SUMMARY
The RNA polymerase II largest subunit C-terminal domain consists of repeated YSPTSPS heptapeptides. The role of tyrosine-1 (Tyr1) remains incompletely understood, as, for example, mutating all Tyr1 residues to Phe (Y1F) is lethal in vertebrates but a related mutant has only a mild phenotype in S. pombe. Here we show that Y1F substitution in budding yeast resulted in a strong slow-growth phenotype. The Y1F strain was also hypersensitive to several different cellular stresses that involve MAP kinase signaling. These phenotypes were all linked to transcriptional changes, and we also identified genetic and biochemical interactions between Tyr1 and both transcription initiation and termination factors. Further studies uncovered defects related to MAP kinase I (Slt2) pathways, and we provide evidence that Slt2 phosphorylates Tyr1 in vitro and in vivo. Our study has thus identified Slt2 as a Tyr1 kinase, and in doing so provided links between stress response activation and Tyr1 phosphorylation.
Graphical Abstract
INTRODUCTION
In eukaryotes, the multisubunit enyzme RNA polymerase II (RNAP II) is responsible for transcription of all mRNAs as well as numerous noncoding RNAs. The largest subunit of RNAP II, Rpb1, contains a C-terminal domain (CTD) consisting of 26 (in yeast) to 52 (in vertebrates) heptad repeats, with the consensus Tyr1-Ser2-Pro3-Thr4-Ser5-Pro6-Ser7 (reviewed by Hsin and Manley 2012; Eick and Geyer 2013). The non-proline residues are all subject to phosphorylation and de-phosphorylation during the transcription cycle, and the different residues, and their modification, appear to serve distinct roles related to the stage of transcription. The CTD thus functions in essentially all aspects of the synthesis and processing of RNAP II transcripts.
Of the phosphorylatable CTD residues, serines 2 and 5 have received the most attention. Ser5 phosphorylation is concentrated near the 5′ end of genes, primarily as a result of the TFIIH-associated kinase Kin28 (Feaver et al. 1994), where it functions in recruitment of capping enzyme and a number of factors important for transcriptional elongation (e.g., Cho et al. 1997; Ng et al. 2003). Additionally, the multisubunit Mediator takes advantage of CTD flexibility and aids in Kin28 phosphorylation of all CTD heptads by guiding it through the Mediator-PIC (pre-initiation complex) head module (Robinson et al. 2016). Ser5P is also directly recognized by Nrd1 of the NNS complex, which also consists of Nab3 and Sen1, to terminate transcription of short RNAP II products, including cryptic unstable transcripts (CUTs), stable uncharacterized transcripts (SUTs) and small nucleolar RNAs (Arigo et al. 2006, Vasiljeva et al. 2008). The primary driver of Ser2 phosphorylation in yeast, Ctk1, is responsible for the bulk of Ser2P, which accumulates towards the 3′ end of genes (Mayer et al. 2010). Ser2P levels peak near 3′ cleavage-polyadenylation sites, where termination factors such as Pcf11 and Rtt103 interact directly with the CTD, facilitating 3′ processing and termination (Kim et al. 2004; Luo et al. 2006).
Determining the function of Ser2 and Ser5, as well as other residues in the heptad repeat, has benefited from the strategy of directly modifying the CTD. Besides direct substitutions (e.g., Ser-to-Ala), more recent efforts (Schuller et al. 2016; Suh et al. 2016) have altered the CTD to allow detection of phosphorylation patterns using mass spectrometry. These studies confirmed that all non-proline residues are phosphorylated, although replacing many individual residues (mostly Ser7) with lysine and arginine (enabling trypsin cleavage) produced a slow growth phenotype under cold stress (Suh et al. 2016). Extending the importance of Ser2 and Ser5, these studies found that Tyr1 and Thr4 phosphorylation was much less abundant compared to Ser2/5, although whether or not this was due to an effect of the other CTD modifications or to the relative importance of Tyr1/Thr4 under the growth conditions used is not clear.
The other phosphorylatable CTD residues (Ser7, Thr4 and Tyr1) appear to perform more specialized roles (reviewed in Yurko and Manley 2017). Ser7 phosphorylation is present on RNAP II on active genes (Chapman et al. 2007) and is also important for proper small nuclear RNA gene expression, such as U1/U2 snRNA 3′ end formation, in mammals (Egloff et al. 2007). It also enables better recruitment of the functional human homolog of Ctk1, P-TEFb, to the CTD (Mayer et al. 2010; Czudnochowski et al. 2012). Thr4 has been found to have differing roles depending on the organism. In chicken cells expressing a T4V derivative, histone mRNA 3′ processing was found to be defective (Hsin et al. 2011), and both chicken T4V and human T4A (Hintermair et al. 2012) mutants were lethal. In budding yeast, Thr4 is required for the induction of certain classes of genes, and is necessary for proper histone H2A.Z eviction and induction of PHO and GAL metabolic genes (Rosonina et al. 2014). A role for Thr4 in splicing and Rtt103 recruitment in budding yeast has also been suggested (Harlen et al. 2016).
The function of Tyr1 has only more recently been investigated. Chicken DT40 cells expressing a Y1F derivative were inviable, and Tyr1, and its phosphorylation, are required for CTD stability (Hsin et al. 2014). Tyr1 was also found to be required for efficient turnover of certain unstable transcripts, such as upstream antisense RNAs (uaRNAs), and Tyr1 phosphorylation was elevated on regions encoding uaRNAs. Very similar results were observed in human cells (Descostes et al. 2014). A Y1F derivative was also found to be lethal in budding yeast (West and Corden 1995), and Tyr1P patterns along yeast genes are similar to Ser2P (Mayer et al. 2012). Tyr1 phosphorylation is also important for histone chaperone Spt6 binding (Burugula et al. 2014), and the presence of Tyr1P impairs Nrd1 and Rtt103 interaction with CTD peptides, suggesting a role in modulating termination (Lunde et al. 2010; Mayer et al. 2012). Surprisingly, in fission yeast Tyr1 can be mutated with no loss of viability, although cold- and iron-sensitivity phenotypes were noted (Schwer and Shuman 2011; Schwer et al. 2012, 2014). While evidence exists that Tyr1 can be phosphorylated by the c-Abl kinase in mammals (Baskaran et al. 1993), the responsible kinase(s) in yeast, where tyrosine phosphorylation is rare, is unknown.
Here, we investigate the role of Tyr1 and Tyr1P in S. cerevisiae. We first generate a strain that expresses a full-length Y1F form of the CTD and show that the strain is viable but very slow growing under normal conditions. We identify genetic links between the Y1F mutant and Mediator, and found that the Y1F phenotype can be partially suppressed by removing Cdk8/CycC subunits. We also found that several stress-response genes dealing with cell wall, oxidative, and related stress responses, as well as iron response (Schwer et al. 2014), are dysregulated in Y1F cells, and that the cells are hypersensitive to stresses that specifically target these pathways. Bringing these observations together, we demonstrate that the specific MAP kinase involved in these processes, Slt2, phosphorylates Tyr1 in vitro, and that in vivo Slt2 is necessary for full Tyr1 phosphorylation and modulates Tyr1P levels during stress responses. These effects are accompanied by defects in transcription termination factor recruitment, as Y1F disrupts Rtt103 binding to the CTD and impairs Nrd1 recruitment to chromatin. Our study thus links Tyr1 with Slt2-mediated stress responses, and reveals Slt2 as a Tyr1 CTD kinase.
RESULTS
Mutation of all CTD Tyr1 residues results in severe growth defects
In this study, we wished to gain more insight into the function of CTD Tyr1 in budding yeast. Previously, we found that replacing the CTD of chicken Rpb1 with a Y1F derivative resulted in lethality (Hsin et al. 2014), consistent with an earlier study in budding yeast (West and Corden 1995), but in contrast to similar experiments with S. pombe (Schwer et al. 2014). All of these studies, however, employed truncated CTDs, and we therefore constructed a yeast Y1F strain containing 26 FSPTSPS repeats (Figure 1A). As a control, an all-consensus YSPTSPS CTD was inserted into the same background, as in our previous analysis of Thr4 (Rosonina et al. 2014).
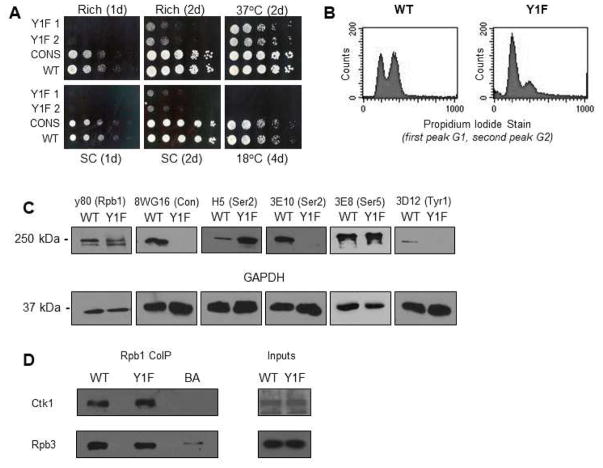
Figure 1. RNAP II CTD Tyr to Phe substitution results in severe growth defects.
(A) Tetrad dissection of heterozygous diploid Y1F CTD strain (Y1F). Y1F CTD in haploid tetrads marked with NAT resistance and is present in all small-size tetrads. Yeast spot assay comparing growth of WT, CONS, and two Y1F clones, with serial fivefold dilutions, on rich or synthetic complete (SC) medium at 30°C, or on rich medium at indicated temperature, for indicated number of days. (B) Cell-cycle assay showing unsynchronized cells stained with propidium iodide, the two peaks representing cells in G1/G0 and G2, respectively. (C) Western blot analysis of cell lysates derived from WT and Y1F strains using antibodies recognizing the N-terminus of Rpb1 (y80), unphosphorylated consensus repeats (8WG16), Ser2P (3E10 and H5), Ser5P (3E8), Tyr1P (3D12) and GAPDH as indicated. (D) Western blot analysis of an Rpb1 co-IP (using y-80), probing for Ctk1 interaction (using 3HA tagged Ctk1 and HA antibody) with RNAP II (normalized to Rpb3). 5% inputs shown. All results shown are representative of three independent experiments.
We next determined several properties of the Y1F strain, which we refer to simply as Y1F. Comparing the growth of the parental strain (WT), consensus (CON), and two separate Y1F (Y1F 2A and 2B) strains revealed that Y1F displayed a severe slow-growth defect on both rich and synthetic complete media (Figure 1A). This phenotype was somewhat alleviated by growing at 37°C, but, consistent with related results in fission yeast (Schwer et al. 2014), Y1F was cold sensitive (Figure 1A). Tetrad analysis showed that the slow growth phenotype was specific to the Y1F mutation (Figure S1A), and the slow growth defect was also observed in liquid media (Figure S1B). Cell-cycle analysis showed that Y1F cells were defective in exiting G1/G0 and entering G2 (Figure 1B).
We next examined accumulation of Rpb1 and CTD phosphorylation status by Western blot (Figure 1C). As expected, the WT strain showed evidence of Tyr1P as well Ser2P and Ser5P. However, Y1F Rpb1, while as expected was not detected by an anti-Tyr1P antibody (3D12), was also not recognized by antibodies specific for unphosphorylated consensus repeats (8WG16) or Ser2P (3E10). This likely indicates an inability of these antibodies to recognize the Y1F epitope (see below). Indeed, a different Ser2P antibody (H5) produced a stronger signal with the Y1F CTD, although whether this represents a bona fide increase in Ser2P, an increase in Ser2P with Ser5P (see Phatnani and Greenleaf 2006), or differential recognition due to an altered epitope, is not known. Ser5P levels appeared unaffected. Finally, analysis with an antibody (y-80) that recognizes an epitope in the first 80 residues of Rpb1 showed that Y1F Rpb1 accumulated to levels comparable to WT and CON Rpb1, and was full length. This contrasts with the situation in vertebrate cells, where Y1F mutations resulted in CTD destabilization (Descostes et al. 2014; Hsin et al. 2014). Levels of the hyperphosphorylated Rpb1 IIo isoform were also similar, suggesting that the apparent differences in Ser2P most likely reflected differences in epitope recognition by the phospho-specific Abs. Supporting the conclusion that Ser2 phosphorylation was unaffected, a co-immunoprecipitation (co-IP) assay with WT or Y1F Rpb1 and Ctk1 revealed no changes in Ctk1 association with Rpb1, providing evidence that Ser2 phosphorylation by Ctk1 was not affected by the Y1F mutation (Figure 1D).
Tyr1 interacts genetically with Mediator subunits MED13/CDK8/CYCC
To investigate what pathways are affected by the Y1F mutation, we carried out a synthetic genetic array assay (SGA) (Tong et al. 2001, Rosonina et al. 2014). We performed the SGA as a suppressor assay, using a 5FOA-URA3 system with a plasmid-borne WT copy of Rpb1, comparing Y1F and CON strains (Poschke et al. 2012). Genes related to transcription found in each of two independent suppressor SGA analyses are listed in Figure 2A (the complete list is in Table S2), and those that best suppressed the Y1F slow growth phenotype are shown in Figure 2B. Sample tetrad dissections are shown in Figure S2A, and suppression of the Y1F growth defect could also be observed in liquid cultures (Figure S2B). Interestingly, subunits of the Cdk8/CycC Mediator kinase module, including a protein required for stable association of Cdk8/CycC to Mediator (Med13), suppressed the Y1F phenotype when deleted, and were responsible for three of the four strongest interactions detected. The Cdk8/CycC kinase/cyclin pair inhibits transcription of numerous genes (many as part of the general stress response) and can phosphorylate transcription factors such as Gcn4 to dampen activation, in tandem with other post-translational modifications such as ubiquitination and SUMOylation (Bose et al. 2005; Rosonina et al. 2012; Allen and Taatjes 2015). The other strongest hit, Ubp8, is a component of the SAGA complex, and is a ubiquitin-specific protease required for deubiquitination of histone H2B (see Koutelou et al. 2010).
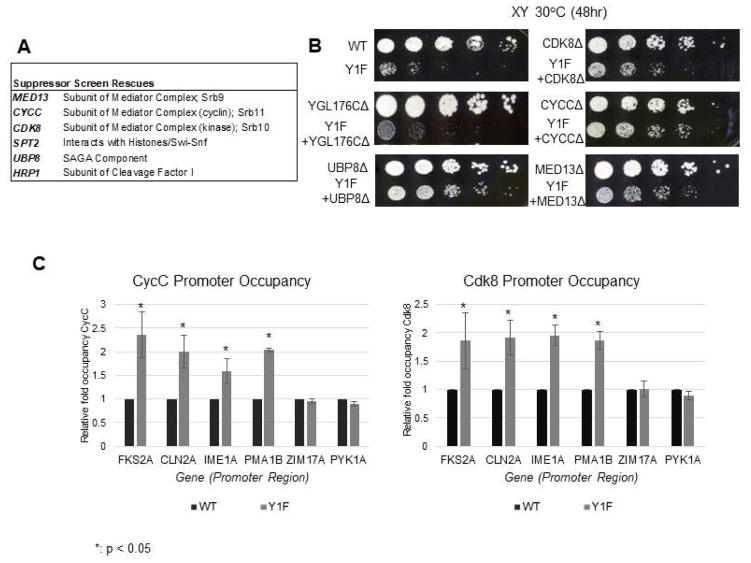
Figure 2. SGA analysis identifies suppressors of Y1F growth defects.
(A) List of genes showing synthetic rescue of Y1F strain in two SGA suppressor screens. Genes displaying strongest interactions and involved in RNAP II transcription are shown (refer to Table S2 for a complete list). (B) Confirmation of genetic interaction between Y1F and MED13/CDK8/CYCC and UBP8. Strains of indicated genotypes were generated by recombinant transformation and growth compared by spot assay on rich medium with serial five-fold dilutions. YGL178C showed no interaction with Y1F in SGA screen and serves as a control. (C) ChIP analysis of Cdk8/CycC occupancy at promoter regions of indicated genes, using 3HA-tagged Cdk8/CycC. Data normalized to WT signal at each gene; P values less than 0.05 are indicated (*). ChIP data is represented as mean +/− SE of three independent experiments.
We next investigated the significance of the Cdk8/CycC SGA suppressor result. For this, we conducted ChIP assays using 3HA-tagged strains in both WT and Y1F backgrounds. Four genes regulated by Cdk8/CycC (FKS2, encoding a cell wall enzyme, cell-cycle coordinator CLN2, meiosis regulator IME1, and PMA1) and two genes not subject to Cdk8/CycC regulation (ZIM17 and PYK1) were analyzed. We detected a roughly two-fold increase in promoter occupancy of Cdk8/CycC on all four target genes in the Y1F strain, while occupancy on the genes without a link to Cdk8/CycC was not affected (Figure 2C). This suggests genes regulated by Cdk8/CycC, which include stress response and cell-cycle genes (Dynlacht 1997; Krasley et al. 2006), are especially sensitive to Y1F, possibly explaining the Y1F cell-cycle defect. We note that although Mediator mutations have long been known to suppress truncated CTD derivatives (Hengartner et al. 1995; Yuryev and Corden 1996), suppression of a full-length Y1F CTD likely reflects a distinct mechanism reflecting enhanced Cdk8/CycC promoter occupancy. These findings are discussed in more detail below.
Tyr1 is required for proper expression of stress-mediated genes
We next extended our analysis by examining directly how Y1F alters global gene expression. To this end, we performed RNA sequencing using 3′READS (Hoque et al. 2013) with WT and Y1F strains grown in rich medium. This method allowed us to determine possible effects of the Y1F on alternative polyadenylation as well as poly(A+) RNA levels. In addition to a general shift favoring distal poly(A) site usage (Figure S3), changes in expression of multiple genes were observed. Expression of 5,925 genes is tabulated in Table S3. Transcript levels of 306 genes were significantly increased, while 329 were reduced (adjusted P-values < 0.05). Correlations between samples were as high as 0.94 for significant genes (Figures S4A and S4B). GO analysis was then performed, with the top associated terms listed in Figure 3A. Consistent with the SGA and/or previous work (Schwer et al. 2014), terms enriched from this analysis include cell wall stress, iron homeostasis, and processes related to oxidoreductive stress. Additionally, a significant upregulation of both CUTs (P-value of 7.49 × 10−12) and SUTs (P-value of 2.23 × 10−5) was detected (Figure S4C). RT-PCR analysis of a sample (12) of the genes with altered expression validated the RNA-seq results (Figure 3B). Among these were FKS2 (associated with multiple stress responses), SIT1 (iron stress response), and CAP2 (DNA damage response). We note that while the changes we observed in relative expression levels of individual genes between WT and Y1F strains was robust, our analysis did not address the possibility that there may have been differences in absolute transcript levels due to the Y1F mutation. Additionally, some of these changes may be due to secondary effects reflecting for example the lower growth rate of the Y1F cells.
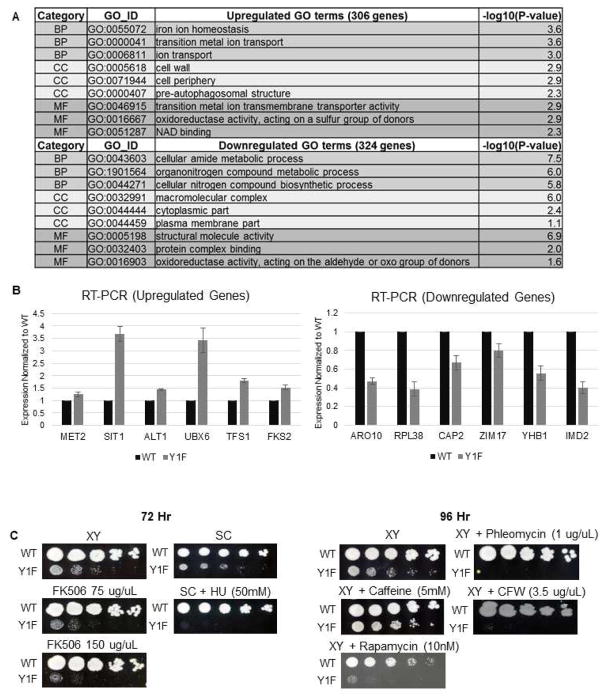
Figure 3. Expression of multiple genes is altered in Y1F.
(A) List of enriched GO terms through Gene Ontology analysis. The numbers of significantly altered genes used for GO analysis were 306 up-regulated, 324 down-regulated. (B) RT-PCR analysis confirming changes in RNA levels for indicated genes between WT and Y1F strains. (IMD2 was not detected in RNA-seq analysis). (C) Spot assays comparing growth of WT and Y1F strains, using serial five-fold dilutions, on rich and synthetic media with media containing indicated stress-inducing compounds.
We next compared our data with an existing Tyr1P ChIP dataset (Mayer et al. 2012). Out of our 630 genes displaying altered expression in Y1F cells, 498 were also found in this dataset (222 up-regulated, 276 down-regulated). Strikingly, the up-regulated genes had Tyr1P ChIP signals weaker than 4032 unaffected genes (P-value of 4.45 × 10−8) whereas the down-regulated genes had stronger Tyr1P signals (P-value of 6.60 × 10−10) (Figure S5). Similar results were obtained when Tyr1P values were normalized to total RNAP II levels (Rpb3; Mayer et al. 2010) (Figure S6A). It is also notable that both up- and down-regulated genes were on average expressed at higher levels in WT yeast (Nagalakshmi et al. 2008) than were the unaffected genes (Figure S6B).
The GO analysis suggested that expression of genes involved in several stress responses was altered in Y1F cells. Notably, however, the expression changes detected were not due to a generalized stress response (Gasch et al. 2000; O’Duibhir et al. 2014), as there was poor overlap between our Y1F data and the Extended Stress Response (ESR) gene set identified by Gasch et al. (11 out of 138 genes from the ESR overlapped). Interestingly, though, we noted that cell wall and iron stress responses both require MAP kinase signaling, specifically the MAPK Slt2, for proper function, and the DNA damage/oxidative stress response does as well (Levin 2011; Soriano-Carot et al. 2012). We therefore next examined possible links between Y1F and stress responses involving MAP kinases. We compared growth of Y1F and WT in medium containing compounds that perturb MAP kinase-associated pathways (Figure 3C). These include calcofluor white (CFW), phleomycin, hydroxyurea, FK506, and rapamycin. Strikingly, with three of the compounds (CFW, phleomycin, and hydroxyurea), Y1F was completely inviable while WT growth was unaffected, and both rapamycin and FK506 slowed growth of Y1F significantly more than WT. Additional stress pathways not known to involve Slt2 (e.g., galactose metabolism, high salt and low phosphate conditions) were also tested and found not to significantly alter growth of Y1F compared to WT (Figure S7A). These observations, together with the RNA analysis above, indicate that although Tyr1 is not required for expression of most genes under normal growth conditions, a subset of MAP kinase-induced genes, and those important for MAP kinase function (such as MKK1), require Tyr1. This in turn implicates Tyr1, and perhaps its phosphorylation, in the response to MAPK-related stresses.
Slt2 phosphorylates Tyr1 in vitro and in vivo
The above data suggesting that MAPK signaling pathways are defective in Y1F cells raise the possibility that a kinase in this pathway may phosphorylate Tyr1. Two candidates were Slt2 and Hog1, both of which are Ser/Thr kinases that have also been shown capable of Tyr phosphorylation (Levin-Salomon et al. 2009; Maayan et al. 2012). Slt2 is a component of the PKC1 signaling pathway (reviewed by Pearson et al. 2011), which regulates the cellular response to several stresses, such as heat shock, cell wall stress and DNA damage, through MAP kinase cascades (Soriano-Carot et al. 2012). Slt2 acts in transcription through phosphorylation of transcription factors (TFs), such as the Swi4/Swi6 complex, also known as SBF (e.g., Kim and Levin 2010). Activated Slt2 is recruited to promoters to increase transcription by phosphorylating TFs as well as by mediating destruction of CycC, thereby further increasing transcription (Levin 2011). Furthermore, Slt2 promotes transcription of stress related genes not only by phosphorylation of SBF and other TFs, but also by blocking association of the NNS complex, preventing early transcription termination (Kim and Levin 2011). Hog1 functions in the cellular response to osmotic stress, and is homologous to p38 and JNK kinases in mammals (reviewed in Brewster and Gustin 2014).
In light of the above, we tested the possibility that Slt2 and/or Hog1 have Tyr1 kinase activity. We first used an in vitro kinase assay (adapted from Campbell 2014) to determine if either kinase can phosphorylate a GST-CTD derivative. For this, we generated 3HA-tagged versions of Slt2 and Hog1 to allow us to immunoprecipitate (IP) these kinases from cell extracts. We IPed HA-tagged Slt2 and Hog1 from WT cells (after activating the kinases through exposure to the appropriate stress conditions, i.e. CFW or NaCl, respectively), incubated them with a purified GST-CTD fusion protein, and analyzed phosphorylation by Western blot with CTD phospho-specific antibodies (Figure 4A). Importantly, we found that Slt2, but not Hog1, can indeed phosphorylate Tyr1. Consistent with previous data (Akhtar et al. 2009; Chasman et al. 2014), Slt2 and Hog1 also phosphorylated Ser2 and Ser5. As controls, strains carrying kinase-dead (KD) HA-tagged derivatives of these two kinases were prepared and used in the in vitro kinase assay. Neither KD derivative showed any kinase activity (Figure 4A). As additional controls for specificity, we constructed and analyzed strains expressing 3HA-tagged Kin28 and Ctk1, which phosphorylated Ser2 and/or Ser5 but not Tyr1 (Figure S7B). Our data thus provide strong evidence that Slt2 possesses Tyr1 kinase activity.
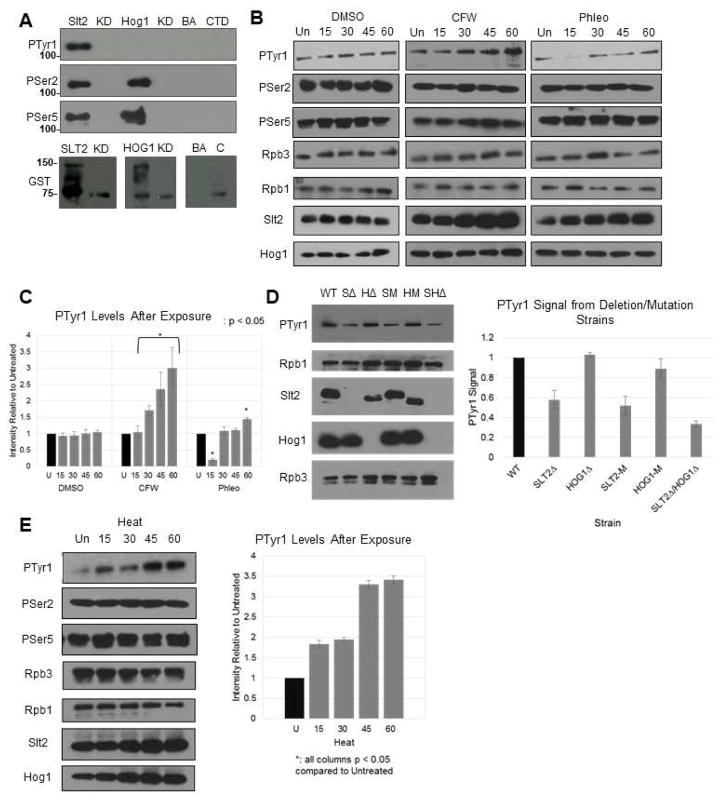
Figure 4. Slt2 phosphorylates Tyr1 in vitro and in vivo.
(A) In vitro kinase assay using 3HA-tagged Slt2 and Hog1 extracted and IPed from activated cells. Following incubation with GST-CTD, proteins were resolved by SDS-PAGE and blots probed with the indicated antibodies. Lanes are Slt2-HA (SLT2) and kinase-dead (KD), Hog1-HA (HOG1) and kinase dead (KD), beads and antibody control (BA) and GST-CTD alone (CTD). (B) Western blot of Tyr1P levels after stress induction. DMSO, calcofluor white (CFW) and phleomycin (Phl) are shown. Rpb1, Slt2, Hog1, and Rpb3 were also probed using their respective antibodies. Time points are untreated (Un), 15, 30, 45 and 60 minutes. (C) Quantification of (B). Signals were normalized individually to Rpb3 levels, then collectively to uninduced control. (D) Western blot and quantification of Tyr1P levels in isogenic deletion strains. Protein extracts from strains with either SLT2/HOG1 deletions (SΔ/HΔ; double, SHΔ) or kinase-dead mutations (SM/HM) were blotted using 3D12 and normalized to WT. (E) Western blot of Tyr1P levels after heat stress (37°C); signals normalized as in (B). All results shown are representative of three independent experiments; data are represented as mean +/− SE of three independent experiments.
We next investigated whether Slt2 can affect Tyr1 phosphorylation status in vivo. For this, we first subjected WT cells to MAPK pathway-related stresses, specifically cell wall stress resulting from exposure to CFW and DNA damage stress from phleomycin exposure. Strikingly, Western blot analysis of cell lysates revealed an increase in Tyr1P following CFW exposure (3.0 fold after one hour, normalized to Rpb3 levels), but not of Ser2P or Ser5P (Figure 4B, quantified in Figure 4C). While phleomycin treatment resulted in a decrease in Tyr1P after 15 minutes, a net 1.5-fold increase was observed after one hour (Figure 4B). Slt2 levels following CFW and phleomycin exposure were increased relative to the DMSO alone control (following a decrease after 15 minutes in the case of phleomycin), while Hog1 levels were unchanged by either treatment (Figure 4B). To determine whether the increased Tyr1P was in fact due to Slt2, we generated strains carrying deletions of SLT2 (SΔ), HOG1 (HΔ), or both genes (SHΔ). We also analyzed the kinase-dead strains described above (SM and HM). WT cells were grown under normal conditions (SLT2 mutant strains are inviable in the presence of CFW [van Voorst et al. 2006]), and lysates analyzed by Western blot (Figure 4D). Importantly, Tyr1P was reduced in the SΔ, S-KD and SH1Δ strains (1.8-, 1.9-, and 3.0-fold, respectively), but not in the HΔ and H-KD strains. These findings strongly support our in vitro data that Slt2 is a Tyr1 kinase, although as Tyr1P was not completely abrogated in the SLT2 mutant strains, another unknown kinase may also target Tyr1.
We extended this analysis to another MAPK-related stress, heat shock. Consistent with earlier studies (Kamada et al. 1995; Kim et al. 2010), and similar to what we observed following CFW exposure, heat shock resulted in an increase in overall Slt2 levels (Figure 4E). Importantly, heat shock induced a 3.4-fold increase in Tyr1P, while Rpb1 levels and Ser2P/Ser5P were unchanged. Due to lethality of heat exposure for SLT2 mutants (Torres et al. 1991), we were unable to measure Tyr1P levels under heat stress in the deletion and kinase-dead strains described above.
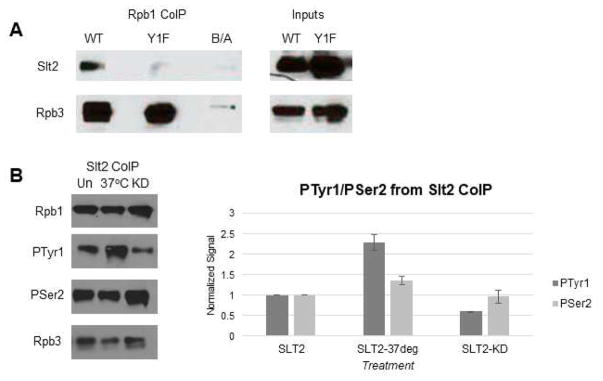
We next examined if Slt2 associates with RNAP II. To this end, we performed coIP assays with extracts from WT and Y1F cells expressing 3HA-tagged Slt2 (Figure 5A). Using y-80 for IP, we found that Slt2 coIPed with RNAP II from WT cell extracts, but not from Y1F extracts. (DNAse treatment did not alter the results; data not shown). To extend these results, we prepared extracts from WT cells expressing 3HA-tagged Slt2, either growing normally or treated with CFW to activate the kinase, and from normally growing cells expressing 3HA-tagged KD Slt2. Extracts were IPed with anti-HA antibodies and Western blots probed with anti-Tyr1P, -Rpb1, -Ser2P and -Rpb3 antibodies. Strikingly, Slt2-associated Tyr1P levels were 2.3-fold higher in the extracts from activated relative to normal cells, while 0.6-fold lower in the kinase-dead strain (Figure 5B); no changes in Rpb1, Ser2P or Ser5P levels were detected. Taken together, our findings establish Slt2 as a CTD Tyr1 kinase, and link Slt2-mediated Tyr1 phosphorylation with the cellular stress response.
Figure 5. Differential association of Slt2 with WT and Y1F RNAP II.
(A) Western blot analysis of a Rpb1 co-IP (using y-80), probing for Slt2 interaction with RNAP II (normalized to Rpb3). 5% inputs shown. (B) Western blot analysis of a Slt2-3HA co-IP, probing for total Rpb1 (y80), Tyr1-P Rpb1 (3D12) and Ser2-P Rpb1 (3E10), using Rpb3 as control for normalization. Untreated (Un), heat treated (37°C) and kinase dead (KD) shown. All results shown are representative of three independent experiments.
Y1F alters Nrd1 and Rtt103 interactions with RNAP II
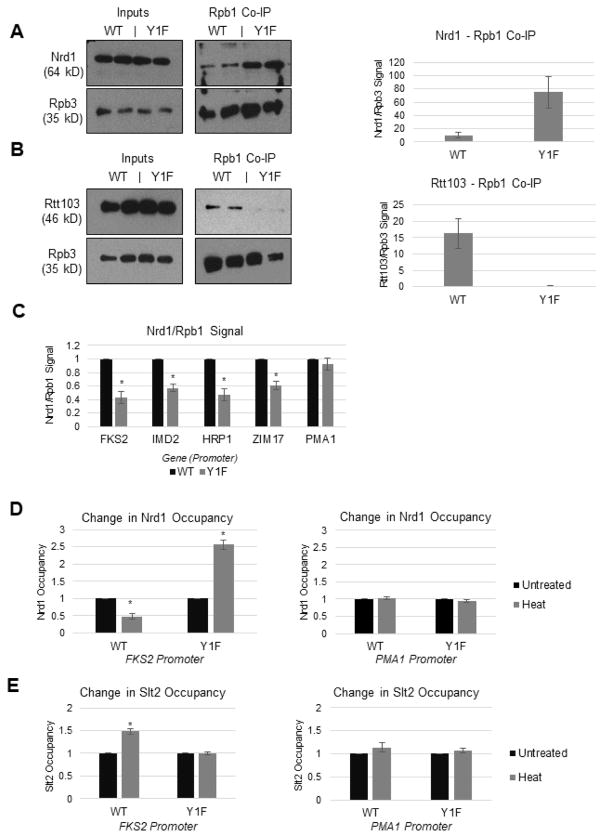
We next examined how Tyr1 and its phosphorylation by Slt2 affect target gene expression. Since Tyr1 phosphorylation and Slt2 activity are both known to influence association of certain termination factors with the transcription machinery (Kim and Levin 2011; Mayer et al. 2012), we examined if recruitment of such factors is altered by Y1F. To do this, we attempted to generate 3HA-tagged versions of Nrd1, Rtt103 and Pcf11, three proteins previously shown to interact with the CTD peptides in a Tyr1P-sensitive manner (Mayer et al. 2012), in WT and Y1F backgrounds. While we were unable to tag Pcf11 in the Y1F strain, we did generate Nrd1- and Rtt103- tagged strains. These were then used in coIP experiments, using y-80. Consistent with earlier predictions (Mayer et al. 2012), Y1F Rpb1 showed a 7.3-fold increase in Nrd1 binding (Figures 6A). In contrast, Rtt103 levels in the co-IP were significantly reduced in the Y1F strain (Figure 6B). Although not entirely as expected (Mayer et al. 2012), a possibility consistent with previous studies (Lunde et al 2010; Mayer et al. 2012) is that Rtt103 requires the hydroxyl group of Tyr1, which phosphorylation as well as Phe substitution disrupts, to bind the CTD efficiently. Ser2 (Lunde et al. 2010) and Thr4 (Jasnovidova et al. 2017) are also required for this association.
Figure 6. RNAP II Tyr1 and Slt2 affect Nrd1 recruitment to RNAP II and chromatin.
(A) Western blot analysis of Nrd1 levels after Rpb1 co-IP (using y-80 antibody), with quantification. Nrd1 interaction with RNAP II quantified using Rpb3. (B) Western blot analysis of Rtt103 levels after Rpb1 co-IP (using y-80 antibody), with quantification. Rtt103 interaction with RNAP II quantified using Rpb3. (C) ChIP analysis of Nrd1 occupancy at promoter regions of indicated genes in WT and Y1F strains. (D) ChIP analysis of Nrd1 occupancy at promoter and body regions of model gene FKS2, before and after heat stress. (E) ChIP analysis of Slt2 occupancy at promoter and body regions of model gene FKS2, before and after heat stress. All data are represented as mean +/− SE of three independent experiments.
We next examined Nrd1 occupancy in WT and Y1F strains on genes known to be Nrd1/NNS targets. Using ChIP, we found unexpectedly that Nrd1 levels, normalized to Rpb1 occupancy, decreased in Y1F near promoters for FKS2, IMD2 and HRP1 (all previously shown to be Nrd1/NNS targets; Kim and Levin 2011, Steinmetz et al. 2006), as well as for ZIM17 (one of the most significantly down-regulated genes in Y1F; see Figure 3A). In contrast, Nrd1 occupancy on PMA1, not known to be an NNS target, was not affected (Figure 6C). Nrd1 was not detected on downstream regions on all genes tested in WT or Y1F cells (results not shown). This decreased Nrd1 occupancy on target promoters is consistent with the general upregulation of CUTs and SUTs we observed in Y1F cells (see above).
To gain more insight into how Y1F effects Nrd1 occupancy on target promoters, we examined Nrd1 and Slt2 occupancy following heat shock. To this end, we generated paired Nrd1 and Rpb1 ChIP samples from control and heat-shocked WT and Y1F cells, and analyzed both Nrd1 and Slt2 occupancy on FKS2, which has served as an important model gene for both NNS function and MAPK signaling (Kim and Levin 2011). We found that Nrd1 occupancy relative to Rpb1 at the promoter region was reduced following heat shock in WT cells, as expected (Kim and Levin 2011). However, upon heat shock Nrd1 occupancy relative to Rpb1 in Y1F cells increased (2.5-fold; Figure 6D), indicating that Tyr1, and likely its phosphorylation, is required for NNS loss following heat shock. Supporting a role for Slt2 in this process, while heat shock resulted in Slt2 recruitment to promoters in WT cells, as observed previously (Kim and Levin 2011), this was not observed in Y1F cells (Figure 6E), consistent with the co-IP results shown above. Together, our results implicate Tyr1 and its phosphorylation by Slt2 in controlling Nrd1/NNS function during the stress response.
DISSCUSION
Our studies have provided new insights into the function of RNAP II CTD Tyr1 residues in budding yeast. While previous analyses of Tyr1 function in vertebrate cells (Descostes et al. 2014; Hsin et al. 2014) indicate significant differences between yeast and higher eukaryotes, one notable similarity is that Tyr1 plays relatively specific roles in gene expression. We have shown here that Tyr1 is required for expression of a subset of stress-inducible genes, notably MAP kinase-associated genes. Importantly, we found that Tyr1 is phosphorylated by Slt2 in vitro, and that the levels of Tyr1P in cells are modulated by exposure to specific stresses known to require Slt2 function, or by inactivating the kinase. Given previously defined roles of both Tyr1 and Slt2 in anti-termination, we provided evidence for Slt2 and Nrd1 co-regulation of stress-related genes, showing that Tyr1 provides a nexus at which stress response and anti-termination meet to influence transcription. Below, we discuss how Slt2 and Tyr1 serve as the connection for these processes in budding yeast, as well as the varied roles Tyr1 and its phosphorylation play throughout evolution.
Our data implicate Tyr1 in the proper function of the cell wall integrity pathway. Upon sensing cell wall stress, the Rho1 effector pathway is activated, triggering a kinase cascade resulting in activation of Slt2 (Levin 2005). Slt2 has a diverse number of nuclear targets, many of them TFs such as Swi4 of the SBF complex (Baetz et al. 2001). While previous investigations of Slt2 focused on serine phosphorylation, removal of the C-terminus of Slt2 enables autophosphorylation of the TxY activation loop itself, a method of regulation that has been observed in higher eukaryotes (Goshen-Lago et al. 2016; Smorodinsky-Atias et al. 2016).
The involvement of Cdk8/CycC in stress responses has been known for some time. The kinase/cyclin pair is a target of Slt2, with Slt2 phosphorylation leading to degradation of CycC (Jin et al. 2014; Strich and Cooper 2014). The association between oxidative and cell wall stresses and CycC has been known longer, since it was found that Ask10 (“Activator of Skn7”) directly mediates the destruction of CycC in response to oxidative stress (Cohen et al. 2003). Additionally, increased expression of certain stress response genes, particularly iron response, upon phosphorylation of Mediator subunit Med2 by Cdk8, has been observed (van de Peppel et al. 2005), which is consistent with our ChIP and RNA analyses. Our data that Tyr1 is required for loss of Cdk8/CycC from target genes extends the role of Slt2 in the cell wall integrity (CWI) pathway, which previously was not known to target RNAP II directly. Previous work established Slt2’s role in facilitating CycC translocation from nucleus to cytoplasm (Jin et al. 2014) subsequent to its degradation by the proteasome (Strich and Cooper 2014). This function is impaired in Y1F cells, as Cdk8/CycC occupancy on several target promoters increased in these cells, and Slt2 was not recruited to at least one of these genes (FKS2), likely reflecting its defective interaction with Y1F RNAP II.
The involvement of Slt2 in the expression of stress response-related genes has been well documented. Our data extend these findings by showing that Tyr1 phosphorylation by Slt2 is critical in several stress responses. Besides the CWI pathway, as exemplified by sensitivity to calcofluor white (Levin 2011), Slt2 is also involved in the response to DNA damaging agents such as hydroxyurea and phleomycin (Soriano-Carot et al. 2012), and functions along with calcineurin upon exposure to FK506 (Mizunuma et al. 1998). Additionally, in concert with TORC1 signaling, Slt2 helps stabilize the cell’s response to rapamycin (Moreno-Torres et al. 2015). Importantly, many of the genes with altered expression in Y1F were already known to be regulated by Slt2, as part of the above stress responses. In a related Y1F S. pombe strain, a similar response to phleomycin was observed, which was the result of iron uptake pathway upregulation (Schwer et al. 2014). Our study extends this finding, not only by expanding the role of Tyr1 in the stress response to most Slt2-dependent responses, but also by involving Tyr1-dependent interactions directly in Slt2 function. We expect additional Slt2-dependent stress responses to involve Tyr1, either through direct Tyr1 phosphorylation or indirectly through regulation of other Slt2 targets.
Previous studies of Tyr1 revealed differences in the distribution of Tyr1P between species, as well as the importance of the residue to termination factor recruitment. Genome-wide ChIP analyses in S. cerevisiae showed that Tyr1P reaches a peak at the 3′ end of genes, much like Ser2P (Mayer et al. 2012). This is in contrast to human cells, which show a peak at the 5′ end of genes as well as in the antisense direction, and at transcriptional enhancers (Descostes et al. 2014). Further differentiating yeast and human cells, there is no clear human Nrd1 homolog, and processing of snRNAs is done differently through the Integrator complex (Baillat et al. 2005). It would make sense, then, that the functions associated with Tyr1 differ between yeast and human cells; the proposed function of Tyr1P in budding yeast (preventing termination factors Nrd1, Pcf11 and Rtt103 from prematurely binding to the CTD) seems to be yeast specific (Mayer et al. 2012). The involvement of Tyr1 in the stress responses we have shown here, mediated in part by the Nrd1/NNS complex, may also be yeast specific, but further studies will be needed to investigate this.
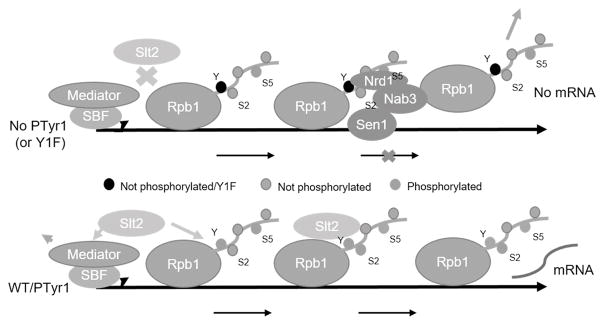
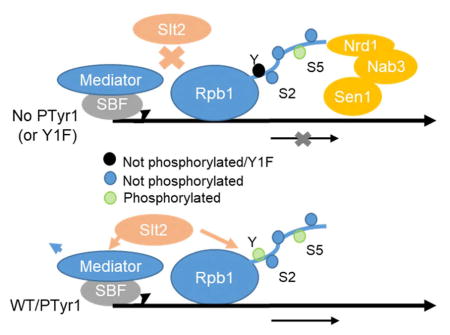
Our data provide new insight into the CTD interaction with the NNS complex. The substantial increase in Nrd1 binding to the Y1F CTD we observed is consistent with previous studies showing that Tyr1P-containing CTD peptides bound Nrd1 less strongly than unmodified peptides (Mayer et al. 2012). The finding that upon cell stress, Slt2 is induced and recruited to coding regions as well as promoters of specific genes links Slt2 with NNS function, as NNS association with elongating RNAP II drops with Slt2 induction (Kim and Levin 2011). While the presence of Slt2 on target genes was suggested to be sufficient for anti-termination (Kim and Levin 2011), our data provide evidence that Slt2 phosphorylation of Tyr1 is critical for this process by facilitating dissociation of NNS. Importantly, our findings that Tyr1P levels increase in response to stress link Tyr1 phosphorylation by Slt2 with activation of stress-related genes. Based on these findings, we propose a model (Figure 7) that incorporates not only Slt2, Tyr1P and NNS, but also Cdk8/CycC and SBF, known targets of Slt2 (Jin et al. 2014) now linked to CTD Tyr1, into activation of stress response genes. While some of our observations were obtained from single-gene experiments, and thus may not apply in all circumstances, we believe the robust responses from our model system are indicative of more general mechanisms.
Figure 7. Role of CTD Tyr1 and Slt2 kinase in activating transcription of stress-inducible genes.
In the absence of activation by a stress-induced signaling cascade, Slt2 is inactive and Tyr1 is not phosphorylated. The Nrd1-containing NNS complex is thus recruited to a primarily Ser5P CTD and transcripts are prematurely terminated and degraded. Activation of Slt2 enables the kinase to associate with RNAP II and Mediator/SBF, promoting Cdk8/CycC degradation and RNAP II Tyr1 phosphorylation. This combination of events facilitates activation and prevents termination factor (e.g., NNS) association with the CTD until the 3′ end, when Tyr1 is dephosphorylated, the transcript 3′ processed and transcription terminated.
The significance of tyrosine phosphorylation in budding yeast is not entirely clear. S. cerevisiae encodes no typical tyrosine kinases, and the relative levels of TyrP are extremely low (<0.1% total phosphorylation; Chi et al. 2007). Tyr phosphorylation does occur in the CWI pathway, as Mkk1 phosphorylates the Thr and Tyr residues of the Slt2 activation loop (Martín et al. 2000). Other dual-specificity kinases have been identified, including Hrr25 (Hoekstra et al. 1994) and Yak1 (Kassis et al. 2000). Our study adds Slt2 to this list, and establishes an important role for it in transcriptional control.
It remains to be seen how our findings on Tyr1 function extend to other organisms. As mentioned above, the corresponding Y1F mutation in S. pombe, despite the truncated CTD, did not cause nearly as strong a growth defect as the S. cerevisiae derivative we analyzed (Schwer and Shuman 2011; Schwer et al. 2014). Notably, the S. pombe Y1F derivative retained a Tyr1-containing 4-repeat “rump,” offering at least a partial explanation for the mild growth defects observed. The S. pombe and S. cerevisiae derivatives did however show similarities, including sensitivity to cold and phleomycin, and it is possible that future studies will reveal defects in additional stress pathways. It will also be of interest to determine whether the S. pombe Slt2 homologue, Pmk1, which functions in pathways similar to Slt2 (Madrid et al. 2006), is a Tyr1 kinase. Regulation of transcription by MAP kinase pathways also extends to higher eukaryotes, as homologs of Slt2, such as Erk1/2, are recruited to chromatin in a manner similar to Slt2, on similar stress-induced genes (Pokholok et al. 2006; Yang et al. 2013). Indeed, Erk1/2 have CTD Ser5 kinase activity (Trigon et al. 1998; Bonnet et al. 1999), although there is no evidence that they also target Tyr1. While there are significant differences between yeast and human cells in their response to stress (Verghese et al. 2012), it will be of interest to determine whether additional mechanisms evolved in human cells to amplify or refine signaling through Tyr1. In any event, our results show that Tyr1 and its phosphorylation by Slt2 play an important role in regulating transcription in response to stress in budding yeast, extending the complexity of CTD function in control of gene expression.
STAR*METHODS
Detailed methods are provided in the online version of this paper and include the following:
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, James Manley (jlm2@columbia.edu).
Experimental Model and Subject Details
Saccharomyces cerevisiae
Yeast strains used are listed in Table S1. Y1F and CONS strains were constructed by PCR amplification of corresponding CTD constructs generated for use in previous studies (Hsin et al. 2011, Hsin et al. 2014), followed by transformation into haploid (for CONS) or diploid (for Y1F) S288C or BY4741/2 strains. The diploid strains carrying the Y1F CTD along with NAT-MX cassette (see Tong and Boone 2006, Rosonina et al. 2014) 3′ of the RPO21 gene were then confirmed by PCR, sporulation and tetrad dissection. The CONS strain was also marked with the NAT-MX cassette 3′ of the RPO21 gene. Media containing FK506 (75 to 150 ug/uL), caffeine (5 mM), rapamycin (10 nM), phleomycin (1 ug/uL), or calcofluor white (3.5 ug/uL, kept in light-block environment) were prepared by adding the indicated amounts to rich media; hydroxyurea (50 mM) was added to synthetic complete media. All strains were grown at 30°C (standard conditions) or 37°C (heat shock conditions) in appropriate media for all experiments. Rich media (XY; YPD with supplemental adenine and tryptophan) and synthetic complete (SC) or synthetic drop-out (SC without selective amino acid) were used. For liquid growth curves, 10 mL cultures were prepared using XY media in triplicate.
SGA screen, yeast spot assays and media used were as previously described (Rosonina et al. 2009, 2010).
Method Details
Chromatin Immunoprecipitation (ChIP) Analyses
For ChIP analyses, 50-mL cultures were grown and cells were lysed by bead-beating three times for one min each with one min in between in a 4°C cold room, as previously described (Rosonina et al. 2014). After centrifugation, extracts were immunoprecipitated with the appropriate antibody overnight, washed and treated with pronase, and DNA was extracted using phenol/chloroform extraction. Radioactive semi-quantitative PCR was performed as previously described (Rosonina et al. 2012), using a region of chromosome VII as internal control and the appropriate gene-specific primers. Statistical analyses were performed using a two-tailed Student’s t-test, with significance (p < 0.05) denoted by an asterisk. Error bars in graphs represent standard errors of at least three experiments.
Western blots and co-immunoprecipitation (co-IP) assays
Western blot and co-IP assays were as previously described (Rosonina et al. 2009, 2010). Cell pellets from 10 mL liquid cultures harvested between 0.5 and 1.0 OD600 were washed with IP buffer (see Rosonina et al. 2009) and cells were lysed using glass beads. Lysates were removed from glass beads then centrifuged at high speed (15,000 RPM, Eppendorf Centrifuge 5424) to pellet chromatin and debris. Supernatants were then centrifuged at high speed again and either diluted with 2X SDS loading buffer (for whole cell extracts) or immunoprecipitated with sepharose beads and the appropriate antibody overnight at 4°C before five washing steps (with IP buffer). DNAse treatment of samples was based on NEB protocol M0303, where 1 unit of DNAse I per 50 uL of protein extract was added, and samples were incubated at 37°C for 10 minutes. This was performed before diluting samples with 2X SDS loading buffer. The reaction was stopped by adding 2X SDS buffer and boiling samples for 5 minutes.
Antibodies used, Primers and RNA
Antibodies used for ChIP and western blotting were HA (ABM; ChIP and CoIP), HA (12AC5, a gift from Elizabeth Miller; Western), GAPDH (Sigma), MPK1 (sc-374434) and HOG1 (sc-165978) (Santa Cruz Biotechnology), Rpb3 (Biolegend), Y1P 3D12 (Active Motif), Ser2P (3E10, a gift from Dirk Eick), Ser5P (3E8; Millipore) and Rpb1 (y-80; Santa Cruz).
Primer sequences used for cloning, ChIP and RT-PCR analyses are available upon request.
All RNA extractions were carried out for total RNA. Random hexamers were used with total RNA for RT-PCR analyses, performed in triplicate. For details on 3′READS analysis, see Quantification and Statistical Analysis.
Cell Cycle Assay
Cell cycle analysis was performed according to Zhang and Siede (2004). 500 uL of actively growing yeast cultures (between 0.5 and 1 OD) were harvested by centrifugation (15,000 RPM, Eppendorf Centrifuge 5424) and resuspended in 500 uL of 70% ethanol. The sample was centrifuged again, 10 minutes at 6,000 RPM, and resuspended in 250μl of Tris (pH 7.4, 50 mM) with RNase A. The cells were incubated at 37°C for 2 hours, centrifuged (10,000 RPM for 5 minutes), and resuspended in 500 Ul sodium citrate (50 mM, pH 7.0). Cells were then sonicated (single pulse, 5 seconds, amplitude 30), and resuspended in 500 ul of sodium citrate (pH 7.0) with propidium iodide (final concentration 12.5 ug/mL) added. This was then incubated for 30 minutes at 4°C, diluted to 105 cells/mL, and analyzed using FACS analysis.
Synthetic Genetic Array (SGA)
SGA analyses were conducted according to Tong and Boone (2006). Strains NYYM206A and NYYM207A were plated on rich media and then mated to the SGA library set and DaMP collection (Dharmacon). Successive matings were conducted to select for diploids carrying both Y1F and consensus CTDs (NAT-associated) and individual deletions (G418-associated). Diploids were then sporulated for 7 days, and haploids bearing both synthetic CTDs and deletions of interest were selected through successive platings on synthetic drop-out media (-His/Arg/Lys/Ura) containing canavanine and thialysine (see Tong and Boone 2006). An additional plating to media containing 5-FOA was performed to select for haploids without the plasmid containing an unmodified RPB1 gene. Results were tabulated using Microsoft Excel.
In vitro kinase assays
In vitro kinase assays were performed according to Campbell (2014). After growing cultures to 0.5 OD, SLT2-3HA cultures were incubated at 37°C for 1 hour, and HOG1-3HA cultures were grown in 0.8 M NaCl for 1 hour, in order to activate the tagged kinases prior to extraction. Cells were then harvested and extracts prepared as described (Rosonina et al. 2009). Briefly, cells were agitated with glass beads in IP buffer for 30 minutes, then washed and pelleted. Cell extracts were immunoprecipitated using HA antibody (ABM) and Sepharose-G beads at 4°C for two hours, then washed three times with lysis buffer and twice with kinase buffer (25 mM Tris pH 7.5, 5 mM β-glycerophosphate, 2 mM DTT, 10 mM MgCl2, 10 mM NaF). Beads were then resuspended in 60 uL kinase buffer, with 200 nM GST-CTD (Hsin et al. 2014) and 10 mM ATP. Reaction mixtures were incubated for two hours at 30°C with agitation every 10 minutes to keep beads suspended. 2X SDS sample buffer was then added and samples were boiled for 5 minutes before analysis by SDS PAGE and Western blot.
Quantification and Statistical Analysis
RNA sequencing and data analysis
RNA sequencing was performed using Y1F and WT samples, in duplicate, and 3′READS data were analyzed as previously described (Hoque et al. 2013). Briefly, 3′READS reads were mapped to the S. cerevisiae genome (sacCer3) using bowtie2 with local mode. Uniquely mapped reads (with MAPQ score > 10) that had at least two additional 5′ Ts after genomic alignments were assigned to genes by gene models from UCSC database (Tyner et al. 2017). CUTs and SUTs were annotated as described (Xu et al. 2009), using existing annotation data. DESeq was used to identify differentially expressed genes (Love et al. 2014). Dysregulated genes were selected by FDR ≤ 0.05. All statistical details can be found in the Results section, as well as in figure legends and supplemental figures/legends.
Gene Ontology analysis
Gene Ontology (GO) annotations were obtained from the Gene Ontology Consortium (Gene Ontology Consortium, 2015). GO entries were tested for significance of association with regulated genes using the hypergeometric test (Grossmann et al. 2007). GO terms associated with more than 2,000 genes were discarded as too generic. To reduce redundancy, each represented GO term was required to have at least 30% of genes that had not been associated with another GO term or any other GO term with a more significant P-value (Ji and Tian, 2009).
Data and Software Availability
Table S2 (Full list of suppressor mutants from the Y1F suppressor SGA. Related to Figure 2.) and Table S3 (Complete results from Y1F RNA sequencing analysis (separated by tabs). Related to Figure 3.) can be found at data.mendeley.com.
Description: Yurko et al 2017 Tables S2 and S3
The above datasets have been deposited in the Mendeley database under DOI number 10.17632/8fvx64vvpg.1.
Key Resource Table
For all S. cerevisiae strains used in this study, please see Table S1.
The table highlights the genetically modified organisms and strains, cell lines, reagents, software, and source data essential to reproduce results presented in the manuscript. Depending on the nature of the study, this may include standard laboratory materials (i.e., food chow for metabolism studies), but the Table is not meant to be comprehensive list of all materials and resources used (e.g., essential chemicals such as SDS, sucrose, or standard culture media don’t need to be listed in the Table). Items in the Table must also be reported in the Method Details section within the context of their use. The number of primers and RNA sequences that may be listed in the Table is restricted to no more than ten each. If there are more than ten primers or RNA sequences to report, please provide this information as a supplementary document and reference this file (e.g., See Table S1 for XX) in the Key Resources Table.
Please note that ALL references cited in the Key Resources Table must be included in the References list. Please report the information as follows:
REAGENT or RESOURCE: Provide full descriptive name of the item so that it can be identified and linked with its description in the manuscript (e.g., provide version number for software, host source for antibody, strain name). In the Experimental Models section, please include all models used in the paper and describe each line/strain as: model organism: name used for strain/line in paper: genotype. (i.e., Mouse: OXTRfl/fl: B6.129(SJL)-Oxtrtm1.1Wsy/J). In the Biological Samples section, please list all samples obtained from commercial sources or biological repositories. Please note that software mentioned in the Methods Details or Data and Software Availability section needs to be also included in the table. See the sample Table at the end of this document for examples of how to report reagents.
SOURCE: Report the company, manufacturer, or individual that provided the item or where the item can obtained (e.g., stock center or repository). For materials distributed by Addgene, please cite the article describing the plasmid and include “Addgene” as part of the identifier. If an item is from another lab, please include the name of the principal investigator and a citation if it has been previously published. If the material is being reported for the first time in the current paper, please indicate as “this paper.” For software, please provide the company name if it is commercially available or cite the paper in which it has been initially described.
-
IDENTIFIER: Include catalog numbers (entered in the column as “Cat#” followed by the number, e.g., Cat#3879S). Where available, please include unique entities such as RRIDs, Model Organism Database numbers, accession numbers, and PDB or CAS IDs. For antibodies, if applicable and available, please also include the lot number or clone identity. For software or data resources, please include the URL where the resource can be downloaded. Please ensure accuracy of the identifiers, as they are essential for generation of hyperlinks to external sources when available. Please see the Elsevier list of Data Repositories with automated bidirectional linking for details. When listing more than one identifier for the same item, use semicolons to separate them (e.g. Cat#3879S; RRID: AB_2255011). If an identifier is not available, please enter “N/A” in the column.
A NOTE ABOUT RRIDs: We highly recommend using RRIDs as the identifier (in particular for antibodies and organisms, but also for software tools and databases). For more details on how to obtain or generate an RRID for existing or newly generated resources, please visit the RII or search for RRIDs.
Please use the empty table that follows to organize the information in the sections defined by the subheading, skipping sections not relevant to your study. Please do not add subheadings. To add a row, place the cursor at the end of the row above where you would like to add the row, just outside the right border of the table. Then press the ENTER key to add the row. Please delete empty rows. Each entry must be on a separate row; do not list multiple items in a single table cell. Please see the sample table at the end of this document for examples of how reagents should be cited.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-HA | ABM | G166 |
| Anti-HA (12AC5) | Liz Miller (gift) | - |
| GAPDH | Sigma | G9545 |
| MPK1 | Santa Cruz | Sc-374434 |
| HOG1 | Santa Cruz | Sc-165978 |
| Anti-Rpb3 | Biolegend | 665004 |
| Anti-Y1P (3D12) | Active Motif | 61384 |
| Anti-Ser2P (3E10) | Dirk Eick (gift) | - |
| Anti-Ser5P (3E8) | Millipore | 04-1572 |
| Anti-Rpb1 (y80) | Santa Cruz | Sc-25758 |
| Anti-Rpb1 (8WG16) | Santa Cruz | Sc-56767 |
| Anti-Ser5P (H5) | Biolegend | 920204 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Nourseothricin (NAT) | Werner Bioagents | 5.001.000 |
| G418 (Geneticin) | Invivogen | Ant-gn-5 |
| Random Hexamer Primer | Thermo Scientific | SO142 |
| GST-CTD | Hirose and Manley 1998 | - |
| Phleomycin | Invivogen | Ant-ph-1 |
| Calcofluor White | Sigma-Aldrich | 18909 |
| FK506 | Invivogen | Tlrl-fk5 |
| Deposited Data | ||
| SGA Dataset (Table S2) | This paper | doi:10.17632/8fvx64vvpg.1 |
| 3′READS Dataset (Table S3) | This paper | doi:10.17632/8fvx64vvpg.1 |
| Experimental Models: Organisms/Strains | ||
| Saccharomyces cerevisiae background BY4741 | ATCC | ATCC: 4040002 |
| Saccharomyces cerevisiae background Y7092 | Boone Lab | - |
| Please see Table S1 for all derivative strains | - | - |
| Software and Algorithms | ||
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| DESeq | Love et al. 2014 | - |
TABLE WITH EXAMPLES FOR AUTHOR REFERENCE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Snail | Cell Signaling Technology | Cat#3879S; RRID: AB_2255011 |
| Mouse monoclonal anti-Tubulin (clone DM1A) | Sigma-Aldrich | Cat#T9026; RRID: AB_477593 |
| Rabbit polyclonal anti-BMAL1 | This paper | N/A |
| Bacterial and Virus Strains | ||
| pAAV-hSyn-DIO-hM3D(Gq)-mCherry | Krashes et al., 2011 | Addgene AAV5; 44361-AAV5 |
| AAV5-EF1a-DIO-hChR2(H134R)-EYFP | Hope Center Viral Vectors Core | N/A |
| Cowpox virus Brighton Red | BEI Resources | NR-88 |
| Zika-SMGC-1, GENBANK: KX266255 | Isolated from patient (Wang et al., 2016) | N/A |
| Staphylococcus aureus | ATCC | ATCC 29213 |
| Streptococcus pyogenes: M1 serotype strain: strain SF370; M1 GAS | ATCC | ATCC 700294 |
| Biological Samples | ||
| Healthy adult BA9 brain tissue | University of Maryland Brain & Tissue Bank; http://medschool.umaryland.edu/btbank/ | Cat#UMB1455 |
| Human hippocampal brain blocks | New York Brain Bank | http://nybb.hs.columbia.edu/ |
| Patient-derived xenografts (PDX) | Children’s Oncology Group Cell Culture and Xenograft Repository | http://cogcell.org/ |
| Chemicals, Peptides, and Recombinant Proteins | ||
| MK-2206 AKT inhibitor | Selleck Chemicals | S1078; CAS: 1032350-13-2 |
| SB-505124 | Sigma-Aldrich | S4696; CAS: 694433-59-5 (free base) |
| Picrotoxin | Sigma-Aldrich | P1675; CAS: 124-87-8 |
| Human TGF-β | R&D | 240-B; GenPept: P01137 |
| Activated S6K1 | Millipore | Cat#14-486 |
| GST-BMAL1 | Novus | Cat#H00000406-P01 |
| Critical Commercial Assays | ||
| EasyTag EXPRESS 35S Protein Labeling Kit | Perkin-Elmer | NEG772014MC |
| CaspaseGlo 3/7 | Promega | G8090 |
| TruSeq ChIP Sample Prep Kit | Illumina | IP-202-1012 |
| Deposited Data | ||
| Raw and analyzed data | This paper | GEO: GSE63473 |
| B-RAF RBD (apo) structure | This paper | PDB: 5J17 |
| Human reference genome NCBI build 37, GRCh37 | Genome Reference Consortium | http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/ |
| Nanog STILT inference | This paper; Mendeley Data | http://dx.doi.org/10.17632/wx6s4mj7s8.2 |
| Affinity-based mass spectrometry performed with 57 genes | This paper; and Mendeley Data | Table S8; http://dx.doi.org/10.17632/5hvpvspw82.1 |
| Experimental Models: Cell Lines | ||
| Hamster: CHO cells | ATCC | CRL-11268 |
| D. melanogaster: Cell line S2: S2-DRSC | Laboratory of Norbert Perrimon | FlyBase: FBtc0000181 |
| Human: Passage 40 H9 ES cells | MSKCC stem cell core facility | N/A |
| Human: HUES 8 hESC line (NIH approval number NIHhESC-09-0021) | HSCI iPS Core | hES Cell Line: HUES-8 |
| Experimental Models: Organisms/Strains | ||
| C. elegans: Strain BC4011: srl-1(s2500) II; dpy-18(e364) III; unc-46(e177)rol-3(s1040) V. | Caenorhabditis Genetics Center | WB Strain: BC4011; WormBase: WBVar00241916 |
| D. melanogaster: RNAi of Sxl: y[1] sc[*] v[1]; P{TRiP.HMS00609}attP2 | Bloomington Drosophila Stock Center | BDSC:34393; FlyBase: FBtp0064874 |
| S. cerevisiae: Strain background: W303 | ATCC | ATTC: 208353 |
| Mouse: R6/2: B6CBA-Tg(HDexon1)62Gpb/3J | The Jackson Laboratory | JAX: 006494 |
| Mouse: OXTRfl/fl: B6.129(SJL)-Oxtrtm1.1Wsy/J | The Jackson Laboratory | RRID: IMSR_JAX:008471 |
| Zebrafish: Tg(Shha:GFP)t10: t10Tg | Neumann and Nuesslein-Volhard, 2000 | ZFIN: ZDB-GENO-060207-1 |
| Arabidopsis: 35S::PIF4-YFP, BZR1-CFP | Wang et al., 2012 | N/A |
| Arabidopsis: JYB1021.2: pS24(AT5G58010)::cS24:GFP(-G):NOS #1 | NASC | NASC ID: N70450 |
| Oligonucleotides | ||
| siRNA targeting sequence: PIP5K I alpha #1: ACACAGUACUCAGUUGAUA | This paper | N/A |
| Primers for XX, see Table SX | This paper | N/A |
| Primer: GFP/YFP/CFP Forward: GCACGACTTCTTCAAGTCCGCCATGCC | This paper | N/A |
| Morpholino: MO-pax2a GGTCTGCTTTGCAGTGAATATCCAT | Gene Tools | ZFIN: ZDB-MRPHLNO-061106-5 |
| ACTB (hs01060665_g1) | Life Technologies | Cat#4331182 |
| RNA sequence: hnRNPA1_ligand: UAGGGACUUAGGGUUCUCUCUAGGGACUUAGGGUUCUCUCUAGGGA | This paper | N/A |
| Recombinant DNA | ||
| pLVX-Tight-Puro (TetOn) | Clonetech | Cat#632162 |
| Plasmid: GFP-Nito | This paper | N/A |
| cDNA GH111110 | Drosophila Genomics Resource Center | DGRC:5666; FlyBase:FBcl0130415 |
| AAV2/1-hsyn-GCaMP6- WPRE | Chen et al., 2013 | N/A |
| Mouse raptor: pLKO mouse shRNA 1 raptor | Thoreen et al., 2009 | Addgene Plasmid #21339 |
| Software and Algorithms | ||
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Samtools | Li et al., 2009 | http://samtools.sourceforge.net/ |
| Weighted Maximal Information Component Analysis v0.9 | Rau et al., 2013 | https://github.com/ChristophRau/wMICA |
| ICS algorithm | This paper; Mendeley Data | http://dx.doi.org/10.17632/5hvpvspw82.1 |
| Other | ||
| Sequence data, analyses, and resources related to the ultra-deep sequencing of the AML31 tumor, relapse, and matched normal. | This paper | http://aml31.genome.wustl.edu |
| Resource website for the AML31 publication | This paper | https://github.com/chrisamiller/aml31SuppSite |
Supplementary Material
Acknowledgments
We thank Elizabeth Miller, Silvere Pagant, Rodney Rothstein and Robert Reid for sharing yeast strains, and members of the labs of JLM, BT, Elizabeth Miller and Songtao Jia for technical help. We also thank David Levin for sharing the SLT2-K54R construct, as well as Yuqi Wang for sharing the HOG1-K52R strain. This work was supported by NIH grants R35 GM118136 to JLM and R01 GM084089 to BT, and NMY was partially supported by NIH training grant 5T32GM008798.
Footnotes
AUTHOR CONTRIBUTIONS
NY performed all the experiments described. XL and TY carried out computational analyses. MH and BT performed RNA-Seq and related analyses. NY wrote the first draft and all authors contributed to manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtar MS, Heidemann M, Tietjen JR, Zhang DW, Chapman RD, Eick D, Ansari AZ. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol Cell. 2009;34:387–93. doi: 10.1016/j.molcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16:155–166. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arigo JT, Eyler DE, Carroll KL, Corden JL. Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol Cell. 2006;23:841–851. doi: 10.1016/j.molcel.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Baetz K, Moffat J, Haynes J, Chang M, Andrews B. Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol Cell Biol. 2001;21:6515–28. doi: 10.1128/MCB.21.19.6515-6528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillat D, Hakimi MA, Naar AM, Shilatifard A, Cooch N, Shiekhattar R. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell. 2005;123:265–276. doi: 10.1016/j.cell.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Baskaran R, Dahmus ME, Wang JY. Tyrosine phosphorylation of mammalian RNA polymerase II carboxyl-terminal domain. Proc Natl Acad Sci USA. 1993;90:11167–71. doi: 10.1073/pnas.90.23.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F, Vigneron M, Bensaude O, Dubois MF. Transcription-independent phosphorylation of the RNA polymerase II C-terminal domain (CTD) involves ERK kinases (MEK1/2) Nucleic Acids Res. 1999;27:4399–4404. doi: 10.1093/nar/27.22.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Dutko JA, Zitomer RS. Genetic factors that regulate the attenuation of the general stress response of yeast. Genetics. 2005;169:1215–1226. doi: 10.1534/genetics.104.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, Gustin MC. Hog1: 20 years of discovery and impact. Sci Signal. 2014;7:re7. doi: 10.1126/scisignal.2005458. [DOI] [PubMed] [Google Scholar]
- Burugula BB, Jeronimo C, Pathak R, Jones JW, Robert F, Govind CK. Histone deacetylases and phosphorylated polymerase II C-terminal domain recruit Spt6 for cotranscriptional histone reassembly. Mol Cell Biol. 2014;34:4115–29. doi: 10.1128/MCB.00695-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PA. IP-Kinase Assay. Bio-protocol. 2014;4:e1059. [Google Scholar]
- Chapman RD, Heidemann M, Albert TK, Mailhammer R, Flatley A, Meisterernst M, Kremmer E, Eick D. Transcribing RNA polymerase II is phosphorylated at CTD residue Serine-7. Science. 2007;318:1780–1782. doi: 10.1126/science.1145977. [DOI] [PubMed] [Google Scholar]
- Chasman D, Ho YH, Berry DB, Nemec CM, MacGilvray ME, Hose J, Merrill AE, Lee MV, Will JL, Coon JJ, Ansari AZ, Craven M, Gasch AP. Pathway connectivity and signaling coordination in the yeast stress-activated signaling network. Mol Syst Biol. 2014;10:759. doi: 10.15252/msb.20145120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi A, Huttenhower C, Geer LY, Coon JJ, Syka JE, Bai DL, Shabanowitz J, Burke DJ, Troyanskaya OG, Hunt DF. Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc Natl Acad Sci USA. 2007;104:2193–8. doi: 10.1073/pnas.0607084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EJ, Takagi T, Moore CR, Buratowski S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal-domain. Genes Dev. 1997;11:3319–3326. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Lee K, Rutkowski LH, Strich R. Ask10p mediates the oxidative stress-induced destruction of the Saccharomyces cerevisiae C-type cyclin Une3p/Srb11p. Eukaryot Cell. 2003;2:962–970. doi: 10.1128/EC.2.5.962-970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czudnochowski N, Bösken CA, Geyer M. Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition. Nat Comm. 2012;3:842. doi: 10.1038/ncomms1846. [DOI] [PubMed] [Google Scholar]
- Descostes N, Heidemann M, Spinelli L, Schüller R, Maqbool MA, Fenouil R, Koch F, Innocenti C, Gut M, Gut I, Eick D, Andrau JC. Tyrosine phosphorylation of RNA polymerase II CTD is associated with antisense promoter transcription and active enhancers in mammalian cells. Elife. 2014;3:e02105. doi: 10.7554/eLife.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht BD. Regulation of transcription by proteins that control the cell cycle. Nature. 1997;389:149–152. doi: 10.1038/38225. [DOI] [PubMed] [Google Scholar]
- Egloff S, O’Reilly D, Chapman RD, Taylor A, Tanzhaus K, Pitts L, Eick D, Murphy S. Serine 7 of the RNA polymerase II CTD is specifically required for snRNA gene expression. Science. 2007;318:1777–9. doi: 10.1126/science.1145989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D, Geyer M. The RNA polymerase II carboxy-terminal domain (CTD) code. Chem Rev. 2013;113:8456–8490. doi: 10.1021/cr400071f. [DOI] [PubMed] [Google Scholar]
- Feaver WJ, Svejstrup JQ, Henry NL, Kornberg RD. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994;79:1103–9. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–57. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43(Database issue):D1049–56. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen-Lago T, Goldberg-Carp A, Melamed D, Darlyuk-Saadon I, Bai C, Ahn NG, Admon A, Engelberg D. Variants of the yeast MAPK Mpk1 are fully functional independently of activation loop phosphorylation. Mol Biol Cell. 2016;27:2771–2783. doi: 10.1091/mbc.E16-03-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann S, Bauer S, Robinson PN, Vingron M. Improved detection of overrepresentation of Gene-Ontology annotations with parent child analysis. Bioinformatics. 2007;23:3024–31. doi: 10.1093/bioinformatics/btm440. [DOI] [PubMed] [Google Scholar]
- Harlen KM, Trotta KL, Smith EE, Mosaheb MM, Fuchs SM, Churchman LS. Comprehensive RNA polymerase II interactomes reveal distinct and varied roles for each phospho-CTD residue. Cell Rep. 2016;15:2147–58. doi: 10.1016/j.celrep.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner CJ, Thompson CM, Zhang J, Chao DM, Liao SM, Koleske AJ, Okamura S, Young RA. Association of an activator with an RNA polymerase II holoenzyme. Genes Dev. 1995;9:897–910. doi: 10.1101/gad.9.8.897. [DOI] [PubMed] [Google Scholar]
- Hintermair C, Heidemann M, Koch F, Descostes N, Gut M, Gut I, Fenouil R, Ferrier P, Flatley A, Kremmer E, Chapman RD, Andrau JC, Eick D. Threonine-4 of mammalian RNA polymerase II CTD is targeted by Polo-like kinase 3 and required for transcriptional elongation. EMBO J. 2012;31:2784–97. doi: 10.1038/emboj.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra MF, Dhillon N, Carmel G, DeMaggio AJ, Lindberg RA, Hunter T, Kuret J. Budding and fission yeast casein kinase I isoforms have dual-specificity protein kinase activity. Mol Biol Cell. 1995;5:877–86. doi: 10.1091/mbc.5.8.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M, Ji Z, Zheng D, Luo W, Li W, You B, Park JY, Yehia G, Tian B. Analysis of alternative cleavage and polyadenylation by 3′ region extraction and deep sequencing. Nat Methods. 2013;10:133–139. doi: 10.1038/nmeth.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin JP, Li W, Hoque M, Tian B, Manley JL. RNAP II CTD tyrosine 1 performs diverse functions in vertebrate cells. eLife. 2014;3:e02112. doi: 10.7554/eLife.02112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin JP, Manley JL. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 2012;26:2119–37. doi: 10.1101/gad.200303.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin JP, Sheth A, Manley JL. RNAP II CTD phosphorylated on threonine-4 is required for histone mRNA 3′ end processing. Science. 2011;334:683–686. doi: 10.1126/science.1206034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnovidova O, Krejcikova M, Kubicek K, Stefl R. Structural insight into recognition of phosphorylated threonine-4 of RNA polymerase II C-terminal domain by Rtt103p. EMBO Rep. 2017;18:906–913. doi: 10.15252/embr.201643723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Tian B. Reprogramming of 3′ untranslated regions of mRNAs by alternative polyadenylation in generation of pluripotent stem cells from different cell types. PLoS One. 2009;4:e8419. doi: 10.1371/journal.pone.0008419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Strich R, Cooper KF. Slt2p phosphorylation induces cyclin C nuclear-to-cytoplasmic translocation in response to oxidative stress. Molecular Biology of the Cell. 2014;25:1396–1407. doi: 10.1091/mbc.E13-09-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–71. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Kassis S, Melhuish T, Annan RS, Chen SL, Lee JC, Livi GP, Creasy CL. Saccharomyces cerevisiae Yak1p protein kinase autophosphorylates on tyrosine residues and phosphorylates myelin basic protein on a C-terminal serine residue. Biochem J. 2000;348:263–72. [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Levin DE. Transcriptional reporters for genes activated by cell wall stress through a non-catalytic mechanism involving Mpk1 and SBF. Yeast. 2010;27:541–548. doi: 10.1002/yea.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Levin DE. Mpk1 MAPK association with the Paf1 complex blocks Sen1-mediated premature transcription termination. Cell. 2011;144:745–56. doi: 10.1016/j.cell.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Truman AW, Caesar S, Schlenstedt G, Levin DE. Yeast Mpk1 cell wall integrity mitogen-activated protein kinase regulates nucleocytoplasmic shuttling of the Swi6 transcriptional regulator. Mol Biol Cell. 2010;21:1609–1619. doi: 10.1091/mbc.E09-11-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, Buratowski S. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature. 2004;432:517–22. doi: 10.1038/nature03041. [DOI] [PubMed] [Google Scholar]
- Koutelou E, Hirsch CL, Dent SY. Multiple faces of the SAGA complex. Curr Opin Cell Biol. 2010;22:374–82. doi: 10.1016/j.ceb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde BM, Reichow SL, Kim M, Suh H, Leeper TC, Yang F, Mutschler H, Buratowski S, Meinhart A, Varani G. Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat Struct Mol Biol. 2010;17:1195–201. doi: 10.1038/nsmb.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Johnson AW, Bentley DL. The role of Rat1 in coupling mRNA 3′-end processing to transcription termination: implications for a unified allosteric-torpedo model. Genes Dev. 2006;20:954–65. doi: 10.1101/gad.1409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan I, Beenstock J, Marbach I, Tabachnick S, Livnah O, Engelberg D. Osmostress induces autophosphorylation of Hog1 via a C-terminal regulatory region that is conserved in p38α. PLoS One. 2012;7:e44749. doi: 10.1371/journal.pone.0044749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid M, Soto T, Khong HK, Franco A, Vicente J, Pérez P, Gacto M, Cansado J. Stress-induced response, localization, and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J Biol Chem. 2006;281:2033–43. doi: 10.1074/jbc.M506467200. [DOI] [PubMed] [Google Scholar]
- Martín H, Rodriguez-Pachón JM, Ruiz C, Nombela C, Molina M. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J Biol Chem. 2000;275:1511–1519. doi: 10.1074/jbc.275.2.1511. [DOI] [PubMed] [Google Scholar]
- Mayer A, Lidschreiber M, Siebert M, Leike K, Söding J, Cramer P. Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Bio. 2010;17:1272–1278. doi: 10.1038/nsmb.1903. [DOI] [PubMed] [Google Scholar]
- Mayer A, Heidemann M, Lidschreiber M, Schreieck A, Sun M, Hintermair C, Kremmer E, Eick D, Cramer P. CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science. 2012;336:1723–5. doi: 10.1126/science.1219651. [DOI] [PubMed] [Google Scholar]
- Mizunuma M, Hirata D, Miyahara K, Tsuchiya E, Miyakawa T. Role of calcineurin and Mpk1 in regulating the onset of mitosis in budding yeast. Nature. 1998;392:303–306. doi: 10.1038/32695. [DOI] [PubMed] [Google Scholar]
- Moreno-Torres M, Jaquenoud M, De Virgilio C. TORC1 controls G1–S cell cycle transition in yeast via Mpk1 and the greatwall kinase pathway. Nat Commun. 2015;6:8256. doi: 10.1038/ncomms9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–9. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HH, Robert F, Young RA, Struhl K. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol Cell. 2003;11:709–19. doi: 10.1016/s1097-2765(03)00092-3. [DOI] [PubMed] [Google Scholar]
- O’Duibhir E, Lijnzaad P, Benschop JJ, Lenstra TL, van Leenen D, Groot Koerkamp MJ, Margaritis T, Brok MO, Kemmeren P, Holstege FC. Cell cycle population effects in perturbation studies. Mol Syst Biol. 2014;10:732. doi: 10.15252/msb.20145172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson TB, Xu B, Karandikar M, Berman K, Cobb MH. Mitogen-Activated Protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Reviews. 2011;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- Poschke H, Dees M, Chang M, Amberkar S, Kaderali L, Rothstein R, Luke B. Rif2 promotes a telomere fold-back structure through Rpd3L recruitment in budding yeast. PLoS Genet. 2012;8:e1002960. doi: 10.1371/journal.pgen.1002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Trnka MJ, Bushnell DA, Davis RE, Mattei PJ, Burlingame AL, Kornberg RD. Structure of a complete Mediator-RNA Polymerase II Pre-Initiation Complex. Cell. 2016;166:1411–1422. doi: 10.1016/j.cell.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosonina E, Duncan SM, Manley JL. SUMO functions in constitutive transcription and during activation of inducible genes in yeast. Genes Dev. 2010;24:1242–1252. doi: 10.1101/gad.1917910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosonina E, Duncan SM, Manley JL. Sumoylation of transcription factor Gcn4 facilitates its Srb10-mediated clearance from promoters in yeast. Genes Dev. 2012;26:350–355. doi: 10.1101/gad.184689.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosonina E, Willis IM, Manley JL. Sub1 functions in osmoregulation and in transcription by both RNA polymerases II and III. Mol Cell Biol. 2009;29:2308–2321. doi: 10.1128/MCB.01841-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosonina E, Yurko N, Li W, Hoque M, Tian B, Manley JL. Threonine-4 of the budding yeast RNAP II CTD couples transcription with Htz1-mediated chromatin remodeling. Proc Natl Acad Sci USA. 2014;111:11924–31. doi: 10.1073/pnas.1412802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller R, Forné I, Straub T, Schreieck A, Texier Y, Shah N, Decker TM, Cramer P, Imhof A, Eick D. Heptad-specific phosphorylation of RNA Polymerase II CTD. Mol Cell. 2016;61:305–14. doi: 10.1016/j.molcel.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Schwer B, Bitton DA, Sanchez AM, Bähler J, Shuman S. Individual letters of the RNA polymerase II CTD code govern distinct gene expression programs in fission yeast. PNAS. 2014;111:4185–4190. doi: 10.1073/pnas.1321842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Sanchez AM, Shuman S. Punctuation and syntax of the RNA polymerase II CTD code in fission yeast. Proc Natl Acad Sci. 2012;109:18024–18029. doi: 10.1073/pnas.1208995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Shuman S. Deciphering the RNA polymerase II CTD code in fission yeast. Mol Cell. 2011;43:311–318. doi: 10.1016/j.molcel.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smorodinsky-Atias K, Goshen-Lago T, Goldberg-Carp A, Melamed D, Shir A, Mooshayef N, Beenstock J, Karamansha Y, Darlyuk-Saadon I, Livnah O, Ahn NG, Admon A, Engelberg D. Intrinsically active variants of Erk oncogenically transform cells and disclose unexpected autophosphorylation capability that is independent of TEY phosphorylation. Mol Biol Cell. 2016;27:1026–1039. doi: 10.1091/mbc.E15-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Carot M, Bañó MC, Igual JC. The yeast mitogen-activated protein kinase Slt2 is involved in the cellular response to genotoxic stress. Cell Division. 2012;7:1. doi: 10.1186/1747-1028-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz EJ, Warren CL, Kuehner JN, Panbehi B, Ansari AZ, Brow DA. Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol Cell. 2006;24:735–46. doi: 10.1016/j.molcel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Strich R, Cooper KF. The dual role of cyclin C connects stress regulated gene expression to mitochondrial dynamics. Microbial Cell. 2014;1:318–324. doi: 10.15698/mic2014.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Ficarro SB, Kang UB, Chun Y, Marto JA, Buratowski S. Direct analysis of phosphorylation sites on the Rpb1 C-terminal domain of RNA polymerase II. Mol Cell. 2016;61:297–304. doi: 10.1016/j.molcel.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AH, Boone C. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol. 2006;313:171–92. doi: 10.1385/1-59259-958-3:171. [DOI] [PubMed] [Google Scholar]
- Torres L, Martín H, García-Saez MI, Arroyo J, Molina M, Sánchez M, Nombela C. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol Microbiol. 1991;5:2845–54. doi: 10.1111/j.1365-2958.1991.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Trigon S, Serizawa H, Conaway JW, Conaway RC, Jackson SP, Morange M. Characterization of the residues phosphorylated in vitro by different C-terminal domain kinases. J Biol Chem. 1998;273:6769–6775. doi: 10.1074/jbc.273.12.6769. [DOI] [PubMed] [Google Scholar]
- Tyner C, Barber GP, Casper J, Clawson H, Diekhans M, Eisenhart C, Fischer CM, Gibson D, Gonzalez JN, Guruvadoo L, Haeussler M, Heitner S, Hinrichs AS, Karolchik D, Lee BT, Lee CM, Nejad P, Raney BJ, Rosenbloom KR, Speir ML, Villarreal C, Vivian J, Zweig AS, Haussler D, Kuhn RM, Kent WJ. The UCSC Genome Browser database: 2017 update. Nucleic Acids Res. 2017;45(Database issue):D626–D634. doi: 10.1093/nar/gkw1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Peppel J, Kettelarij N, van Bakel H, Kockelkorn TT, van Leenen D, Holstege FC. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol Cell. 2005;19:511–22. doi: 10.1016/j.molcel.2005.06.033. [DOI] [PubMed] [Google Scholar]
- van Voorst F, Houghton-Larsen J, Jønson L, Kielland-Brandt MC, Brandt A. Genome-wide identification of genes required for growth of Saccharomyces cerevisiae under ethanol stress. Yeast. 2006;23:351–9. doi: 10.1002/yea.1359. [DOI] [PubMed] [Google Scholar]
- Vasiljeva L, Kim M, Mutschler H, Buratowski S, Meinhart A. The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat Struct Mol Biol. 2008;15:795–804. doi: 10.1038/nsmb.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Abrams J, Wang Y, Morano KA. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev. 2012;76:115–158. doi: 10.1128/MMBR.05018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ML, Corden JL. Construction and analysis of yeast RNA polymerase II CTD deletion and substitution mutations. Genetics. 1995;140:1223–33. doi: 10.1093/genetics/140.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Münster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457:1033–7. doi: 10.1038/nature07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513:1–13. doi: 10.1016/j.gene.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Yurko NM, Manley JL. The RNA polymerase II CTD “orphan” residues: Emerging insights into the functions of Tyr-1, Thr-4 and Ser-7. Transcription. 2017 doi: 10.1080/21541264.2017.1338176. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuryev A, Corden JL. Suppression analysis reveals a functional difference between the serines in positions two and five in the consensus sequence of the C-terminal domain of yeast RNA polymerase II. Genetics. 1996;143:661–71. doi: 10.1093/genetics/143.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Siede W. Analysis of the budding yeast Saccharomyces cerevisiae cell cycle by morphological criteria and flow cytometry. Methods Mol Biol. 2004;241:77–91. doi: 10.1385/1-59259-646-0:77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.