Abstract
Sertraline, a selective serotonin reuptake inhibitor, has been used for the treatment of depression. Although it is generally considered safe, cases of sertraline-associated liver injury have been documented; however, the possible mechanism of sertraline-associated hepatotoxicity is entirely unknown. Here, we report that mitochondrial impairment may play an important role in liver injury induced by sertraline. In mitochondria isolated from rat liver, sertraline uncoupled mitochondrial oxidative phosphorylation and inhibited the activities of oxidative phosphorylation complexes I and V. Additionally, sertraline induced Ca2+-mediated mitochondrial permeability transition (MPT), and the induction was prevented by bongkrekic acid (BA), a specific MPT inhibitor targeting adenine nucleotide translocator (ANT), implying that the MPT induction is mediated by ANT. In freshly isolated rat primary hepatocytes, sertraline rapidly depleted cellular adenosine triphosphate (ATP) and subsequently induced lactate dehydrogenase leakage; both were attenuated by BA. Our results, including ATP depletion, induction of MPT, inhibition of mitochondrial respiration complexes, and uncoupling oxidative phosphorylation, indicate that sertraline-associated liver toxicity is possibly via mitochondrial dysfunction.
Keywords: sertraline, liver toxicity, mitochondrial dysfunction, rat primary hepatocytes, isolated liver mitochondria
Sertraline (1S,4S-N-methyl-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthylamine, Zoloft), a selective serotonin reuptake inhibitor (SSRI), is currently one of the most prescribed antidepressants in Europe and in the United States. It is also approved for the treatment of panic disorder, obsessive–compulsive disorder, and post-traumatic stress disorder (Davies and Kluwe, 1998; DeVane et al., 2002).
Prior to the first SSRI, fluoxetine, launched in the market in 1988, tricyclic antidepressants and monoamine oxidase inhibitors were commonly used to treat depression. Compared with these classical antidepressants, SSRIs were thought to be less hepatotoxic (Biour et al., 1992); the hepatotoxicity incidence of SSRIs is from 1.28 to 3.62 cases per “patient-year” (Collados et al., 2010).
Although adverse event reports of sertraline-associated toxicity are relatively rare, evidence from preclinical and clinical studies indicates that the use of sertraline is not without risk. In a preclinical study, liver was identified as the target organ in mice, rats, and dogs dosed orally with sertraline, and hepatomegaly, hepatocellular hypertrophy, increased serum transaminase activity, and proliferation of smooth endoplasmic reticulum were observed (Davies and Kluwe, 1998). In a premarketing study, elevations in serum transaminase activities occurred in approximately 0.8% of study patients, and the enzyme levels increased from the first week to nine weeks of treatment and generally returned to normal value upon drug withdrawal (Zoloft, 1997). Also, numerous clinical adverse events involving liver toxicity have been reported during sertraline therapy, two of which caused severe hepatitis (Collados et al., 2010; Collados Arroyo et al., 2008; Fartoux-Heymann et al., 2001; Hautekeete et al., 1998; Kim et al., 1999; Martínez Matos, 2002; Persky and Reinus, 2003; Verrico et al., 2000). Extensive hepatocyte necrosis, with formation of bridges linking portal tracts together and to terminal hepatic venules, was found in one fatal case (Fartoux-Heymann et al., 2001); another case of severe hepatitis was due to a hypersensitivity reaction cholestasis that appeared 2 months after taking sertraline with a recovery time of 6 months (Galán Navarro, 2001).
We have been studying liver toxicants using high-throughput assays and have applied the Cellular Systems Biology platform (http://www.cellumen.com/) to evaluate and identify the risks for about 200 FDA approved drugs (unpublished data). This platform is designed to monitor the effects of test compounds on many cellular system responses known to be correlated with toxic challenge, such as oxidative stress, organelle dysfunction, stress pathway activation, damage to cytoskeletal integrity and the cell cycle, and DNA damage (Vernetti et al., 2008). Using rat primary hepatocytes, sertraline was classified as a “toxic” compound based on changes of two toxicity indicators: decrease in cell viability and nuclear size (Supplementary file 1). An extensive literature search showed that the mechanism of sertraline-induced toxicity has been rarely documented and unclearly presented, prompting us to confirm further the data generated by the high-throughput assay and to investigate the possible mechanisms of sertraline-related hepatotoxicity. We, therefore, evaluated the toxic effects of sertraline in rat primary hepatocytes and performed a set of mitochondrial assays including oxygen consumption, mitochondrial membrane potential, and measurements of individual complex activities in isolated mitochondria. Data from our study indicate that sertraline disrupts liver mitochondria, and mitochondrial dysfunction may be the underlying mechanism that contributes to sertraline-associated liver toxicity.
MATERIALS AND METHODS
Chemicals and reagents
Sertraline, William’s E medium, penicillin, streptomycin, bongkrekic acid (BA), cyclosporine A, hexokinase, glucose-6-phosphate dehydrogenase, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), linoleic acid-albumin, insulin, holo-transferrin bovine, dexamethasone, sodium selenous acid, dimethysulfoxide (DMSO), and Percoll were from Sigma-Aldrich (St Louis, MO). Atractyloside was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, GA). Nembutal was purchased from Lundbeck Inc. (Deerfield, IL).
Animal care
Male Sprague Dawley rats (6–8 weeks old) were obtained from the breeding colony of the FDA’s National Center for Toxicological Research (NCTR). Rats were anesthetized by ip injection of 1.5 ml/kg of Nembutal containing 50 mg/ml of pentobarbital sodium prior to undergoing liver perfusion. All animals used in this study were handled in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Institutes of Health, and the experimental procedures were approved by the NCTR Institutional Animal Care and Use Committee.
Rat primary hepatocyte isolation and cell culture
Rat primary hepatocytes were isolated by a two-stage collagenase perfusion process according to methods as previously described with some modifications (Guo et al., 2006a; Kreamer et al., 1986; Seglen, 1976). Cell viability was determined with trypan blue exclusion using a hemocytometer. Isolated hepatocytes with a cell viability great than 90% were used for experiments. The isolated hepatocytes were suspended in William’s E medium supplemented with 10% FBS, 10mM HEPES, 1% linoleic acid-albumin, 5 μg/ml insulin, 5 μg/ml holo-transferrin, 25nM dexamethasone, 5 ng/ml sodium selenous acid, 50 U/ml penicillin, and 50 μg/ml streptomycin and were seeded at a cell density of 2 × 104 per well in 100 μl media in 96-well plates that were precoated with 1 mg/ml bovine collagen I (PureCol, Advanced BioMatrix, San Diego, CA). Cells were cultured for 4–6 h prior to the treatment, allowing cell attachment. They were then treated with control DMSO or sertraline in DMSO at various concentrations up to 100μM and time periods specified in the serum-free medium. The final DMSO concentration in the medium was 0.1%. Cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Cellular adenosine triphosphate level measurement
Adenosine triphosphate (ATP) content was quantified using the CellTiter-Glo Luminescent Cell Viability Assay (Promega Corporation, Madison, WI) and measured with a Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT). The cellular ATP content was calculated by comparing the luminescence of the treated cells with that of the DMSO controls.
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) release from cultured cells was used as a measure of cell death. LDH activity was measured in culture medium and whole cell lysates (Korzeniewski and Callewaert, 1983). In brief, after treatment, 10 μl medium (supernatant) was collected, and the cells remaining in each well were lysed with 10 μl of 10% Triton X-100. After 2-h incubation, 10 μl lysates were collected, and 240 μl reaction buffer (81mM Tris, 204mM NaCl, 0.2mM NADH, and 1.7mM monosodium pyruvate, pH 7.2) was added to 10 μl of both cell lysate and supernatant in clear 96-well plates. Absorption was measured at 340 nm for 5 min at 1-min intervals with a microplate reader (BioTek). The LDH release was calculated according the following formula: (%) = (decrease in supernatant absorption/decrease in cell lysate absorption) * 100.
Rat liver mitochondria isolation
Rat liver mitochondrial fraction was prepared as described by Hogeboom et al. (1948) with some modifications. In brief, rat liver was washed with ice-cold PBS and minced in ice-cold buffer A (210mM mannitol, 70mM sucrose, 5mM HEPES, and 1.0mM ethylene glycol tetraacetic acid, pH 7.2) with the ratio of liver (w)/medium (w) being 1:9 and homogenized three times with a Potter homogenizer. The homogenates were centrifuged at 900 × g for 10 min at 4°C to remove nuclear debris. The supernatant was then transferred to a fresh tube and centrifuged at 10,000 × g for 10 min 4°C. The supernatant was decanted, and the pellet was then suspended in 20 ml of ice-cold buffer B (25mM potassium phosphate and 5mM MgCl2, pH 7.2) and centrifuged at 10,000 × g for 10 min at 4°C. The resulting pellet was then resuspended in buffer B and centrifuged again at 10,000 × g for 10 min at 4°C and the final mitochondrial pellet was resuspended in 1 ml buffer B. The protein concentration was determined by a bicinchoninic acid protein assay (Bio-Rad Life Science, Hercules, CA) with bovine serum albumin (BSA) as the standard. The final protein concentration of the mitochondrial suspension was adjusted to 1.0 mg/ml.
Measurement of oxygen consumption
Oxygen consumption of isolated liver mitochondria was measured polarographically with a Clark-type oxygen electrode (Hansatech Instruments Ltd., Norfolk, England) following the method described by Frezza et al. (2007). For each reaction, mitochondria at a protein concentration of 1.0 mg/ml were used. To initiate basal activity of respiration (state 2), complex I substrate glutamate/malate (5mM of each) or complex II substrate succinate (5mM) was incubated with mitochondria. ADP (100μM) was added to stimulate state 3 respiration. Sertraline or DMSO was preincubated with mitochondria for 3 min before the addition of the respiratory substrate (the final concentration of DMSO was 0.1% in the reaction buffer). The respiration control ratio (RCR) was calculated as the ratio of oxygen uptake in state 3 (with ADP) to that in state 4 (without ADP). The ADP/O was calculated as the number of nanomoles of ADP phosphorylated by nanomole of oxygen atoms of oxygen consumed during ADP phosphorylation.
Measurement of individual mitochondrial complex activity
The activities of mitochondrial oxidative phosphorylation complexes were measured according to the methods described by Kirby et al. (2007). All enzymatic assays were performed at room temperature in a final volume of 0.2 ml or 1 ml with a Synergy 2 Multi-Mode Microplate Reader (BioTek) or a Beckman DU640B spectrophotometer (Brea, CA). In this set of experiments, sertraline at the concentration specified or DMSO control was incubated with mitochondria for 5 min before analysis.
Complex I (NADH-ubiquinone oxidoreductase) activity was measured as the rate of NADH oxidation by recording a decrease in absorbance at 340 nm with 425 nm as the reference wavelength (ε = 6.81mM−1 cm−1) for 5 min at 30-s intervals. Mitochondria (25 μg of protein) were added to the 0.2 ml reaction mixture of 50mM potassium phosphate buffer (pH 7.4), 2mM potassium cyanide (KCN), 2.5 mg/ml BSA, 0.13mM NADH, 2 μg/ml antimycin A, and 65μM decylubiquinone. The reaction was performed in the presence and absence of 2 μg/ml rotenone because complex I activity is rotenone sensitive.
Complex II (succinate-ubiquinone oxidoreductase) activity was measured by recording a decrease in absorbance due to the reduction of 2,6-dichlorophenolindophenol (DCPIP) at 600 nm, with 750 nm as the reference wavelength (ε = 19.1mM−1 cm−1). Mitochondria (10 μg of protein) were preincubated in 0.2 ml reaction mixture of 50mM potassium phosphate buffer (pH 7.4), 5mM MgCl2, and 20mM sodium succinate at room temperature for 10 min. Then, 2 μg/ml antimycin A, 2 μg/ml rotenone, 2mM KCN, and 50μM DCPIP were added, and the baseline rate was recorded for 3 min. The reaction was started by the addition of 65μM decylubiquinone, and the enzyme-catalyzed reduction of DCPIP was measured for 5 min at 30-s intervals.
Complex III (ubiquinol-ferricytochrome c oxidoreductase) activity was measured by the reduction of cytochrome c at 550 nm, with 540 nm as the reference wavelength (ε = 19mM−1 cm−1). Mitochondria (20 μg of protein) were incubated with the 1 ml reaction mixture of 50mM potassium phosphate buffer (pH 7.4), 5mM MgCl2, BSA (2.5 mg/ml), 2mM KCN, 50μM cytochrome c, 2 μg/ml rotenone, and 0.6mM dodecyl-β-D-maltoside at room temperature for 10 min. The reaction was initiated upon addition of 50μM decylubiquinone, and the increase in absorbance was measured for 1 min at 10-s intervals.
Complex IV (cytochrome c oxidase) activity was measured by following the oxidation of reduced cytochrome c at 550 nm, with 540 nm as the reference wavelength (ε = 19mM−1 cm−1). Mitochondria (20 μg of protein) was preincubated in 1 ml of 10mM Tris-HCl buffer (pH 7.4) containing 250mM sucrose and 1mM dodecyl-β-D-maltoside at room temperature for 10 min. Reduced cytochrome c was added to 20μM in 50mM Tris-HCl (pH 7.4) containing 600mM KCl, and the stability of the absorbance was checked for 1 min. The reaction was begun by adding the preincubated mitochondria, and the decrease in absorbance was measured for 1 min at 10-s intervals.
Complex V (ATPase) activity was determined by following the reduction of NADH with LDH and pyruvate kinase as coupling enzymes. The reduction of NADH was monitored at 340 nm, with 425 nm as the reference wavelength (ε = 6.81mM−1 cm−1) for 3 min at 20-s intervals. The reaction mixture (1 ml) of 50mM Tris-HCl (pH 7.4), 300mM sucrose, 5mM MgCl2, 0.2mM NADH, 4mM ATP, 0.6mM phosphoenolpyruvate, 1 unit of LDH, 1 unit of pyruvate kinase, 2mM KCN, 2 μg/ml antimycin A, and BSA (2.5 mg/ml) was incubated at room temperature for 15 min. Mitochondria (25 μg of protein) were added, and the reaction was followed for 3 min with and without 2μM oligomycin. The oligomycin-sensitive activity was the complex V activity.
Induction of the mitochondrial permeability transition
The mitochondrial permeability transition (MPT) was determined by measuring mitochondrial swelling, which was measured spectrophotometrically. The liver mitochondrial preparation (1.0 mg/ml) was incubated with sertraline (37.5, 50, 75, and 100μM) in a reaction buffer containing 210mM mannitol, 70mM sucrose, 2μM rotenone, and 5mM HEPES, pH 7.4 at 30°C in the presence of 20μM CaCl2. In order to determine the effect of MPT inhibitors, liver mitochondrial preparations were preincubated with BA (10μM) or cyclosporine A (1μM) for 2 min before addition of sertraline. Mitochondrial swelling was measured spectrophotometrically using a Synergy 2 Multi-Mode Microplate Reader (BioTek) by monitoring the decrease in absorbance at 540 nm for a period of 20 min.
Assay of adenine nucleotide translocator activity
After exposure of mitochondria to various experimental conditions, adenine nucleotide translocator (ANT) activities were evaluated according to Atlante et al. (2006). Briefly, mitochondria (1 mg protein/ml) were suspended at 25°C in 1 ml of a standard reaction buffer (200mM sucrose, 75mM KCl, 20mM HEPES—Tris pH 7.2, 1mM MgCl2, 1mM Pi-Tris) in the presence of an ATP detection system (2.5mM glucose, 0.5 E.U. hexokinase, 0.5 E.U. glucose-6-phosphate dehydrogenase, and 200μM NADP+). Externally added ADP started an ADP exchange with intramitochondrial ATP through ANT. NADPH formation (ε340 nm = 6.2mM−1 cm−1), which is proportional to ATP efflux, was monitored continuously for 5 min by spectrophotometry at 340 nm. The rate of absorbance increase was used to calculate the amount of exchanged ADP (nmol min−1 mg protein−1). Control experiments were carried out in the presence of atractyloside (ATR), a substrate of ANT, to ensure that the ADP/ATP exchange was mediated by the ADP/ATP carrier (Atlante et al., 2003; Rossignol et al., 2000; Wanders et al., 1984).
Statistical analysis
Data are presented as mean ± SD of at least three independent experiments (n ≥ 3). Analyses were performed using GraphPad Prism 5 (La Jolla, CA). Statistical significance was determined by one-way ANOVA followed by the Dunnett’s tests for pairwise comparisons or two-way ANOVA followed by the Bonferroni’s posttest. The difference was considered statistically significant when the p < 0.05.
RESULTS
Sertraline Causes the Cellular Damage in Primary Rat Hepatocytes
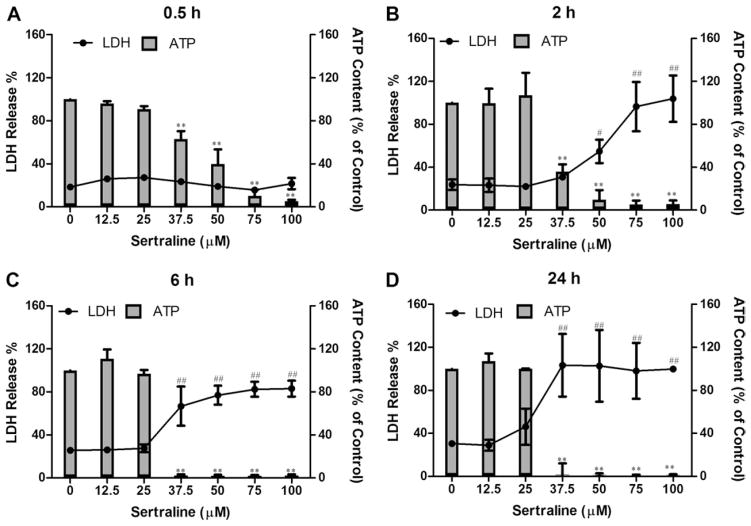
Sertraline-induced cytotoxicity to rat primary hepatocytes was assessed by LDH release at the time points of 0.5, 2, 6, and 24 h. As shown in Figure 1 (lines), there was no significant LDH release observed for all concentrations tested for 0.5 h treatment compared with the DMSO control. Significant cytotoxic effect began to be seen with 50μM sertraline treatment at 2 h; about 55% of LDH was released into the culture medium with 50μM treatment, whereas only 24% of LDH was released with the control. At 6 and 24 h, sertraline caused profound cell damage, starting at 37.5μM with LDH release of about 77 and 100%, respectively. Our data show that sertraline at 37.5μM and higher caused extensive cell death as measured by LDH release.
FIG. 1.
Effects of sertraline on cellular ATP level (bars) and cell death (lines) in rat primary hepatocytes. Rat primary hepatocytes were treated with DMSO as vehicle control and sertraline at the concentrations of 0–100μM for 0.5, 2, 6, and 24 h. Cellular ATP content and LDH release were measured as described in the Materials and Methods section. #p < 0.01 and ##p < 0.001 represent LDH release is significantly different from the control for each time point; **p < 0.001 represents ATP depletion is significantly different from the control for each time point. Data are represented as mean ± SD from at least three independent experiments.
Sertraline Depletes Cellular ATP Content in Rat Primary Hepatocytes
LDH release assesses the permeability of cell membrane and is an indication of necrotic cell death; other functional parameters are useful for exploring the possible mechanisms of cytotoxicity. We therefore measured cellular ATP levels to determine if sertraline disrupted cellular energy metabolism. For this purpose, rat primary hepatocytes were treated with sertraline at various concentrations from 12.5 to 100μM, and the cellular ATP level was measured. As indicated in Figure 1 (bars), sertraline began to decrease ATP levels at as early as 0.5 h, a time point at which no cell death was observed as measured by LDH release. At the concentration of 37.5μM, approximately 37, 64, and 90% of ATP was depleted at 0.5, 2, and 6 h, respectively. Nearly complete ATP depletion was observed at 0.5 h for sertraline at a concentration of 100μM, whereas no obvious cell death was detected by LDH release at this early time.
Sertraline Uncouples Oxidative Phosphorylation in Isolated Liver Mitochondria
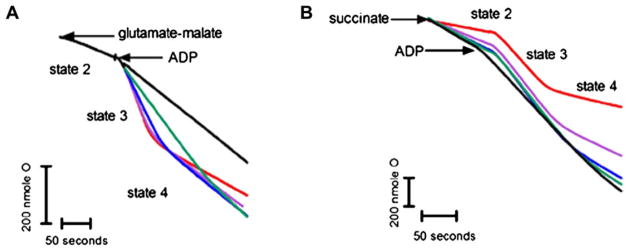
The rapid ATP depletion caused by sertraline may imply mitochondrial impairment because mitochondria are the “powerhouses” of the cells. Accordingly, we performed a set of mitochondrial function-related assays to evaluate whether or not sertraline has an effect on liver mitochondria. We first assessed the effect of sertraline on mitochondrial oxygen consumption both with complex I substrate glutamate/malate and with complex II substrate succinate (Fig. 2). The results are tabulated in Table 1. The values of the RCR of control samples were 5.6 ± 0.4 for glutamate/malate and 3.6 ± 1.5 for succinate, indicating good mitochondrial preparations.
FIG. 2.
Representative recording of the effect of sertraline on mitochondrial oxygen consumption in isolated liver mitochondria. The assessment of oxygen consumption was conducted as described in the Materials and Methods section. Mitochondrial respiration was initiated by adding complex I substrate 5mM glutamate/malate (A) and by complex II substrate 5mM succinate (B). ADP (100μM) was used to start the state 3 respiration. Sertraline (0–100μM) were incubated with mitochondria (1.0 mg/ml) for 3 min before the reaction. Red: DMSO, purple: 25μM sertraline, blue: 50μM sertraline, green: 75μM sertraline, and black: 100μM sertraline.
TABLE 1.
Effects of Sertraline on Oxygen Consumption and Oxidative Phosphorylation
| Sertraline (μM) | Substrate | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Glutamate/malate | Succinate | |||||||
|
|
|
|||||||
| State 3 | State 4 | RCR | ADP/O | State 3 | State 4 | RCR | ADP/O | |
| 0 | 76.4 ± 3.3 | 13.6 ± 1.2 | 5.6 ± 0.4 | 3.0 ± 0.6 | 87.0 ± 2.7 | 24.2 ± 0.6 | 3.6 ± 1.5 | 2.1 ± 0.5 |
| 25 | 71.4 ± 1.7* | 23.8 ± 1.0*** | 3.0 ± 0.3 | 3.2 ± 0.7 | 88.3 ± 2.8 | 42.0 ± 2.1*** | 2.1 ± 0.8 | 1.5 ± 0.4 |
| 50 | 51.5 ± 1.1*** | 23.4 ± 0.9*** | 2.2 ± 0.3 | 2.7 ± 0.8 | 90.0 ± 3.4 | 47.4 ± 4.0*** | 1.9 ± 0.5 | 1.1 ± 0.2 |
| 75 | 27.6 ± 1.3*** | 25.1 ± 2.1*** | 1.1 ± 0.3* | n.m. | 90.4 ± 0.8 | 56.5 ± 2.4*** | 1.6 ± 0.6 | 0.9 ± 0.1 |
| 100 | 24.5 ± 0.1*** | 24.2 ± 0.4*** | 1.0 ± 0.0* | n.m. | 82.9 ± 4.8 | 55.3 ± 7.5*** | 1.5 ± 0.5 | 0.8 ± 0.2 |
Note. Respiration of state 3 and state 4 were expressed as absolute values of nanomole of oxygen atoms consumed per minutes per mg protein. RCR (respiratory control ratio) was calculated as the ratio between state 3 and state 4 respirations. The ADP/O was calculated as the number of nanomoles of ADP phosphorylated per nanomole of oxygen atoms consumed during ADP phosphorylation. All the values are presented as absolute mean values ± SD of at least three independent experiments. n.m. not measurable.
p < 0.05 and
p < 0.001 compared with control.
When mitochondria were energized by complex I substrates, 25μM sertraline inhibited state 3 respiration and stimulated state 4 respiration with an RCR of 3.0 ± 0.3. Higher concentrations of sertraline dramatically decreased RCR (1.1 ± 0.3 for 75μM and 1.0 ± 0.0 for 100μM compared with 5.6 ± 0.4 for control). The ADP/O ratio was not affected for complex I substrate except for the higher concentrations of 75 and 100μM. When mitochondria were energized with complex II substrate, unlike by complex I substrate energizing, state 3 respiration was not inhibited. However, state 4 respiration was again stimulated by sertraline and the ADP/O ratio decreased in a concentration-dependent manner (Fig. 2 and Table 1). Our results indicate that sertraline inhibited mitochondrial function by uncoupling oxidative phosphorylation (stimulating state 4, decreasing ADP/O) and inhibiting the activity of complex (inhibited state 3).
Sertraline Inhibits the Activities of Complexes I and V in Isolated Liver Mitochondria
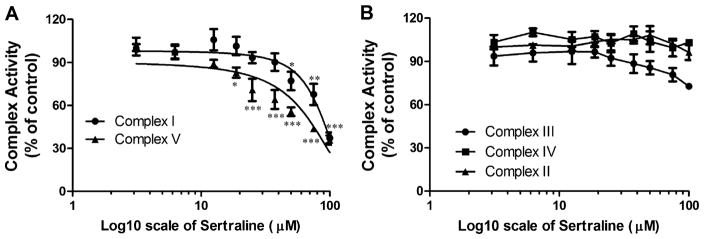
The perturbation of the activity for each individual mitochondrial complex (I–V) was further investigated. In isolated mitochondria, sertraline displayed concentration-dependent inhibitory effects on complexes I and V with IC50s of 88.6 and 69.0μM, respectively (Fig. 3A). Although a slight inhibitory effect on complex III at 100μM was observed, it was not statistically significant compared with control (Fig. 3B). The activity of complex II or IV was not affected even by 100μM sertraline, the highest concentration tested in our study (Fig. 3B).
FIG. 3.
Effects of sertraline on mitochondrial electron transport complex activity in isolated liver mitochondria. Activities of complexes I–V were assessed by spectrophotometric assay described in the Materials and Methods section. (A) Inhibitory effects of sertraline (0–100μM) on complex I and complex V. Concentrations of 50% inhibitory effects on complexes I and V are 88.6 and 69.0μM. (B) Effects of sertraline (0–100μM) on complexes II, III, and IV. Data are presented as % of control. The values are the means ± SD collected from at least three mitochondrial preparations. *p < 0.05, **p < 0.01, and ***p < 0.001, significantly different from the control.
Sertraline Induces the MPT in Isolated Liver Mitochondria
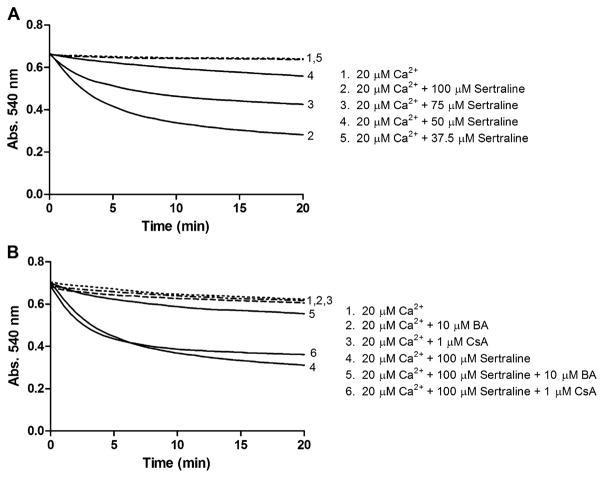
The MPT, characterized by a progressive permeabilization of the inner mitochondrial membrane, leads to the disruption of the mitochondrial membranes, causes mitochondrial swelling, and ultimately triggers to cell death. The MPT is normally determined by examining calcium-dependent mitochondrial swelling, as indicated by the rate of change in absorbance at 540 nm. As Figure 4A shown, sertraline elicited a concentration-dependent mitochondrial swelling from 37.5 to 100μM in the presence of 20μM Ca2+, shown by the reduction in the absorbance of 540 nm. When the concentration was ≤ 37.5μM, sertraline caused no significant induction of MPT.
FIG. 4.
Effects of sertraline on MPT induction in isolated liver mitochondria. Mitochondrial (1.0 mg/ml) was incubated with sertraline (0–100μM) in the presence of 20μM Ca2+. (A) Induction of sertraline on mitochondrial swelling. (B) BA but not cyclosporine A (CsA) protected mitochondrial swelling induced by sertraline. Data are represented as mean from at three independent experiments.
Sertraline Inhibits ANT Activity in Isolated Liver Mitochondria
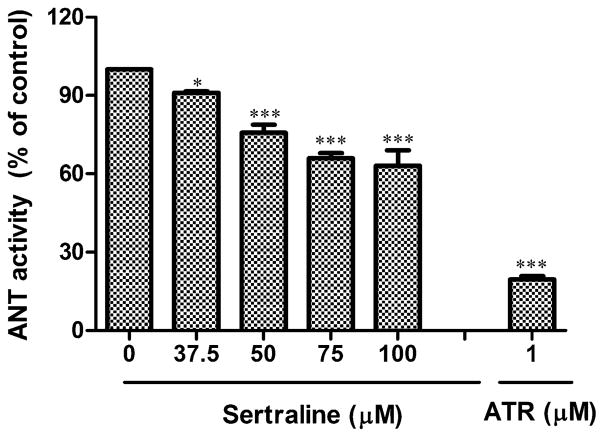
It is known that the induction of MPT results from the opening of the MPT pore (MPTP), a protein pore consisting of numerous components. ANT, also as known as ADP/ATP antiporter (imports ADP to the matrix and exports ATP to the cytosol) which is located in the mitochondrial inner membrane, is a critical structural component of the pore (Crompton, 1999). The activity of ANT can be monitored spectrophotometrically by measuring the formation of NADPH because NADPH formation in the extramitochondrial phase is proportional to ATP efflux. Two ANT regulators have been used to study MPTP. BA, a specific inhibitor of ANT (Klingenberg and Buchholz, 1973), suppresses the opening of the MPTP, whereas an agonist of ANT, atractyloside (ATR), opens the MPTP (Imai et al., 2003). In our study, we used atractyloside as a positive control to ensure that the assay system worked. Figure 5 shows that the MPTP opener atractyloside significantly decreases the activity of ANT. Under the same experimental conditions, sertraline similarly inhibits ANT activity, in a concentration-dependent manner. At the highest concentration (100μM) used in the study, ANT inhibition was approximately 40% (Fig. 5).
FIG. 5.
Effect of sertraline on ANT activity in isolated liver mitochondria. ANT activity was measured by ADP-induced ADP/ATP exchange. The results represented as percent of control. Sertraline of 0–100μM were analyzed in this assay. Atractyloside (ATR, 1μM) was used as positive control. *p < 0.05 and ***p < 0.001, significantly different from the control. Data are represented as mean ± SD from at least three independent experiments.
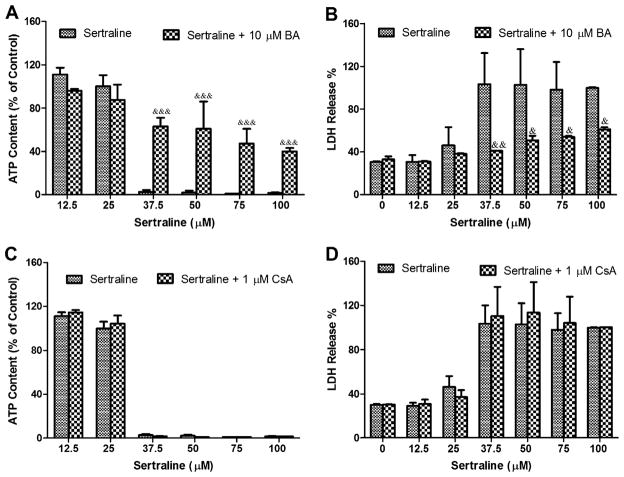
BA but Not Cyclosporine A Protects Liver Injury in Both Isolated Mitochondria and Primary Hepatocytes
Besides ANT, cyclophilin D (CyPD), located in mitochondrial matrix, is another component important in assembling the MPTP (Connern and Halestrap, 1994). Cyclosporine A (CsA), a pseudosubstrate of CyPD (Lin and Lechleiter, 2002), keeps CyPD from interacting with MPTP then acts as an MPT inhibitor (Broekemeier et al., 1989). To explore further mechanisms of sertraline-associated mitochondrial toxicity and identify the target of MPTP, MTP inhibitors were used to look for protective effects against sertraline-associated mitochondrial inhibition. BA and cyclosporine A, two MPT inhibitors with different targets, as discussed above, were used in the current study. In isolated mitochondria, BA attenuated the mitochondrial swelling caused by 100μM sertraline (Fig. 4B). In contrast, cyclosporine A did not prevent sertraline-induced MPT (Fig. 4B). These results demonstrated that sertraline may target ANT primarily rather than CyPD for the opening of MPTP and consequently induce MPT. In rat primary hepatocytes, when coculturing sertraline for 24 h with BA or cyclosporine A, BA prevented ATP depletion (Figs. 6A and 6B) and LDH release (Figs. 6C and 6D), whereas cyclosporine A poorly prevented either ATP depletion or LDH release.
FIG. 6.
Effects of BA and cyclosporine A (CsA) against sertraline-induced ATP depletion and LDH leakage in rat primary hepatocytes. BA (10μM) and CsA (1μM) was cocultured with sertraline (0–100μM) in rat primary hepatocytes. BA protected ATP depletion (A) and LDH leakage (B). CsA failed to prevent ATP depletion (C) and LDH leakage (D). The ATP content result was represented as percentage of DMSO-treated negative control. &&p < 0.01 and &p < 0.001, significantly different from the treatment without BA. Data are represented as mean ± SD from at least three independent experiments.
DISCUSSION
Drug-induced liver injury (DILI) is a frequent cause for the failure of a drug to get approved or for the withdrawal of many drugs from the market post-FDA approval. Although drugs can cause hepatotoxicity through different mechanisms (Lee, 2003), a growing body of literature reports that mitochondria is a primary or secondary drug target and that the impairment of mitochondria is one of the major contributors to DILI (Dykens et al., 2007; Scatena et al., 2007). The mechanisms of drug-induced mitochondrial dysfunction include uncoupling of electron transport from ATP synthesis, inhibition of mitochondrial complexes, opening of the MPT pore, and inhibition of mitochondrial DNA polymerase (Dykens et al., 2007; Scatena et al., 2007). For instance, troglitazone, approved by the FDA to treat type 2 diabetes, was withdrawn from the market after reports of severe liver failure (Guo et al., 2006b; Watkins and Whitcomb, 1998). Subsequent in vitro and in vivo studies demonstrated that troglitazone induced MPT, uncoupled oxidative phosphorylation, and inhibited the activities of complexes (Masubuchi et al., 2006; Ong et al., 2007; Tirmenstein et al., 2002). The reason for withdrawing nefazodone, an antidepressant agent, in the United States in 2004 was also liver failure. The malfunction of mitochondria in nefazodone-induced hepatotoxicity was attributed to the inhibition of mitochondrial respiratory complexes I and IV activity and depolarization of the mitochondrial membrane (Dykens et al., 2008).
In the present study, we examined liver toxicity induced by sertraline, an SSRI antidepressant, and investigated the possible underlying mechanisms, focusing on mitochondrial dysfunction. We initially measured cellular ATP content of hepatocytes, and ATP depletion by sertraline was clearly observed in a time- and concentration-dependent manner (Fig. 1). ATP depletion occurred within 0.5 h, whereas other toxicity parameters such as LDH release (Fig. 1) were not altered. ATP depletion is an early event of mitochondrial dysfunction. With prolonged or worsening ATP depletion, irreversible mitochondrial damage and necrotic cell death occur (Kristensen, 1989). Because we observed ATP depletion in our initial experiment, we conducted more experiments to investigate the role of mitochondrial dysfunction.
The mitochondrial inner membrane permeability transition (MPT) is one of main changes in mitochondria that leads to cell death (Al-Nasser and Crompton, 1986; Hunter and Haworth, 1979). The MPT is a transition in the permeability of the inner membrane that results from the MPT pore opening when extracellular calcium (Ca2+) or other stimuli presents (Haworth and Hunter, 1979). The MPT pore is a protein pore formed by structural molecules such as voltage-dependent anion channel, ANT, and cyclophilin D (CyPD) (Tsujimoto et al., 2006). Changes of the MPT pore cause mitochondrial swelling and uncoupling of oxidative phosphorylation, leading to interrupted ATP generation and further inducing cytotoxicity (Kroemer et al., 2007). Because we found that sertraline induced concentration-dependent MPT (with mitochondrial swelling as the indicator) (Fig. 4A), we were interested in identifying the target responsible for MPT induction and used two common MPT blockers (BA and cyclosporine A) targeting different components of MPT pore. Mitochondrial swelling was prevented by the ANT inhibitor BA but not by the CyPD inhibitor cyclosporine A (Fig. 4B), implying that ANT maybe the primary target for MPT induction. Moreover, in rat primary hepatocytes, BA attenuated both ATP depletion and LDH release caused by sertraline, whereas cyclosporine A had little effect (Fig. 6), indicating further that MPT induction through the interaction with ANT is the most likely mechanism of action in sertraline-associated liver toxicity.
Multiple mechanisms and targets are often reported to be involved in drug- or xenobiotic-associated mitochondrial impairment (Labbe et al., 2008). In our study, we found that sertraline inhibited the activities of complexes I and V with IC50s of 88.6 and 69μM, respectively, indicating that the inhibitory potency is greater for complex V than that for complex I (Fig. 3). In intact mitochondria, proton gradient was transferred from complex I to complex IV and generates ATP through complex V (ATP synthase). During the process of ATP synthesis, ANT, as the transporter protein, is important for transmembrane change between ATP produced via oxidative phosphorylation and cytosolic ADP (Fiore et al., 1998). It is interesting that in our study, sertraline inhibited both complex V and ANT, suggesting that inhibition of mitochondrial respiratory complex and induction of MPT via ANT inhibition are two possible mechanisms for the decrease in energy status observed in rat primary hepatocytes. In our oxygen consumption study (Fig. 2), it is clear that irrespective of the substrate used (glutamate/malate for complex I or succinate for complex II), state 4 respiration was stimulated, which characterized sertraline as an uncoupler of mitochondrial oxidative phosphorylation (Masubuchi et al., 1999).
Clinically, single oral doses of 400 mg sertraline administered to healthy volunteers gave maximum plasma concentrations of 253.20 ± 112.29 ng/ml (~0.74μM) (Saletu et al., 1986), and plasma concentrations were higher in young females and in elderly patients of both sexes (Warrington, 1991). Thus, concentrations of sertraline used in our study were higher than clinical concentrations. It is known that many variables, such as genetic variability, race, sex, age, metabolic capacity, drug-drug interactions, and preexisting diseases, contribute to individual susceptibility and toxicity of idiosyncratic drugs (Lee, 2003). It has been suggested that to identify an idiosyncratic hepatotoxic drug, it should be tested at an in vitro dose that is 100-fold of the Cmax value reported in humans (Xu et al., 2008). Since the toxic concentration of sertraline in our study started at 37.5μM, which is close to 100 times of reported Cmax of sertraline (~74μM), the concentrations used in our study were meaningful.
In our study, the mitochondria impairments induced by sertraline have been observed which supported by a battery of assays, we further performed previously established glucose/galactose assay (Marroquin et al., 2007) to examine if there is any different toxic effects between two cell culture conditions. As shown in Supplementary figure 2, our preliminary result indicated that sertraline caused similar toxicity in galactose-grown HepG2 cells and glucose-grown HepG2 cells, which illuminated that mitochondrial impairment may not be sole but one of the important contributors to sertraline-induced hepatotoxicity.
It should be noted that this work was designed to elucidate the previously unknown mechanism of sertraline-associated liver toxicity; it is important to identify the risk factors that contribute to individual susceptibility. In future studies, we plan to investigate the effect of altered drug metabolic ability on sertraline-induced liver toxicity because (1) there are interindividual differences in the expression of drug metabolizing enzymes, affecting drug clearance and toxicity (Guo et al., 2011); (2) it is likely that other drugs or dietary supplements will be taken with sertraline, possibly, leading to potential drug-drug interactions due to the modulation of drug metabolizing enzymes; and (3) in an attempt to study sertraline metabolite-associated toxicity, we found that sertraline-associated toxicity assessed by ATP depletion and LDH release were enhanced by SKF 525-A, a general inhibitor of CYP 450, indicating that the parent form of sertraline is more toxic than its metabolites. Therefore, it is reasonable to suspect that any defects in CYP 450 include CYP2D6, 2C9, 2B6, 2C19, and 3A4 involving in sertraline metabolism will enhance its parent drug toxicity. Although the contributions of the individual CYP 450s need to be detailed, CYP2C19 will be a first candidate to be studied because in a pharmacokinetics study it was reported that poor CYP2C19 metabolizers had a higher level of sertraline than normal metabolizers (Wang et al., 2001).
In conclusion, our study, using an in vitro model, showed that sertraline caused mitochondrial dysfunction. Although sertraline is considered relatively safe, it should be noted that the use of sertraline may be not without risk.
Supplementary Material
Acknowledgments
FUNDING
Y.L. was supported by the appointment to the Postgraduate Research Program at the National Center for Toxicological Research administered by Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. FDA.
We thank Drs William Melchior, Baitang Ning, Varsha Desai, and Fred Beland for their critical review of this manuscript. We also thank Drs Weida Tong and Leming Shi for their support and collaboration.
This article is not an official guidance or policy statement of the U.S. FDA. No official support or endorsement by the U.S. FDA is intended or should be inferred.
Footnotes
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
References
- Al-Nasser I, Crompton M. The reversible Ca2+-induced permeabilization of rat liver mitochondria. Biochem J. 1986;239:19. doi: 10.1042/bj2390019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlante A, Bobba A, De Bari L, Fontana F, Calissano P, Marra E, Passarella S. Caspase dependent alteration of the ADP/ATP translocator triggers the mitochondrial permeability transition which is not required for the low potassium dependent apoptosis of cerebellar granule cells. J Neurochem. 2006;97:1166–1181. doi: 10.1111/j.1471-4159.2006.03820.x. [DOI] [PubMed] [Google Scholar]
- Atlante A, de Bari L, Bobba A, Marra E, Calissano P, Passarella S. Cytochrome c, released from cerebellar granule cells undergoing apoptosis or excytotoxic death, can generate protonmotive force and drive ATP synthesis in isolated mitochondria. J Neurochem. 2003;86:591–604. doi: 10.1046/j.1471-4159.2003.01863.x. [DOI] [PubMed] [Google Scholar]
- Biour M, Poupon R, Grange JD, Chazouilleres O, Levy VG, Bodin F, Cheymol G. Hépatotoxicité des médicaments: Mise à jour du fichier bibliographique des atteintes hépatiques et des médicaments responsables. Gastroenterol Clin Biol. 1992;16:64–88. [PubMed] [Google Scholar]
- Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem. 1989;264:7826–7830. [PubMed] [Google Scholar]
- Collados V, Hallal H, Andrade RJ. Sertraline hepatotoxicity: Report of a case and review of the literature. Dig Dis Sci. 2010;55:1806–1807. doi: 10.1007/s10620-010-1192-7. [DOI] [PubMed] [Google Scholar]
- Collados Arroyo V, Plaza Aniorte J, Hallal H, Pérez Cuadrado E. Hepatotoxicidad asociada a sertralina. Farm Hosp. 2008;32:60–61. doi: 10.1016/s1130-6343(08)72814-9. [DOI] [PubMed] [Google Scholar]
- Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994;302(Pt 2):321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- Davies TS, Kluwe WM. Preclinical toxicological evaluation of sertraline hydrochloride. Drug Chem Toxicol. 1998;21:521–537. doi: 10.3109/01480549809002220. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41:1247–1266. doi: 10.2165/00003088-200241150-00002. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Jamieson JD, Marroquin LD, Nadanaciva S, Xu JJ, Dunn MC, Smith AR, Will Y. In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone, and buspirone. Toxicol Sci. 2008;103:335–345. doi: 10.1093/toxsci/kfn056. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Marroquin LD, Will Y. Strategies to reduce late-stage drug attrition due to mitochondrial toxicity. Expert Rev Mol Diagn. 2007;7:161–175. doi: 10.1586/14737159.7.2.161. [DOI] [PubMed] [Google Scholar]
- Fartoux-Heymann L, Hezode C, Zafrani ES, Dhumeaux D, Mallat A. Acute fatal hepatitis related to sertraline. J Hepatol. 2001;35:683–684. doi: 10.1016/s0168-8278(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Fiore C, Trezeguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noel F, Lauquin GJ, Brandolin G, Vignais PV. The mitochondrial ADP/ATP carrier: Structural, physiological and pathological aspects. Biochimie. 1998;80:137–150. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Galán Navarro J. Hepatitis aguda colestásica probablemente causada por sertralina. Rev Esp Enferm Dig. 2001;93:822. [PubMed] [Google Scholar]
- Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos. 2011;39:528–538. doi: 10.1124/dmd.110.035873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Fang H, Collins J, Fan X, Dial S, Wong A, Mehta K, Blann E, Shi L, Tong W. Differential gene expression in mouse primary hepatocytes exposed to the peroxisome proliferator-activated receptor α agonists. BMC Bioinformatics. 2006a;7:S18. doi: 10.1186/1471-2105-7-S2-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhang L, Sun Y, Muskhelishvili L, Blann E, Dial S, Shi L, Schroth G, Dragan YP. Differences in hepatotoxicity and gene expression profiles by anti-diabetic PPAR gamma agonists on rat primary hepatocytes and human HepG2 cells. Mol Divers. 2006b;10:349–360. doi: 10.1007/s11030-006-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautekeete ML, Colle I, van Vlierberghe H, Elewaut A. Symptomatic liver injury probably related to sertraline. Gastroenterol Clin Biol. 1998;22:364–365. [PubMed] [Google Scholar]
- Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- Hogeboom GH, Schneider WC, Pallade GE. Cytochemical studies of mammalian tissues: I. Isolation of intact mitochondria from rat liver; some biochemical properties of mitochondria and submicroscopic particulate material. J Biol Chem. 1948;172:619. [PubMed] [Google Scholar]
- Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- Imai H, Koumura T, Nakajima R, Nomura K, Nakagawa Y. Protection from inactivation of the adenine nucleotide translocator during hypoglycaemia-induced apoptosis by mitochondrial phospholipid hydroperoxide glutathione peroxidase. Biochem J. 2003;371:799–809. doi: 10.1042/BJ20021342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Hwang W, Narendran R. Acute liver damage possibly related to sertraline and venlafaxine ingestion. Ann Pharmacother. 1999;33:381–382. doi: 10.1345/aph.18155. [DOI] [PubMed] [Google Scholar]
- Kirby D, Thorburn D, Turnbull D, Taylor R. Biochemical assays of respiratory chain complex activity. In: Wilson L, Matsudaira P, editors. Methods in Cell Biology. Academic Press; San Diego, CA: 2007. pp. 93–119. [DOI] [PubMed] [Google Scholar]
- Klingenberg M, Buchholz M. On the mechanism of bongkrekate effect on the mitochondrial adenine-nucleotide carrier as studied through the binding of ADP. Eur J Biochem. 1973;38:346–358. doi: 10.1111/j.1432-1033.1973.tb03067.x. [DOI] [PubMed] [Google Scholar]
- Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MTS, Pitot HC. Use of a low-speed, iso-density Percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol Plant. 1986;22:201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- Kristensen SR. A critical appraisal of the association between energy charge and cell damage. Biochim Biophys Acta Mol Cell Res. 1989;1012:272–278. doi: 10.1016/0167-4889(89)90108-0. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: Mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335–353. doi: 10.1111/j.1472-8206.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- Lin DT, Lechleiter JD. Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem. 2002;277:31134–31141. doi: 10.1074/jbc.M112035200. [DOI] [PubMed] [Google Scholar]
- Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: Replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97:539–547. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- Martínez Matos Y. Mujer de mediana edad con prurito y patrón analítico de colestasis aguda. SEMERGEN. 2002;28:105–107. [Google Scholar]
- Masubuchi Y, Kano S, Horie T. Mitochondrial permeability transition as a potential determinant of hepatotoxicity of antidiabetic thiazolidinediones. Toxicology. 2006;222:233–239. doi: 10.1016/j.tox.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Yamada S, Horie T. Diphenylamine as an important structure of nonsteroidal anti-inflammatory drugs to uncouple mitochondrial oxidative phosphorylation. Biochem Pharmacol. 1999;58:861–865. doi: 10.1016/s0006-2952(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007;97:205–213. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- Persky S, Reinus JF. Sertraline hepatotoxicity: A case report and review of the literature on selective serotonin reuptake inhibitor hepatotoxicity. Dig Dis Sci. 2003;48:939–944. doi: 10.1023/a:1023007831047. [DOI] [PubMed] [Google Scholar]
- Rossignol R, Letellier T, Malgat M, Rocher C, Mazat JP. Tissue variation in the control of oxidative phosphorylation: Implication for mitochondrial diseases. Biochem J. 2000;347(Pt 1):45–53. [PMC free article] [PubMed] [Google Scholar]
- Saletu B, Grunberger J, Linzmayer L. On central effects of serotonin re-uptake inhibitors: Quantitative EEG and psychometric studies with sertraline and zimelidine. J Neural Transm. 1986;67:241–266. doi: 10.1007/BF01243351. [DOI] [PubMed] [Google Scholar]
- Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B. The role of mitochondria in pharmacotoxicology: A reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol. 2007;293:C12–C21. doi: 10.1152/ajpcell.00314.2006. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Hu CX, Gales TL, Maleeff BE, Narayanan PK, Kurali E, Hart TK, Thomas HC, Schwartz LW. Effects of troglitazone on HepG2 viability and mitochondrial function. Toxicol Sci. 2002;69:131–138. doi: 10.1093/toxsci/69.1.131. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006;1757:1297–1300. doi: 10.1016/j.bbabio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Vernetti L, Irwin W, Giuliano KA, Gough A, Johnston K, Taylor D. Cellular systems biology applied to preclinical safety testing: A case study of CellCiphrTM profiling. In: Ekins S, Xu JJ, editors. Drug Efficacy, Safety, and Biologics Discovery: Emerging Technologies and Tools. John Wiley & Sons, Inc; Hoboken, NJ: 2008. pp. 53–73. [Google Scholar]
- Verrico MM, Nace DA, Towers AL. Fulminant chemical hepatitis possibly associated with donepezil and sertraline therapy. J Am Geriatr Soc. 2000;48:1659–1663. doi: 10.1111/j.1532-5415.2000.tb03879.x. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Groen AK, Van Roermund CW, Tager JM. Factors determining the relative contribution of the adenine-nucleotide translocator and the ADP-regenerating system to the control of oxidative phosphorylation in isolated rat-liver mitochondria. Eur J Biochem. 1984;142:417–424. doi: 10.1111/j.1432-1033.1984.tb08303.x. [DOI] [PubMed] [Google Scholar]
- Wang JH, Liu ZQ, Wang W, Chen XP, Shu Y, He N, Zhou HH. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;70:42–47. doi: 10.1067/mcp.2001.116513. [DOI] [PubMed] [Google Scholar]
- Warrington SJ. Clinical implications of the pharmacology of sertraline. Int Clin Psychopharmacol. 1991;6(Suppl 2):11–21. doi: 10.1097/00004850-199112002-00004. [DOI] [PubMed] [Google Scholar]
- Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med. 1998;338:916–917. doi: 10.1056/NEJM199803263381314. [DOI] [PubMed] [Google Scholar]
- Xu JJ, Henstock PV, Dunn MC, Smith AR, Chabot JR, de Graaf D. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol Sci. 2008;105:97–105. doi: 10.1093/toxsci/kfn109. [DOI] [PubMed] [Google Scholar]
- Zoloft. Zoloft (Sertraline Hydrochloride) Package Insert. Roering Division of Pfizer, Inc; New York, NY: 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.