Investigators. Mol Aspects Med. 1994;15(suppl):S287-S294. [DOI] [PubMed] [Google Scholar]
- 19. Greenlee H, Shaw J, Lau YK, Naini A, Maurer M. Lack of effect of coenzyme q10 on doxorubicin cytotoxicity in breast cancer cell cultures. Integr Cancer Ther. 2012;11:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conklin KA. Coenzyme q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther. 2005;4:110-130. [DOI] [PubMed] [Google Scholar]
- 21. Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem Biophys Res Commun. 1997;234:296-299. [DOI] [PubMed] [Google Scholar]
- 22. Folkers K, Shizukuishi S, Takemura K, et al. Increase in levels of IgG in serum of patients treated with coenzyme Q10. Res Commun Chem Pathol Pharmacol. 1982;38:335-338. [PubMed] [Google Scholar]
- 23. Rusciani L, Proietti I, Paradisi A, et al. Recombinant interferon alpha-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: a 3-year trial with recombinant interferon-alpha and 5-year follow-up. Melanoma Res. 2007;17:177-183. [DOI] [PubMed] [Google Scholar]
- 24. Akihama T, Nakamoto Y, Shindo T, Nakayama Y, Miura A. Protective effects of coenzyme Q10 on the adverse reactions of anthracycline antibiotics: using double blind method—with special reference to hair loss. Gan To Kagaku Ryoho. 1983;10:2125-2129. [PubMed] [Google Scholar]
- 25. Okuma K, Furuta I, Ota K. Protective effect of coenzyme Q10 in cardiotoxicity induced by adriamycin. Gan To Kagaku Ryoho. 1984;11:502-508. [PubMed] [Google Scholar]
- 26. Takimoto M, Sakurai T, Kodama K, et al. Protective effect of CoQ 10 administration on cardial toxicity in FAC therapy. Gan To Kagaku Ryoho. 1982;9:116-121. [PubMed] [Google Scholar]
- 27. Iarussi D, Auricchio U, Agretto A, et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med. 1994;15(suppl):S207-S212. [DOI] [PubMed] [Google Scholar]
- 28. Lesser GJ, Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. 2013;11(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977;197:165-167. [DOI] [PubMed] [Google Scholar]
- 30. Tsubaki K, Horiuchi A, Kitani T, et al. Investigation of the preventive effect of CoQ10 against the side-effects of anthracycline antineoplastic agents. Gan To Kagaku Ryoho. 1984;11:1420-1427. [PubMed] [Google Scholar]
- 31. Bold RJ, Ishizuka J, Townsend CM, Jr, Thompson JC. All-trans-retinoic acid inhibits growth of human pancreatic cancer cell lines. Pancreas. 1996;12:189-195. [DOI] [PubMed] [Google Scholar]
- 32. Frey JR, Peck R, Bollag W. Antiproliferative activity of retinoids, interferon alpha and their combination in five human transformed cell lines. Cancer Lett. 1991;57:223-227. [DOI] [PubMed] [Google Scholar]
- 33. Tsurui K, Kosakai Y, Horie T, Awazu S. Vitamin A protects the small intestine from methotrexate-induced damage in rats. J Pharmacol Exp Ther. 1990;253:1278-1284. [PubMed] [Google Scholar]
- 34. Israel L, Hajji O, Grefft-Alami A, et al. Vitamin A augmentation of the effects of chemotherapy in metastatic breast cancers after menopause: randomized trial in 100 patients. Ann Med Interne (Paris). 1985;136:551-554. [PubMed] [Google Scholar]
- 35. Dagdemir A, Yildirim H, Aliyazicioglu Y, Kanber Y, Albayrak D, Acar S. Does vitamin A prevent high-dose-methotrexate-induced D-xylose malabsorption in children with cancer? Support Care Cancer. 2004;12:263-267. [DOI] [PubMed] [Google Scholar]
- 36. Meyskens FL, Jr, Kopecky KJ, Appelbaum FR, Balcerzak SP, Samlowski W, Hynes H. Effects of vitamin A on survival in patients with chronic myelogenous leukemia: a SWOG randomized trial. Leuk Res. 1995;19:605-612. [DOI] [PubMed] [Google Scholar]
- 37. Khor SC, Abdul Karim N, Wan Ngah WZ, Mohd Yusof YA. Vitamin E in sarcopenia: current evidences on its role in prevention and treatment. Oxid Med Cell Longev. 2014;2014:914853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Felemovicius I, Bonsack ME, Baptista ML, Delaney JP. Intestinal radioprotection by vitamin E (alpha-tocopherol). Ann Surg. 1995;222:504-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar KS, Srinivasan V, Toles R, Jobe L, Seed TM. Nutritional approaches to radioprotection: vitamin E. Mil Med. 2002;167(2, suppl):S57-S59. [PubMed] [Google Scholar]
- 40. Bairati I, Meyer F, Gelinas M, et al. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J Clin Oncol. 2005;23:5805-5813. [DOI] [PubMed] [Google Scholar]
- 41. Pathak AK, Bhutani M, Guleria R, et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. J Am Coll Nutr. 2005;24:16-21. [DOI] [PubMed] [Google Scholar]
- 42. Meyer F, Bairati I, Fortin A, et al. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int J Cancer. 2008;122:1679-1683. [DOI] [PubMed] [Google Scholar]
- 43. Fuchs-Tarlovsky V, Bejarano-Rosales M, Gutierrez-Salmean G, Casillas MA, Lopez-Alvarenga JC, Ceballos-Reyes GM. Effect of antioxidant supplementation over oxidative stress and quality of life in cervical cancer. Nutr Hosp. 2011;26:819-826. [DOI] [PubMed] [Google Scholar]
- 44. Suhail N, Bilal N, Khan HY, et al. Effect of vitamins C and E on antioxidant status of breast-cancer patients undergoing chemotherapy. J Clin Pharm Ther. 2012;37:22-26. [DOI] [PubMed] [Google Scholar]
- 45. Ferreira PR, Fleck JF, Diehl A, et al. Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: a double-blind randomized trial. Head Neck. 2004;26:313-321. [DOI] [PubMed] [Google Scholar]
- 46. Pace A, Savarese A, Picardo M, et al. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. J Clin Oncol. 2003;21:927-931. [DOI] [PubMed] [Google Scholar]
- 47. Kottschade LA, Sloan JA, Mazurczak MA, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Support Care Cancer. 2011;19:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Argyriou AA, Chroni E, Koutras A, et al. Preventing paclitaxel-induced peripheral neuropathy: a phase II trial of vitamin E supplementation. J Pain Symptom Manage. 2006;32:237-244. [DOI] [PubMed] [Google Scholar]
- 49. Argyriou AA, Chroni E, Koutras A, et al. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: final results. Support Care Cancer. 2006;14:1134-1140. [DOI] [PubMed] [Google Scholar]
- 50. Pace A, Giannarelli D, Galie E, et al. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology. 2010;74:762-766. [DOI] [PubMed] [Google Scholar]
- 51. Wadleigh RG, Redman RS, Graham ML, Krasnow SH, Anderson A, Cohen MH. Vitamin E in the treatment of chemotherapy-induced mucositis. Am J Med. 1992;92:481-484. [DOI] [PubMed] [Google Scholar]
- 52. Chitra S, Shyamala Devi CS. Effects of radiation and alpha-tocopherol on saliva flow rate, amylase activity, total protein and electrolyte levels in oral cavity cancer. Indian J Dent Res. 2008;19:213-218. [DOI] [PubMed] [Google Scholar]
- 53. Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release. 2006;113:189-207. [DOI] [PubMed] [Google Scholar]
- 54. Halperin EC, Gaspar L, George S, Darr D, Pinnell S. A double-blind, randomized, prospective trial to evaluate topical vitamin C solution for the prevention of radiation dermatitis: CNS Cancer Consortium. Int J Radiat Oncol Biol Phys. 1993;26:413-416. [DOI] [PubMed] [Google Scholar]
- 55. Al-Delaimy WK, Ferrari P, Slimani N, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr. 2005;59:1387-1396. [DOI] [PubMed] [Google Scholar]
- 56. Schabath MB, Grossman HB, Delclos GL, et al. Dietary carotenoids and genetic instability modify bladder cancer risk. J Nutr. 2004;134:3362-3369. [DOI] [PubMed] [Google Scholar]
- 57. Ansari MS, Gupta NP. Lycopene: a novel drug therapy in hormone refractory metastatic prostate cancer. Urol Oncol. 2004;22:415-420. [DOI] [PubMed] [Google Scholar]
- 58. Guttenplan JB, Chen M, Kosinska W, Thompson S, Zhao Z, Cohen LA. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett. 2001;164:1-6. [DOI] [PubMed] [Google Scholar]
- 59. Fuchs-Tarlovsky V, Rivera MA, Altamirano KA, Lopez-Alvarenga JC, Ceballos-Reyes GM. Antioxidant supplementation has a positive effect on oxidative stress and hematological toxicity during oncology treatment in cervical cancer patients. Support Care Cancer. 2013;21:1359-1363. [DOI] [PubMed] [Google Scholar]
- 60. Maestroni GJ, Covacci V, Conti A. Hematopoietic rescue via T-cell-dependent, endogenous granulocyte-macrophage colony-stimulating factor induced by the pineal neurohormone melatonin in tumor-bearing mice. Cancer Res. 1994;54:2429-2432. [PubMed] [Google Scholar]
- 61. Ghielmini M, Pagani O, de Jong J, et al. Double-blind randomized study on the myeloprotective effect of melatonin in combination with carboplatin and etoposide in advanced lung cancer. Br J Cancer. 1999;80:1058-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186-195. [DOI] [PubMed] [Google Scholar]
- 63. Lissoni P. Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol Biol (Paris). 2007;55:201-204. [DOI] [PubMed] [Google Scholar]
- 64. Lissoni P, Chilelli M, Villa S, Cerizza L, Tancini G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003;35:12-15. [DOI] [PubMed] [Google Scholar]
- 65. Lissoni P, Tancini G, Barni S, et al. Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Support Care Cancer. 1997;5:126-129. [DOI] [PubMed] [Google Scholar]
- 66. Lissoni P, Barni S, Mandala M, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 1999;35:1688-1692. [DOI] [PubMed] [Google Scholar]
- 67. Cerea G, Vaghi M, Ardizzoia A, et al. Biomodulation of cancer chemotherapy for metastatic colorectal cancer: a randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticancer Res. 2003;23:1951-1954. [PubMed] [Google Scholar]
- 68. Lissoni P. Biochemotherapy with immunomodulating pineal hormones other than melatonin: 5-methoxytryptamine as a new oncostatic pineal agent. Pathol Biol (Paris). 2007;55:198-200. [DOI] [PubMed] [Google Scholar]
- 69. Lissoni P, Paolorossi F, Ardizzoia A, et al. A randomized study of chemotherapy with cisplatin plus etoposide versus chemoendocrine therapy with cisplatin, etoposide and the pineal hormone melatonin as a first-line treatment of advanced non-small cell lung cancer patients in a poor clinical state. J Pineal Res. 1997;23:15-19. [DOI] [PubMed] [Google Scholar]
- 70. Karasek M, Winczyk K. Melatonin in humans. J Physiol Pharmacol. 2006;57(suppl 5):19-39. [PubMed] [Google Scholar]
- 71. Ibrahimpasic K. Alpha lipoic acid and glycaemic control in diabetic neuropathies at type 2 diabetes treatment. Med Arch. 2013;67:7-9. [DOI] [PubMed] [Google Scholar]
- 72. Guo Y, Jones D, Palmer JL, et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial. Support Care Cancer. 2014;22:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim J, Cho HJ, Sagong B, et al. Alpha-lipoic acid protects against cisplatin-induced ototoxicity via the regulation of MAPKs and proinflammatory cytokines. Biochem Biophys Res Commun. 2014;449:183-189. [DOI] [PubMed] [Google Scholar]
- 74. Pantuck AJ, Leppert JT, Zomorodian N, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018-4026. [DOI] [PubMed] [Google Scholar]
- 75. Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75-100. [DOI] [PubMed] [Google Scholar]
- 76. Ertam I, Mutlu B, Unal I, Alper S, Kivcak B, Ozer O. Efficiency of ellagic acid and arbutin in melasma: a randomized, prospective, open-label study. J Dermatol. 2008;35:570-574. [DOI] [PubMed] [Google Scholar]
- 77. Amakura Y, Umino Y, Tsuji S, et al. Constituents and their antioxidative effects in eucalyptus leaf extract used as a natural food additive. Food Chem. 2002;77:47-56. [Google Scholar]
- 78. Smith WA, Gupta RC. Determining efficacy of cancer chemopreventive agents using a cell-free system concomitant with DNA adduction. Mutat Res. 1999;425:143-152. [DOI] [PubMed] [Google Scholar]
- 79. Falsaperla M, Morgia G, Tartarone A, Ardito R, Romano G. Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. Eur Urol. 2005;47:449-454, discussion 454-455. [DOI] [PubMed] [Google Scholar]
- 80. Witaicenis A, Seito LN, da Silveira Chagas A, et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. 2014;21:240-246. [DOI] [PubMed] [Google Scholar]
- 81. Rahimtula AD, Zachariah PK, O’Brien PJ. The effects of antioxidants on the metabolism and mutagenicity of benzo[a]pyrene in vitro. Biochem J. 1977;164:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Loprinzi CL, Kugler JW, Sloan JA, et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med. 1999;340:346-350. [DOI] [PubMed] [Google Scholar]
- 83. Marshall ME, Butler K, Fried A. Phase I evaluation of coumarin (1,2-benzopyrone) and cimetidine in patients with advanced malignancies. Mol Biother. 1991;3:170-178. [PubMed] [Google Scholar]
- 84. Grotz KA, Wustenberg P, Kohnen R, et al. Prophylaxis of radiogenic sialadenitis and mucositis by coumarin/ troxerutine in patients with head and neck cancer: a prospective, randomized, placebo-controlled, double-blind study. Br J Oral Maxillofac Surg. 2001;39:34-39. [DOI] [PubMed] [Google Scholar]
- 85. Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappa B-regulated gene products. Cancer Res. 2007;67:3853-3861. [DOI] [PubMed] [Google Scholar]
- 86. Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1-75. [DOI] [PubMed] [Google Scholar]
- 87. Ryan JL, Heckler CE, Ling M, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013;180:34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kanai M, Yoshimura K, Asada M, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157-164. [DOI] [PubMed] [Google Scholar]
- 89. Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137-1141. [DOI] [PubMed] [Google Scholar]
- 90. Bayet-Robert M, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8-14. [DOI] [PubMed] [Google Scholar]
- 91. Kasperczyk S, Dobrakowski M, Kasperczyk A, Ostalowska A, Birkner E. The administration of N-acetylcysteine reduces oxidative stress and regulates glutathione metabolism in the blood cells of workers exposed to lead. Clin Toxicol (Phila). 2013;51:480-486. [DOI] [PubMed] [Google Scholar]
- 92. Lin PC, Lee MY, Wang WS, et al. N-acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Support Care Cancer. 2006;14:484-487. [DOI] [PubMed] [Google Scholar]
- 93. Ralph AP, Waramori G, Pontororing GJ, et al. l-Arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8:e70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang B, Liu Y, Feng L, et al. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015;167:91-99. [DOI] [PubMed] [Google Scholar]
- 95. Heys SD, Ogston K, Miller I, et al. Potentiation of the response to chemotherapy in patients with breast cancer by dietary supplementation with L-arginine: results of a randomised controlled trial. Int J Oncol. 1998;12:221-225. [DOI] [PubMed] [Google Scholar]
- 96. Arai S, Abe K, Kanazawa K, Yoshikawa T, Watanabe S. Encyclopedia of Functional Food. Tokyo, Japan: Asakura; 2007. [Google Scholar]
- 97. Masayuki Y, Osamu M. Development of Medicinal Foods. Tokyo, Japan: CMC; 2007. [Google Scholar]
- 98. Inoue M. Active Oxygen and Eat Well and to Be Well. Tokyo, Japan: Kyoritu Shuppan; 1996. [Google Scholar]
- 99. Zubair H, Khan HY, Sohail A, et al. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: putative anticancer mechanism of antioxidants. Cell Death Dis. 2013;4:e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rayman MP. Selenium and human health. Lancet. 2012;37:1256-1268. [DOI] [PubMed] [Google Scholar]
- 101. Muecke R, Micke O, Schomburg L, et al. Impact of treatment planning target volumen (PTV) size on radiation induced diarrhoea following selenium supplementation in gynecologic radiation oncology: a subgroup analysis of a multicenter, phase III trial. Radiat Oncol. 2013;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Muecke R, Micke O, Schomburg L, et al. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology: follow-up analysis of the survival data 6 years after cessation of randomization. Integr Cancer Ther. 2014;13:463-467. [DOI] [PubMed] [Google Scholar]
- 103. Buntzel J, Riesenbeck D, Glatzel M, et al. Limited effects of selenium substitution in the prevention of radiation-associated toxicities: results of a randomized study in head and neck cancer patients. Anticancer Res. 2010;30:1829-1832. [PubMed] [Google Scholar]
- 104. Hu YJ, Chen Y, Zhang YQ, et al. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol Trace Elem Res. 1997;56:331-341. [DOI] [PubMed] [Google Scholar]
- 105. Sieja K, Talerczyk M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol Oncol. 2004;93:320-327. [DOI] [PubMed] [Google Scholar]
- 106. Elsendoorn TJ, Weijl NI, Mithoe S, et al. Chemotherapy-induced chromosomal damage in peripheral blood lymphocytes of cancer patients supplemented with antioxidants or placebo. Mutat Res. 2001;4:145-158. [DOI] [PubMed] [Google Scholar]
- 107. Weijl NI, Elsendoorn TJ, Lentjes EG, et al. Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: a randomised, double-blind, placebo-controlled study. Eur J Cancer. 2004;40:1713-1723. [DOI] [PubMed] [Google Scholar]
- 108. Truong-Tran AQ, Carter J, Ruffin R, Zalewski PD. New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol. 2001;79:170-177. [DOI] [PubMed] [Google Scholar]
- 109. Sangthawan D, Phungrassami T, Sinkitjarurnchai W. A randomized double-blind, placebo-controlled trial of zinc sulfate supplementation for alleviation of radiation-induced oral mucositis and pharyngitis in head and neck cancer patients. J Med Assoc Thai. 2013;96:69-76. [PubMed] [Google Scholar]
- 110. Ripamonti C, Zecca E, Brunelli C, et al. A randomized, controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer. 1998;82:1938-1945. [DOI] [PubMed] [Google Scholar]
- 111. Lyckholm L, Heddinger SP, Parker G, et al. A randomized, placebo controlled trial of oral zinc for chemotherapy-related taste and smell disorders. J Pain Palliat Care Pharmacother. 2012;26:111-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ertekin MV, Koc M, Karslioglu I, Sezen O, Taysi S, Bakan N. The effects of oral zinc sulphate during radiotherapy on anti-oxidant enzyme activities in patients with head and neck cancer: a prospective, randomised, placebo-controlled study. Int J Clin Pract. 2004;58:662-668. [DOI] [PubMed] [Google Scholar]
- 113. Ertekin MV, Uslu H, Karslioglu I, Ozbek E, Ozbek A. Effect of oral zinc supplementation on agents of oropharyngeal infection in patients receiving radiotherapy for head and neck cancer. J Int Med Res. 2003;31:253-266. [DOI] [PubMed] [Google Scholar]
- 114. Ertekin MV, Koc M, Karslioglu I, Sezen O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys. 2004;58:167-174. [DOI] [PubMed] [Google Scholar]
- 115. Halyard MY, Jatoi A, Sloan JA, et al. Does zinc sulfate prevent therapy-induced taste alterations in head and neck cancer patients? results of phase III double-blind, placebo-controlled trial from the North Central Cancer Treatment Group (N01C4). Int J Radiat Oncol Biol Phys. 2007;67:1318-1322. [DOI] [PubMed] [Google Scholar]
- 116. DeVita VT, Lawrence ST, Rosenberg SA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 117. Wilson MK, Baguley BC, Wall C, Jameson MB, Findlay MP. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac J Clin Oncol. 2014;10:22-37. [DOI] [PubMed] [Google Scholar]
- 118. van Staden SR, Groenewald M, Engelbrecht R, Becker PJ, Hazelhurst LT. Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. S Afr Med J. 2013;103:865-868. [DOI] [PubMed] [Google Scholar]
- 119. Zhang J, Cao J, Ma S, et al. Tumor hypoxia enhances non-small cell lung cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014;5:9664-9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ren T, Zhang W, Liu X, et al. Discoidin domain receptor 2 (DDR2) promotes breast cancer cell metastasis and the mechanism implicates epithelial-mesenchymal transition programme under hypoxia. J Pathol. 2014;234:526-537. [DOI] [PubMed] [Google Scholar]
- 121. Mizutani H. Mechanism of DNA damage and apoptosis induced by anticancer drugs through generation of reactive oxygen species. Yakugaku Zasshi. 2007;127:1837-1842. [DOI] [PubMed] [Google Scholar]
- 122. Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000;37:1-18. [DOI] [PubMed] [Google Scholar]
- 123. Hrelia S, Bordoni A, Angeloni C, Leoncini E, Biagi P. Nutritional interventions to counteract oxidative stress in cardiac cells. Ital J Biochem. 2004;53:157-163. [PubMed] [Google Scholar]
- 124. Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107-121. [DOI] [PubMed] [Google Scholar]
- 125. Jung M, Hotter G, Vinas JL, Sola A. Cisplatin upregulates mitochondrial nitric oxide synthase and peroxynitrite formation to promote renal injury. Toxicol Appl Pharmacol. 2009;234:236-246. [DOI] [PubMed] [Google Scholar]
- 126. Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H. Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice. Am J Respir Cell Mol Biol. 2009;41:661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rockwell S, Sartorelli AC, Tomasz M, Kennedy KA. Cellular pharmacology of quinone bioreductive alkylating agents. Cancer Metastasis Rev. 1993;12:165-176. [DOI] [PubMed] [Google Scholar]
- 128. Chandel NS, Tuveson DA. The promise and perils of antioxidants for cancer patients. N Engl J Med. 2014;371: 177-178. [DOI] [PubMed] [Google Scholar]
- 129. Nakayama A, Alladin KP, Igbokwe O, White JD. Systematic review: generating evidence-based guidelines on the concurrent use of dietary antioxidants and chemotherapy or radiotherapy. Cancer Invest. 2011;29:655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. Am Soc Clin Oncol Educ Book. 2014:e478-e486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.