Abstract
OBJECTIVE
This study examines off-label medication prescribing use and trends in children on Medicaid with ADHD with particular focus on the very young (under age 6 years).
METHODS
This was an observational cohort study and retrospective analysis of ADHD medication prescriptions from Oregon Medicaid records (N = 83,190) in 2012. Manufacturer prescribing information was used to determine off-label designation. Children ages 3 to 18 years at the time of prescription who had continuous Medicaid enrollment of at least 10 months during the index year of 2012 were included in the sample frame.
RESULTS
Children with ADHD were prescribed off-label medications primarily at the ages of 5 years and younger. Among children ages 3 to 5 years, 91.4% of prescriptions were off-label. After the age of 5 years, the percentage of off-label prescriptions dropped notably to 21%, reflecting the increase in availability of approved medications for the treatment of ADHD starting at age 6 years. In the 3- to 5-year-old age group, specific off-label and concerning medication-related observations included a high frequency of alpha agonist (e.g., guanfacine, clonidine) prescribing; the prescribing of untested formulations such as clonidine patches; prescribing of atomoxetine; and prescribing of large doses of stimulant medications.
CONCLUSIONS
Most ADHD drugs prescribed for very young children are off-label, which is concerning owing to lack of safety and efficacy data in this vulnerable population.
Keywords: attention deficit disorder, drug prescribing, off-label use, preschool child
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is currently diagnosed in approximately 11% of United States children ages 4 to 17 years.1 From 2007 to 2011 the number of children ages 2 to 5 years taking medication to treat ADHD increased by 2-fold.2 Practices changed significantly within the last decade to favor pharmacologic treatment in very young children, despite a consensus in guidelines recommending non-pharmacologic therapy as the primary treatment approach. In 2007, a total of 97,583 children ages 2 to 5 years diagnosed with ADHD were identified as being untreated or treated without medication, while 51,409 children were identified as taking an ADHD medication, a ratio of approximately 2:1 in favor of not using medication.2 By contrast, in 2011, there were 103,562 children 2 to 5 years old on medication and 49,839 diagnosed and not receiving medication, a ratio of approximately 3:1 in favor of taking medication for ADHD.3 Also in 2011, over half of preschool-aged children diagnosed with ADHD were not receiving behavioral therapy even though there is significant evidence demonstrating its effectiveness for the treatment of ADHD symptoms.3
The American Academy of Pediatrics (AAP) guidelines recommend behavioral therapy as first-line treatment for preschool-aged 4- to 5-year-old children.4 The American Academy of Child and Adolescent Psychiatry recommends behavioral therapy as required adjunctive therapy in children 3 to 5 years of age.5 If medication is indicated, methylphenidate is the treatment of choice for young children.4 Methylphenidate should be initiated at a small dose and titrated up slowly. Since ADHD is a condition that often manifests most prominently in the young, it is recommended that children be frequently monitored when initiating therapy and be evaluated at least twice a year once maintained. For young children who are not successfully maintained on methylphenidate, treatment alternatives used in practice include other stimulants, the alpha agonists, and atomoxetine.
Background
Prescribing for Children on Medicaid. Medicaid claims data have been used to examine prescribing patterns for children with ADHD in multiple studies.6–8 Oregon Medicaid, unlike other state programs, funds mental health medications as a carve-out, which allows for individualized prescribing. The Oregon Medicaid formulary system uses a preferred drug list for ADHD medications but does not require prior authorization for non-preferred mental health medications.9 Both on-label and off-label prescribing patterns among Oregon nurse practitioner prescribers and physicians (the most prevalent type of prescribers for children on Medicaid) are similar for the treatment of ADHD,10 and their ability to prescribe controlled substances is similar on a state level in scope and breadth.10
Off-label Prescribing in Children. Off-label prescribing is described by the US Food and Drug Administration (FDA) as the “unapproved use of an approved drug […] for a disease or a medical condition.”11 When a new medication is approved, the FDA-approved labeling is based on the indication and age group treated during clinical trials conducted for the approval process. The labeling cannot contain suggestions for use in an unstudied population because of lack of supporting clinical evidence.12 In 2014, the AAP issued a policy statement regarding the off-label use of medications in children. It recommended prescribing decisions be made on the basis of product labeling, clinical evidence, expert judgment, and published literature. AAP further stated that off-label prescribing is considered reasonable if based on sound clinical evidence.13
Purpose. The purpose of this study was to examine current trends of off-label prescribing practices for children diagnosed with ADHD, with a particular focus on very young children under 6 years of age.
Methods
This observational cohort study was deemed exempt by the institutional review boards at Washington State University and Pacific Lutheran University. The Oregon Health Authority provided de-identified Medicaid encounter and pharmacy claims data. The period covered by this study included records with prescribing dates of service from January 1, 2012, to December 31, 2012.
The sample was restricted to children ages 3 to 18 years with a provider diagnosis of ADHD within the prior 730 days, and continuous Medicaid eligibility, defined as at least 10 of 12 months in a given year. Diagnosis of ADHD was broadly defined by using ICD-9 diagnosis codes (314.0, 314.00, 314.01, 314.1, 314.2, 314.8, 314.9, V62.3, 313.83). All prescription drug event records (i.e., pharmacy claims) with prescribing dates of service from January 1, 2012, to December 31, 2012, were requested. The Oregon Health Authority stores prescription records by the year of the date of fill. To obtain all prescription drug events written in 2012, pharmacy claims records from 2012 and 2013 were requested, allowing for capture of prescriptions written in 2012 and filled in 2013. Data about prescriptions that were written, but not filled, were not available. Prescriptions for non-ADHD medications were excluded.
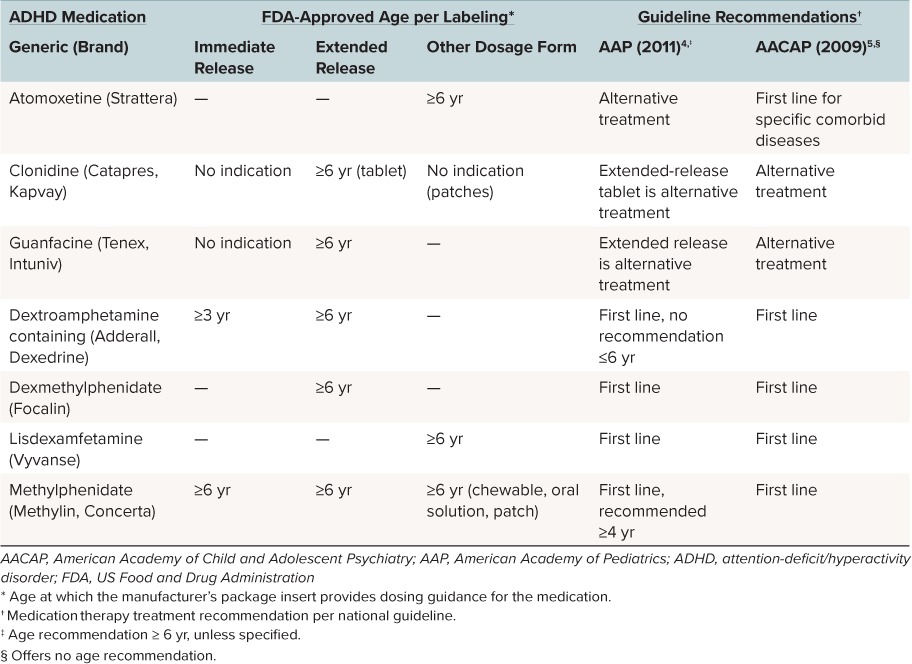
Classification of a prescription national drug code as an ADHD medication was based on the Healthcare Effectiveness Data and Information Set 2013 measure for follow-up care for children prescribed ADHD medication. ADHD medications examined included methylphenidate, amphetamine-dextroamphetamine mixed salts and dextroamphetamine as a combined category, lisdexamfetamine, dexmethylphenidate (controlled substances) and guanfacine, clonidine, and atomoxetine (non-controlled substances). On-label and off-label indications were determined by researcher review of the FDA-approved manufacturer prescribing information (Table 1).
Table 1.
Comparison of FDA Labeling of Medications for ADHD and Guidelines for Recommended Pharmacotherapy by Age4,5,14–29
Records available for analysis were age of the child at the time the prescription was filled, sex, date the prescription was written, date filled, the 11-digit National Drug Code for the medication prescribed, supply prescribed, formulation (immediate or extended release), and the prescribing provider's national provider identifier taxonomies. Records were analyzed on the basis of a 30-day supply (28–31 days) being dispensed. The unit of analysis used was the number of 30-day supply prescriptions. A 30-day supply represents the minimum amount for which a typical prescriber would initiate or refill a prescription. Use of this unit of analysis allowed for inclusion of all prescriptions written for varying number of days' supply. For example, a prescription written for a 90-day supply was counted as three 30-day supply prescriptions. A full description of study methods has been published previously.10
Statistical Analysis. Descriptive statistics, including the mean, standard deviation, frequency, and percentage, were used to characterize all children, the subset of children receiving at least 1 off-label prescription, and the subset of children receiving at least 1 on-label prescription. Frequency of off-label and on-label prescription use was stratified by the age of the child at the time the prescription was written (3 to 5; 6 to 11; and 12 to 18 years) and by sex. Off-label prescription use by provider type (nurse practitioner, physician, and other) and by age group and sex of the child was examined. We summarized off-label prescribing rates, using 30-day supply prescriptions characterized by active ingredient and formulation (7 ADHD medication categories) and release (immediate or extended). Data were summarized by using reports from a structured query language server database.
Statistical analyses comparing off-label to on-label prescribing by age group, sex, and provider type were not performed. Non-unique subsets of children among levels of the comparator groups (e.g., age group) violate the assumptions of mutually exclusive groups required for statistical testing of group differences. Our dataset violated the mutually exclusive comparator group requirement in several ways. For example, a child could have received both off-label and on-label prescriptions, resulting in inclusion in more than 1 subset such that the sum of the number of children in these subsets exceeded the total number of children. Similarly, a child could have been counted in more than 1 age group based on the age of the child at the time the prescription was written, or a child could have been prescribed both on-label and off-label medications by different provider types.
Results
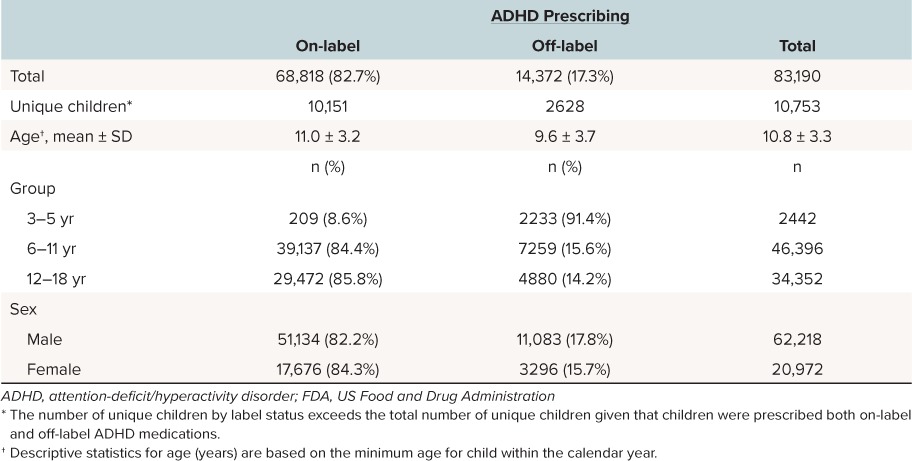
The dataset contained 197,364 prescriptions written for children ages 3 to 18 years in 2012, of which 108,775 prescriptions were excluded because these prescriptions were for non-ADHD medications, and 3892 prescriptions were excluded owing to the absence of an encounter claim with a provider within 730 days with an ADHD diagnosis. As a result, 84,697 eligible prescription records were available for analysis. Prescription records were further reduced to the number of 30-day supply prescriptions (N = 83,190) among 10,753 unique children. A total of 721 children were treated by at least 2 providers for a total of 11,448 prescriptions (13.8%).
Prescribers. Prescribers were identified by National Provider Identification to represent a total of 1799 providers (381 nurse prescribers, 1404 physician prescribers, and 14 other [non-nurse and non-physician] prescribers). Physicians wrote 67,346 total prescriptions for ADHD medications and accounted for 80.9% of the 83,190 prescriptions; nurse practitioners wrote 15,408 (18.5%) of the total prescriptions; and other prescribers wrote 436 (0.6%) of the total prescriptions. Forty-four percent (n = 791) of the 1799 providers wrote off-label prescriptions to children 3 to 18 years of age. Rates of off-label prescribing by age group and sex use were similar across provider types. In the youngest age group (ages 3 to 5 years), 13.6% (398/2928) of prescriptions written by nurse prescribers were classified as off-label, while 16.2% (1834/11,308) of prescriptions written by physician prescribers were classified as off-label.
There were no discernable differences in the rate of off-label prescribing to children, based on the sex of the child: 17.8% of males received off-label prescriptions compared to 15.7% of females (Table 2). Of the 83,190 prescriptions written for a 30-day supply, 14,372 (17.3%) were considered to be off-label. Of all the prescriptions written for children in the 3- to 5-year-old category, 91.4% (2233/2442) were prescribed off-label (3 years of age, 87.3%; 4 years of age, 93.8%; and 5 years of age, 90.9%). By comparison, rates of off-label prescribing dropped starting at age 6 years. For children in the 6- to 11-year-old category, 15.6% received off-label prescriptions for ADHD (7259/46,396). For children in the 12- to 18-year-old category, 14.2% received off-label prescriptions for ADHD (4880/34,352).
Table 2.
Demographic Characteristics of Children (3–18 Years) With an ADHD Diagnosis Who Were Prescribed ADHD Medications by FDA Label Status
Medications. The most commonly prescribed off-label prescriptions for children ages 3 to 5 years were methylphenidate and alpha agonist formulations (Table 3). Nine hundred sixteen of 2233 off-label prescriptions (41.0%) were written for formulations of methylphenidate (immediate and extended release combined). Methylphenidate records for 3- to 5-year-old children in our dataset included 166 prescriptions for doses larger than 25 mg per day and 13 prescriptions for doses as large as 54 mg. Clonidine and guanfacine (immediate and extended release combined) accounted for 42.6% (952/2233) of the off-label prescriptions written for the 3- to 5-year-old age group. Clonidine immediate-release tablets at 97.6% (363/372) were prescribed more often than the extended-release tablets at 1.2% (4/372) and patches at 1.2% (5/372).
Table 3.
Off-label Prescribing of ADHD Medications (N = 83,190; 30-Day Supply Scripts)
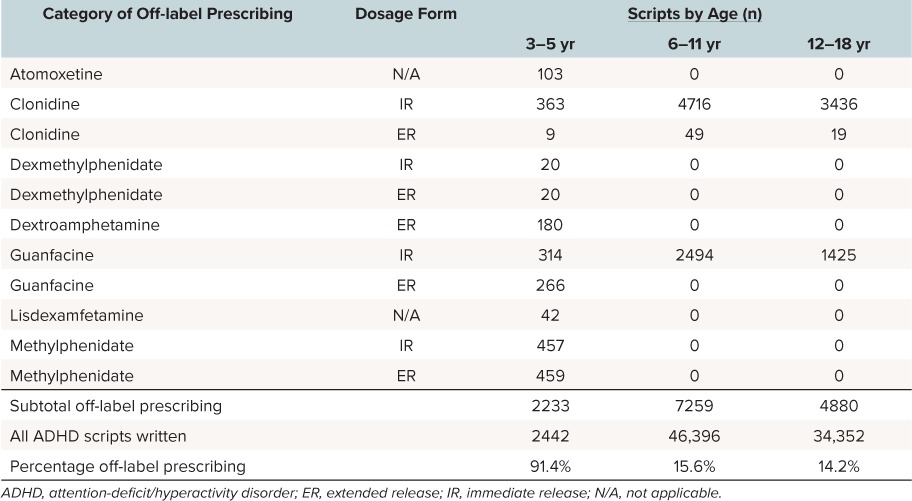
Prescribing of immediate- and extended-release guanfacine was similar at 54.1% (314/580) and 45.9% (266/580), respectively. Clonidine (immediate and extended release) and guanfacine (immediate release) prescribing accounted for all off-label prescribing to children 6 to 11 years of age (n = 7259) and to children 12 to 18 years of age (n = 4880). Not reported in Table 2, 73 of 77 off-label prescriptions (children aged 3 to 18 years) for clonidine extended release were written for the clonidine patch. Dextroamphetamine-containing extended-release formulations (n = 180) and atomoxetine (n = 103) were also frequently prescribed off-label medications for children ages 3 to 5 years (8.1% and 4.6%, respectively). The 2 other medications for which off-label prescriptions were written for children ages 3 to 5-years included dexmethylphenidate, both immediate (20/2233) and extended release (20/2233), and lisdexamfetamine (42/2233).
Discussion
Most medications used to treat ADHD are considered off-label for children younger than age 6 years.30 Few studies exist concerning the prescribing of ADHD medications to preschool-aged children; therefore, current research provides limited evidence to inform guidelines and FDA labeling. Our discussion adds beneficial information to the currently available data by providing insights regarding specific medications being used off-label in this age group.
Methylphenidate. Methylphenidate is the only ADHD medication recommended for use in children as young as 4 years by AAP guidelines.4 Studies have demonstrated that children ages 4 to 5 years are to receive a weight-based dose of 0.7 ± 0.4 mg/kg/day of methylphenidate.31 From this dosing guidance, most children under the age of 5 years should be receiving doses smaller than 25 mg per day, and yet our dataset revealed 166 prescriptions written for methylphenidate doses larger than 25 mg per day and 13 prescriptions written for doses as large as 54 mg per day in the 3- to 5-year-old age group. Larger doses of methylphenidate are associated with an increased incidence of sleep disturbances and related issues in children.32 It is uncertain what other adverse medication-related events are occurring as a result of using large doses of methylphenidate in children 3 to 5 years old. However, studies of preschool-aged children have revealed that 30% of the children will experience side effects when prescribed a stimulant to treat ADHD and 10% will choose to discontinue the medication.6
Dextroamphetamine. The immediate-release formulations of dextroamphetamine-containing medications are FDA approved for use in children starting at age 3 years.20,22 However, according to the AAP, the approval of immediate-release dextroamphetamine was made during a time of less stringent approval processes, and therefore the AAP does not recommend dextroamphetamine in 3- to 5-year-old children.4 In contrast, the extended-release formulations of dextroamphetamine-containing medications are not FDA approved until the age of 6 years. If used in children younger than 6 years, general dosing recommendations made by the prescribing information for dextroamphetamine and amphetamine mixed salts suggest beginning with 2.5 mg/day, which is half of the starting dose for a 6-year-old child and then titrating up to the desired effect.20,22 In our study, extended-release dextroamphetamine-containing medications represented 8% (180/2233) of the overall off-label prescribing in children 3 to 5 years old and, as with methylphenidate, larger doses (25–30 mg) of dextroamphetamine were noted.
Atomoxetine. In our dataset, 103 prescriptions for atomoxetine were dispensed for children ages 3 to 5 years. The use of atomoxetine prescriptions in very young children may be concerning owing to limited research demonstrating efficacy in very young children.33 To our knowledge, atomoxetine has not been studied in children ages 3 to 4 years and only 1 study of 101 children (80% power) has been reported investigating the effectiveness of atomoxetine in children ages 5 to 6 years. The study concluded atomoxetine was effective in treating ADHD, compared to placebo, in 5- to 6-year-old children, but no other studies have been reported that reproduce or validate this study's findings.34
Also of concern are data suggesting a potentially higher incidence of adverse events with atomoxetine in children younger than 6 years. A safety data extrapolation analysis of the atomoxetine study in children ages 5 to 7 years found that 5 year olds are more likely than older children to experience labile moods from the medication. Irritability was reported 36.8% of the time for 5-year-old children versus 3.6% in the 6 to 7 year olds. Mood disorders such as tearfulness, mood swings, and emotional disorders were twice as likely in 5 year olds than in 6 to 7 year olds (less than 3% versus 6.9%, respectively).33
Alpha Agonists. Alpha agonist medications accounted for 42% of the off-label prescriptions for children ages 3 to 5 years, which was more than those written for all methylphenidate formulations combined (41%) even though methylphenidate is the first-line medication recommended by the AAP guidelines for use in children starting at age 4 years. The role of alpha agonists as adjunctive therapy and, in recent studies, monotherapy, has brought them to the forefront of treatment plans for ADHD in children.4,35 Extended-release oral formulations of these medications are FDA approved as monotherapy or adjunct therapy for ADHD in children 6 years and older and are routinely used as a treatment for ADHD.4,35 In this study, immediate-release formulations of clonidine and guanfacine were prescribed more often for preschoolers than the extended-release forms (363 and 314 versus 9 and 266, respectively).
The AAP guidelines (2011) note efficacy, but limited supporting evidence for the extended-release formulation of the alpha agonist medications in children older than preschool-age, and do not support immediate-release use for any age group and do not mention the use of other dosage forms such as the clonidine patch. The American Academy of Child and Adolescent Psychiatry guidelines (2007) acknowledge the prevalence of alpha agonist prescriptions for ADHD particularly when comorbid conditions are involved, but note that extensive controlled trials are lacking and published data are variable in methods and outcomes.5 Regardless, in practice, the immediate-release alpha agonists are often used to help counteract the side effects of stimulant medications such as sleep disturbances.36 And, while not the focus of this research, the potential for coprescribing of an alpha agonist with a stimulant medication was observed and noted as occurring at 22.9% of the time for all children and 26.9% of the time in children ages 3 to 5 years. The impact of continued or long-term use of this medication class, originally developed as antihypertensive medications, not for psychiatric use, is unknown and unstudied in the pediatric population.
Other Observations. One final concern observed was the use of Institute for Safe Medication Practices (ISMP) “Do Not Crush” medications. Three of the highlighted medications, atomoxetine and each of the extended-release alpha agonists, are solid oral dosage forms, which cannot be opened and sprinkled on food.14,16,19,37 While some very young children can swallow pills whole, potential administration concerns exist for those who cannot.
Limitations. Study limitations include the following: the dataset was processed on the basis of the diagnosis of ADHD. It is unknown what, if any, comorbid conditions existed in the children analyzed in our dataset. The use of the alpha agonists was common for children 3 to 5 years old. Medication dosing frequency was not available in the dataset, and medication regimens were not connected to an individual patient. Therefore, it is unknown if the alpha agonists were being coprescribed to treat side effects of stimulant medications or to treat comorbid conditions such as Tourette's syndrome.38 Prescriptions written, but not dispensed, were not included, as these data were not available. This report includes 1 year of data from a Medicaid population in 1 state and may not be reproducible in other states. Finally, it is unknown which, if any, children received cognitive behavioral counseling.
Conclusion
The practice of off-label prescribing is considered reasonable if based on sound clinical evidence; however, the current literature, regulatory approvals, and guidance for the use of ADHD medications in children ages 3 to 5 years are lacking. Our findings support prioritization of studying and appropriately relabeling ADHD medications for pediatric patients, particularly for the very young, while acknowledging that behavioral therapy strategies should continue to be first-line and adjunctive treatment in this age group.
Acknowledgment
Information contained in this manuscript was previously presented as a symposium presentation titled “Examining Patterns in Prescribing ADHD Medications” at the Western Institute of Nursing Meeting; April 2016; Anaheim, California.
Abbreviations
- AAP
American Academy of Pediatrics
- ADHD
attention-deficit hyperactivity disorder
- FDA
US Food and Drug Administration
- ISMP
Institute for Safe Medication Practices
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org.
REFERENCES
- 1. Visser SN, Danielson ML, Bitsko RH, . et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014; 53 1: 34– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Indicator 2.7: how many children currently have ADD/ADHD and take medication for this condition? National Survey of Children's Health 2007. http://childhealthdata.org/browse/survey/results?q=241&r=1&g=64. Accessed October 19, 2017.
- 3. Indicator 2.7: how many children currently have ADD/ADHD and take medication for this condition? National Survey of Children's Health 2011. http://childhealthdata.org/browse/survey/results?q=2482&r=1&g=451. Accessed October 19, 2017.
- 4. Subcommittee on Attention-Deficit. . ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011; 125 5: 1007– 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pliszka S, Steven, AACAP Work Group on Quality Issues. . Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007; 46 7: 894– 921. [DOI] [PubMed] [Google Scholar]
- 6. Visser SN, Danielson ML, Wolraich ML, . et al. Vital signs: national and state-specific patterns of attention deficit/hyperactivity disorder treatment among insured children aged 2–5 years—United States, 2008–2014. Morb Mortal Wkly Rep. 2016; 65 17; 443– 450. [DOI] [PubMed] [Google Scholar]
- 7. Hoagwood KE, Kelleher K, Zima BT, . et al. Ten-year trends in treatment services for children with attention deficit hyperactivity disorder enrolled in Medicaid. Health Aff. 2016; 35 7: 1266– 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garfield LD, Brown DS, Allaire BT, . et al. Psychotropic drug use among preschool children in the Medicaid program from 36 states. Am J Public Health. 2015; 105 3: 524– 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oregon Health Plan. Questions and answers about the preferred drug list. http://www.oregon.gov/OHA/healthplan/pages/pdl.aspx. Accessed October 19, 2017.
- 10. Klein TA, Panther S, Woo T, . et al. Childhood attention-deficit/hyperactivity disorder prescribing by prescriber type and specialty in Oregon Medicaid. J Child Adolesc Psychopharmacol. 2016; 26 6: 548– 554. [DOI] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration. . Understanding unapproved use of approved drugs “off label.” http://www.fda.gov/forpatients/other/offabel/default.htm. Accessed October 19, 2017.
- 12. Requirements on content and format of labeling for human prescription drug and biological products. Fed Regist. 2014. Codified at 21 CFR §201.56(a)(3). [PubMed] [Google Scholar]
- 13. Nelville KA, Frattarelli DA, Galinkin JL, . et al. Off-label use of drugs in children. Pediatrics. 2014; 133 3: 563– 567. [DOI] [PubMed] [Google Scholar]
- 14. Atomoxetine hydrochloride [package insert]. Indianapolis, IN: Eli Lilly and Company; April 2015. [Google Scholar]
- 15. Clonidine hydrochloride immediate release [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; October 2009. [Google Scholar]
- 16. Clonidine hydrochloride extended release [package insert]. Atlanta, GA: Shionogi Pharma Inc; 2010. [Google Scholar]
- 17. Clonidine transdermal therapeutic system [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; August 2016. [Google Scholar]
- 18. Guanfacine hydrochloride immediate release [package insert]. Bridgewater, NJ: Promius Pharma LLC; July 2013. [Google Scholar]
- 19. Guanfacine hydrochloride extended release [package insert]. Wayne, PA: Shire US Manufacturing Inc; August 2013. [Google Scholar]
- 20. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate immediate release [package insert]. Pomona, NY: Barr Laboratories Inc; March 2007. [Google Scholar]
- 21. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate extended release [package insert]. Wayne, PA: Shire US Inc; November 2013. [Google Scholar]
- 22. Dextroamphetamine sulfate immediate release [package insert]. Research Triangle Park, NC: GlaxoSmithKline; June 2006. [Google Scholar]
- 23. Dextroamphetamine sulfate extended release [package insert]. Research Triangle Park, NC: GlaxoSmithKline; March 2007. [Google Scholar]
- 24. Dexmethylphenidate hydrochloride immediate release [package insert]. East Hanover, NJ: Novartis; April 2015. [Google Scholar]
- 25. Dexmethylphenidate hydrochloride extended release [package insert]. East Hanover, NJ: Novartis; April 2015. [Google Scholar]
- 26. Lisdexamfetamine dimesylate [package insert]. Lexington, MA: Shire Inc; December 2015. [Google Scholar]
- 27. Methylphenidate hydrochloride oral solution [package insert]. St. Louis, MO: Mallinckrodt Inc; May 2007. [Google Scholar]
- 28. Methylphenidate hydrochloride chewable [package insert]. St. Louis, MO: Mallinckrodt Inc; May 2007. [Google Scholar]
- 29. Concerta ER (methylphenidate hydrochloride) [prescribing information]. Auckland, New Zealand: Janssen-Cilag Pty Ltd; October 2015. [Google Scholar]
- 30. Centers for Disease Control and Prevention. . Data & statistics: attention-deficit/hyperactivity disorder (ADHD). 2016. http://www.cdc.gov/ncbddd/adhd/data.html. Accessed October 19, 2017.
- 31. Greenhill L, Kollins S, Abikoff H, . et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006; 45 11: 1284– 1294. [DOI] [PubMed] [Google Scholar]
- 32. Becker SP, Froehlich TE, Epstein JN.. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016; 37 5: 395– 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Upadhyaya H, Kratochvil C, Ghuman J, . et al. Efficacy and safety extrapolation analysis for atomoxetine in young children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015; 25 10: 799– 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kratochvil CJ, Vaughan BS, Stoner JA, . et al. A double-blind, placebo-controlled study of atomoxetine in young children with ADHD. Pediatrics. 2011; 127 4: e862– e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirota T, Schwartz S, Correll CU.. Alpha-2 agonists for attention deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014; 53 2: 153– 173. [DOI] [PubMed] [Google Scholar]
- 36. Dodson WW. Reduce appetite suppression, insomnia in ADHD treatment. Curr Pyschiatry. 2005; 4 7: 61– 62. [Google Scholar]
- 37. Institute for Safe Medication Practices. . Oral dosage forms that should not be crushed. 2015. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwiF8K_8jvLRAhUU4GMKHd6ADFQQFggaMAA&url=http%3A%2F%2Fwww.ismp.org%2FTools%2FDoNotCrush.pdf&usg=AFQjCNEAGT-7DHY4N39dANzVaK5O_uom9w&sig2=ut3NtlQfWBtyCoVYTcaR5w. Accessed October 19, 2017.
- 38. Fiks AG, Mayne SL, Song L, . et al. Changing patterns of alpha-agonist medication use in children and adolescents 2009–2011. J Child Adolesc Psychopharmacol. 2015; 25 4: 362– 367. [DOI] [PMC free article] [PubMed] [Google Scholar]


