Abstract
European honey bees (Apis mellifera) are critically important to global food production by virtue of their pollination services but are severely threatened by deformed wing virus (DWV) especially in the presence of the external parasite Varroa destructor. DWV exists as many viral strains with the two major variants (DWV-A and DWV-B) varying in virulence. A single plasmid standard was constructed containing three sections for the specific determination of DWV-A (VP2 capsid region), DWV-B (IRES) and a conserved region suitable for total DWV (helicase region). The assays were confirmed as specific and discriminatory with limits of detections of 25, 25 and 50 genome equivalents for DWV-A, DWV-B and total-DWV, respectively. The methods were successfully tested on Apis mellifera and V. destructor samples with varying DWV profiles. The new method determined a more accurate total DWV titre in samples with substantial DWV-B than the method currently described in the COLOSS Beebook. The proposed assays could be utilized for the screening of large quantities of bee material for both a total DWV load overview along with more detailed investigations into DWV-A and DWV-B profiles.
Introduction
The European Honeybee (Apis mellifera) is the most economically important global pollinator, estimated to pollinate crops worth $10billion in the United States of America every year, while in the European Union insect pollination is worth around €14.6billion annually [1,2]. In 2005, insect pollination represented 9.5% of the total global value of agricultural production, which equated to €153billion [3]. Colony numbers across Central Europe and the USA have been declining since the mid-1980s [4,5]. One of the main factors that impacts colony loss are viral infections and their vector, Varroa destructor. V. destructor is an ectoparasite of A. mellifera which jumped host from the Asian Honeybee (Apis cerana) to A. mellifera in the 1960s and subsequently rapidly spread globally [6–8]. At an individual level, V. destructor infestation has been shown to cause immunosuppression and weight loss due to the mites haemolymph feeding activity [9,10]. At a colony level workers, from infested colonies have reduced vitellogenin levels, which is used as a protein store for overwintering bees resulting in lower survival to spring [11]. High individual DWV titres have been found to be associated with overwinter colony losses [12,13].
Twenty four viruses have been identified to date in A. mellifera, eight of which have been found to be associated with V. destructor-vectored transmission, including deformed wing virus (DWV) [14,15]. DWV is a member of the picorna-like insect virus family Iflaviridae, consisting of a 30nm icosahedral particle with a single, positive strand RNA genome [15–17]. Prior to the arrival of V. destructor DWV existed as an endemic covert infection with no visible symptoms in A. mellifera [15,16,18,19]. However, in the presence of V. destructor, overt DWV infections occur giving rise to A. mellifera with deformed wings, bloated, shortened and discoloration of the abdomen and shortened life spans [15]. DWV has been demonstrated to replicate within whole V. destructor [20,21] and isolated tissues [22] rather than acting simply as a mechanical vector. Several studies have highlighted that after V. destructor’s arrival in a new location the DWV viral diversity reduces and viral loads increase [23,24]. Like many RNA viruses, DWV exists as many related variant strains [16,23,25]. Historically, the main variants discussed when considering DWV infection are: the classical DWV strain (DWV-A) and the Varroa destructor virus-1 (VDV-1) strain (DWV-B) and recombinants between the these two variants [16,23,25,26]. Recent molecular analysis of A. mellifera samples from across the UK, has shown DWV-B to be widespread [26]. Detailed global distribution of DWV-B is currently unknown, with a study in the USA not detecting significant amounts of DWV-B [27].
The main method currently utilized in viral load molecular analysis is quantitative PCR (qPCR). Viral loads can be compared by using the Cq (quantification cycle) value; with low Cq values resulting from large amounts of the amplified product indicating higher viral loads [28]. Viral loads of four A. mellifera viruses have been calculated in qPCR assays by the use of standard curves produced from cDNA amplicons following spectrophotometric quantifications [29,30]; however this method requires positive samples to naturally occur during sample collection. To increase the reproducibility and the absolute quantification in qPCR assays plasmid standards containing target sequences can be employed. External plasmid standards are routinely used for the absolute quantification of genes and infection status [31–34]. Currently, there are individual external plasmid standards designed for three honeybee viruses: Chronic bee paralysis virus, Sacbrood virus and DWV [35–38]. Plasmid standards have also been designed for the analysis of DWV-B IRES activity and DWV-B/DWV-A recombination [39,40]. A DWV plasmid assay is the current standard method for DWV quantification detailed in the COLOSS Beebook [37]. A plasmid standard has also been designed to provide in vitro transcription of DWV-specific ssRNA for single strand RT-qPCR analysis, and a linearized RNA transcript [41,42]. Recently, a standard reference plasmid has been designed containing fragments of six A. mellifera viruses (Acute bee paralysis virus, Black queen cell virus, DWV, Israeli acute paralysis virus, Kashmir bee virus, and Sacbrood bee virus) [43].
Most studies looking at DWV infection do not consider the impact that the different variants have on viral load and virulence. In the present study, a new method of quantifying levels of the DWV strains using SYBR Green qPCR has been developed. A single external plasmid standard combining three target areas was designed for the quantification of DWV-A and DWV-B variants, along with the total DWV strain loads. The new assays were assessed on Apis melifera and V. destructor samples and compared favourably with the standard in the COLOSS Beebook method [34].
Methods and materials
DWV variant plasmid quantification primer design
Three sections within the DWV genome were identified to allow quantification of both the DWV-A and DWV-B variants, and a conserved section for multiple DWV variants (Pan-DWV). All primers designed (S1 and S2 Tables) for this plasmid assembly were designed in Primer3Plus [44]. Seven DWV-A and DWV-B strain sequences from multiple countries (JX878305.1, JX878304.1, GU109335.1, AY292384.1, JQ413340.1, AY251269.2, and HM162354.1) were aligned in Mega 6.06 and Clustal Omega [45–47], to aid in the design of the variant specific and conserved sections for use in plasmid assembly. These sequences from a range of countries were chosen to aid the capture of the most viral diversity. The DWV-A variant specific section is located in a conserved region across DWV-A variants (1427-1895nt, AY292384.1, within the VP2 capsid protein region); a larger (468bp) section was designed for plasmid insertion, containing a smaller section (211bp) for quantification primers (DWV-A) (Fig 1). A large DWV-B variant insert (357bp) (7-364nt, AY251269.2, within the nontranslated region (NTR) containing the internal ribosome entry site (IRES) region) was designed to include an 116bp section used for quantification (DWV-B) (VDV-1 IRES primers taken from [25] (Fig 1). A completely conserved region (Pan-DWV section) across all seven sequences within the helicase protein region was used in primer design to allow maximum variant capture. A large insert (375bp) (4883-5257nt, AY292384.1) was designed to include a 179bp section used for quantification (Pan-DWV) (Fig 1). All three sequences were aligned using Mega version 6.06 [47] and analysed in silco to ensure that they were unlikely to cross-hybridise.
Fig 1. Location of quantification viral primers.
1) DWV-B primer location, 2) DWV-A primer location, 3) Pan-DWV primer location, shown on all seven viral sequences used. Bold and Underlined sequences identify which sequence was used for primer design, numbers correspond to location on genome sequences (Forward primers on the left, Reverse on the right), blue highlighted (*) areas indicate consensus and red areas indicate mismatch locations, diagonal double line break indicates removed sequence section between primer locations.
Variant-specific primer design
To test for variant specificity, tagged primers were created for the variant specific DWV-A and DWV-B primer sites, by designing primers that would amplify the opposite variant to the primers intended target (DWV-A variant specific (DWVA_VS) and DWV-B variant specific (DWVB_VS) (S1 Table). These primers were designed due to the close nucleotide identity between DWV-A and DWV-B, to ensure the variant specificity of the assay. The primers were designed to detect the mismatched sequences in the quantification primer sites (Fig 1). The DWV-A variant specific primers were designed to match the sequence 6 (Panel 2, Fig 1) and the DWV-B variant specific primers were designed to match sequence 5 (Panel 1, Fig 1). These variant-specific primers were used to create variant-specific plasmids to allow the assay quantification primers to be tested. RNA was extracted using TRIzol® Reagent (Sigma-Aldrich, Gillingham, UK) from nine individual worker A. mellifera collected from the University of Aberdeen apiary. cDNA was synthesized using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, USA) from 500ng RNA per sample, and then pooled to allow the viral primers to capture as many variants as possible for inserts. PCR was performed for each primer set using 25μl BioMix Red (Bioline Reagents Limited, London, UK), 1μl 10μM primer sets, 22μl H2O and 2μl template cDNA (1/5 dilution). The following PCR profile was used: 15 minutes at 95°C, followed by 36 cycles of 30 seconds at 94°C, 1 minute at 58°C, 30 seconds at 72°C, followed by 10 minutes at 72°C. PCR products were analysed by gel electrophoresis on 1% agarose Tris-borate-EDTA (TBE) gel with SYBR® safe DNA gel stain (ThermoFisher Scientific, Loughborough, UK). The resultant PCR products (DWVA_VS and DWVB_VS) were subsequently used in a TOPO® TA Cloning reaction for plasmid insertion using the pCR®4-TOPO plasmid (Invitogen, Life technologies, UK). Plasmids were transfected into Escherichia coli competent cells (JM109) (Promega, Southampton, UK), and bacterial colonies grown overnight on LB agar with ampicillin (100mg/ml). Colony picks and PCR was performed to confirm plasmid insert uptake using insert primers (DWVA_VS and DWVB_VS primers, respectively) (S1 Table). Positive colonies were grown overnight in LB broth with ampicillin and the plasmid preps were subsequently isolated using a QIAprep® Spin Miniprep Kit (Qaigen, Manchester, UK). PCR was performed using the variant specific primers to ensure plasmid preps contained the correct insert (S1 Table).
To check the specificity of the assay quantification primers (DWV-A and DWV-B) (S2 Table), qPCR was carried out using the variant-specific plasmids and A. mellifera cDNA (1/100 dilution) as positive controls for the assay primers. The DWV-A variant-specific plasmid (1/50 dilution) was tested against the DWV-A primers, and the DWV-B variant-specific plasmid (1/50 dilution) was tested against the DWV-B primers. If assay primers were specific as designed then no amplification product would be expected with the variant-specific plasmids as template.
All qPCRs were carried out using the Bio-Rad CFX Touch Real-Time PCR detection system, and all analyses performed with the Bio-Rad FCX Manager (Bio-Rad). SYBR Green fluorescence detection was used and all samples were done in triplicate in 20μl reactions containing 10μl iTaq Universal SYBR Green Supermix (Bio-Rad), 1μl of 10mM primer sets, 4μl H2O and 5μl template cDNA, per primer using the following qPCR profile: 3 minutes at 95°C, followed by 36 cycles of 15 seconds 94°C, 30 seconds of 58°C, and 15 seconds of 72°C, followed by 10 minutes at 72°C, and a melt curve from 65°C to 95°C increasing 0.5°C every 5 seconds.
DWV variant plasmid assembly
Following the successful testing of the primer specificity through the production of variant specific plasmids, three individual plasmids (DWV-A, DWV-B and Pan-DWV) were created using the cloning protocol described above. These three plasmids were created to allow all three primer sets to be used with each plasmid to ensure there was no cross-reactivity prior to DWV variant plasmid construction. Custom T3 and T7 forward and reverse DNA sequencing (Eurofins Genomics, Germany) was carried out on all three plasmids. Sequence identity was confirmed to be either DWV-A (Pan-DWV and DWV-A plasmid) or DWV-B (DWV-B plasmid) following BLASTn searches. Sequences were aligned in MEGA 6.06 to ensure they matched predicted sequences prior to DWV variant plasmid assembly. The three inserts were arranged using the NEBuilder Assembly Tool (New England BioLabs (UK), Hitchin, UK) to enable a Gibson assembly reaction to be used combing all three sections in a pCR®4-TOPO plasmid (S1 Fig). Inserts were generated using Gibson assembly PCR primers (S1 Table) and annealing temperature (60°C) using the PCR profile given above. Following insert generation the Gibson Assembly reaction was carried out following the manufacturer’s instructions for 2–3 fragment assembly (New England BioLabs). This initial product was then used as template in a subsequent PCR reaction to increase the starting material (DWVL_F and DWVBL _R). The amplified PCR product was then cloned into JM109 E.coli competent cells and plasmid preps were produced as described above. PCR was carried out on plasmid preps using DWVL_F and DWVBL_R primers along with variant specific qPCR primers, with positive samples being confirmed via DNA sequencing (Eurofins Genomics) and qPCR analysis. Positive plasmid prep samples were pooled and concentration quantified using a Nanodrop ND-1000 microspectrophotometer (Thermoscientfific, Loughborough, UK). Exact genome equivalents (ge) were calculated using the following formula (http://www6.appliedbiosystems.com/support/tutorials/pdf/quant_pcr.pdf); based on molecular weight of the plasmid and plasmid DNA concentration, to produce standard stock solutions of known genome equivalent concentration. To test the linearity of the single construct plasmid assay, standard curves for all three primer pairs were created ranging from 100,000,000 to 100 plasmid copies per qPCR reaction. Linearity was also tested using A. mellifera cDNA to create a serial dilution containing six dilutions of pooled A. mellifera (1/5 dilution) of cDNA. All linearity test qPCRs were performed in 10ul reactions (5μl iTaq Universal SYBR Green Supermix (Bio-Rad), 0.5μl of 10μl primer sets, 2μl H2O, 2.5μl template cDNA). All standards and cDNA samples were assayed in quadruple using the thermocycler profile conditions, described above.
The limit of detection (LOD) for each primer set was calculated via the LOD95% method, using 75, 50, and 25 plasmid copies per qPCR reaction based on the lowest quantity that can be detected in at least 95% of the wells [48]. LOD was tested on 10 samples of each dilution quantity. PCR products from A. mellifera cDNA from the University of Aberdeen Apiary using the Pan-DWV, DWV-A or DWV-B primers (12 samples per primer pair). PCR products were purified using a QIAquick PCR Purification Kit (Qiagen, Manchester, UK) and were sent for primer specific-sequencing (Eurofins Genomics, Germany), to confirm all three primer pairs were only detecting DWV variants.
DWV variant plasmid external standard qPCR assessment on biological samples
To assess the plasmid and assays for absolute quantification in biological samples, A. mellifera worker samples were collected from the University of Aberdeen Apiary (Newburgh, Aberdeenshire, NJ9926), two apiaries in Aberdeenshire (Inverurie, Aberdeenshire), an apiary in the V. destructor-free Beinn Eighe National Nature Reserve (Anancaun, Ross-shire, NH0262), and four apiaries in South East England (Essex, Bedfordshire, and two locations in Suffolk). V. destructor samples were collected from brood combs from the University of Aberdeen apiary. All samples from the Aberdeen area were collected and stored at -80°C within hours. All other samples were transported to Aberdeen in RNAlater (Sigma-Aldrich) and then stored at -80°C. Heads were removed from A. mellifera prior to extraction as bee heads are reported to contain PCR inhibitors [49,50].Bees underwent RNA extraction individually, as described above. A. mellifera samples were standardised prior to cDNA synthesis to give 500ng RNA per reverse transcription reaction, V. destructor samples were extracted in pools of three individuals, then standardised prior to cDNA synthesis to give 1000ng RNA per reverse transcription reaction. cDNA was synthesized using iScript™ cDNA Synthesis Kit (Bio-Rad). Sample cDNA (1/5 dilution) was pooled by location for A. mellifera prior to qPCR analysis. Three pools of three individuals were created for the University Aberdeen, two pools of 4 individuals for Aberdeenshire, and two pools of four individuals for Beinn Eighe. For South East England each location resulted in one pooled sampled, four individuals from Essex, Bedfordshire, and Mid-Suffolk, and two individuals from East-Suffolk. The V. destructor samples remained separate as three samples of three individuals (1/15 dilution). Comparison of the DWV loads determined by the Pan-DWV assay and the method described in the Beebook [37] based on primers from Genersch, [51] was performed on eight University of Aberdeen samples and eight South East England samples (four from Essex, and four from Mid-Suffolk).
All qPCRs were performed in 20μl reactions (10μl iTaq Universal SYBR Green Supermix (Bio-Rad), 1μl of 10μM primer sets, 4μl H2O, 5μl template cDNA (1/5 dilution of cDNA for all A. mellifera samples, or 1/15 dilution of cDNA for all V. destructor samples). Standards and unknown samples were assayed in triplicate using the thermocycler profile conditions described above. New dilutions for plasmid standard curves were prepared from stocks for each assay. Viral loads of samples were calculated using the linear regression equation from the standard curve (Log10.ge vs Cq value) from the DWV variant plasmid. Five individuals from the University of Aberdeen were analysed individually to examine the variation between individual A. mellifera samples.
Data analysis
All statistical analyses were performed on log10 transformed viral titre data, but are presented graphically and in the text in untransformed format. Regression analyses were performed on the data for the plasmid standard curve and serially diluted A. mellifera cDNA to assess the linear relationship across the range for Pan-DWV, DWV-A and DWV-B. The mean bias and linearity uncertainty (ULINi) of the assay were determined for each variant section following the methods of Blanchard et al. [35].The relationship between DWV-A and Pan-DWV titres in five A. mellifera from the University of Aberdeen was determined by calculating the Pearson’s correlation coefficient. Comparison of the DWV titres determined by the Beebook Method [34] and the Pan-DWV method was assessed by a two-tailed 1-sample Z-test of the relative DWV titre [(Pan-DWV / Beebook Method)*100]. Statistical tests were performed in Minitab version 18.
Results
Specificity, linearity and sensitivity of the DWV-A, DWV-B, and DWV assays
In silco analysis was performed on all three variant qPCR amplicons to ensure all sequence similarities were within DWV variants. The seven DWV sequences presented in Fig 1 and a further seven complete DWV sequences (KX373899.1, KY909333.1, AJ489744.2, KT004425.1, KU847397.1, MF036686.1, KX783225.1) were analysed in silco for primer specificity. The Pan-DWV primer sites were found in all 14 sequences, the DWV-A primer sites were found in all DWV-A sequences either as a perfect match or with one base mismatches (none within the 3’ end) with the exception of one sequence which had two mismatches. The DWV-B primer sites were conserved in all the DWV-B sequences.
When the DWV-A-specific primers were tested with the plasmid construct containing the DWV-B variant of that VP2-capsid region, there was no detection of any product (Ct > 35 cycles, no determinable melting point). Likewise, the DWV-B specific primers detected nothing with the plasmid containing the DWV-A variant of that IRES region. Conversely, the DWV-A specific primers readily amplified a product from the plasmid containing the DWV-A variant of the VP2-capsid region and the DWV-B primers amplified a product from the plasmid containing the DWV-B variant of the IRES region. Thus, the DWV-A and DWV-B primers demonstrated specificity for the appropriate DWV strain.
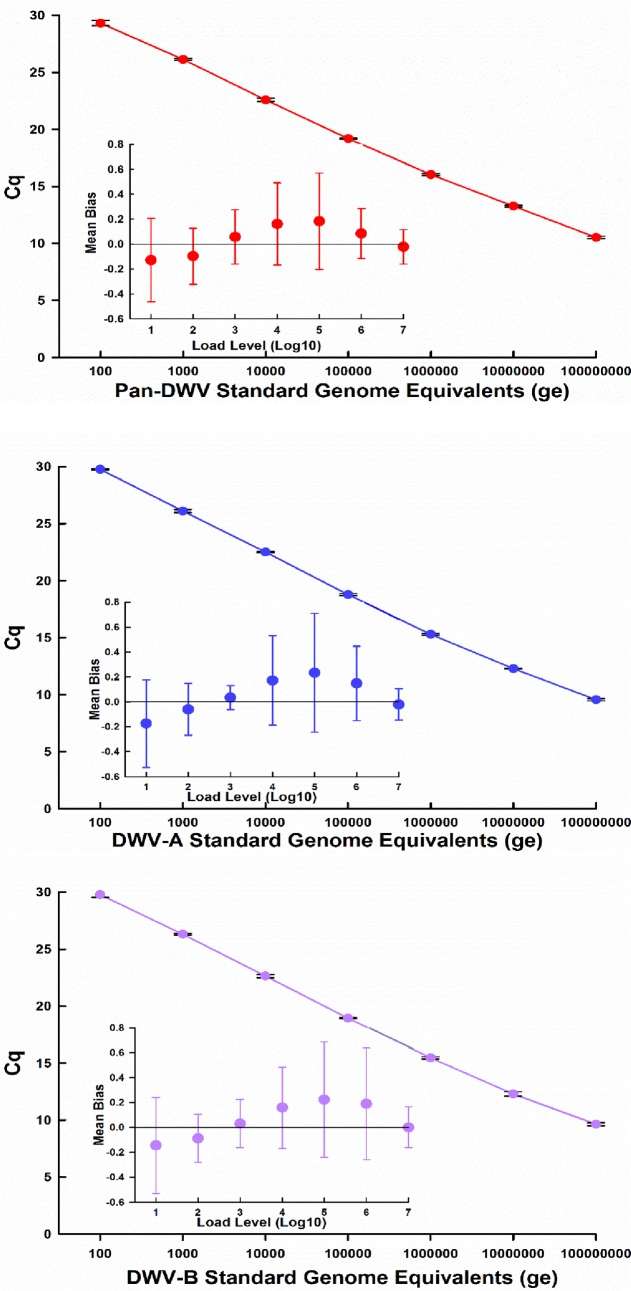
The plasmid sections were determined to have good efficiencies (95.7%-109%) producing dynamic linear curves across a large range (100–100,000,000 plasmid copies per qPCR reaction) for all three variants DWV-A, DWV-B and Pan-DWV contained within the single constructed plasmid (Pan-DWV R2 = 0.997, DWV-A R2 = 0.997, and DWV-B R2 = 0.996, Fig 2). The mean bias and linearity uncertainty (ULINi) of the assay were determined for each variant section, as described by Blanchard et al. [35]. For all three qPCRs, the absolute mean bias values at all concentrations tested were ≤ 0.25 log10.
Fig 2. Standard curves and linear uncertainty plots for Pan-DWV, DWV-A and DWV-B assays.
Standard curves and linearity uncertainty (ULINi) plots for each primer pair using the single constructed plasmid external standard containing fragments for A.) Pan-DWV, B.) DWV-A and C.) DWV-B. Data for the standard curve represent mean ± SE performed in quadruple. Linear regression performance was determined by calculating the mean bias for each load, with bars representing the linearity uncertainty (ULINi).
The limit of detection for each DWV variant was empirically determined [48], indicating the lowest amount of copies per qPCR reaction which can be detected in at least 95% wells. The lowest viral loads that can be reproducibly determined were: Pan-DWV = 50, DWV-A and DWV-B both = 25 plasmid copies per qPCR reaction. Each primer set gave a specific melt curve following qPCR, these temperatures were different for each amplicon (DWV-A = 77 ± 0.042°C; DWV-B = 74.8 ± 0.071°C; Pan-DWV = 80.2 ± 0.74°C), allowing each section to be easily identified during analysis.
The assay’s linearity was also assessed with a serial dilution series (neat to1:100,000 dilution) of 1/5 dilution of cDNA produced from a pool of nine University of Aberdeen A. mellifera. The serial dilution series displayed linearity (S2 Fig) for all three variant targets over a wide dynamic range (Pan-DWV R2 = 0.998, DWV-A R2 = 0.998, and DWV-B R2 = 0.996). PCR products from A. mellifera cDNA from the University of Aberdeen Apiary using the Pan-DWV, DWV-A or DWV-B primers (12 samples per primer pair) were sequenced and homologues in GenBank retrieved by BLASTn and aligned. In all three cases, the primer pair used generated an amplicon whose sequence matched the expected target confirming discriminatory specificity for the DWV-A and–B qPCR assays and no false negatives with the Pan-DWV primers.
DWV variant composition in individual A. mellifera and V. destructor
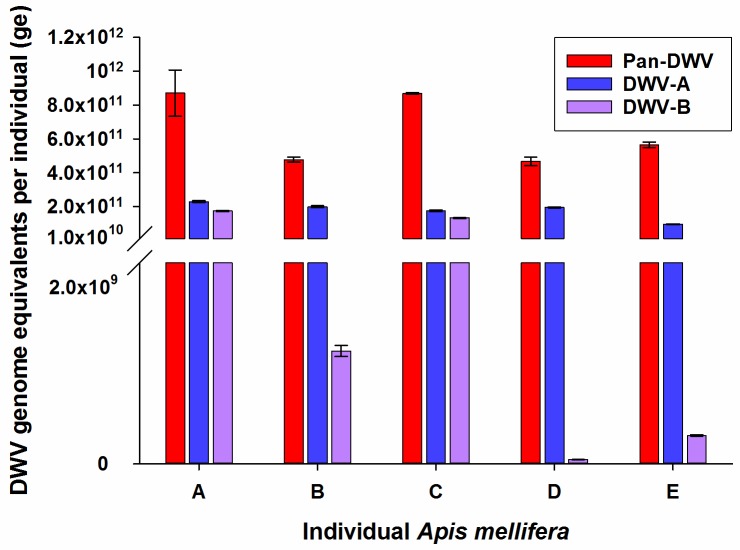
Five individual A. mellifera workers from the University of Aberdeen apiary were analysed to determine the level of variation in the DWV variant composition in individual bees (Fig 3). Even within this single colony there was great variation in the relative proportion that the DWV-A and DWV-B contributed to the total Pan-DWV titre. For example, individuals A and C had almost equal DWV-A and DWV-B loads, while D and E had much higher proportions of DWV-A than DWV-B (Fig 3). The total Pan-DWV titre was always greater than the sum of the variant restricted DWV-A and DWV-B titres with approximately 40% of the total Pan-DWV not accounted for by the sum of DWV-A and DWV-B (Fig 3). Importantly, we determined that total DWV viral load could not be extrapolated from the DWV-A variant specific primer (Pearson’s coefficient correlation r = 0.199, P = 0.749).
Fig 3. DWV-A, DWV-B and Pan-DWV in A. mellifera from heavily Varroa-infested hives.
The DWV-A, DWV-B and Pan-DWV titres in five individual A. mellifera from the University of Aberdeen apiary employing the single plasmid construct as the external standard. Data presented are DWV genome equivalents per individual (total extracted RNA) mean ± SE performed in triplicate, n = 5.
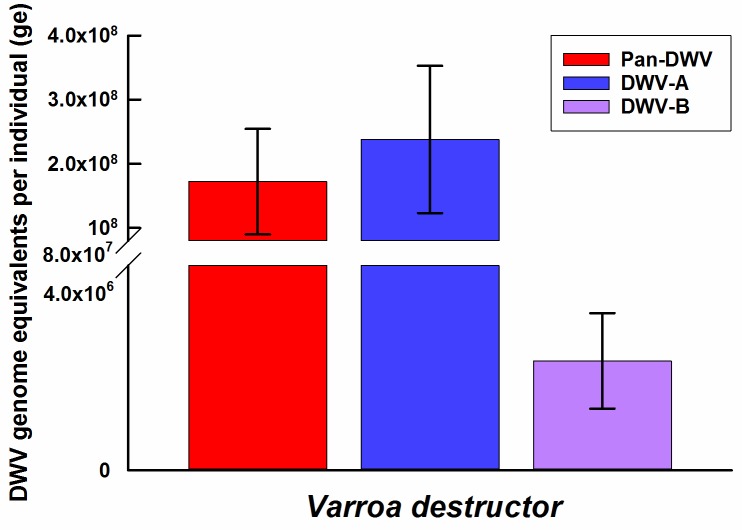
V. destructor collected from the University of Aberdeen apiary were analysed to assess the applicability of the DWV-A, DWV-B and Pan-DWV primers and constructed plasmid external standard to determine the variant composition of DWV within the mites. All three DWV variants were readily detectable within the V. destructor and the total viral load determined with the Pan-DWV primers accounted for the sum of the DWV-A and DWV-B loads (Fig 4). DWV-A was far more predominant than DWV-B within these V. destructor samples.
Fig 4. DWV-A, DWV-B and Pan-DWV in Varroa destructor.
The average DWV-A, DWV-B and Pan-DWV titres in V. destructor from the University of Aberdeen apiary employing the single plasmid construct as the external standard. Data presented are DWV genome equivalents per mite (total RNA from pooled extractions), mean ± SE performed in triplicate of three pools each containing three mites.
Determining DWV composition in A. mellifera in different regions and V. destructor infestation levels
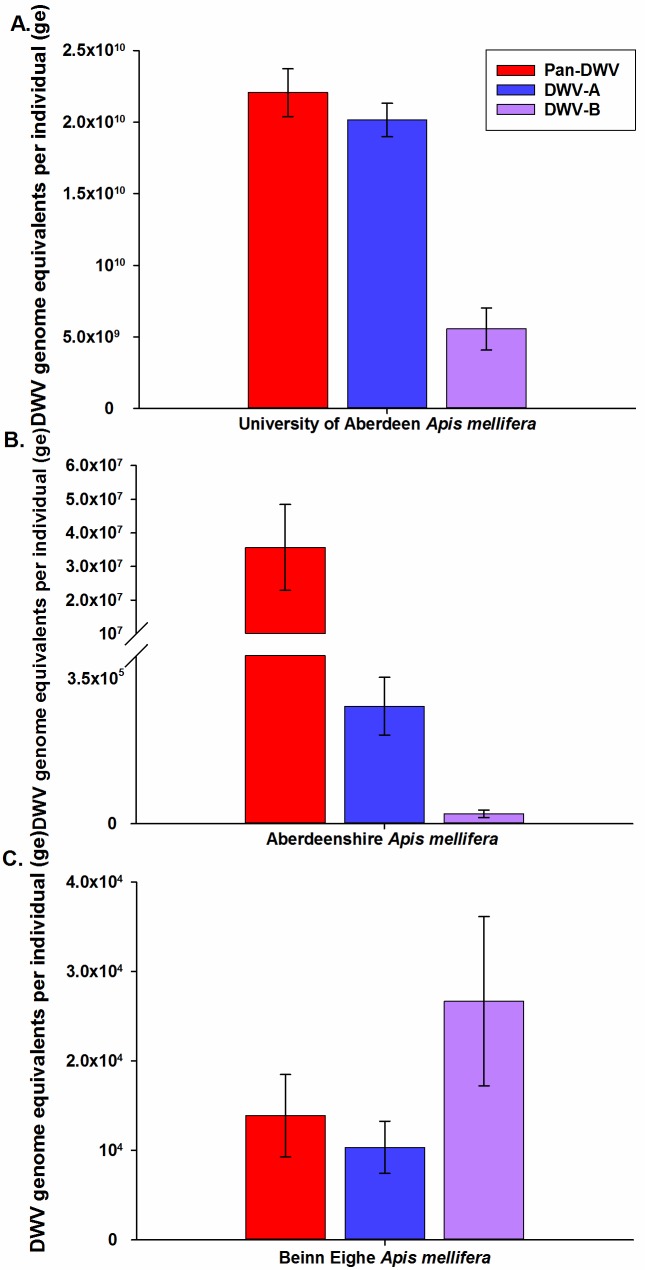
Viral loads were determined in adult worker A. mellifera from different regions within the UK and apiaries containing different infestation levels of V. destructor with all three newly developed assays. In every A. mellifera analysed, both DWV-A and DWV-B were detected, but in widely varying proportions (Fig 5). The Pan-DWV primers consistently detected the highest level of viral load and, in general, this was equal to or greater than the sum of the DWV-A and DWV-B viral loads. A. mellifera from the heavily Varroa-infested colonies at the University of Aberdeen apiary which undergo minimal V. destructor control had extremely high levels of total Pan-DWV, which largely consisted of the DWV-A variant (91%) (Fig 5A), which was also seen in the V. destructor data from this apiary (Fig 4). The Aberdeenshire A. mellifera which are of the same origin and stock as the University of Aberdeen samples, but undergo standard V. destructor treatment and have relatively low V. destructor infestation levels, contained 500X less Pan-DWV (>40,000,000ge vs <20,000,000,000ge, T(df = 1) = 4.98, P = 0.126) compared to the University of Aberdeen samples (Fig 5B). A. mellifera from Beinn Eighe, a totally V. destructor-free region, had extremely low total Pan-DWV consisting of approximately equal levels of DWV-A and DWV-B (Fig 5C). Pan-DWV levels were approximately 1.5 million times lower in the V. destructor-free A. mellifera than the University of Aberdeen samples (>14,000ge vs <20,000,000,000ge, T(df = 1) = 29.5, P = 0.022). Interestingly, the V. destructor-free A. mellifera had relatively higher proportions of DWV-B than DWV-A than V. destructor infested A. mellifera (Fig 5A–5C).
Fig 5. DWV-A, DWV-B and Pan-DWV levels in A. mellifera from hives with differing Varroa infestations.
DWV-A, DWV-B and Pan-DWV titres in A. mellifera from three apiaries in Scotland, A) Heavily Varroa-infested colonies at the University of Aberdeen apiary (n = 9), B) Lowly Varroa-infested colonies at an Aberdeenshire apiary (n = 8), and C) Varroa-free colonies at the Beinn Eighe National Park (n = 8). Data presented are DWV genome equivalents per individual (total extracted RNA), mean ± SE performed in triplicate.
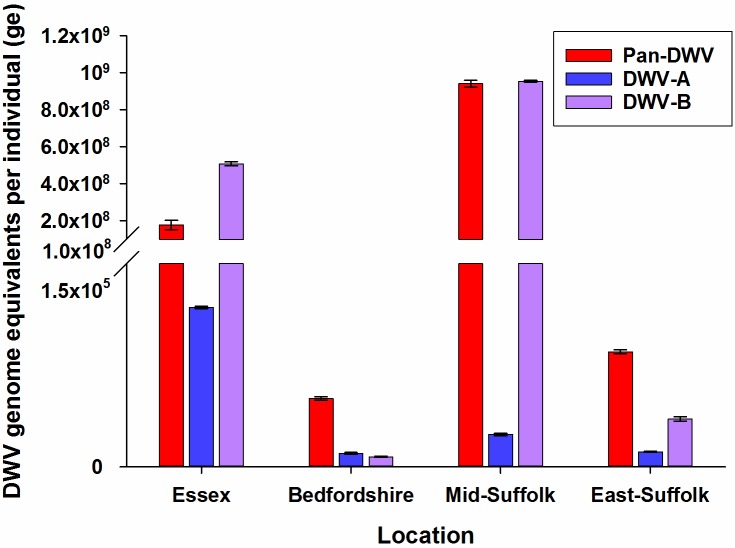
Additionally, we determined the total and variant specific DWV loads in A. mellifera workers in four locations in South Eastern England in apiaries which undergo standard V. destructor treatments and to maintain low V. destructor levels. Across the four locations, total Pan-DWV loads varied considerably and all colonies contained both the DWV-A and DWV-B variants (Fig 6), with both Essex and Mid-Suffolk containing proportionally higher DWV-B levels. Notably, it was obvious that determining only the DWV-A variant would have greatly underestimated the total DWV load in all of the South East England A. mellifera samples (Fig 6).
Fig 6. DWV-A, DWV-B and Pan-DWV levels in A. mellifera in some English colonies.
DWV-A, DWV-B and Pan-DWV titres in A. mellifera from four apiaries in South East England (Essex n = 4, Bedfordshire n = 4, Mid-Suffolk n = 4, East-Suffolk n = 2) employing the single plasmid construct as the external standard. Data presented are DWV genome equivalents per individual (total extracted RNA), mean ± SE performed in triplicate.
Standard Beebook Method vs new Pan-DWV method
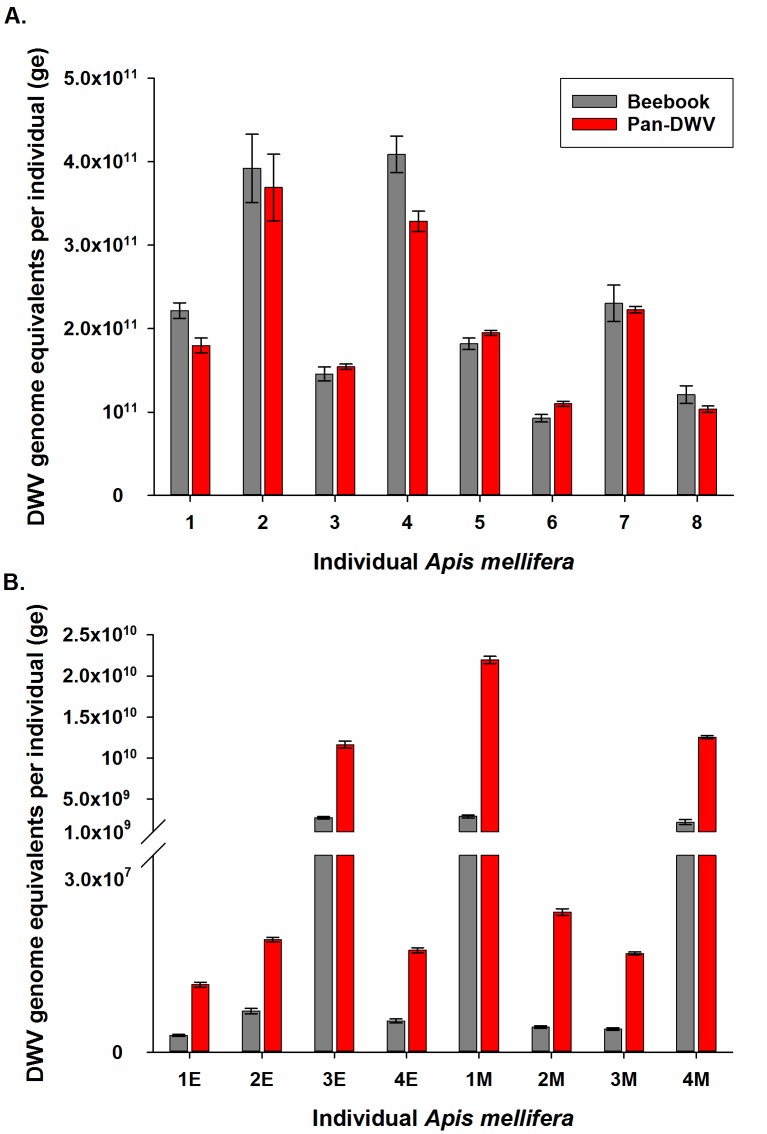
We compared the Beebook Method to the Pan-DWV primer within our assay by analysing eight individual University of Aberdeen A. mellifera and eight individuals from South East England (four from Essex, and four from Mid-Suffolk) (Fig 7). These locations were selected to highlight the impact of strain composition (DWV-A dominant at the University of Aberdeen (Fig 3), and DWV-B dominant in the South East England sites (Fig 6)) on the estimated viral titres using the Beebook method and Pan-DWV primers. In all University of Aberdeen samples from a DWV-A dominant hive, similar viral loads were determined using both the Beebook Method and Pan-DWV primer (Fig 7A) (96.2 ± 4.9% (95% CI 86.7–105.7%), Z = -1.41, P = 0.160). However, in the South East England samples from DWV-B dominant hives, the Pan-DWV primer determined consistently and significantly higher viral loads compared to the Beebook Method (Fig 7B) (471.4 ± 55.8% (95% CI 362–581%), Z = 14.60, P < 0.0001).
Fig 7. Comparison of the Beebook Method and the Pan-DWV method total DWV titre determination.
Total DWV titres in individual A. mellifera from two locations with different dominant strains as determined by the Beebook Method and the Pan-DWV method. A) University of Aberdeen apiary (DWV-A dominant, n = 8) and B) South East England (E = Essex, n = 4; and M = Mid-Suffolk, n = 4) (DWV-B dominant). Data presented are DWV genome equivalents per individual (total extracted RNA), mean ± SE performed on triplicates.
Discussion
As for most viruses, DWV’s existence as variant strains proves a challenge for the accurate quantification of viral levels with A. mellifera and V. destructor. Within the literature, research into DWV can be broadly split, with some focusing solely on detecting titres of specific variant strains, but the majority of studies are interested in reporting total DWV titres [52–54]. Here we report on the development and field-testing of assays for the analysis and quantification of the total DWV (Pan-DWV) and the variant specific DWV-A and DWV-B titres. All assays utilized the same plasmid as the external standard for absolute quantification which contained three DWV viral sequences that can be used to discriminate between DWV-A and DWV-B populations, while also suitable for detecting the majority of the total DWV population (Pan-DWV). The proposed assays have low limits of detection; DWV-A and DWV-B (LOD95%≥25 plasmid copies per qPCR reaction), and Pan-DWV (LOD95%≥50 plasmid copies per qPCR reaction), a wide dynamic range (100–100,000,000 plasmid copies per qPCR reaction) with each of the three amplicons having distinct melting points allowing easy identification of the product. Despite the high nucleotide identity (~84%) that occurs within DWV [16]; each variant-specific section selected and the corresponding primer pairs designed did not cross-react with the other variant. The ability to join multiple viral strains in one combined plasmid has been previously shown [43], with a plasmid combining six different viral sections together, indicating the suitability of this method especially for viral strain investigations. The assay gave similar total viral titres at the individual level, to previously published data on DWV viral titres in A. mellifera with varying levels of V. destructor infestation [23,28].
All three assays rely on a single plasmid construct, resulting in only one standard source having to be prepared, purified, stored and diluted, while allowing it to remain equimolar across assays. Further plasmid or transfected E. coli cells can be readily generated in any lab from the easily shipped original plasmid and shared between researchers. If desired, it should be possible to design a probe-based, multiplexed assay to allow DWV-A, DWV-B and the total DWV titre to be determined in a single qPCR reaction using the regions of conservation and the external plasmid identified in this report. Its demonstrated success in samples from across the whole UK and even in A. mellifera jemenitica in Saudi Arabia (A. Hroobi, unpublished observations) suggest it will be applicable over a very wide geographic range, if not world-wide.
The currently described method for DWV titre determination [37] is presented in the COLOSS Beebook chapter “Standard methods for molecular research in Apis mellifera”. The Beebook Method utilizes primers that were designed in 2005 [51] and have been employed in several studies [20,55]. However, much research in the area of DWV has taken place since 2005 and our understanding of the DWV variants has increased [15,16,23,25,26,28]. It is now accepted that there are two clear DWV variants, the classic DWV (DWV-A) and the VDV-1 strain (DWV-B), and recombinants between these two main variants [16,23,25,26]. DWV-B has been reported to be far more virulent to A. mellifera than DWV-A [26] and it is clearly important in modern DWV studies to be able to consider the tires of DWV-A, DWV-B and the total DWV. The assays presented in this paper have been demonstrated to be able to deliver this requirement of separating the two main variants. While the assays are not designed for recombinant quantification, the Pan-DWV section will detect any recombinants which possess that section of the genome.
The performances of the new assay were compared to the Beebook Method on A. mellifera samples from two geographic locations with different dominant strains, the University of Aberdeen apiary (DWV-A dominant) and two locations in South East England (DWV-B dominant). For the Beebook Method, a plasmid standard was created and primers used as described in the Beebook [37]. In DWV-A dominant hives similar viral titres were obtained with both methods, while in DWV-B dominant hives the Pan-DWV method gave significantly higher viral titres. The differences in viral titres from the DWV-B dominant hives show that the Pan-DWV primers are designed in a highly conserved region of both the DWV-A and DWV-B genomes, while the Beebook primers are located in a region that is conserved within the DWV-A genome but not the DWV-B genome. These differences in conserved regions explain why the Pan-DWV primer is more efficient at detecting DWV-B, when compared to the Beebook Method. The Beebook Method primers were designed in 2005, when we had a less complete picture of the DWV strain diversity, resulting in an understandable DWV-A bias. However, following more recent discoveries into the relationship between DWV-A and DWV-B, methods for total viral titres need to take both strains into account. These results indicate that while the Beebook Method works well in DWV-A dominant samples, in DWV-B dominant samples this method will underestimate total viral titres. Sequences of the amplicons generated by the Pan-DWV primers confirmed that this method was only detecting DWV variants.
The assays described in this report provides a more in-depth analysis of viral infection allowing DWV variant-specific information and total DWV titres to be collected by a qPCR approach. Though the qPCR approach does not provide anywhere near the information that deeper sequencing can provide [25–28], it does allow far more samples to be analysed at a fraction of the cost or time and is, thus, suited to bigger surveys. The DWV-B variant is proposed to be the more pathogenic DWV strain [26], therefore it is important to be able to determine the DWV-A: DWV-B proportions and to investigate DWV-B’s virulence in multi-apiary and multi-colony wide surveys in the field. The titres of DWV-A, DWV-B, and the Pan-DWV were determined in individual A. mellifera at the University of Aberdeen apiary and showed a wide variation between the levels of DWV-A and DWV-B. The DWV variant profile was similarly determined in V. destructor at this apiary, with similar dominant variant distribution (DWV-A dominant variant) and high viral titres to the A. mellifera at this site. These similar relationships in DWV structure between A. mellifera and associated V. destructor has been previously shown, while the V. destructor used in this study did not come from the same hive as the A. mellifera samples, they were from the same apiary [56]. Though not the focus of the present study, it would be interesting to use these DWV assays to investigate possible causes of the varying DWV-A and DWV-B titres in individuals within the same colony relative to age, V. destructor-status or other variables and the subsequent effect on fitness and mortality. The variation in DWV loads in individual A. mellifera within the same colony (as seen in this study) have been shown to be related to caste, age and visible condition [57], but these new variant-specific assays would allow deeper investigations.
The multiple UK locations were selected to allow the assays to be tested on samples with different levels of V. destructor infestation. The University of Aberdeen apiary is minimally treated for V. destructor and has very high infestation levels, whereas the two sites in Aberdeenshire and the four locations in South East England are regularly treated and have relatively low levels of Varroa infestation. Previous research into V. destructor infestation levels on DWV titres has highlighted the complex relationship between V. destructor and DWV. DWV titres were much lower in all the sites with relatively low Varroa infestation levels which regularly undergo V. destructor treatment when compared to the University of Aberdeen apiary which has very high levels of Varroa infestation. Treated hives have lower viral loads when compared to untreated hives [58,59] though V. destructor treatment does not cause an immediate reduction in DWV titres [60]. Additionally, the assay’s suitability for analysis of low viral titre samples was confirmed by analysing bees from a V. destructor-free location within the Beinn Eighe National Nature Reserve, in the Highlands of Scotland [61]. As expected the DWV viral titre of the Beinn Eighe A. mellifera was much lower than that found at other sites.
Conclusion
In conclusion, the three new assays for DWV variant strain quantification described in this report allow for more in-depth analysis of the proposed lesser and more virulent variants (DWV-A and DWV-B, respectively) viral titres in both A. mellifera and V. destructor all using the same plasmid construct for the external standard. We have shown that the current standard method, the Beebook method for DWV quantification, underestimates total viral loads when DWV-B strains are substantial within the hives, when compared to the Pan-DWV method. The method for DWV variant strain viral quantification allows the complicated nature of DWV infections to be teased apart at much lower costs and, thus, permitting a much higher number of samples to be processed than more complex deep sequencing approaches.
Supporting information
(DOCX)
(DOCX)
Forward primers are indicated by black arrows and reverse primers with orange arrows. Viral sections and length are shown above the corresponding sections, and primer product length is given within each section. The grey section shows the location of the T7 binding site, and the green section shows the location of the T3 binding site, to indicate where the inserted sections are location in relation to other features of the pCR4 plasmid.
(DOCX)
Linearity plot created using serial diluted pooled A. mellifera cDNA from the University of Aberdeen apiary, using the single plasmid construct as the external standard. Data presented are DWV genome equivalents per qPCR reaction, mean ± SE performed in quadruple.
(DOCX)
Acknowledgments
The authors wish to thank Mr W. Thrale, Mr Z. Blackmore, Mr J. Quinlan, and Mr J. Palombo for sample collection from the South East of England and Margie Ramsey for Beinn Eighe National Nature Reserve sample collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
ELB was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) EASTBIO Doctoral Training Partnership (http://www.bbsrc.ac.uk) [grant number BB/J01446X/1] and an Eastern Association Regional Studentship (EARS) and The Morley Agricultural Foundation awarded to ASB. CRC was supported by a KTN BBSRC CASE studentship (BB/M503526/1) (http://www.bbsrc.ac.uk), part-funded by the Scottish Beekeeping Association (https://www.scottishbeekeepers.org.uk/) and the Animal Health - Disease Prevention, Scottish Government awarded to ASB CRC. This project received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 613960 (SMARTBEES) (http://www.smartbees-fp7.eu/) awarded to ASB. The funders had no role in study design, data collection and analysis decision to publish, or preparation of the manuscript.
References
- 1.Leonhardt SD, Gallai N, Garibaldi LA, Kuhlmann M, Klein A. Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl Ecol 2013;14(6):461–471. [Google Scholar]
- 2.Watanabe ME. Pollination worries rise as honey bees decline. Science 1994;265(5176):1170 doi: 10.1126/science.265.5176.1170 [DOI] [PubMed] [Google Scholar]
- 3.Gallai N, Salles J, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 2009;68(3):810–821. [Google Scholar]
- 4.Lee KV, Steinhauer N, Rennich K, Wilson ME, Tarpy DR, Caron DM, et al. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 2015;46(3):292–305. [Google Scholar]
- 5.Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, Jones R, et al. Declines of managed honey bees and beekeepers in Europe. J Apic Res 2010;49(1):15–22. [Google Scholar]
- 6.Anderson DL, Trueman JWH. Varroa jacobsoni (Acari: Varroidae) is more than one species. Experimental and Applied Acarology 2000;24(3):165–189. [DOI] [PubMed] [Google Scholar]
- 7.De Jong D, Morse R, Eickwort G. Mite Pests of Honey Bees. Annu Rev Entomol 1982;27:229–252. [Google Scholar]
- 8.Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. J Invertebr Pathol 2010;103(SUPPL. 1):S96–S119. [DOI] [PubMed] [Google Scholar]
- 9.Duay P, De Jong D, Engels W. Weight loss in drone pupae (Apis mellifera) multiply infested by Varroa destructor mites. Apidologie 2003;34(1):61–65. [Google Scholar]
- 10.Yang X, Cox-Foster DL. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci U S A 2005;102(21):7470–7475. doi: 10.1073/pnas.0501860102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amdam GV, Hartfelder K, Norberg K, Hagen A, Omholt SW. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): A factor in colony loss during overwintering? J Econ Entomol 2004;97(3):741–747. [DOI] [PubMed] [Google Scholar]
- 12.Dainat B, Evans JD, Chen YP, Gauthier L, Neumanna P. Dead or alive: Deformed wing virus and varroa destructor reduce the life span of winter honeybees. Appl Environ Microbiol 2012;78(4):981–987. doi: 10.1128/AEM.06537-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Highfield AC, El Nagar A, Mackinder LCM, Noël LM-J, Hall MJ, Martin SJ, et al. Deformed wing virus implicated in overwintering honeybee colony losses. Appl Environ Microbiol 2009;75(22):7212–7220. doi: 10.1128/AEM.02227-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Miranda JR, Bailey L, Ball BV, Blanchard P, Budge GE, Chejanovsky N, et al. Standard methods for virus research in Apis mellifera. J Apic Res 2013;52(4). [Google Scholar]
- 15.Genersch E, Aubert M. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet Res 2010;41(6):54 doi: 10.1051/vetres/2010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Miranda JR, Genersch E. Deformed wing virus. J Invertebr Pathol 2010;103(SUPPL. 1):S48–S61. [DOI] [PubMed] [Google Scholar]
- 17.Ongus JR, Peters D, Bonmatin J, Bengsch E, Vlak JM, van Oers MM. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J Gen Virol 2004;85(12):3747–3755. [DOI] [PubMed] [Google Scholar]
- 18.Bowen-Walker PL, Martin SJ, Gunn A. The Transmission of Deformed Wing Virus between Honeybees (Apis melliferaL.) by the Ectoparasitic Mite Varroa jacobsoni Oud. J Invertebr Pathol 1999;73(1):101–106. doi: 10.1006/jipa.1998.4807 [DOI] [PubMed] [Google Scholar]
- 19.Wilfert L, Long G, Leggett HC, Schmid-Hempel P, Butlin R, Martin SJM, et al. Deformed wing virus is a recent global epidemic in honeybees driven by Varroamites. Science 2016;351(6273):594–597. doi: 10.1126/science.aac9976 [DOI] [PubMed] [Google Scholar]
- 20.Gisder S, Aumeier P, Genersch E. Deformed wing virus: Replication and viral load in mites (Varroa destructor). J Gen Virol 2009;90(2):463–467. [DOI] [PubMed] [Google Scholar]
- 21.Yue C, Genersch E. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J Gen Virol 2005;86(12):3419–3424. [DOI] [PubMed] [Google Scholar]
- 22.Campbell EM, McIntosh CH, Bowman AS. A toolbox for quantitative gene expression in varroa destructor: RNA degradation in field samples and systematic analysis of reference gene stability. PLoS ONE 2016;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin SJ, Highfield AC, Brettell L, Villalobos EM, Budge GE, Powell M, et al. Global Honey bee viral landscape altered by a parasitic mite. Science 2012;336(6086):1304–1306. doi: 10.1126/science.1220941 [DOI] [PubMed] [Google Scholar]
- 24.Mondet F, de Miranda JR, Kretzschmar A, Le Conte Y, Mercer AR. On the Front Line: Quantitative Virus Dynamics in Honeybee (Apis mellifera L.) Colonies along a New Expansion Front of the Parasite Varroa destructor. PLoS Pathog 2014;10(8):e1004323 doi: 10.1371/journal.ppat.1004323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore J, Jironkin A, Chandler D, Burroughs N, Evans DJ, Ryabov EV. Recombinants between Deformed wing virus and Varroa destructor virus-1 may prevail in Varroa destructor-infested honeybee colonies. J Gen Virol 2011;92(1):156–161. [DOI] [PubMed] [Google Scholar]
- 26.McMahon DP, Natsopoulou ME, Doublet V, Fürst M, Weging S, Brown MJF, et al. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc R Soc B Biol Sci 2016;283(1833). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornman RS, Boncristiani H, Dainat B, Chen Y, vanEngelsdorp D, Weaver D, et al. Population-genomic variation within RNA viruses of the Western honey bee, Apis mellifera, inferred from deep sequencing. BMC Genomics 2013;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryabov EV, Wood GR, Fannon JM, Moore JD, Bull JC, Chandler D, et al. A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis mellifera) Prevails after Varroa destructor-Mediated, or In Vitro, Transmission. PLoS Pathogens 2014;10(6):e1004230 doi: 10.1371/journal.ppat.1004230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kukielka D, Esperón F, Higes M, Sánchez-Vizcaíno JM. A sensitive one-step real-time RT-PCR method for detection of deformed wing virus and black queen cell virus in honeybee Apis mellifera. J Virol Methods 2008;147(2):275–281. doi: 10.1016/j.jviromet.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 30.Kukielka D, Sánchez-Vizcaíno JM. One-step real-time quantitative PCR assays for the detection and field study of Sacbrood honeybee and Acute bee paralysis viruses. J Virol Methods 2009;161(2):240–246. doi: 10.1016/j.jviromet.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 31.Li X, Wang X. Application of real-time polymerase chain reaction for the quantitation of interleukin-1ß mRNA upregulation in brain ischemic tolerance. Brain Res Protoc 2000;5(2):211–217. [DOI] [PubMed] [Google Scholar]
- 32.Mengelle C, Pasquier C, Rostaing L, Sandres-Sauné K, Puel J, Berges L, et al. Quantitation of human cytomegalovirus in recipients of solid organ transplants by real-time quantitative PCR and pp65 antigenemia. J Med Virol 2003;69(2):225–231. doi: 10.1002/jmv.10277 [DOI] [PubMed] [Google Scholar]
- 33.Polanco JC, Rodríguez JA, Corredor V, Patarroyo MA. Plasmodium vivax: Parasitemia determination by real-time quantitative PCR in Aotus monkeys. Exp Parasitol 2002;100(2):131–134. doi: 10.1016/S0014-4894(02)00010-3 [DOI] [PubMed] [Google Scholar]
- 34.Yun JJ, Heisler LE, Hwang IIL, Wilkins O, Lau SK, Hyrcza M, et al. Genomic DNA functions as a universal external standard in quantitative real-time PCR. Nucleic Acids Res 2006;34(12):e85 doi: 10.1093/nar/gkl400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanchard P, Regnault J, Schurr F, Dubois E, Ribière M. Intra-laboratory validation of chronic bee paralysis virus quantitation using an accredited standardised real-time quantitative RT-PCR method. J Virol Methods 2012;180(1–2):26–31. doi: 10.1016/j.jviromet.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 36.Blanchard P, Guillot S, Antùnez K, Köglberger H, Kryger P, de Miranda JR, et al. Development and validation of a real-time two-step RT-qPCR TaqMan® assay for quantitation of Sacbrood virus (SBV) and its application to a field survey of symptomatic honey bee colonies. J Virol Methods 2014;197:7–13. doi: 10.1016/j.jviromet.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 37.Evans JD, Schwarz RS, Chen YP, Budge G, Cornman RS, De La Rua P, et al. Standard methods for molecular research in Apis mellifera. J Apic Res 2013;52(4). [Google Scholar]
- 38.Shah KS, Evans EC, Pizzorno MC. Localization of deformed wing virus (DWV) in the brains of the honeybee, Apis mellifera Linnaeus. Virology Journal 2009;6:182 doi: 10.1186/1743-422X-6-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ongus JR, Roode EC, Pleij CWA, Vlak JM, van Oers MM. The 5' non-translated region of Varroa destructor virus 1 (genus Iflavirus): Structure prediction and IRES activity in Lymantria dispar cells. J Gen Virol 2006;87(11):3397–3407. [DOI] [PubMed] [Google Scholar]
- 40.Zioni N, Soroker V, Chejanovsky N. Replication of varroa destructor virus 1 (VDV-1) and a varroa destructor virus 1-deformed wing virus recombinant (VDV-1-DWV) in the head of the honey bee. Virology 2011;417(1):106–112. doi: 10.1016/j.virol.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 41.Boncristiani HF, Di Prisco G, Pettis JS, Hamilton M, Chen YP. Molecular approaches to the analysis of deformed wing virus replication and pathogenesis in the honey bee, Apis mellifera. Virol J 2009;6:221 doi: 10.1186/1743-422X-6-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazzei M, Carrozza ML, Luisi E, Forzan M, Giusti M, Sagona S, et al. Infectivity of DWV associated to flower pollen: Experimental evidence of a horizontal transmission route. PLoS ONE 2014;9(11):e113448 doi: 10.1371/journal.pone.0113448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrillo-Tripp J, Dolezal AG, Goblirsch MJ, Miller WA, Toth AL, Bonning BC. In vivo and in vitro infection dynamics of honey bee viruses. Scientific Reports 2016. February 29;6:22265 doi: 10.1038/srep22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 2007;35(SUPPL.2):W71–W74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res 2005;33(DATABASE ISS.):D34–D38. doi: 10.1093/nar/gki063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 2011;7:539 doi: 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 49.Beckmann JF, Fallon AM. Decapitation improves detection of Wolbachia pipientis (Rickettsiales: Anaplasmataceae) in Culex pipiens (Diptera: Culicidae) mosquitoes by the polymerase chain reaction. J Med Entomol 2012;49(5):1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boncristiani H, Li J, Evans JD, Pettis J, Chen Y. Scientific note on PCR inhibitors in the compound eyes of honey bees, Apis mellifera. Apidologie 2011;42(4):457–460. [Google Scholar]
- 51.Genersch E. Development of a rapid and sensitive RT-PCR method for the detection of deformed wing virus, a pathogen of the honeybee (Apis mellifera). Veterinary Journal 2005;169(1):121–123. [DOI] [PubMed] [Google Scholar]
- 52.Barriga GP, Cifuentes-Muñoz N, Rivera PA, Gutierrez M, Shmaryahu A, Valenzuela PDT, et al. First detection and complete genome sequence of Deformed wing virus in Chilean honeybees. Virus Genes 2012;45(3):606–609. doi: 10.1007/s11262-012-0791-0 [DOI] [PubMed] [Google Scholar]
- 53.Genersch E, Von Der Ohe W, Kaatz H, Schroeder A, Otten C, Büchler R, et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010;41(3):332–352. [Google Scholar]
- 54.Tentcheva D, Gauthier L, Zappulla N, Dainat B, Cousserans F, Colin ME, et al. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl Environ Microbiol 2004;70(12):7185–7191. doi: 10.1128/AEM.70.12.7185-7191.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genersch E, Yue C, Fries I, De Miranda JR. Detection of Deformed wing virus, a honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum) with wing deformities. J Invertebr Pathol 2006;91(1):61–63. doi: 10.1016/j.jip.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 56.Gauthier L, Tentcheva D, Tournaire M, Dainat B, Cousserans F, Colin ME, et al. Viral load estimation in asymptomatic honey bee colonies using the quantitative RT-PCR technique. Apidologie 2007;38(5):426–435. [Google Scholar]
- 57.Chen YP, Higgins JA, Feldlaufer MF. Quantitative real-time reverse transcription-PCR analysis of deformed wing virus infection in the honeybee (Apis mellifera L.). Appl Environ Microbiol 2005;71(1):436–441. doi: 10.1128/AEM.71.1.436-441.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francis RM, Nielsen SL, Kryger P. Varroa-Virus Interaction in Collapsing Honey Bee Colonies. PLoS ONE 2013;8(3):e57540 doi: 10.1371/journal.pone.0057540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin SJ, Ball BV, Carreck NL. Prevalence and persistence of deformed wing virus (DWV) in untreated or acaricide-treated Varroa destructor infested honey bee (Apis mellifera) colonies. J Apic Res 2010;49(1):72–79. [Google Scholar]
- 60.Locke B, Forsgren E, Fries I, de Miranda JR. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl Environ Microbiol 2012;78(6):2073–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sunderland S, Knox A, Highet F. The 2012 Scottish honey Bee Health Surveillance Report—SASA. 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Forward primers are indicated by black arrows and reverse primers with orange arrows. Viral sections and length are shown above the corresponding sections, and primer product length is given within each section. The grey section shows the location of the T7 binding site, and the green section shows the location of the T3 binding site, to indicate where the inserted sections are location in relation to other features of the pCR4 plasmid.
(DOCX)
Linearity plot created using serial diluted pooled A. mellifera cDNA from the University of Aberdeen apiary, using the single plasmid construct as the external standard. Data presented are DWV genome equivalents per qPCR reaction, mean ± SE performed in quadruple.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.