Abstract
The aim of this study was to investigate expression of cyclin D1, bcl-2, p53, Ki-67 and HER-2 proteins in 14 cases of non-small cell lung cancer and to establish their correlation to classical clinico-pathological findings, and alleged prognostic value to estimate biological potential of tumor. Retrospective pilot study of the surgically treated non-small cell lung cancer biopsy specimen, paraffin embedded, used immu-nohistochemical method to demonstrate expression of cyclin D1, bcl-2, p53, Ki-67 and HER-2. Protein quantification was performed by the semi-quantitative method. Achieved results were correlated with classical clinico-pathological parameters, like tumor size, histological type, differentiation level, presence ofvascular invasion and metastasis in regional lymph nodes. Out of 14 cases of non-small cell lung cancer, squamous cell carcinoma was found in 7 patients, giant cell carcinoma in 3, adenocarcinoma in 2, and 1 case of pleomorphic and mucoepidermoid carcinoma. Expression of cyclin D1 was not found, while expression of HER-2 and bcl-2 protein was established in one cases each. p53 expression was noted in 8 cases (57,1%). Statistically positive significant correlation (p<0,05) was found among: presence of lympho-vascular invasion to tumor tissue and appearance of nodal metastasis; proliferation Ki-67 index and level of tumor differentiation, i.e. size of tumor. Other investigated parameters showed no significant statistically dependence. p53 expression was not correlated to any of the investigated parameters what might imply the possibility that there is an independent pathway of this protein expression. Negative expression of bcl-2 protein points out to possibility that it is not included into process of tumor apoptosis, as well as that proteins cyclin D1 and HER-2 are not included into processes of the tumor genesis. Since the prolif-erative activity of the tumor, measured by the expression of Ki-67, is correlated to the gradus and size of the tumor mass, Ki-67 protein can be of a prognostic value to determine biological potential of non-small cell lung cancer.
Keywords: cyclin D1, bcl-2, p53, Ki-67, HER-2, immunohistochemistry, non-small cell lung cancer
INTRODUCTION
Non-small cell lung cancer up to date has been a subject to many epidemiological, etiological, pathohis-tological and other aspects of investigation. All these investigation demonstrated that in prognosis of this cancer type classical clinico-pathological parameters are no longer sufficient since the proteins, which are products of different types of oncogenes and suppressor genes, dictate biological behavior of neoplasm (1-5). Apoptosis is a genetically programmed cell death regulated by the various gene actions. The cell survival is prolongated and the development of neoplasm is supported by the inhibition of apoptosis via dissregulation of responsible genes (6). Apoptosis can be induced by the various stimuli actions, including p53 gene expression. Proto-oncogene bcl-2 codes protein, which inhibits apoptosis. Apoptosis induced by the p53 gene actions can be blocked by the actions of protein bcl-2. Bcl-2 oncogene and p53 suppressor gene are included in that manner in process of cell death and tumor proliferation. Suppressor gene p53 mutation accompanies cancer of many tissue types (7-10), including non-small cell lung cancer (1-2). Family of bcl-2 protein is present in many solid tumors (11-14). Receptor to human epidermal growth factor HER-2 is cell membrane 185-kd oncoprotein, coded by the c-erb-B2 gene, located on chromosome 17q12-21.32 (15). This receptor shows activity of tyrosine kinase and has a key role in growth regulation, survival and differentation of epithelial cells (16-18). Mutation (amplification) of gene HER-2 leads to increased cells growth and its reproduction, whose final result is a cancer development. Overexpression of HER-2 protein leads to incresed cell growth and thier reproduction, whose final result is cancer development. Overexpression of HER-2 in membranes of tumor cells is a part of malignant transformation and tumor progression processes (20-21). Activation of this receptor stimulates signal trans-duction what leads to increased level of intra-cellular calcium and increased plasma membrane potential what has mitogen effect (22). Nuclear protein Ki-67 is associated protein of cell cycle, which shows expression in all phases of cell cycle, except for resting phase (G0) (23). A number Ki-67 positive cell is a demonstrator of proliferating potential and level of tumor differentiation (23-24). Cyclin D1 is, beside cyclin D2 and D3, member of cyclin D-group, which represent regulating proteins of cell cycle. Cyclin D1 has a key role in control of progression of cell from G1 to S phase of cell cycle. Cyclin D1 is one of most often expressed oncogenes in breast cancer, lung cancer, urinary bladder cancer, head and neck cancer (11-14). Beside classical clinical-pathological parameters-size, histological form, level of tumor differentiation, presence of vascular invasion within tumor and presence of metastasis in regional lymph nodes, also have been the investigated immunohistochemical status of protein cyclin D1, bcl-2, p53, Ki.67 and HER-2, as well as correlation of these proteins with mentioned clinical-pathological parameters, with special review of appearance of metastasis in lymph nodes. Hypothesis is that distribution and intensity of above-mentioned protein can be important factor of differentiation and metastatic potential, and can be of help in prognosis of non-small cell lung cancers.
MATERIAL AND METHODS
Tissue samples of non-small cell lung cancer of 14 patients diagnosed at the Institute for Pathology Faculty of Medicine University of Sarajevo have been investigated in period January 2004 to January 2005. All tissue samples have been fixed in 10% buffered formaldehyde, paraffin embedded, and afterwards cut into 5μ sections, stained with standard hematox-illine eosin (HE) method. Classification and grading of lung cancers was done in accordance to widely accepted international classification recommended by the World Health Organization. Tumor status was determined by the recommendation of American Joint Committee on Cancer (AJCC) in cooperation with TNM Committee of the International Union Against Cancer (UICC). In all cases intrathoracal-mediastinal (paratraheal, prae- and retrotracheal, aortal, subcarinal, perioesophageal and inside lower lung ligament) and intrapulmonal (hilar, peribronchial, interlobar, lobar and segmental) regional lymph nodes were examined. The antibodies specific for Cyclin D1 were used (1:100, DAKO, Glostrup, Denmark), bcl-2 (1:40, DAKO, Glostrup, Denmark), p53 (1:5, DAKO, Glostrup, Denmark), Ki-67 (1:200, DAKO, Glostrup, Denmark) and HercepTest ™ (DAKO, Glostrup, Denmark). Immunohistochemical evaluation of investigated protein expression was performed based on the range and intensity of cell (citoplasmatic or nuclear) positivity. Cy-clin D1 was negative (score 1) in cases when less than 5% tumor cells expressed positivity; it was positive in cases where more than 5% of cells expressed positivity (score 2). Bcl-2 was negative (score 0) in cases where tumor cell shown no nuclear positivity; in cases where 10-50% of cells shown positive staining, it was marked as score 1; score 2 was in cases when more than 51% showed positivity. Protein p53 was negative (score 0) in cases when less than 10 of tumor cells showed nuclear expression of protein, score 1 was marked in cases when 10-30% of tumor cells were stained, score 2 when 3150% of cells were stained; score 3 when more than 50% of cells were stained. Ki-67 expression was negative (score 0) in cases when less than 25% of cells showed nuclear positivity of protein; score 1 in cases when 2650% of cells were positive, score 2 when 51-75% tumor cells showed positivity; score 3 when more than 75% of cells showed positivity. HER-2 expression was calculated as negative (score 0) in cases where there was no membrane staining of tumor cells; as score 1 the cases are calculated when positivity was found in up to 25% of cells; score 2 when 26-50% tumor cells were positive and score 3 when 51-75% of cells were positive; score 4 when more than 75% of cells were stained. The relation of investigated proteins expression and clinical-pathological parameters were analyzed with Mann-Whitney test. Statistical significance was determined up to p<0,05.
RESULTS
Classical clinico-pathological parameters and joint im-munohistochemical expression of investigated proteins is presented at the Table 1.
TABLE 1.
Clinicopathological and immunohistochemical features for 14 non-small cell lung cancer cases.
Among 14 investigated patients with lung cancer, there was 11 men (78,5%) and 3 women (21,5%), age range 32 to 70 years (average age 51,6), out of which 13 (92,86%) was older than 41.
Out of 14 cases of non-small cell lung cancer, squamous cell carcinoma was found in 7 patients (50%), giant cell carcinoma in 3 (21,4%), adenocarcinoma in 2 (14,2%), and 1 case (7,1%) of pleomorphic and mucoepidermoid carcinoma. In 4 cases (28,5%) tumor size was less than 3 cm and surrounded by the lung parenchyma (pT1), in 7 cases (50%) tumor size was larger than 3 cm (pT2), in 2 cases (14,2%) it was a microscopic infiltration of visceral pleura (pT3), and in one case (7,1%) it was bilocular in one lobi (pT4).
In 7 cases (50%) tumor was weakly (G3) and 3 cases (21,4%) moderate (GII) and well differentiated (GI). In 11 cases (78,5%) there was no metastasis in regional lymph nodes. In 3 cases (21,4%) the metastasis were found in regional lymph nodes out of which one case (33,3%) was pN1, and 3 cases (66,6%) were pN2.
Intratumor lymphovascular invasion was determined in 6 cases (42,8%).
Immunohistochemical expression of investigated proteins in each single case of tumor was presented in Table 2.
TABLE 2.
Histolohical types and immunohistochemical characteristics of 14 non-small cells lung cancers.
Cyclin D1 in all cases showed very weak nuclear expression in less than 5% cells (score 1).
Nuclear expression of bcl-2 was established in only one case of moderate differentiated squamous cell carcinoma (score 1).
In most of the cases (42,8%) nuclear expression of protein p53 was negative. In 3 cases (21,4%) positivity was established among 31-50% of tumor cells (score 2), and in 5 cases (35,7%) positivity was established in more than 51% (score 3) tumor cells (Figure 1).
FIGURE 1.
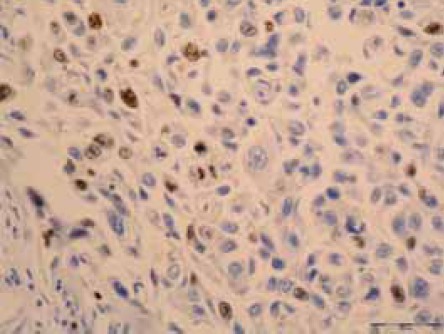
Ki-67 positive nuclear staining in squamous cell carcinoma of lung (IH, X40).
Ki-67 nuclear positivity which is an index of prolifera-tive activity of cells, in 9 cases (62,4%) was easy (score 1), in 2 cases (14,2%) moderate intensity (score 2) (Figure 2) while in 3 cases (21,4%) less than 25% cells showed expression of protein (score 0).
FIGURE 2.
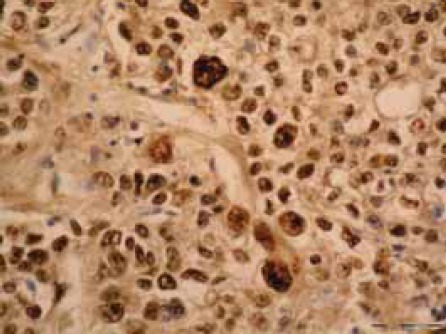
p53 positive nuclear staining in squamous cell carcinoma of lung (IH, X40).
Expression of protein HER-2 was found in only one case (7,5%) moderate differentiated squamous cell cancer (grade II). Statistically positive significant correlation (p<0,05) was found between: presence of lymphovascular invasion in tumor tissue and appearance of nodal metastasis, proliferating Ki-67 index and level of differentiation of tumor that is a size of a tumor. Other investigated parameters did not show mutual dependence.
DISCUSSION
In a pilot study of 14 cases of non-small cell lung cancer expression of cyclin D1, p53, bcl-2, Ki-67 and HER-2 were investigated, which are correlated with classical clinical-pathological parameters, like size and level of differentiation of tumor, presence of lymphovascular invasion in cancer cells of tumor tissue and surrounding tissue, and presence of metastasis in regional lymph nodes. Statistically positive significant correlation (p<0,05) was established between: presence of lymphovascu-lar invasion in tumor tissue and appearance of nodal metastasis; proliferating Ki-67 index and level of tumor differentiation that is a size of tumor. Other investigated parameters showed no mutual dependence. Non-small cell cancer appears in 80% of cases of all lung cancer and almost in every case have a fatal outcome. They present a group of heterogeneous clinical entities, of a different cellular origin, with different trail of molecular events and with different clinical behavior and prognosis. And in cases of early diagnosis and adequate tumor resection, 5-year survival of patient with I stage is about 60-70% of cases. To determine a prognosis for each single patient is very hard, specially having in mind a high potential of different clinical manifestation of primary tumor, site of metastasis appearance and appearance of paraneoplastic syndrome. After the surgery mostly it is hard to predict further outcome of the disease based only on conventional statistical methods and standard clinico-path-ological parameters. Stage of the disease in non-small lung cancer is still the most important feature in patient prognosis. There are other important factors that determine the biology of these tumors, and specially refer to gene who are participating in appearance of metastasis. The role of potentially critical proteins that have undeniable influence to biological behavior of tumors is not widely known and there is a need to define as soon as possible additional, obviously potent prognostic factors that can be of a help to choose the best possible therapy (24). Potentially these factors could be markers of high-risk group of patients in the future what would ask for a different treatment. Some studies of non-small cell lung cancer evaluated actions of different proteins to prognosis but the clinical importance of their expression is still very controversial. Mitsudoni (25) in patients with non-small cell lung cancer points out to bad prognosis in cases of p53 protein expression. Esposito (2) quotes that p53 mutation is not included in forming investigated cases of non-small cell lung cancer. Segawa (3) noticed p53 positivity in cases of higher grade of tumor (II and III), i.e. in cases of more aggressive tumors. In investigating of correlation of protein p53 and survival period 156 patients with non-small cell lung cancer, Lee (26) found positive correlation of investigated parameters. Strong p53 expression in early stage of the disease is linked to significantly worse outcome (27). It is believed that in different types of non-small cell lung cancer p53 has a different role (27). In our investigation appearance of p53 expression of different intensity was established in 8 cases (57,1%), but statistically is not correlated to any of the investigated parameters. This points out to possibility that there is an independent pathway of this protein appearance. Bcl-2 family plays a crucial role in regulation of apoptosis and consequent forming of tumor, as well as appearance of tumor resistance to treatment. Pezzella (28) in his investigation concludes that bcl-2 is not involved in process of apoptosis suppression in cases of non-small cell lung cancer. Neither did Jaren (29) found a correlation between expression of bax, bcl-2 and c-kit in investigated cases of non-small cell lung cancer, and believes that bcl-2 is probably independent in regulation of apop-tosis. As we established bcl-2 expression only in one case (7,1%) we believe that this protein is not likely to be involved in process of tumor apoptosis, what was demonstrated by investigation of some other authors (30). Beside proteins of rhetinoblastoma, cyclin D1 protein acts as a controller of a cell cycle progression in transition from D1 to S step. Observation that expression of this protein in tumor tissue has an important role in development of cancer in human. Cyclin D1 expression is noted in about 50% of cases of non-small cell lung cancer (31). In 60 patients with non-small cell lung cancer who underwent surgery, Caputi (32) found no statistically significant correlation between expression of cyclin D1 and histological grade, status and TNM status of tumor, but has found that these patients have longer survival period. According to his opinion cy-clin D1 expression is common (it was established in all 60 cases) in non-small cell lung cancer and can have a prognostic meaning. Michima (33) in a study of 111 patients with non-small cell lung cancer also finds a longer survival in patients whose tumor showed expression of cyclin D1, what speaks out for participation of this protein in development and progression of tumor, its proliferative activity and clinical behavior. Cyclin D1 was found in cases of vascular invasion and invasion of visceral pleura, chronical obstructive lung diseases and bad tumor differentiation, with emphasize that research is still needed to be done on a large patient groups (34). There are also opposite opinions. In investigating prognostic importance and correlation of cyclin D1 expression and clinical pathological parameters, Keum (35) found abbreviation of survival period, appearance of metastasis in regional lymph nodes and higher histological grade in patients with cyclin D1 posi-tivity of non-small cell lung cancer. In investigated series of non-small cell lung cancer, only one case we noticed expression of cyclin D1 in small percent of tumor cells, and is obvious that this protein was not in investigated cases included in the process of tumor development. HER-2 gene, known as c-erbB-2 or HER-2/neu is protooncogene that codes membrane-linked receptor tyrosine kinase from family of epithelial growth factor receptors (EGFR). It is considered that possible role in proliferation of tumor cells, tumor invasion and metastizing as well as resistance to drugs. In immuno-histochemical study of 88 non-small cell lung cancers, in which a correlation of HER-2 expression and tumor pathology was performed, Ugoscai et al (36) found relatively low concentration of this protein, and believe that it is not justified to include trastuzumab in lung cancer treatment. Some other authors’ experiences (37) are opposite to this and state that HER-2 expression in early stage of tumor points out to its aggression and that relapse period in patients who expressed this protein is shorter. In a study of prognostic meaning of laminin, HER-2 and Ki-67 protein, Szelachowksa (38) founds that patients with high HER-2 expression have shorter survival, while Ki-67 expression has no influence to survival period. In our investigation expression was found in only one case, where less that 25% of cells were positive to HER-2. Nuclear antigen Ki-67 is a protein of cell cycle that is present in all phases of cell growth except for resting phase (G0). The range of proliferative activity of tumor, which points out to its aggresivity, is determined immunohistochemically by numerical value of Ki-67 positive cells. As for above investigated proteins, experiences on importance of Ki-67 protein expression in non-small cell lung cancer. Maddau (27) found in lung adenocarcinoma that exceptionally high risk of repeated appearance of malignoma is carried by the strong expression of protein Ki-67in early cases of disease compared to II and III stage of the disease. Szelakowska (38) in study of 64 non-small cell lung cancer founds no correlation between Ki-67 expression and survival period length. Niemic (37) while investigating same protein in 78 cases of squamous lung cancer found that Ki-67 nuclear expression is more emphasized in cases of moderate and weak differentiation (G2 and G3, respectively) compared to well differentiated cancer (G1) and in pT1+pT2 compared to pT3 stage. Niemec (39) concludes that Ki-67 is independent prognostic marker in survival of patients with non-small cell lung cancer. Maddau (27) founds that high expression of protein Ki-67 in stage I, unlike stages II and III is very risky only in cases of adenocarcinoma, but non in other non-small cell lung cancer. Meert (40) while analyzing different markers quotes possibility that beside protein p53 and Ki-67 potential prognostic factor that was diagnosed in 32% of cases of non-small cell lung cancer. In our investigation size and level of tumor differentiation were in statistically significant correlation to Ki-67 protein expression, what speaks out for the proliferative index to be of a direct influence to size and grade of tumor mass.
The results achieved in our investigation, that mostly fit in those of other investigators, point out to need for a further investigation of protein markers in non-small cell lung cancer, that should be done in the future on large number of patients. In this study small specimen represented a limitation factor, so it was not possible to identify significant difference when it exists.
CONCLUSION
This study showed that in different histological types of non-small cell lung cancer, there are significant variations in expression of cell proteins that can vary individually. These differences can be of a clinical importance so that each form of non-small cell lung cancer can be observed as special entity. In each case, there is a need for further immu-nohistochemical studies for numerous prognostic markers in large number of cases. These markers can serve to form a prognostic model in identifying patients with high risk to relapse the disease, who need specific therapy.
REFERENCES
- 1.Rom W.N, Hay J.G, Lee T.C, Jiang Y, Tchou-Wong K.M. Molecular and genetic aspects of lung cancer. J. Respir. Crit. Care. Med. 2000;161(14 Pt 1):1355–1367. doi: 10.1164/ajrccm.161.4.9908012. [DOI] [PubMed] [Google Scholar]
- 2.Esposito V, Baldi A, De Luca A, Micheli P, Mazzarella G, Baldi F, Caputi M, Giordano A. Prognostic value of p53 in non-small cell lung cancer: relationschip with proliferating cell nuclear antigen and cigarette smoking. Hum. Pathol. 1977;28(2):233–237. doi: 10.1016/s0046-8177(97)90112-x. [DOI] [PubMed] [Google Scholar]
- 3.Segawa Y, Kageyama M, Suzuki S, Jinno K, Takigawa N, Fujim-oto N, Hotta K, Eguchi K. Measurement and evaluation of serum anti.p53b antibody levels in patienst with lung cancer at its initial presentation: a prospective study. Br. J. Cancer. 1987;8(5):667–672. doi: 10.1038/bjc.1998.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patz E.F, Goodman P.C, Bepler G. Screening for lung cancer. N. Engl. J. Med. 2000;30(343(22)):1627–1622. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- 5.Hughes J.H, Cohen M.B. Nuclear matrix proteins and their ppo-tential applications to diagnostic pathology. Am. J. Clin. Pathol. 1999;111(1):267–274. doi: 10.1093/ajcp/111.2.267. [DOI] [PubMed] [Google Scholar]
- 6.Lu Q, Abel P, Foster C.S, Lalani E. Bcl-2; role in epithelial differentiation and oncogenesis. Hum. Pathol. 1996;27:102–110. doi: 10.1016/s0046-8177(96)90362-7. [DOI] [PubMed] [Google Scholar]
- 7.Bronner P.M, Culin C, Reed J.C, Furth E.E. The bcl-2 proto-oco-gene and the gastrointestinal epithelial tumor proliferation using the monoclonal antibody Ki-67. Am. J. Pathol. 1995;146:20–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Chang F, Syrjanen S, Tervahauta A, Syrjanen K. Tumorigenesis associated with the p53 tumour suppressor gene. Br J Cancer. 1993;68:653–661. doi: 10.1038/bjc.1993.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridrich K, Dimmer V, Haroske G, Meyer W, Thessíng F, Thí-eme B, Kunze KD. Morphological heterogeneity f p53 positive and p53 negative nuclei in breast cancer stratified by clinico-pathological variables. Analytical Cell. Path. 1997;14:111–123. doi: 10.1155/1997/619309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinicrope F.A, Ruan S.B, Cleary K.R. Bcl-2 and p53 oncopro-tein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 11.Sutherland R.L, Musgrove E.A. Cyclin D1 and mammary carcinoma: new insights from transgenic muse models. Brest Cancer Res. 2002;4:14–17. doi: 10.1186/bcr411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berenson J.R, Koga H, Yang J, et al. Frequent amplification of the bcl-1 locus in poorly differentiated squamous cell carcinoma of the lung. Oncogene. 1990;5:1343–1348. [PubMed] [Google Scholar]
- 13.Proctor A.J, Coombs L.M, Cairns J.P, Knowles M.A.A. Amplification at chromosome 11q13 in transitional cell tumors of the bladder. Oncogene. 1991;6:789–795. [PubMed] [Google Scholar]
- 14.Berenson J.R, Yang J, Mickel R.A. Frequent amplification of the bcl-1 locus in head and neck squamous cell carcinomas. Oncogene. 1989;4:1111–1116. [PubMed] [Google Scholar]
- 15.Popescu N.C, King C.R, Kraus M.H. Location of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12-21.32. Genomics. 1989:362–366. doi: 10.1016/0888-7543(89)90343-1. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann C, Hung M.C, Weinber R.A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 17.King C.R, Kraus M.H, Aaronson S.A. Amplification of novel V-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 18.Menard S, Fortis S, Castiglioni F, Agresti R, Balsari A. HER2 as a Prognostic Factor in Breast Cancer. Oncology. 2001;61:57–72. doi: 10.1159/000055404. [DOI] [PubMed] [Google Scholar]
- 19.Slamon D.J, Godolphin W, Jones L.A, et al. Studies of the HER-2/neu protooncogenie in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 20.Press M.F, Pike M.C, Chazin V.R, et al. Her-2/neu expression in node-negative breast cancer: Direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of current disease. Cancer Res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- 21.Hynes N.E, Stern D.E. The biology of erbB-2/neu/HER-2 and its role in cancer. Biophym.Biophys. Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 22.Soomoro I.N, Jholmes J, Whimster W.F. Predicting prognosis in lung cancer use of proliferation marker, Ki67 monoclonal antibody. J Pak Med Assoc. 1998;48(3):66–69. [PubMed] [Google Scholar]
- 23.Theile A, Muller K.M. Proliferation kinetics of Bronchioalveolar tumorlets. Pathologe. 1996;17(2):163–170. doi: 10.1007/s002920050152. [DOI] [PubMed] [Google Scholar]
- 24.Brundage M.D, Davies D, Mackillop J. Prognostic factors in non-small cell lung cancer. Chest. 2002;122:1037–1057. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- 25.Mistudoni T, Hamajima N, Ogawa M, Takahashi T. Prognostic significance of p53 alteration in patients with non-small cell lung cancer: a meta-analysis. Clin Cancer Res. 2000;6:4055–4063. [PubMed] [Google Scholar]
- 26.Lee J.S, Yoon A, Kalapurakal S.K. Expression of p53 oncoprotein in non-small cell lung cancer: a favourable prognostic factor. J. Clin. Oncol. 1995;113:1893–1903. doi: 10.1200/JCO.1995.13.8.1893. [DOI] [PubMed] [Google Scholar]
- 27.Maddau C, Confortini M, Bisanzi S, Janni A, Montinaro F, Paci E, et al. Prognostic significance of p53 and Ki-67 antigen expression in surgically treated non-small cell lung cancer: immunohis-tochemical detection with imprint cytology. Am. J. Clin. Pathol. 2006;125(3):425–431. [PubMed] [Google Scholar]
- 28.Pezzella F, Turley H, Kuzu I, Tungekar M.F, Dunnill M.S, Pierce C.B, Harris A, Gatter K.C, Mason D.Y. bcl-2 protein in non-small-cell lung carcinoma. N. Engl. J. Med. 1993;2(329):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- 29.Jaren A, Oztop I, Kargi A, et al. Bax, bcl-2, and C-kit expression in non.small cell lung cancer and their effects on prognosis. Int. J.Clin. Pract. 2006;60:675. doi: 10.1111/j.1368-5031.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 30.Laudanski J, Niklinska W, Burzykovski T, Chyczewski L, Niklin-ski J. Prognostic significance of p53 and bcl-2 abnormalities in operable non small. Cell lung cancer. Eur Respir J. 2001;17:660–666. doi: 10.1183/09031936.01.17406600. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti A, Doglioni C, Barbareschi M, Buttitta F, Pellegrini S, Gaeta P, et al. Cyclin D1 and retinoblastoma susceptibility gene alterations in non-small cell lung cancer. Int. J. Cancer. 1998;19:187–192. doi: 10.1002/(sici)1097-0215(19980119)75:2<187::aid-ijc4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Caputi M, De Luca L, Papaccio G, D’Aponte A, Cavallotti I, Scalpa P, et al. Prognostic role of cyclin D1 in non small lung cancer: an immunohistochemical analysis. Eur. J. Histochem. 1997;41(2):133–138. [PubMed] [Google Scholar]
- 33.Michima T, Dosaka-Akita H, Kinoshita I, Hommura F, Morika-wa T, Kotoh H, et al. Cyclin D1 expression in non-small cell lung cancers: its association with alteder p53 expression, cell proliferation and clinical outcome. British J. Cancer. 1999;80:1289–1295. doi: 10.1038/sj.bjc.6990500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayed A.K, Adesina H. Prognostic significance of Cyclin D1 expression in resected stage I II non small lung cancer in Arabs. Interactive Cardio Vasc. Thorac. Surg. 2005;5:47–51. doi: 10.1510/icvts.2005.120030. [DOI] [PubMed] [Google Scholar]
- 35.Keum J.S, Kong G, Yang S.C, Shin D.H, Park S.S, Lee J.H, Lee J.D. Cyclin D1 overexpression is an indicator of poor prognosis in respectable non-small cell lung cancer. British J. Cancer. 1999;81:127–132. doi: 10.1038/sj.bjc.6690661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ugoscai K, Mandoky L, Tiszlavicz L, Molnar J. Investigation og HER-2 everexpression in non-small cell lung cancer. Anticancer Res. 2005;25(4):3061–3066. [PubMed] [Google Scholar]
- 37.Chih-Cheng H, Kuan-Chih C, Huci-Jyh F, Chun-Ming T, Wing-Yin L, Min-Hsiung H, et al. Prognostic significance of HER-2/neu overexpression in stage I adenocarcinoma of lung. Ann. Thorac. Surg. 1998;66:1159–1163. doi: 10.1016/s0003-4975(98)00792-9. [DOI] [PubMed] [Google Scholar]
- 38.Szelachowska J, Jelen M. Laminin, HER2/neu and Ki-67 as prognostic factors in non-small cell lung cancer. Rocz Akad Med Bia-lymst. 2004;49:256–61. [PubMed] [Google Scholar]
- 39.Niemiec J, Kolodziejski L, Dyczek S. EGFR and Ki-67 are independent prognostic parameters influencing survivals of surgically treated squamous cell lung cancer patients. Neoplasma. 2005;52:231–7. [PubMed] [Google Scholar]
- 40.Meert A.P, Martin B, Verdebout J.M, Paesmans M, Berghmans T, Ninane V, Sculier J.P. Correlation of different markers (p53, EGFR, c-erB-2, Ki-67) expression in the diagnostic biopsies and the corresponding resected tumors in non-small cell lung cancer. Lung Cancer. 2004;44(3):295–301. doi: 10.1016/j.lungcan.2003.12.009. [DOI] [PubMed] [Google Scholar]


