Abstract
Plethodontid salamanders exhibit biphasic, larval form paedomorphic, and direct developing life cycles. This diversity of developmental strategies exceeds that of any other family of terrestrial vertebrate. Here we compare patterns of larval development among the three divergent lineages of biphasic plethodontids and other salamanders. We discuss how patterns of life-cycle evolution and larval ecology might have produced a wide array of larval life histories. Compared with many other salamanders, most larval plethodontids have relatively slow growth rates and sometimes exceptionally long larval periods (up to 60 mo). Recent phylogenetic analyses of life-cycle evolution indicate that ancestral plethodontids were likely direct developers. If true, then biphasic and paedomorphic lineages might have been independently derived through different developmental mechanisms. Furthermore, biphasic plethodontids largely colonized stream habitats, which tend to have lower productivity than seasonally ephemeral ponds. Consistent with this, plethodontid larvae grow very slowly, and metamorphic timing does not appear to be strongly affected by growth history. On the basis of this, we speculate that feeding schedules and stress hormones might play a comparatively reduced role in governing the timing of metamorphosis of stream-dwelling salamanders, particularly plethodontids.
Keywords: Corticosterone, Direct development, Growth, Larva, Paedomorphosis, Thyroid hormones
There are more species of plethodontid salamanders than of all other salamander families combined (Frost 2016). The diversification of plethodontids during the Cenozoic has been manifested by both lineage proliferation and morphological disparity (Rabosky and Adams 2012; Shen et al. 2016). Plethodontids also exhibit a diversity of life-cycle strategies that include: (1) a biphasic life cycle with an aquatic larval stage followed by metamorphosis into a more-terrestrial adult, (2) larval-form paedomorphosis through the loss of metamorphosis and the terrestrial adult stage, and (3) direct development with transformation to a terrestrial form before hatching. Biphasic life cycles occur in three different clades (the subfamily Spelerpinae, Hemidactylium and some Desmognathus). Several spelerpine lineages express obligate paedomorphosis (Bonett et al. 2014a,b), but facultative paedomorphosis is uncommon. Direct-developing lineages collectively represent the greatest diversity of salamanders (Wake and Hanken 1996), and range from western North America into South America, with relict species in the Mediterranean and Asia (Dunn 1926; Wake 1966; Min et al. 2005). Lineages with a biphasic life cycle and larval-form paedomorphosis are restricted to eastern North America, especially within the Appalachians, Interior Highlands (Ozark and Ouachita Mountains), and Edwards Plateau (central Texas).
Classical interpretations of plethodontid relationships (Wilder and Dunn 1920; Dunn 1926; Wake 1966) were based on the notion that ancestral life cycles were biphasic, and subsequent life-cycle evolution only occurred through simplification (to direct development or larval-form paedomrophosis). Analyses based on molecular phylogenies have indicated that life-cycle evolution is reversible, however, with complex life cycles secondarily derived from direct-developing and paedomorphic ancestors (Titus and Larson 1996; Chippindale et al. 2004; Mueller et al. 2004; Bonett et al. 2014a,b). With regard to direct development, there are either (1) multiple episodes of direct development from biphasic ancestors, or (2) re-evolution of free-living larvae from direct-developing ancestors, including the possibility that a direct-developing life cycle could be the ancestral life cycle for plethodontids (Chippindale et al. 2004; Mueller et al. 2004). Indeed, ancestral-state reconstruction suggests the possibility that the ancestral plethodontid was direct developing (Bonett et al. 2014b). Also, over a much shorter time frame there is evidence of a reversal from paedomorphosis to a biphasic life cycle in one clade of Eurycea from the Edwards Plateau of central Texas (Bonett et al. 2014a).
The possibility that larval periods have been re-evolved from direct-developing ancestors now seems probable (Wake 2009; Bonett et al. 2014a,b). Regardless of the directionality, life-cycle transitions can be explained by heterochrony (i.e., that the timing of metamorphosis, maturation, and hatching is shifted, with the result being a new life-cycle mode; Ryan and Bruce 2000; Bonett et al. 2014b). What remains unresolved is the source of the susceptibility to express significant life-cycle evolution. Ecology, phylogeny, and endocrinology can shed light on how changes in developmental timing have produced the unusual larval forms of plethodontids. By drawing on both classic and recent work in several disparate fields, we postulate several explanations for the unusual patterns of metamorphosis in plethodontids. In particular, we describe aspects of metamorphic biology that differ more in plethodontids than in any other group of amphibians. Specifically, we discuss (1) the natural history of the larval period, (2) experiments on larval growth and metamorphosis, and (3) what is known about the endocrinology of plethodontid metamorphosis. We discuss how these observations fit with the most likely path of life-cycle evolution in this family.
How Is the Larval Life History Different in Plethodontids?
The two principal ways in which the larval life history of plethodontids is different from other amphibians are that plethodontid larvae grow slowly and the larval period is very long (Bruce 1980; Voss 1993). There is an abundant literature on growth in larval amphibians (e.g., Alford 1999; Harris 1999). In their first 30 d of larval life, tadpoles can experience a 40-fold increase in mass (Beachy et al. 1999; Beachy 2001), and larval ambystomatids can increase mass by 25–125 times during this period (Clarkson and Beachy 2015; Ihli and Beachy 2016). In contrast, larval plethodontids do not even double their mass during this period, even when fed ad libitum (Beachy 1995a; O’Laughlin and Harris 2000). Field observations verify the idea that larval growth is slow in plethodontids. For example, both desmognathan larvae (e.g., Beachy 1995a; Bruce 2016a,b) and spelerpine larvae (Bruce 1980; Voss 1993) grow less than 1 mm/mo. These low rates of larval growth also appear to hold for paedomorphic spelerpines (Niemiller et al. 2016; Bendik 2017).
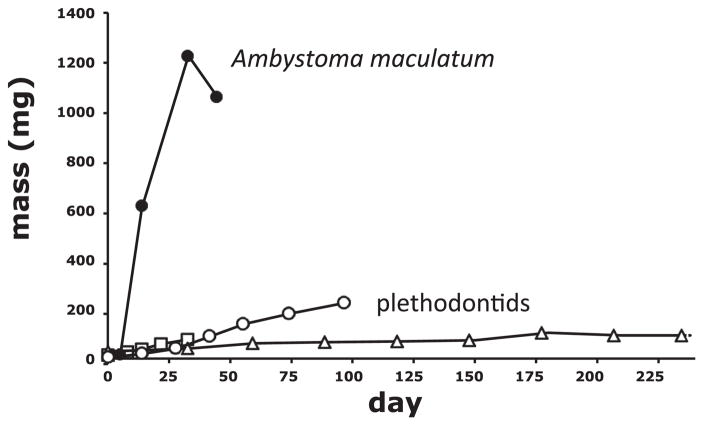
It is possible that these low growth rates are determined by the larval habitat. Most plethodontid larvae occupy streams. These habitats are often characterized by substantially lower productivity (principally just allochthonous leaf fall) than temporary pools and eutrophic lakes (Hynes 1970; Wetzel 1983). Several species of larval plethodontids use productive temporary pools for larval development, yet these species (e.g., Eurycea quadridigitata [Semlitsch 1980], Hemidactylium scutatum [Blanchard 1923], and Pseudotriton montanus [Bruce 1978]) still do not exhibit the remarkable rapid larval growth seen in syntopic amphibians (e.g., tadpoles and larval ambystomatids). In addition, even in growth experiments where larval growth history is directly manipulated (e.g., Alford and Harris 1988), plethodontids fail to grow rapidly. In experiments where at least one growth treatment is to provide ad libitum food, the slowest-growing ambystomatid, Ambystoma maculatum, grows an order of magnitude faster than the fastest-growing plethodontid, Hemidactylium (Ihli and Beachy 2016; O’Laughlin and Harris 2000; Fig. 1). Interestingly, Hemidactylium larvae fed ad libitum (O’Laughlin and Harris 2000) did not grow substantially faster than the stream-dwelling larval Desmognathus ocoee fed ad libitum (Beachy 1995b). This indicates that genetics (rather than environment alone) shapes the major differences in larval growth of plethodontids compared with pond-dwelling salamanders from other families.
Fig. 1.
Larval growth profiles of several amphibians. Growth profiles compare the larval growth and metamorphosis of the plethodontid salamanders Desmognathus ocoee (triangles), Eurycea quadridigitata (open circles), and Hemidactylium scutatum (squares) with the ambystomatid salamander, Ambystoma maculatum. Each profile begins at first mass taken after hatching, and terminates at the mean time and size at metamorphosis in each taxa. Ambystoma maculatum has the slowest growth for any larval ambystomatid. In contrast, E. quadridigitata and H. scutatum have the fastest growth for any larval plethodontid. The profiles presented here are those taken from fastest-growing treatment groups in each experiment. Growth treatments were initiated when hatchling larvae were placed in individual containers and fed prey items ad libitum. Profiles begin with the first measurement of mass and terminate at the mean time of metamorphosis, save for E. quadridigitata, where the experiment was terminated before the completion of metamorphosis. The profiles are taken from Beachy (1995b) for D. ocoee, T.J. Ryan and B. Rothermel (personal observations) for E. quadridigitata, O’Laughlin and Harris (2000) for H. scutatum, and Ihli and Beachy (2016) for A. maculatum.
In addition, larval periods are generally longer in plethodontids than in other amphibians (Beachy and Bruce 1992; Ruben et al. 1993). The long larval period has been associated with several other life-history attributes, including large genome size (Sessions and Larson 1987), long generation times (Hairston 1987), low metabolic scope (Feder 1983), and delayed maturation (Beachy 1995a; Ryan and Bruce 2000; Beachy and Bruce 2003). In any case, the longest larval periods in plethodontids covary with delays in maturation age and consequent large adult body size (Bruce 2016a). This is most clearly seen in the desmognathan plethodontids: the largest, Desmognathus quadramaculatus, has the longest larval period (up to 48 mo) and the smallest, D. wrighti and D. aeneus, are direct developers. Although the spelerpines do not exhibit direct development, the largest species, Gyrinophilus porphyriticus, has a larval period that may extend to 60 mo (Bruce 1980). It is important to note that other salamanders with mountain brook (stream-dwelling) larvae from other families such as Rhyacotriton also have extended multiyear larval periods (36 to 48 mo; Nussbaum and Tait 1977). Explicit macroevolutionary analyses to test the ecological associations of larval durations have yet to be performed. In fact, the cause–effect relationships involved in the pattern of life-history associations among egg size, larval growth, larval period, juvenile growth, age at metamorphosis, age at maturation, and adult body size among salamanders remain unresolved (Bruce 2016b).
How Is Metamorphosis Different in Plethodontids?
Another remarkable pattern is that plethodontid larvae of several species have been shown not to vary in metamorphic timing when grown at different rates (Table 1). When exposed to variable growth schedules (via different feeding treatments), amphibian larvae can metamorphose at different times (Alford and Harris 1988; Beachy et al. 1999; Beachy 2001). Every kind of animal, except for plethodontids, treated in similar growth experiments will metamorphose at different times (Table 1). Generally, larvae grown rapidly metamorphose early (e.g., Hensley 1993; Leips and Travis 1994; Beachy et al. 1999), but there are cases (restricted to the spadefoots, Spea and Scaphiopus) where rapidly growing larvae have metamorphosed later than slow growers (Newman 1988, 1998; Morey and Reznick 2000; Boorse and Denver 2004). This last observation is noteworthy given that spadefoot tadpoles inhabit the most ephemeral of pools and metamorphose to inhabit a hostile terrestrial habitat. The emphasis on taking advantage of larval growth opportunity, if available, in this case behaves as optimality theory predicts (Wilbur and Collins 1973; Alford and Harris 1988). Alternatively, the fixed-rate model describes patterns of metamorphic timing that are independent of environmental factors such as growth. In other words, the age of metamorphosis is fixed regardless of growth rate or body size. Larvae of some species show transitions between growth rate-dependent and fixed-rate patterns during ontogeny (Hentschel 1999; Rose 2005).
Table 1.
Growth experiments on various taxa wherein growth of individual larvae was manipulated and individual responses including metamorphic timing were recorded and evaluated.
| Taxon | Growth treatment affected metamorphic timing? | Reference |
|---|---|---|
| Caudata | ||
| Plethodontidae | ||
| Desmognathus quadramaculatus | No | Hickerson et al. 2005 |
| Desmognathus ocoee | No | Beachy 1995b |
| Hemidactylium scutatum | No | O’Lauglin and Harris 2000 |
| Eurycea wilderae | No | C.K. Beachy, personal observation |
| Ambystomatidae | ||
| Ambystoma maculatum | Yes | Ihli and Beachy 2016 |
| Ambystoma tigrinum | Yes | Ihli and Beachy 2016 |
| Ambystoma mexicanuma | Yes | Clarkson and Beachy 2015 |
| Ambystoma talpoideum | Yes | Ryan and Semlitsch 2003 |
| Salamandridae | ||
| Salamandra infraimmaculata | Yes | Marburg 2009 |
| Neurergus microspilotus | Yes | Vaissi and Sharifi 2016 |
| Anura | ||
| Bufonidae | ||
| Anaxyrus americanus | Yes | Beachy 2001 |
| Anaxyrus woodhousei | Yes | Alford and Harris 1988 |
| Hylidae | ||
| Hyla versicolor | Yes | Beachy et al. 1999 |
| Hyla chrysoscelis | Yes | Audo et al. 1995 |
| Hyla squirella | Yes | Beck 1997 |
| Hyla gratiosa | Yes | Leips and Travis 1994 |
| Hyla cinerea | Yes | Leips and Travis 1994 |
| Pseudacris crucifer | Yes | Hensley 1993 |
| Pelobatidae | ||
| Spea hammondii | Yes | Morey and Reznick 2000; Denver et al. 1998 |
| Scaphiopus couchii | Yes | Newman 1989, 1998; Morey and Reznick, 2000 |
| Spea intermontana | Yes | Morey and Reznick, 2000 |
| Pipidae | ||
| Xenopus laevis | Yes | McCoy et al. 2007 |
| Arthropoda | ||
| Crustacea | ||
| Mesocyclops edax | Yes | Twombly 1996 |
| Balanus glandula | Yes | Hentschel and Emlet, 2000 |
| Petrolisthes cabrilloi | Yes | Howard and Hentschel 2005 |
| Insecta | ||
| Aedes aegypti | Yes | Zeller and Koella 2016 |
| Oncopeltus fasciatus | Yes | Blakley 1981 |
| Oncopeltus cingulifer | Yes | Blakley 1981 |
| Lestes viridis | Yes | Rolff et al. 2004 |
Ambystoma mexicanum are obligately paedomorphic. Larvae in this experiment were induced to metamorphose by immersion in 5 nM T4.
The timing of metamorphosis is not affected by larval growth in Desmogathus ocoee (Beachy 1995b), Hemidactylium scutatum (O’Laughlin and Harris 2000), D. quadramaculatus (Hickerson et al. 2005), and Eurycea wilderae (C.K. Beachy, personal observation). These studies include representative species in all three lineages with biphasic life cycles (i.e., Hemidactylium, Desmognathus, and the Spelerpinae). Even H. scutatum, which has secondarily invaded the more productive pond larval habitat, follows this pattern (O’Laughlin and Harris 2000). This apparent decoupling of growth rate and metamorphic timing is thus far unique to plethodontid larvae, and we suggest that this might be associated with their historical patterns of life-cycle evolution. If ancestral plethodontids were direct developers, then biphasic lineages are derived decelerations of an ancestrally nonfeeding form. Direct developers transform into a fully terrestrial salamander within the egg, and with yolk as a sole resource. Perhaps during their history as direct developers, feeding-based signals (e.g., those produced by fat reserves such as leptin; Crespi and Denver 2006) were lost, played a reduced role in stimulating morphogenesis, or were overridden by other processes. Therefore, the fixed-rate metamorphic pattern predominates. Metamorphosis of the re-evolved free-living larvae of some biphasic plethodontids might be governed by a signaling system more similar to their direct-developing ancestors. A wide range of manipulation and comparative experiments could test this hypothesis.
Is the Metamorphic Response to Thyroid Hormones and Corticosterone Different in Plethodontids?
In amphibians, metamorphosis is regulated by the hypothalamus–pituitary–thyroid (HPT) axis via the production of thyroid hormones (THs), specifically thyroxine (T4) and triiodothyronine (T3; Rose 2005, 2009; Brown and Cai 2007; Bonett 2016). Most of what is known has been derived from work with frogs, principally Xenopus and Rana (e.g., Buchholz et al. 2007), although recent work with ambystomatid salamanders (especially the axolotl, Ambystoma mexicanum) has also added to this information (Boorse and Denver 2002; Page et al. 2008, 2009). T3 is the more active form that regulates transcription and morphogenesis; the conversion of T4 to the more active T3 is controlled by deiodinases in the cytoplasm of target tissues. The role of deiodinases has been intensively explored in frogs (e.g., Brown 2006), but not in salamanders (but see Galton 1992). Because simply immersing most larval amphibians in THs induces transcription and morphogenesis, this hormone is an excellent tool for research programs in vertebrate developmental biology.
The influences of TH on plethodontid metamorphosis have been investigated in many species (Table 2; recently reviewed in Bonett 2016), but mechanistic details are limited, especially compared with the metamorphic models of Xenopus and axolotl. In most of these cases, TH treatment was only performed to determine if a species could be induced to metamorphose (e.g., Kezer 1952; Dundee 1957, 1962). The early TH treatments were often based on crude doses, limited replication, small sample sizes, and no statistics. Furthermore, it is difficult to compare the responses of different species and life histories because the studies were based on different (1) durations of exposure, (2) TH variants (i.e., T3 or T4), (3) concentrations of TH, (4) laboratory conditions, and (5) the assay to determine TH efficacy (e.g., transcriptional response in a target tissue vs. complete metamorphosis by the individual; Table 2). Nevertheless, these early studies demonstrate that several species of Eurycea can be at least partially transformed with thyroid hormones. However, not all exogenous treatments of larval plethodontids have produced transformation. A study of young larval Desmognathus quadramaculatus treated with T4 failed to metamorphose (Hickerson et al. 2005).
Table 2.
Summary of studies on plethodontids treated with thyroid hormone (TH). T3 = triiodothyronine; T4 = thyroxine.
| Taxon | TH | Dosage (nM) | Duration (d) | Metamorphic response? | Reference |
|---|---|---|---|---|---|
| Larvae | |||||
| Desmognathus quadramaculatus | T4 | 1.2–4.8 | 62 | No | Hickerson et al. 2005 |
| Eurycea bislineata | T4 | 50 | 1–2 | Yes | Alberch et al. 1985 |
| Eurycea bislineata | T4 | 0.05 | 84–168 | No | Rose 1995a |
| Eurycea bislineata | T4 | 0.5 | 42 | Partial | Rose 1995a |
| Eurycea bislineata | T4 | 5–50 | 21 | Yes | Rose 1995a |
| Eurycea tynerensis | T3 | 25 | 21 | Yes | Aran et al. 2014 |
| Paedomorphs | |||||
| Eurycea neotenes | T4 | 1200–2400 | 19 | Yes | Kezer 1952 |
| Eurycea rathbuni | T4 | 1200–12,000 | 53 | Yes | Dundee 1957 |
| Eurycea tynerensis | T3 | 25 | 21 | No | Aran et al. 2014 |
| Eurycea tynerensi | T4 | 2400 | 17 | Yes | Kezer 1952 |
| Eurycea wallacei | T4 | 600–12,000 | 15–25 | Yes | Dundee 1962 |
| Gyrinophilus palleucu | T4 | 2400 | 120–470 | Yes | Dent and Kirby-Smith 1963 |
| Direct developers | |||||
| Plethodon cinereus | T4 | 6000–12,000 | 6 | Yes | Dent 1942 |
Analyses of plasma hormones in Eurycea bislineata have shown an increase in the number of individuals with elevated levels of circulating TH during natural metamorphosis (Alberch et al. 1986). However, neither T3 nor T4 was detected in many individuals that were in the process of metamorphosis. The reason for the lack of TH detection in some individuals was discussed (Alberch et al. 1986), but has not been resolved. Exogenous T4 treatments of larval E. bislineata have demonstrated size- and dose-dependent responses in cranial development (Rose 1995a,b, 1996). Eurycea bislineata showed more abrupt changes in the transformation of cranial components compared with larvae of nonplethodontids. One interpretation is that other families have a more gradual increase in plasma TH concentration throughout larval development that is associated with a gradual cranial metamorphosis (Rose 1996, 1999). More recently, a study has shown T3 sensitivity differences in transcription and metamorphic timing among biphasic and paedomorphic populations of E. tynerensis (Aran et al. 2014). It is tempting to suggest an important regulatory role for deiodinases that could account for differences in metamorphic competence among structures, species, and life histories. Perhaps some tissues in premetamorphic-age plethodontid larvae do not produce deiodinases at sufficient concentrations to allow for T4 to induce metamorphosis early.
The hypothalamus–pituitary–interrenal (HPI) axis can provide a mechanism of detection of environmental stressors (e.g., habitat desiccation). The HPT and HPI axes interact in complex ways to influence development, growth, and metamorphosis (Kulkarni and Buchholz 2012). For example, the release of corticosterone (CORT) from the interrenal glands can alter the effects of TH on morphogenesis and synergistically influence the rate of metamorphosis (Kikuyama et al. 1983; Bonett et al. 2010). The utility of cross-talk between these axes allows aquatic larvae under environmental stress to accelerate its transition to land. However, most studies that have evaluated the influence of the HPI axis on amphibian metamorphosis have been based on pond-dwelling species.
Analyses of CORT in plethodontids have largely dealt with physiological (rather than developmental) responses in paedomorphic adults (Gabor et al. 2016) and terrestrial adults (Wack et al. 2013; Thomas et al. 2017). Recent experiments indicate synergistic influences of CORT on T3-induced metamorphosis in Eurycea tynerensis, (R.M. Bonett, personal observations). We predict, however, that a metamorphic response to CORT will be less potent in stream-dwelling compared with pond-dwelling larvae from seasonally ephemeral environments. Most larval plethodontids have multiyear stream-dwelling larvae, even though many live in environments with strong seasonal variation. It is necessary that metamorphosis is not triggered under moderate stress (e.g., brief temperature or water-level changes). A reduced reliability on the stress axis in stream-dwelling plethodontids (compared with other salamanders) might also explain the relative paucity of plethodontids that exhibit facultative life cycles. The context of a direct-developing ancestor and the evolution of a stream-dwelling larval ecology can provide useful framework for designing comparative experiments and further testing the developmental drivers of plethodontid metamorphosis.
Models for Plethodontid Life History and Plasticity
Ryan and Bruce (2000) provided a model of life-cycle evolution in the spelerpine plethodontids that emphasized heterochronic shifts in three key life-history events: hatching, metamorphosis, and maturation. The importance was that the timing of these events can be either accelerated or decelerated, and these episodes of heterochrony in one of these events can be considered either independent or dependent of each of the others. Although this model was focused on the spelerpines (i.e., Eurycea, Gyrinophilus, Pseudotriton, and Stereochilus), it can work equally well to understand life-cycle diversity across Plethodontidae.
Bonett (2016) extended this model by incorporating what is known about the endocrinology of amphibian metamorphosis. The transitions between metamorphic and direct-developing life cycles are predicted to be based on genetic changes in baseline TH release or responsiveness (Bonett 2016). Other endocrine axes might also play a role in influencing variation around baseline HPT patterns (Bonett 2016), but these factors have yet to be tested. Furthermore, it is reasonable to consider that endocrine patterns have been optimized to match diverse larval habitats. Determining the relative importance of HPT and HPI regulation of metamorphic timing among lineages with different ecologies and life-history modes will enlighten our understanding of the developmental patterns that have shaped plethodontid life-cycle evolution.
Acknowledgments
These ideas and this manuscript have been a bit like the larval period of Gyrinophilus porphyriticus: very long with very slow growth. We hope this manuscript represents a full metamorphosis. Some of these ideas were presented in 2007 at the 5th Conference on the Biology of Plethodontid Salamanders; some were presented in 2014 at 6th Conference in Tulsa, OK; and some were presented in the meetings that culminated in this issue. CKB and TJR owe a debt to R.C. Bruce for brooding our interest in plethodontid biology. During this long larval development, CKB has been supported by ND INBRE, North Dakota Department of Game and Fish, and the Edward G. Schlieder Professorship of Southeastern Louisiana University, and RMB has been supported by grants from the National Science Foundation (DEB 1050322 and OK EPSCoR IIA-1301789).
Literature Cited
- Alberch P, Lewbert GA, Gale EA. The fate of larval chondrocytes during the metamorphosis of the epibranchial in the salamander, Eurycea bislineata. Journal of Embryology and Experimental Morphology. 1985;88:71–83. [PubMed] [Google Scholar]
- Alberch P, Gale EA, Larsen PR. Plasma T4 and T3 levels in naturally metamorphosing Eurycea bislineata (Amphibia; Plethodontidae) General and Comparative Endocrinology. 1986;61:153–163. doi: 10.1016/0016-6480(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Alford RA. Ecology: Resource use, competition, and predation. In: McDiarmid RW, Altig R, editors. Tadpoles: The Biology of Anuran Larvae. University of Chicago Press; USA: 1999. pp. 240–278. [Google Scholar]
- Alford RA, Harris RN. Effects of larval growth history on anuran metamorphosis. American Naturalist. 1988;131:91–106. [Google Scholar]
- Aran RP, Steffen MA, Martin SD, Lopez OI, Bonett RM. Reduced effects of thyroid hormone on gene expression and metamorphosis in a paedomorphic plethodontid salamander. Journal of Experimental Zoology (Molecular and Developmental Evolution) 2014;322B:294–303. doi: 10.1002/jez.b.22580. [DOI] [PubMed] [Google Scholar]
- Audo MC, Mann TM, Polk TL, Loudenslager CM, Diehl WJ, Altig R. Food deprivation during different periods of tadpole (Hyla chrysoscelis) affects metamorphic performance differently. Oecologia. 1995;103:518–522. doi: 10.1007/BF00328691. [DOI] [PubMed] [Google Scholar]
- Beachy CK. Age at maturation, body size, and life-history evolution in the salamander family Plethodontidae. Herpetological Review. 1995a;26:179–181. [Google Scholar]
- Beachy CK. Effects of larval growth history on metamorphosis in a stream-dwelling salamander (Desmognathus ochrophaeus) Journal of Herpetology. 1995b;29:375–382. [Google Scholar]
- Beachy CK. Effects of growth history and thyroxine-induced acceleration on metamorphic timing in the toad Bufo americanus (Amphibia, Anura) Copeia. 2001;2001:829–834. [Google Scholar]
- Beachy CK, Bruce RC. Lunglessness in plethodontid salamanders is consistent with the hypothesis of a mountain stream origin: A response to Ruben and Boucot. American Naturalist. 1992;139:839–847. [Google Scholar]
- Beachy CK, Bruce RC. Life history of a small form of the plethodontid salamander Desmognathus quadramaculatus. Amphibia-Reptilia. 2003;24:13–26. [Google Scholar]
- Beachy CK, Surges TH, Reyes M. Effects of developmental and growth history on metamorphosis in the gray treefrog, Hyla versicolor (Amphibia, Anura) Journal of Experimental Zoology. 1999;283:522–530. doi: 10.1002/(sici)1097-010x(19990501)283:6<522::aid-jez3>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- Beck CW. Effect of changes in resources level on age and size at metamorphosis in Hyla squirrela. Oecologia. 1997;112:187–192. doi: 10.1007/s004420050299. [DOI] [PubMed] [Google Scholar]
- Bendik NF. Demographics, reproduction, growth, and abundance of Jollyville Plateau salamanders (Eurycea tonkawae) Ecology and Evolution. 2017;2017:1–14. doi: 10.1002/ece3.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley N. Life history significance of size-triggered metamorphosis in milkweed bugs (Oncopeltus) Ecology. 1981;62:57–64. [Google Scholar]
- Blanchard FN. The life history of the four-toed salamander. American Naturalist. 1923;57:262–268. [Google Scholar]
- Bonett RM. An integrative endocrine model for the evolution of developmental timing and life history of plethodontids and other salamanders. Copeia. 2016;104:209–221. [Google Scholar]
- Bonett RM, Hoopfer ED, Denver RJ. Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. General and Comparative Endocrinology. 2010;168:209–219. doi: 10.1016/j.ygcen.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonett RM, Steffen MA, Lambert SM, Wiens JJ, Chippindale PT. Evolution of paedomorphosis in plethodontid salamanders: Ecological correlates and re-evolution of metamorphosis. Evolution. 2014a;68:466–482. doi: 10.1111/evo.12274. [DOI] [PubMed] [Google Scholar]
- Bonett RM, Steffen MA, Robinson GA. Heterochrony repolarized: A phylogenetic analysis of developmental timing in plethodontid salamanders. EvoDevo. 2014b;5:27. doi: 10.1186/2041-9139-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorse GC, Denver RJ. Acceleration of Ambystoma tigrinum metamorphosis by corticotropin-releasing hormone. Journal of Experimental Zoology. 2002;293:94–98. doi: 10.1002/jez.10115. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Denver RJ. Endocrine mechanisms underlying plasticity in metamorphic timing in spadefoot toads. Integrative and Comparative Biology. 2004;43:646–657. doi: 10.1093/icb/43.5.646. [DOI] [PubMed] [Google Scholar]
- Brown DD. The role of iodinases in amphibian metamorphosis. Thyroid. 2006;15:815–821. doi: 10.1089/thy.2005.15.815. [DOI] [PubMed] [Google Scholar]
- Brown DD, Cai L. Amphibian metamorphosis. Developmental Biology. 2007;306:20–33. doi: 10.1016/j.ydbio.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RC. A comparison of the larval periods of Blue Ridge and Piedmont Mud Salamanders (Pseudotriton montanus) Herpetologica. 1978;34:325–332. [Google Scholar]
- Bruce RC. A model of the larval period of the Spring Salamander, Gyrinophilus porphyriticus, based on size-frequency distributions. Herpetologica. 1980;36:78–86. [Google Scholar]
- Bruce RC. Application of the Gompertz function in studies of growth in dusky salamanders (Plethodontidae: Desmognathus) Copeia. 2016a;104:94–100. [Google Scholar]
- Bruce RC. Relative growth rates in three species of Desmognathus (Amphibia: Plethodontidae) Herpetologica. 2016b;72:174–180. [Google Scholar]
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Developmental Biology. 2007;303:576–590. doi: 10.1016/j.ydbio.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Chippindale PT, Bonett RM, Baldwin AS, Wiens JJ. Phylogenetic evidence for a major reversal of life-history evolution in plethodontid salamanders. Evolution. 2004;58:2809–2822. doi: 10.1111/j.0014-3820.2004.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Beachy CK. Induction of metamorphosis causes sex-specific allocation patterns in axolotls (Ambystoma mexicanum) that have different growth histories. Journal of Herpetology. 2015;49:621–626. doi: 10.1670/14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ. Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10092–10097. doi: 10.1073/pnas.0507519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JN. The embryonic development of Plethodon cinereus as correlated with the differentiation and functioning of the thyroid gland. Journal of Morphology. 1942;71:577–601. [Google Scholar]
- Dent JN, Kirby-Smith JS. Metamorphic physiology and morphology of the Cave Salamander, Gyrinophilus palleucus. Copeia. 1963;1963:119–130. [Google Scholar]
- Denver RJ, Mirhadi N, Phillips M. Adaptive plasticity in amphibian metamorphosis: Response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology. 1998;79:1859–1872. [Google Scholar]
- Dundee HA. Partial metamorphosis induced in Typhlomolge rathbuni. Copeia. 1957;1957:52–53. [Google Scholar]
- Dundee HA. Response of the neotenic salamander Haideotriton wallacei to a metamorphic agent. Science. 1962;135:1060–1061. doi: 10.1126/science.135.3508.1060. [DOI] [PubMed] [Google Scholar]
- Dunn ER. The Salamanders of the Family Plethodontidae. Smith College; USA: 1926. [Google Scholar]
- Feder ME. Integrating the ecology and physiology of plethodontid salamanders. Herpetologica. 1983;39:291–310. [Google Scholar]
- Frost DR. Amphibian species of the world: an online reference, version 6.0.c. American Museum of Natural History; USA: 2016. Available at http://research.amnh.org/herpetology/amphibia/index.html. Archived by WebCite at http://www.webcitation.org/6s6MQODSa on 20 July 2017. [Google Scholar]
- Gabor CR, Zabierek KC, Kim DS, da Barbiano LA, Mondelli MJ, Bendik NF, Davis DR. A non-invasive water-borne assay of stress hormones in aquatic salamanders. Copeia. 2016;104:172–181. [Google Scholar]
- Galton VA. Thyroid hormone receptors and iodothyronine deiodinases in the developing Mexican Axolotl, Ambystoma mexicanum. General and Comparative Endocrinology. 1992;85:62–70. doi: 10.1016/0016-6480(92)90172-g. [DOI] [PubMed] [Google Scholar]
- Hairston NG. Community Ecology and Salamander Guilds. Cambridge University Press; UK: 1987. [Google Scholar]
- Harris RN. The anuran tadpole: Evolution and maintenance. In: McDiarmid RW, Altig R, editors. Tadpoles: The Biology of Anuran Larvae. University of Chicago Press; USA: 1999. pp. 279–294. [Google Scholar]
- Hensley FR. Ontogenetic loss of phenotypic plasticity of age at metamorphosis in tadpoles. Ecology. 1993;74:2405–2412. [Google Scholar]
- Hentschel BT. Complex life cycles in a variable environment: Predicting when the timing of metamorphosis shifts from resource dependent to developmentally fixed. American Naturalist. 1999;154:549–558. doi: 10.1086/303263. [DOI] [PubMed] [Google Scholar]
- Hentschel NT, Emlet RB. Metamorphosis of barnacle nauplii: Effects of food variability and a comparison with amphibian models. Ecology. 2000;81:3495–3508. [Google Scholar]
- Hickerson CAM, Barker EL, Beachy CK. Determinants of metamorphic timing in the black-bellied salamander, Desmognathus quadramaculatus. Southeastern Naturalist. 2005;4:33–50. [Google Scholar]
- Howard SC, Hentschel BT. Effects of short-term food variability on the plasticity of age and size at metamorphosis of porcelain crab larvae. Limnology and Oceanography. 2005;50:1960–1971. [Google Scholar]
- Hynes HBN. The Ecology of Running Waters. Liverpool University Press; UK: 1970. [Google Scholar]
- Ihli LS, Beachy CK. Experimental analysis of allocation during larval development in ambystomatid salamanders. Herpetologica. 2016;72:1–5. doi: 10.1655/HERPETOLOGICA-D-15-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezer J. Thyroxin-induced metamorphosis of the neotenic salamanders Eurycea tynerensis and Eurycea neotenes. Copeia. 1952;1952:234–237. [Google Scholar]
- Kikuyama S, Niki K, Mayumi M, Shibayama R, Nishikawa M, Shintake N. Studies on corticoid action on the toad tadpole tail in vitro. General and Comparative Endocrinology. 1983;52:395–399. doi: 10.1016/0016-6480(83)90178-8. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Buchholz DR. Beyond synergy: Corticosterone and thyroid hormone have numerous interaction effects on gene regulation in Xenopus tropicalis tadpoles. Endocrinology. 2012;153:5309–5324. doi: 10.1210/en.2012-1432. [DOI] [PubMed] [Google Scholar]
- Leips J, Travis J. Metamorphic responses to changing food levels in two species of hylid frogs. Ecology. 1994;75:1345–1356. [Google Scholar]
- Marburg MR. Age and size at metamorphosis of half-sib larvae of Salamandra infraimmaculata born in the laboratory and raised singly under three different food regimes. Belgian Journal of Zoology. 2009;139:156–165. [Google Scholar]
- McCoy KA, McCoy MW, Amick A, Guillette LG, Jr, St Mary CM. Tradeoffs between somatic and gonadal investments during development in the African Clawed Frog (Xenopus laevis) Journal of Experimental Zoology. 2007;307A:637–646. doi: 10.1002/jez.417. [DOI] [PubMed] [Google Scholar]
- Min MS, Yang SY, Bonett RM, Vieites DR, Brandon RA, Wake DB. Discovery of the first Asian plethodontid salamander. Nature. 2005;435:87–90. doi: 10.1038/nature03474. [DOI] [PubMed] [Google Scholar]
- Morey S, Reznick D. A comparative analysis of plasticity in larval development in three species of spadefoot toads. Ecology. 2000;81:1736–1749. [Google Scholar]
- Mueller RL, Macey JR, Jaekel M, Wake DB, Boore JL. Morphological homoplasy, life history evolution, and historical biogeography of plethodontid salamanders inferred from complete mitochondrial genomes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13820–13825. doi: 10.1073/pnas.0405785101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RA. Adaptive plasticity in development of Scaphiopus couchii tadpoles in desert ponds. Evolution. 1988;42:774–783. doi: 10.1111/j.1558-5646.1988.tb02495.x. [DOI] [PubMed] [Google Scholar]
- Newman RA. Developmental plasticity of Scaphiopus couchii in an unpredictable environment. Ecology. 1989;70:1775–1787. [Google Scholar]
- Newman RA. Ecological constraints on amphibian metamorphosis: Interactions of temperature and larval density with responses to changing food level. Oecologia. 1998;115:9–16. doi: 10.1007/s004420050485. [DOI] [PubMed] [Google Scholar]
- Niemiller ML, Glorioso BM, Fenolio DB, Reynolds RG, Taylor SJ, Miller BT. Growth, survival, longevity, and population size of the Big Mouth Cave Salamander (Gyrinophilus palleucus necturoides) from the type locality in Grundy County, Tennessee, USA. Copeia. 2016;104:35–41. [Google Scholar]
- Nussbaum RA, Tait CK. Aspects of the life history and ecology of the Olympic salamander, Rhyacotriton olympicus (Gaige) American Midland Naturalist. 1977;98:176–199. [Google Scholar]
- O’Laughlin BE, Harris RN. Models of metamorphic timing: An experimental evaluation with the pond-dwelling salamander Hemidactylium scutatum (Caudata: Plethodontidae) Oecologia. 2000;124:343–350. doi: 10.1007/s004420000392. [DOI] [PubMed] [Google Scholar]
- Page RB, Voss SR, Samuels AK, Smith JJ, Putta S, Beachy CK. Effect of thyroid hormone concentration on the transcriptional response underlying induced metamorphosis in the Mexican Axolotl (Ambystoma) BMC Genomics. 2008;9:78. doi: 10.1186/1471-2164-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RB, Monaghan JR, Walker JA, Voss SR. A model of transcriptional and morphological changes during thyroid-induced metamorphosis of the axolotl. General and Comparative Endocrinology. 2009;162:219–232. doi: 10.1016/j.ygcen.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL, Adams DC. Rates of morphological evolution are correlated with species richness in salamanders. Evolution. 2012;66:1807–1818. doi: 10.1111/j.1558-5646.2011.01557.x. [DOI] [PubMed] [Google Scholar]
- Rolff J, Van de Muetter F, Stoks R. Time constraints decouple age and size at maturity and physiological traits. American Naturalist. 2004;164:559–565. doi: 10.1086/423715. [DOI] [PubMed] [Google Scholar]
- Rose CS. Skeletal morphogenesis in the urodele skull: I. Postembryonic development in the Hemidactyliini (Amphibia: Plethodontidae) Journal of Morphology. 1995a;223:125–148. doi: 10.1002/jmor.1052230203. [DOI] [PubMed] [Google Scholar]
- Rose CS. Skeletal morphogenesis in the urodele skull: III. Effect of hormone dosage in TH-induced remodeling. Journal of Morphology. 1995b;223:243–261. doi: 10.1002/jmor.1052230303. [DOI] [PubMed] [Google Scholar]
- Rose CS. An endocrine-based model for the developmental and morphogenetic diversification in metamorphic and paedomorphic urodeles. Journal of Zoology. 1996;239:253–284. [Google Scholar]
- Rose CS. Hormonal control in larval development and evolution. In: Hall BK, Wake MH, editors. The Origin and Evolution of Larval Forms. Academic Press; USA: 1999. pp. 167–216. [Google Scholar]
- Rose CS. Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends in Ecology and Evolution. 2005;20:129–135. doi: 10.1016/j.tree.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Rose CS. Generating, growing and transforming skeletal shape: Insights from amphibian pharyngeal arch cartilages. BioEssays. 2009;31:287–299. doi: 10.1002/bies.200800059. [DOI] [PubMed] [Google Scholar]
- Ruben JA, Reagan NL, Verrell PA, Boucot AJ. Plethodontid salamander origins: A response to Beachy and Bruce. American Naturalist. 1993;142:1038–1051. [Google Scholar]
- Ryan TJ, Bruce RC. Life history evolution and adaptive radiation of hemidactyliine salamanders. In: Bruce RC, Jaeger RG, Houck LD, editors. The Biology of Plethodontid Salamanders. Kluwer Academic/Plenum Press; USA: 2000. pp. 303–323. [Google Scholar]
- Ryan TJ, Semlitsch RD. Growth and the expression of alternative life cycles in the salamander Ambystoma talpoideum (Caudata: Ambystomatidae) Biological Journal of the Linnean Society. 2003;80:639–646. [Google Scholar]
- Semlitsch RD. Growth and metamorphosis of larval dwarf salamanders (Eurycea quadridigitata) Herpetologica. 1980;36:138–140. [Google Scholar]
- Sessions SK, Larson A. Developmental correlates of genome size in plethodontid salamanders and their implications for genome evolution. Evolution. 1987;41:1239–1251. doi: 10.1111/j.1558-5646.1987.tb02463.x. [DOI] [PubMed] [Google Scholar]
- Shen XX, Liang D, Chen MY, Mao RL, Wake DB, Zhang P. Enlarged multilocus data set provides surprisingly younger time of origin for the Plethodontidae, the largest family of salamanders. Systematic Biology. 2016;65:66–81. doi: 10.1093/sysbio/syv061. [DOI] [PubMed] [Google Scholar]
- Thomas JR, Magyan AJ, Freeman PE, Woodley SK. Testing hypotheses about individual variation in plasma corticosterone in free-living salamanders. Journal of Experimental Biology. 2017;220:1210–1221. doi: 10.1242/jeb.149765. [DOI] [PubMed] [Google Scholar]
- Titus TA, Larson A. Molecular phylogenetics of desmognathine salamanders (Caudata: Plethodontidae): A reevaluation of evolution in ecology, life history, and morphology. Systematic Biology. 1996;45:451–472. [Google Scholar]
- Twombly S. Timing of metamorphosis in a freshwater crustacean: Comparison with anuran models. Ecology. 1996;77:1855–1866. [Google Scholar]
- Vaissi S, Sharifi M. Changes in food availability mediate the effects of temperature on growth, metamorphosis and survival in endangered yellow spotted mountain newt: Implications for captive breeding programs. Biologia. 2016;71:444–451. doi: 10.1002/zoo.21327. [DOI] [PubMed] [Google Scholar]
- Voss SR. Relationship between stream order and length of larval period in the salamander Eurycea wilderae. Copeia. 1993;1993:736–742. [Google Scholar]
- Wack CL, Ratay MK, Woodley SK. Effects of corticosterone on locomotory activity in Red-legged Salamanders. Herpetologica. 2013;69:118–126. [Google Scholar]
- Wake DB. Comparative osteology and evolution of the lungless salamanders, Family Plethodontidae. Memoirs of the Southern California Academy of Science. 1966;4:1–111. [Google Scholar]
- Wake DB. What salamanders have taught us about evolution. Annual Review of Ecology, Evolution, and Systematics. 2009;40:333–352. [Google Scholar]
- Wake DB, Hanken J. Direct development in the lungless salamanders: What are the consequences for developmental biology, evolution and phylogenesis? International Journal of Developmental Biology. 1996;40:859–869. [PubMed] [Google Scholar]
- Wetzel RG. Limnology. Saunders Publishing; USA: 1983. [Google Scholar]
- Wilbur HM, Collins JP. Ecological aspects of amphibian metamorphosis. Science. 1973;182:1305–14. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]
- Wilder IW, Dunn ER. The correlation of lunglessness in salamanders with a mountain brook habitat. Copeia. 1920;84:63–68. [Google Scholar]
- Zeller M, Koella JC. Effects of food variability on growth and reproduction of Aedes aegypti. Ecology and Evolution. 2016;6:552–559. doi: 10.1002/ece3.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]