Abstract
In this study we examined motor and sensory conduction velocities in right median and ulnar nerves in diabetic patients. Control group consisted of 25 healthy volunteers (13 males) with average age of 52 years. The first examined group consisted of 25 diabetic patients (13 males) without retinal changes, 59,6 years old on average, and the second group consisted of 40 patients (15 males) 59,4 years old on average: 22 of them with type 1, and 18 with type 2 retinal changes. The motor and sensory conduction velocities in right median nerve in the control group were significantly higher than those measured in the first group (p<0,0005 for motor, and p=0,0027 for sensory velocity), and the second group (p<0,0001 for motor, and p=0,0001 for sensory velocity). Significantly higher conduction velocities in sensory median nerve were compared between the examined groups (p<0,001), but motor conduction velocity was not significantly higher (p=0,09). The motor conduction velocity in ulnar nerve in the control group was significantly higher in comparison with the patients of first (p=0,0027) and second examined group (p=0,0001). The sensory conduction velocity in ulnar nerve was not significantly higher compared with the first (p=0,081), and significantly higher compared with the second examined group (p<0,0001). The sensory conduction velocity of ulnar nerve was significantly higher (p=0,019) in diabetic patients without retinopathy compared with patients with retinopathy.
Diabetic patients with retinal changes have higher risk of developing more severe neurophysiologic signs of neuropathy. So, simple observation with ophthalmoscope may be useful diagnostic tool in its determination and may be the target of further therapeutic strategy.
Keywords: diabetic neuropathy, diabetic retinopathy, n.medianus, n.ulnaris, motoric and sensory velocity
INTRODUCTION
Diabetes mellitus (DM) is metabolic disorder with numerous and serious complications. Basic metabolic disorder in this disease is breach in tissue oxygenation, which is very important factor for the appearance and progression of microangiopathy (1). Autonomous nervous system and peripheral nervous system (PNS) are included as one of four main DM complications together with retinopathy, nephropathy and vascular disease (2). In diabetic neuropathy metabolic mechanisms cause neural degeneration with regeneration problems, especially in tiny myelinic fibers, as opposed to microvascular changes that are essential in diabetic retinopathy (3). Therefore, the time, importance and microangiopathy stadium are controversial in diabetic neuropathy development. There is no generally accepted standard definition of diabetic neuropathy or even diagnostic criteria. Therefore, the prevalence among diabetics is ranges from 0% to 93% (4, 5). Electrophysiological criteria are basic in its evaluation. Diabetic retinopathy is ocular manifestation of this systemic disease, being the primary cause of blindness at the age from 20 to 65 years in United States (6). The basic changes are in pre-capillary and capillary vascular net. Usual vascular changes include microvascular occlusion with increased vascular. According to histopathological changes in diabetic retinopathy there are three stadiums:
unprolipherative diabetic retinopathy
praeprolipherative diabetic retinopathy
prolipherative diabetic retinopathy (7).
In one study, diabetic retinopathy prevalence was 39,9% with 5,4% in prolipherative diabetic retinopathy (8). In Chinese study on 219 diabetics, retinopathy prevalence was 24%, neuropathy 23,5% (9) with obvious time of disease and glicaemic control. Research results of Schmid et al. showed connection between cardiovascular autonomic neuropathy with diabetic retinopathy. There are too many studies that separately target diabetic retinopathy and neuropathy, but there are less studies that target the connections between these two diseases. Therefore the aim of this study is to understand this problem.
AIMS OF STUDY
The first aim was to evaluate right n. medianus and n. ulnaris motoric and sensory velocities in diabetic patients with and without retinopathy and to compare the results with those in the control group of healthy patients. The second aim was to analyze correlation in neurophisiological parameters between the diabetics without retinopathy and diabetics with retinopathy.
MATERIAL AND METHODS
This study was realized at the Neurophisiological and Ophthalmological Departments in Public Health Service Tuzla from January 2005 to December 2006. The control group consisted of 25 healthy patients (13 males) 52 years old on average free of diabetes mellitus and clinical signs of neuropathy. Based on ophthalmological examinations two groups of patients were formed without consideration of diabetes mellitus type or neuropathy symptoms. The first examined group included 25 patients (13 males) without retinal changes 59,6 years old on average suffering from diabetes for 8,2±4,8 years. The second examined group included 40 patients (15 males) 59,4 years of average age suffering from diabetes for 12,5±5,5 years: 22 with type I and 18 with type II retinop-athy. The type of retinopathy was diagnosed at Ophthalmology Department Public Health Service Tuzla following ophthalmoscopic examination. The patients in the control group were not ophthalmologicaly examined. All patients were electroneurogicaly (ENG) tested. Elec-troneurogical evaluation was conducted at room temperature and physiological skin temperature in resting position. EMNG apparatus Medelec Synergy (EMNG and EP Systems-OXFORD INSTRUMENTS 2004) was used for neurograph parameters evaluation. Superficial stimulated and bipolar registration electrodes were used (Large touchproof). Measurement of n. medianus motor conduction velocity was done with n. medianus stimulation in wrist and elbow with m. abductor pollicis brevis registration. N. medianus sensory velocity was measured with n. medianus stimulation in wrist and in connection between the first and second phalanges of the second finger. N. ulnaris motoric speed was measured with wrist and elbow stimulation and with m.abductor pollicis brevis registration. N. ulnaris sensory speed was measured with wrist stimulation and registration in place of the first and second phalanges connection. Therefore, in ENG analysis, sensory velocity in wrist and motor velocity in upper hand was registered in meter per seconds (m/s) from the primary place of sensory nerve action potential (SNAP) and complex motoric action potential (CMAP) to isoelectric line after so called stimulation artifact. Stimulation with registration of motoric and sensory response was done until increased amplitude was stopped (CMAP and SNAP). If there was no response in sensory reaction velocity evaluation was accepted as 0 m/s. Standard statistical parameters were used: statistic means, standard deviation with t-test) (t-test and linear correlation analysis.
RESULTS AND DISCUSSION
It was expected that patients with retinopathy have longer history of diabetes mellitus, in comparison with the diabetic patients without it considering the fact that the period of suffering from the disease is one of the risk factors for neuropathy and retinopathy. The quoted published results show that patients with diabetes mel-litus type 1 develop retinopathy after ten years as well as almost 60% of patients with diabetes mellitus type 2. The duration of the disease is probably the strongest factor in the prediction of development and progression of retinopathy. Among patients suffering from diabetes mellitus for shorter period prevalence of retinopathy any type was 8% for tree years, 25% for 5 years, 60% for ten years and 80% for 15 years (11).
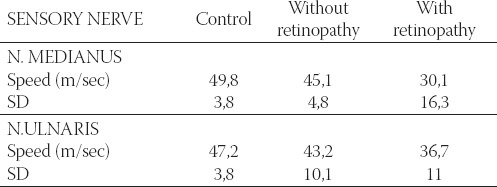
The patients with diabetes mellitus have lower motoric and sensory velocity in n. medianus and n.ulnaris compared with healthy persons (Table 1 and 2).
TABLE 1.
Mean values of motoric velocity in right n. medianus and n. ulnaris in the control group and diabetic patients, with and without retinopathy
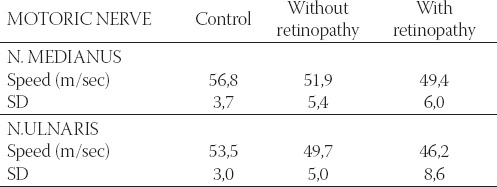
TABLE 2.
Mean values of sensory velocity in right n. medianus and n. ulnaris in the control group and diabetic patients, with and without retinopathy
Results from literature show: diabetic neuropathy is the second most common cause of PNS damage following the traumatic neuropathy (12).
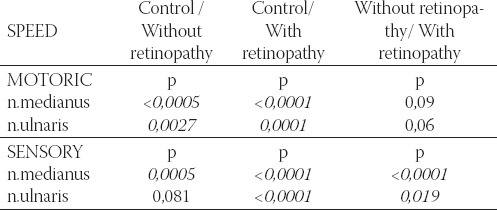
It is obvious that the decrease in motoric and sensory velocity is the greatest in patients with retinopathy. Therefore it is logical to assume that among various risk factors for diabetic neuropathy development, retin-opathy is one of the most important, much more than nephropathy for example. As many as 8 patients in the group with retinopathy (5 with type I and 3 with type II) had no n. medianus sensory response. It means that if ENG shows lack of n. medianus sensory response, these patients already have retinal changes. Two patients in the group with retinopathy, and one without retinopathy could not obtain n. ulnaris sensory response. Statistically, motoric and sensory velocities are much higher in n.medianus, motoric velocity in n. ulnaris in the control group comparing with diabetics group without retinopathy. Comparing control group with retinopathy group showed that both measured velocities (motoric and sensory) of the two nerves were significantly higher. Diabetic patients with retinopa-thy have significantly lower sensory velocity comparing with patients without retinopathy (Table 3).
TABLE 3.
Important difference in motoric and sensory velocity in n.medianus and n. ulnaris in the control group and diabetic patients, with and without retinopathy
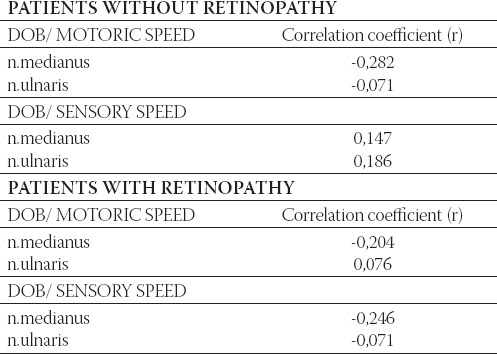
Correlative analyze of diabetes disease treatment period in patients with and without retinopathy shows weak expecting negative connection motoric speed velocity of n. medianus in patients without retinopathy and motoric and sensory speed velocity n. medianus in patients with retinopathy (Table 4).
TABLE 4.
Correlation between the duration of treatment in diabetes mellitus and motoric and sensory velocity in right n. medianus and n. ulnaris in patients with and without retinopathy
Analysis of the group of patients with retinopathy and comparison with the groups with type I and type II changes in retina did not show significant change in motoric and sensory velocity in n.medianus and n. ulnaris (Table 5 and 6). There was no significant difference between patients without sensory reaction n. medianus in different retinopathy types (p=0,205). Although study on larger sample is necessary, it is evident that retinal examination (retinoscopy) may be simple, fast and useful diagnostic procedure and therapeutical approach to retinopathy and neuropathy as well. Simple, fast and almost painless procedure of evaluation in motoric and sensory conduction velocity in one hand may provide information about peripheral nervous system and diabetics’ retina. All this facts may be important in diagnostic and therapeutic strategy.
TABLE 5.
Mean values and motoric velocity in n. medianus and n. ulnaris in diabetic patients with retinopathy correlated with types of changes in eye fundus.
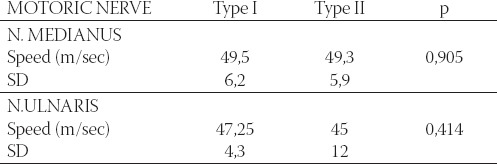
TABLE 6.
Mean values and sensory velocity in right n. medianus and n. ulnaris in diabetic patients with retinopathy correlated with types of changes in eye fundus.
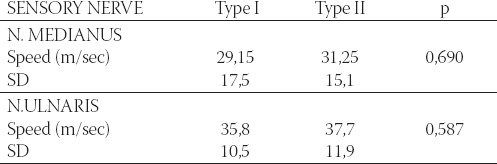
CONCLUSION
◊ Motoric and sensory conduction velocity in n. medianus and n. ulnaris in diabetes mellitus patients are lower in patients with retinal changes.
◊ Retinal changes in diabetes mellitus patients may indicate existence of neuropathy as well.
◊ If it is impossible to get n. medianus sensory potential in the patient with diabetes mellitus the retinal changes already exist.
REFERENCES
- 1.Nikolić L.J. Dijabetička retinopatija Zavod za udžbenike i nastavna sredstva. Beograd. 1999:66–72. [Google Scholar]
- 2.Foster D.W. Diabetes mellitus. In: Petersdorf R.G, Adams R.D, Braunwald E, et al., editors. Harrison’s Principles of Internal Medicine. ed 10. New York: McGraw-Hill; 1983. pp. 661–679. [Google Scholar]
- 3.Harati Y. Diabetic peripheral neuropathies. Ann. Intern. Med. 1987;107:546–559. doi: 10.7326/0003-4819-107-4-546. [DOI] [PubMed] [Google Scholar]
- 4.Thomas P.K, Eliasson S.G. Diabetic neuropathy. In: Dyck P.J, Thomas P.K, Lambert E.H, editors. Peripheral neuropathy. 2nd ed. Philadelphia: WB Saunders; 1984. pp. 1773–1810. [Google Scholar]
- 5.Melton L.J, Dyck P.J. Epidemiology. In: Dyck P.J, Thomas P.K, Asbury A.K, et al., editors. Diabetic neuropathy. Phyladelphia: WB Saunders; 1987. pp. 27–36. [Google Scholar]
- 6.Duh E.J, Aiello L.P. Basic Pathobiology of the eye and its complications. In: Porte J, editor. Ellenberg & Rifkin’s Diabetes Mellitus. New York: McGraw-Hill; 2002. [Google Scholar]
- 7.Rogulja-Pepeonik Ž, Šikić J. Komplikacije na očima u bolesnika sa šećernom bolešću. Medicus. 1997;6(2):197–202. [Google Scholar]
- 8.Manaviat M.R, Afkhami M, Shoja M.R. Retinopathy and mi-croalbuminuria in type II diabetic patients. BMC Ophthalmology. 2003;4:9. doi: 10.1186/1471-2415-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai T.Y, Tseng C.H, Sung S.M, Huang R.F, Chen C.Z, Tsai S.H. J. Retinopathy, neuropathy and nephropathy in non insulin dependent diabetic patients. Formos Med Assoc. Book of abstracts. 1991;90(10):936–40. [PubMed] [Google Scholar]
- 10.Schmid H, Schaan B, Cecconello F, Maestri T, Neumann C. Proliferative retinopathy is related to cardiovascular autonomic neuropathy in non insulin dependant diabetes mellitus. Diabetes Res. Clin. Pract. 1995;29(3):163–168. doi: 10.1016/0168-8227(95)01120-x. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein B.E, Moss S.E, Davis M.D, DeMets D.L. The Wisconsin epidemiologic study of diabetic rRetinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 12.Thomas P.K. Differential diagnosis of peripheral neuropathies. In: Refsum S, Boils C.L, Portera Sanchez A, editors. International Conference on Peripheral Neuropathies. Amsterdam: Excerpta Medica; 1982. pp. 76–84. [Google Scholar]


