Abstract
The use of usnic acid as a weight loss agent is a safety concern due to reports of acute liver failure in humans. Previously we demonstrated that usnic acid induces apoptosis and cytotoxicity in hepatic HepG2 cells. We also demonstrated that usnic acid induces autophagy as a survival mechanism against its cytotoxicity. In this study, we investigated and characterized further molecular mechanisms underlying the toxicity of usnic acid in HepG2 cells. We found that usnic acid causes endoplasmic reticulum (ER) stress demonstrated by the increased expression of typical ER stress markers, including CHOP, ATF-4, p-eIF2α, and spliced XBP1. Usnic acid inhibited the secretion of Gaussia luciferase measured by an ER stress reporter assay. An ER stress inhibitor 4-phenylbutyrate attenuated usnic acid-induced apoptosis. Moreover, usnic acid significantly increased the cytosolic free Ca2+ concentration. Usnic acid increased the expression of calcium release-activated calcium channel protein 1 (CRAM1 or ORAI1) and stromal interaction molecule 1, two key components of store-operated calcium entry (SOCE), which is the major Ca2+ influx pathway in non-excitable cells, this finding was also confirmed in primary rat hepatocytes. Furthermore, knockdown of ORAI1 prevented ER stress and ATP depletion in response to usnic acid. In contrast, overexpression of ORAI1 increased ER stress and ATP depletion caused by usnic acid. Taken together, our results suggest that usnic acid disturbs calcium homeostasis, induces ER stress, and that usnic acid-induced cellular damage occurs at least partially via activation of the Ca2+ channel of SOCE.
Keywords: usnic acid, liver toxicity, ER stress, apoptosis, store operated calcium entry
Usnic acid is a prominent metabolite of Usnea lichen species. Both extracts and purified usnic acid have been used as traditional medicines for antimicrobial, antiviral, antiparasitic, anti-mycotic, and antiproliferative purposes (Guo et al., 2008). Usnic acid alone or as an ingredient of some dietary supplements has been marketed for weight loss in the United States; however, severe hepatotoxicity associated with the use of usnic acid or usnic acid containing products has been reported in humans (Durazo et al., 2004; Favreau et al., 2002; Neff et al., 2004; Yellapu et al., 2011). Despite the reported adverse events and warnings from the U.S. Food and Drug Administration (FDA) on the use of usnic acid-related products (CFSAN, 2001), usnic acid containing products are still available on the market. Therefore, evaluating toxicity caused by usnic acid and studying underlying mechanisms are important. Several studies have revealed that reactive oxygen species (ROS) production and mitochondrial impairment, such as mitochondrial swelling, oxidative phosphorylation uncoupling and inhibition, are associated with the hepatotoxicity of usnic acid (Abo-Khatwa et al., 1996; Han et al., 2004; Pramyothin et al., 2004; Sahu et al., 2012; Sonko et al., 2011). Most recently, we demonstrated that usnic acid induced autophagy (Chen et al., 2014a) as a survival mechanism (Chen et al., 2014c), we also reported that usnic acid induced apoptosis in HepG2 cells and uncoupled oxidative respiration in isolated rat mitochondria (Chen et al., 2014a). Collectively, these results indicate that mitochondria are the likely targets for usnic acid’s toxicity. Pramyothin et al. (2004) noted that usnic acid treatment led to enlargement of the endoplasmic reticulum (ER) in a study using isolated rat hepatocytes and Liu et al. (2012b) reported that usnic acid altered the expression of ER proteins in the livers of usnic acid-treated rats. These results led us to consider the possibility that the ER might be a potential target for usnic acid. The ER is an organelle that performs two crucial roles: facilitating the folding and procession of newly synthesized membrane and secretory proteins and maintaining a crucial Ca2+ storage depot necessary for intracellular signaling (Michalak et al., 2002; Paschen and Doutheil, 1999; Smaili et al., 2013). Disturbance of ER function by toxic stimuli leads to accumulation of unfolded proteins and causes ER stress. Excessive or sustained ER stress can promote cell death (Chen et al., 2014b).
As a strategic intracellular messenger, Ca2+ plays a vital regulatory role that affects critical cellular functions such as gene regulation, cell growth, and cell proliferation. Calcium regulation is a complex process that involves multiple cellular compartments including mitochondria, cytoplasm, and the ER. Disturbed function of these compartments such as ER would result in unbalanced Ca2+ homeostasis. Regulating the flux of Ca2+ between each of these pools is important for maintaining calcium homeostasis (Paschen and Doutheil, 1999; Smaili et al., 2013).
Store-operated calcium entry (SOCE) is a major Ca2+ influx pathway in non-excitable cells such as hepatocytes (Barritt et al., 2009). The depletion of Ca2+ stores in the ER stimulates Ca2+ influx through store-operated channels (SOCs) into the cytoplasm from extracellular pools (Targos et al., 2005). It is reasonable to hypothesize that usnic acid may disrupt Ca2+ homeostasis via SOCs because the ER stress response is Ca2+-dependent and because the ER plays major roles in Ca2+ homeostasis and Ca2+ signaling (Paschen and Doutheil, 1999).
The flow of excess Ca2+ into the cytoplasm via SOCs then creates optimal conditions for Ca2+ refilling into the ER (Parekh and Putney, 2005). The stromal interaction molecules 1 (STIM1) embedded in the ER membrane and the calcium release-activated calcium channel protein 1 (CRAM1 or ORAI1) located in the plasma membrane are two key components responsible for SOCE. STIM1 serves as an ER Ca2+ sensor whereas ORAI1 is an essential pore-forming component of the SOC channel. In response to diminished concentrations of Ca2+ within the ER, STIM1 proteins aggregate and then translocate close to the plasma membrane and interact directly with ORAI1 to form open SOCs that cause Ca2+ influx into the cytoplasm to ultimately trigger the uptake of Ca2+ by the ER (Parekh, 2010). The two constituents of SOCE, ORAI1, and STIM1, have been detected in liver cells and SOCE has been reported to be involved in perturbations of Ca2+ homeostasis and hepatocyte damage caused by ethanol or other hepatotoxicants (Liu et al., 2012a).
Mitochondrial impairment is an important mechanism of usnic acid induced liver toxicity. ER stress is known to be one of the main sources of mitochondrial dysfunction. The induction of ER stress can activate mitochondria-dependent apoptosis by inducing mitochondrial Ca2+ overload and ATP depletion (Raturi and Simmen, 2013). In this study, we investigated the role of ER stress and the perturbation of intracellular calcium homeostasis in the cytotoxicity of usnic acid, particularly the influence of usnic acid on Ca2+ influx via SOCs. We demonstrated that usnic acid induced apoptosis and ER stress; and also disrupted calcium homeostasis. We studied the roles of STIM1 and ORAI1 in usnic acid’s cytotoxicity using gene silencing and over-expression experiments. Our results indicate that ORAI1 is the major player in usnic acid-induced ER stress and cytotoxicity.
MATERIALS AND METHODS
Chemicals and reagents
Usnic acid, William’s E medium, Dulbecco’s Modified Eagle Medium (DMEM), 2-aminoethyl diphenylborinate (2-APB) and dimethysulfoxide (DMSO) were from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA). Antibiotic-antimycotic and puromycin were from Life Technologies (Grand Island, NY). 4-Phenylbutyrate (4-PBA) was from BioVision (Milpitas, CA).
Cell culture
Human hepatoma cell line HepG2 was purchased from the American Type Culture Collection (ATCC; Manassas, VA). HepG2 cells were grown in Williams’s E medium supplemented with 10% FBS and 100 U penicillin/ml, 100 µg streptomycin/ml, and 0.25 µg fungizone/ml at 37°C in a humidified atmosphere with 5% CO2. Cells were seeded at a density of 2–5 × 105 cells/ml in volumes of 100 µl per well in 96-well plates or in volumes of 5 ml in 60-mm tissue culture plates or in volumes of 10 ml in 100 mm tissue culture plates. Cells were cultured for 24h prior to treatment with usnic acid or the DMSO vehicle control.
The 293T cell line used for lentivirus packaging was obtained from Biosettia (San Diego, CA) and maintained in DMEM supplemented with 10% FBS, 1mM sodium pyruvate, and nonessential amino acids.
Rat primary hepatocyte isolation and culture
Primary hepatocytes were isolated by a two-stage collagenase perfusion process as described previously (Shi et al., 2011; Li et al., 2012). The cells were cultured in William’s E medium supplemented with 10mM HEPES, 1% linoleic acid-albumin, 5 µg/ml insulin, 5 µg/ml holo-transferrin, 25nM dexamethasone, 5 ng/ml sodium selenous acid, and 5% FBS. The cells were seeded at a density of 6 × 105 cells per well in six-well plates that were coated with 1 mg/ml bovine collagen I (PureCol, Advanced BioMatrix, San Diego, CA). Cells were cultured for 6h prior to the treatment. All animals used in this study were handled in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Institutes of Health, and the experimental procedures were approved by the NCTR Institutional Animal Care and Use Committee.
Vector construction and stable cell line establishment
The gene expression lentiviral plasmids pLV-EF1a-hORAI1-IRES-Puro, and pLV-EF1a-hSTIM1-IRES-Puro, and doxycycline-inducible RNAi vector pLV-H1tetO-GFP-Bsd were purchased from Biosettia. A specific target site for silencing human ORAI1 was designed using the RNAi designer available at the Biosettia’s website (http://biosettia.com/support/shrna-designer/). The shRNAs-encoding DNA oligo containing inner palindromic sequences were synthesized (Biosythesis, Inc., Lewisville, Texas): sh-ORAI1 (5’-AAAAGCACCTGTTTGCGCTCATGTTGGATCCAACATGAGCGC AAACAGGTGC-3’) and the scrambled shRNA (5’-AAAAGCTACA CTATCGAGCAATTTTGGATCCAAAATTGCTCGATAGTGTAGC-3’), which did not contain significant homology to known genes, was used as a negative control in silencing experiments. The generation of the lentivirus-shRNA plasmid and the establishment of the stable cell lines were described previously (Chen et al., 2014d, 2014e).
Cellular ATP level measurement
The ATP content was assessed using the CellTiter-Glo Luminescent Cell Viability Assay (Promega Corporation, Madision, WI) as described previously (Li et al., 2012).
Caspase-3/7 activity measurement
The enzymatic activity of caspase-3/7 (Promega Corporation) was measured using luminescent assay kits as described previously (Chen et al., 2014e).
RNA isolation and real-time PCR assay
HepG2 cells were seeded in 6-well plates and treated with various concentration of usnic acid. Total RNA was isolated using the RNeasy system (Qiagen, Germantown, MD). Total RNA (2 µg) was reverse transcribed with high capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Quantitative real-time PCR was performed as described previously to evaluate relative gene expression (Chen et al., 2014d) using the following primers: human CHOP (5′-TT GCCTTTCTCCTTCGGGAC-3′ and 5′-CAGTCAGCCAAGCCAGAGA A-3′), human ORAI1 (5′-GCCAGAGTTACTCCGAGGTG-3′ and 5′-CTGGAGGCTTTAAGCTTGGC-3′), and human GAPDH (5′-AGAAG GCTGGGGCTCATTTG-3′ and 5′-AGGGGCCATCCACAGTCTTC-3′).
Gaussia luciferase activity
Gaussia luciferase activity was measured using Gaussia luciferase assay reagent (Nanolight Technology, Pinetop, AZ) as described previously (Chen et al., 2014d).
Western blot analysis
Cells were grown and treated with usnic acid in 60-mm tissue culture plates. Standard western blots were performed and three or four bolts were performed for each treatment. The following antibodies were used: CHOP (C/EBP CCAAT/enhancer binding protein homologous protein), phospho-eIF2α (Ser 51; eukaryotic initiation factor 2), eIF2α, and caspase 4 (Cell Signaling Technology, Danvers, MA), ORAI1, and STIM1 (ProSci Incorporated, Poway, CA), XBP-1 (X-box binding protein 1), ATF-4 (activation of transcription factor 4), and GAPDH as internal control (Santa Cruz Biotechnology, Santa Cruz, CA) followed by a secondary antibody conjugated with horseradish peroxidase (HRP; Santa Cruz Biotechnology).
Determination of intracellular calcium content
The content of Ca2+ was measured as fluorescence intensity of Fluo-4/AM (Life Technologies) detected by flow cytometry. Fluo-4/AM and pluronic F-127 were added into Hanks’ Balanced Salt Solution (HBSS) just before incubation as the Fluo-4/AM loading solution (the final concentrations of Fluo-4/AM and pluronic F-127 were 3 µM and 0.1%, respectively; Kreitzer et al., 2000). At the end of treatment, HepG2 cells were trypsinized and incubated with Fluo-4/AM loading solution at room temperature for 30 min in the dark. The Fluo-4 loaded cells were washed and resuspended with HBSS. The analysis of green fluorescence was performed using a FACSCanto II flow cytometer (BD Biosciences, San Jose CA). Data were acquired and analyzed using FACSDiva software.
Statistical analyses
Data are presented as the mean ± standard deviation (SD) of at least three independent experiments. Analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). Statistical significance was determined by one way analysis of variance (ANOVA) followed by the Dunnett’s test for pairwise-comparisons or two way ANOVA followed by the Bonferroni post-test. The difference was considered statistically significant when p was less than .05.
RESULTS
Usnic acid causes a decrease in ATP level and triggers ER stress in HepG2 cells
In our previous study, we demonstrated that usnic acid induced cytotoxicity in HepG2 cells as assessed by the MTT and LDH assays (Chen et al., 2014a). It also has been reported that usnic acid caused an inhibition of mitochondrial respiration. Considering the fact that mitochondria are the principal sites for ATP generation, we evaluated if usnic acid causes a decline in cellular energy level in HepG2 cells. Up to 50 µM of usnic acid was tested in this present study because this is the maximum concentration used in our previous study and it is also clinically relevant (Chen et al., 2014a).
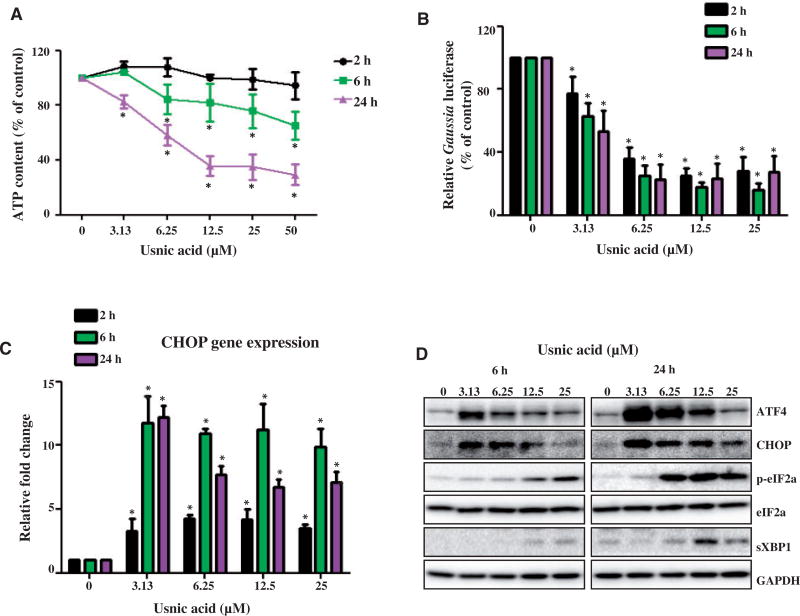
ATP content was measured after cells were treated with increasing concentrations of usnic acid for 2, 6, and 24 h. As shown in Figure 1A, ATP levels decreased significantly in cells exposed to 6.25 µM usnic acid for 6 h; however, neither the MTT or LDH assays showed evidence of cell death using the same usnic acid treatment conditions in prior study (Chen et al., 2014a).
FIG. 1.
Usnic acid causes a decrease in ATP level and triggers ER stress in HepG2 cells. A, HepG2 cells were exposed to increasing concentrations (3.13, 6.25, 12.5, 25, and 50 µM) of usnic acid, with DMSO as the vehicle control for 2, 6, and 24 h. The ATP content measured using CellTiter-Glo Luminescent Cell Viability Assays is expressed as % of control which is calculated by comparing the averaged luminescence of treated cells with that of control cells treated with DMSO alone. B, HepG2-Gluc-Fluc cells were treated with various concentrations of usnic acid for 2, 6, and 24 h. The activity of Gaussia luciferase was measured *P<0.05 as compared with the control for each time point. C, The gene expression level of CHOP was determined by qPCR. Values were mean6SD of three individual experiments *P<0.05 as compared with the control for each time point. D, Total cell lysates were isolated at 6 and 24h after usnic acid treatment. The expression level of ER stress related proteins including ATF-4, CHOP, phospho-eIF2α, eIF2α, spliced XBP1 was determined by Western blotting. GAPDH was used as internal control.
ATP depletion is an early event during cellular damage. With persistent or worsening ATP depletion, subsequent events such as ER stress and necrotic cell death may occur. To determine whether or not usnic acid triggers ER stress in HepG2 cells, a set of ER stress tests was performed. These tests included: inhibition of secreted Gaussia luciferase, induction of CHOP mRNA transcripts, and expression of ER stress-related proteins. We previously established an in vitro system for monitoring ER stress quantitatively using secreted Gaussia luciferase as an ER stress reporter (Chen et al., 2014d); a reduction of protein secretion (in our system, a decrease in Gaussia luciferase) indicates an ER stress response. As shown in Figure 1B, exposure to 3.13 µM usnic acid for 2h inhibited the secretion of Gaussia luciferase significantly. Treatments with 6.25 µM usnic acid for 2, 6, and 24 h caused secreted luciferase levels to decrease to 35%, 25%, and 23% of the amount secreted by untreated cells, respectively. The expression of CHOP mRNA transcripts, a hallmark of ER stress, increased significantly in HepG2 cells treated with 3.13 µM usnic acid at 2h (Fig. 1C). Increased level of CHOP protein was also detected in cells exposed to 3.13 µM usnic acid using western blots (Fig. 1D). Other ER stress related markers were also induced, including ATF4, p-eIF2a, and spliced XBP1 (sXBP1; Fig. 1D; Densitometic analysis is shown in Supplementary Fig. 1). There was a trend of decline in expression of CHOP and ATF4 in the treatments with higher concentrations or longer time periods (Fig. 1C and 1D), possibly due to the increased toxicity. These data indicate that ER stress was triggered in HepG2 cells by usnic acid.
ER stress response contributes to usnic acid-induced apoptosis
In our previous study, we reported that usnic acid induced apoptosis in HepG2 cells as the activity of caspase-3/7 (a key player in the execution of apoptosis) was increased, and the sub-G1 population of apoptotic cells was increased in a concentration-dependent manner (Chen et al., 2014a). Both ER stress and apoptosis are important mechanisms in drug-induced liver toxicity and the two processes are closely related (Chen et al., 2014d, 2014e). Thus, it was of great interest to examine whether usnic acid-induced apoptosis is coupled to ER stress.
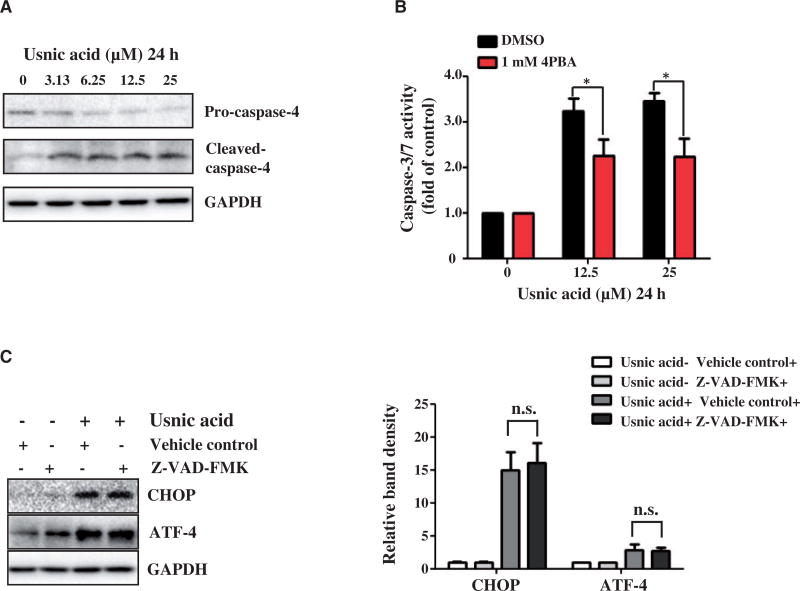
Caspase-4 is reported to be specifically associated with ER stress-induced apoptosis (Hitomi et al., 2004); therefore, we studied the activation status of caspase-4. Both pro- and cleaved- caspase-4 proteins were examined by Western blotting. An obvious decrease in the level of pro-caspase-4 was observed in cells treated with 3.13 µM usnic acid for 24 h; and correspondingly, increase in cleaved-caspase-4 was observed (Fig. 2A; Densitometic analysis is shown in Supplementary Fig. 2), consistent with the usnic acid concentration that caused a significant increase of sub-G1 apoptotic cells in our earlier study (Chen et al., 2014a). The association of ER stress and apoptosis (activation of caspase-3/7) was investigated with an addition of 4-phenylbutyrate (4-PBA), an ER stress inhibitor. Pre-treatment with 4-PBA significantly attenuated caspase-3/7 activation induced by 12.5 or 25 µM usnic acid treatment for 24h compared with treatment with usnic acid alone (Fig. 2B). In contrast, pre-treatment with a general caspase inhibitor (10 µM Z-VAD-FMK) did not alter CHOP induction, an indication of ER stress (Fig. 2C). These data suggest that ER stress contributes to the usnic acid associated apoptotic process.
FIG. 2.
Effect of usnic acid on caspase-4 expression and the activity of caspase-3/7 in HepG2 cells with or without pre-treatment with 4-PBA. A, Total cellular proteins were extracted at 24 h after usnic acid treatment. The expression level of ER stress related caspase protein caspase-4 was determined by Western blotting. B, The HepG2 cells were pretreated with 1mM 4-PBA for 2h prior to 12.5 and 25 µM of usnic acid treatment for additional 24 h. Apoptosis was measured by caspase-3/7 activity. The bar graph shows the mean6SD from four independent experiments. *P<0.05 versus treatment of usnic acid alone. C, Cells were pretreated with 10 µM Z-VAD-FMK, a general caspase inhibitor, for 1h prior to 25 µM of usnic acid treatment for additional 24 h. The expression level of CHOP and ATF-4 was determined by Western blotting. n.s., no significant difference
Usnic acid induces an increase in cytosolic Ca2+
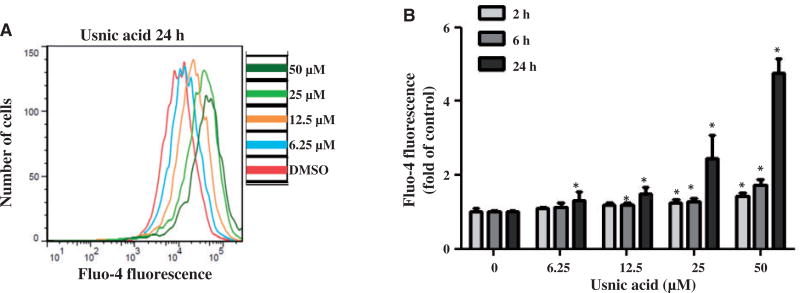
It has been reported that the ER stress response is strictly Ca2+ dependent (Paschen and Doutheil, 1999). To determine whether or not usnic acid alters intracellular Ca2+ levels, HepG2 cells were treated with increasing concentration of usnic acid for 2, 6, and 24h and cellular Ca2+-dependent Fluo-4 fluorescence was measured using flow cytometry. As shown in Figure 3, usnic acid treatment increased the calcium-dependent fluorescence in a concentration- and time-dependent manner. Significant increases in Ca2+-dependent fluorescence occurred at 6h with 12.5 µM usnic acid treatment.
FIG. 3.
Effect of usnic acid on the fluorescence of the calcium indicator Fluo-4 in HepG2 cells. HepG2 cells were exposed to increasing concentrations (3.13, 6.25, 12.5, 25, and 50 µM) of usnic acid, with DMSO as the vehicle control for 2, 6, and 24 h. A, Average Ca2+ level of a single HepG2 cells was determined by flow cytometry. Histogram represents Fluo-4 fluorescence intensities of cells treated with indicated concentrations of usnic acid for 24 h. B, The bar graph shows the mean6SD from three independent experiments. *P<0.05 as compared with the control for each time point.
SOCE influences usnic acid-induced ATP depletion and ER stress
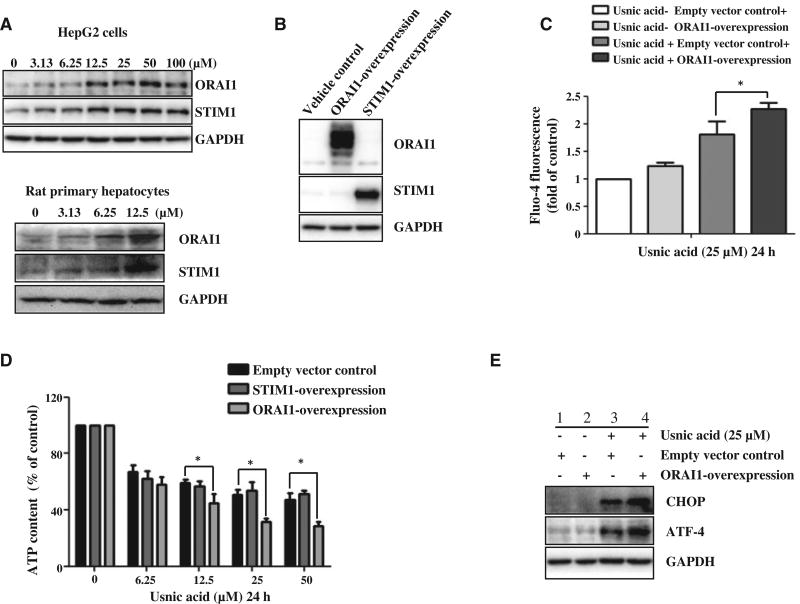
SOCE activity contributes to the perturbed intracellular Ca2+ signal and altered SOCE eventually leads to cell death (Selvaraj et al., 2012). We suspected that SOCE might be involved in usnic acid induced cytotoxicity because of the observed increase in cytosolic Ca2+ (Fig. 3). Because STIM1 and ORAI1 are two key components of the SOCE system (Chen et al., 2013b), we first measured the expression of these two proteins to characterize the possible involvement of SOCE in usnic acid-induced cytotoxicity. As expected, usnic acid markedly increased protein levels of STIM1 and ORAI1 in response to the 2h usnic acid treatment in a concentration-dependent fashion in HepG2 cells (Fig. 4A; Densitometic analysis is shown in Supplementary Fig. 3). Although HepG2 cells offer certain advantages in mechanistic studies of liver toxicity, especially in-depth molecular studies; lack of activities of some metabolizing enzyme is a disadvantage. Of interest, we examined the changes of STIM1 and ORAI1 using freshly isolated rat primary hepatocytes. Similarly as observed in HepG2 cells, usnic acid also induced the expression of both STIM1 and ORAI1 in response to the 2h usnic acid treatment in rat primary hepatocytes (Fig. 4A). To study further the specific role of SOCE in usnic acid-induced ATP depletion and ER stress, ORAI1 or STIM1 were overexpressed in HepG2 cells. The over-expression of ORAI1 or STIM1 was confirmed by Western blotting (Fig. 4B). It is worth noting that the broadening of the ORAI1 band is likely due to the protein glycosylation (Fukushima et al., 2012; Numaga-Tomita and Putney, 2013). Over-expressing of ORAI1 slightly increased Ca2+-dependent fluorescence comparing the empty vector control cells in the absent of usnic acid; however, Ca2+-dependent fluorescence was significantly increased in ORAI1 overexpressed cells comparing to the empty vector control cells in the present of usnic acid (Fig. 4C). Over-expressing ORAI1 increased usnic acid-induced ATP depletion compared with that of control cells (Fig. 4D). However, over-expressing STIM1 did not alter usnic acid-elicited ATP depletion (Fig. 4D). Furthermore, ORAI1 over-expression increased the ER stress response induced by 25 µM usnic acid treatment for 24 h as demonstrated by the further induction of CHOP and ATF-4 (Lane 4 vs Lane 3, Fig. 4E; Densitometic analysis is shown in Supplementary Fig. 3). Taken together, ORAI1 is likely to be involved in ER stress and ATP depletion caused by usnic acid.
FIG. 4.
Effect of usnic acid on the expression of SOCE component proteins (ORAI1 and STIM1) in HepG2 cells. A, HepG2 cells or rat primary hepatocytes were treated with usnic acid at the indicated concentrations for 2 h. Western blotting analysis was performed using antibodies against ORAI1 and STIM1 in whole cell lysates. GAPDH was used as a loading control. B, Confirmation of the overexpression ORAI1 and STIM1 in HepG2 cells (ORAI1-Overexpression and STIM1-Overexpression) by Western blotting. C, D, HepG2-empty vector control cells, STIM-overexpression, and ORAI1-overexpression cells were treated with usnic acid at indicated concentrations for 24 h, then Ca2+ level of a single HepG2 cells was determined (C) and the ATP content was measured (D); the results shown are mean6SD of three separate experiments. *P<0.05 compared with the empty vector control. E, Western blotting analysis was performed with antibodies against CHOP and ATF-4 using lysates from ORAI-overexpression cells exposed to usnic acid at 25 µM for 24 h. GAPDH was used as a loading control.
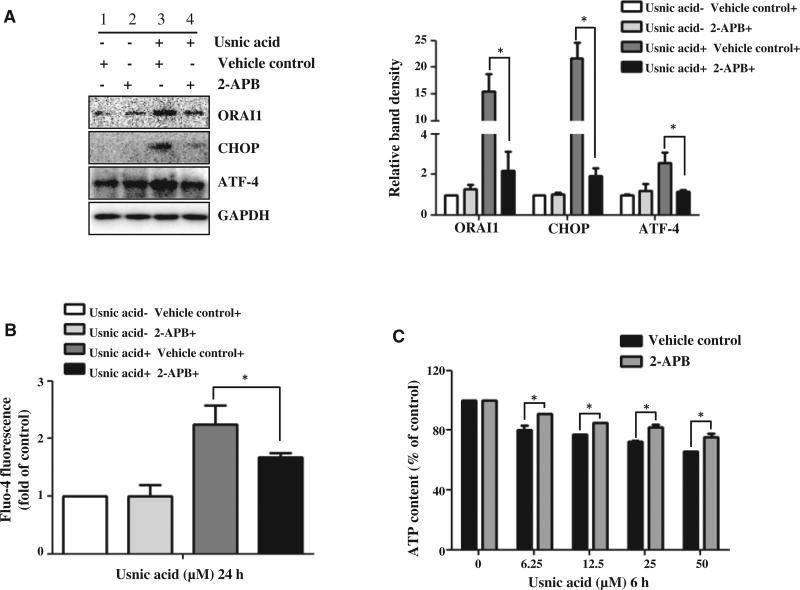
2-APB attenuates usnic acid-induced ER stress and ATP depletion
2-Aminoethoxydiphenyl borate (2-APB) has been reported to influence store-operated calcium channels. 2-APB at low concentrations (1–20 µM) can enhance the function of SOCs, whereas 2-APB at high concentrations (25–100 µM) acts as an antagonist of SOCs by blocking ORAI1-mediated Ca2+ entry (Liu et al., 2012a; Putney, 2010). To elucidate the role of ORAI1, a high concentration of 2-APB 30 µM, was used to block ORAI1 function. HepG2 cells were pretreated with 30 µM 2-APB for 30 min before the addition of 25 µM usnic acid and the protein level of ORAI1 (Fig. 5A) and intensity of Fluo-4 fluorescence (Fig. 5B) was measured at 24 h. The results indicate that the increased expression of ORAI1 in response to usnic acid treatment was markedly reduced by 2-APB (Lane 4 vs Lane 3, Fig. 5A) and the increased usnic acid induced Ca2+ influx was reduced (Fig. 5B). The induction of ER stress markers CHOP and ATF-4 by usnic acid was also reduced by pretreatment with 2-APB (Lane 4 vs Lane 3, Fig. 5A). Moreover, usnic acid-induced ATP depletion was also significantly attenuated by pretreatment with 2-APB (Fig. 5C).
FIG. 5.
Effect of 2-aminoethoxydiphenyl borate (2-APB, SOC blocker) on usnic acid-induced ER stress and ATP depletion and in HepG2 cells. A, HepG2 cells were pre-treated with 30 µM 2-APB for 30 min prior to 25 µM usnic acid treatment for additional 24 h. The expression of ORAI1, CHOP, and ATF-4 were detected by Western blotting; GAPDH was used as a loading control. B, HepG2 cells were pretreated with 30 µM 2-APB for 30min prior to usnic acid treatment for additional 6h and then the intensity of Fluo-4 was measured. C, HepG2 cells were pretreated with 30 µM 2-APB for 30 min prior to indicated concentration of usnic acid treatment for additional 6h and then ATP content was measured. The bar graphs show the mean6SD of three experiments. *P<0.05 versus treatment of usnic acid alone.
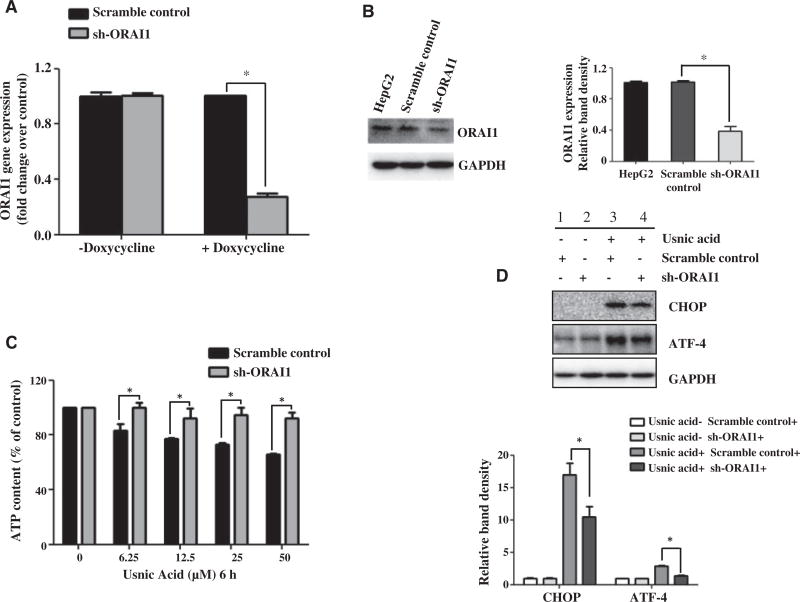
Knockdown of ORAI1 prevents usnic acid-induced ATP depletion and ER stress
To confirm further that ORAI1 is directly associated with usnic acid-induced toxicity, ORAI1 was genetically silenced in HepG2 cells using sh-RNA via a doxycycline-induced lentivirus system. Real-time PCR and Western blotting were carried out to verify the efficiency of ORAI1 knockdown. As shown in Figure 6, sh-RNA targeting ORAI1 markedly down-regulated ORAI1 at both the transcriptional level (Fig. 6A) and the protein level (Fig. 6B). Knockdown of ORAI1 significantly prevented the ATP depletion induced by usnic acid (Fig. 6C) and suppressed the induction of CHOP caused by 25 µM usnic acid for 24 h (Lane 4 vs Lane 3, Fig. 6D). The results from these ORAI1 suppression experiments using both chemical and genetic methods and the results from ORAI1 overexpression experiments show that ORAI1 plays a major role in usnic acid induced ATP depletion and ER stress.
FIG. 6.
Effect of ORAI1 knockdown on usnic acid-induced ATP depletion and ER stress in HepG2 cells. HepG2 cells stably expressing doxycycline-inducible sh-ORAI1 or a scramble control were used. These two cell lines were incubated with doxycycline for 72h followed by continued culture for another 24 h without doxycycline; then the effect of ORAI1 silencing was assessed by real-time PCR (A) and Western blotting (B). Values were mean6SD of three individual experiments *P<0.05 as compared with the SC. C, sh-ORAI1 and scramble control cells were incubated with doxycycline for 72h and then treated with usnic acid at indicated concentration for another 6h without doxycycline. Treated cells were then analyzed for ATP content. The bar graph represents the mean6SD of three separate experiments.*P<0.05 compared with the SCs. D, sh-ORAI1 and scramble control cells were treated with usnic acid at 25 µM for 24 h. Total cellular proteins were extracted. Western blotting was performed using antibodies for CHOP and GAPDH.
DISCUSSION
The ER is a critical organelle and plays roles in many cellular processes such as protein synthesis, protein folding, protein assembly, lipid synthesis, and intracellular calcium homeostasis regulation. ER stress occurs under various circumstances such as unbalanced calcium homeostasis, oxidative stress, hypoxia, energy deprivation, or accumulation of unfolded proteins in the ER (Chen et al., 2014b). A variety of human diseases including cancer, neurological, lung, kidney, inflammatory, and cardiovascular diseases have been reported to be linked to ER stress (Mekahli et al., 2011; Wang and Kaufman, 2012; Yoshida, 2007). ER stress provoked by toxicants or the accumulation of misfolded proteins within the ER and underlying molecular mechanisms involved in the cytotoxic response have been investigated in various experimental systems such as neuroblastoma cells and hepatic cells (Chen et al., 2014d; Hossain et al., 2015; Li et al., 2014b; Salminen et al., 2009).
The role of ER stress in drug-induced liver toxicity has been underestimated in the past; however, it has been receiving increased attention in recent years. The ER stress response participates in a complex interplay of multiple cellular processes such as apoptosis, ATP depletion, oxidative stress, and mitochondrial dysfunction (Apostolova et al., 2011; Chen, et al., 2014d; Kaplowitz et al., 2007; Malhi and Kaufman, 2011; Nagy et al., 2007; Naranmandura et al., 2012; Uzi et al., 2013). Because apoptosis, ATP depletion (Fig. 1A), and mitochondrial dysfunction have been observed in the livers of rodents or in cultured hepatic cells in response to challenge with usnic acid (Abo-Khatwa et al., 1996; Chen et al., 2014a; Han et al., 2004; Pramyothin et al., 2004; Sahu et al., 2012), it was important to explore the association of ER stress to usnic acid’s toxicity. Using our previously established ER stress Gaussia luciferase reporter assay (Chen et al., 2014d), we first measured usnic acid triggered ER stress (Fig. 1B). The induction of ER stress by usnic acid was demonstrated by the upregulation of ER stress markers at the gene expression level (CHOP) using real-time qPCR and at the protein expression level (ATF4, CHOP, p-eIF2α, and sXBP1) using Western blot analysis (Figs. 1C and 1D). The inhibition of secreted protein (reporter gene assay) and upregulation of ATF4, CHOP, p-eIF2α, and sXBP1 clearly demonstrate that usnic acid causes ER stress in hepatic cells. It is reasonable to hypothesize that Ca2+ homeostasis is disrupted by usnic acid because the ER is a major site for maintaining Ca2+ homeostasis and regulating Ca2+ signaling (Paschen and Doutheil, 1999; Smaili et al., 2013); and the ER stress response is Ca2+-dependent (Paschen and Doutheil, 1999). Not surprisingly, a usnic acid concentration-dependent increase was also observed in the fluorescence intensity for the calcium indicator Fluo-4 in treated cells (Fig. 3). Ca2+ regulation is a complex mechanism involving various organelles and different Ca2+ channels. It is generally considered that elevated influx of extracellular Ca2+ into cells is triggered by the depletion of Ca2+ stores within the ER. The Ca2+ influx for replenishing depleted ER Ca2+ is initiated by SOCE with STIM1 and ORAI1 being the two critical constituents in SOCs. When ER Ca2+ is reduced, either by inhibition of ER/SR Ca2+ pump (SERCA) or release Ca2+ through IP3 receptors, the Ca2+ sensor STIM1 is activated. Subsequently, ORAI1 is activated via aggregation with activated STIM1, thus leading an opening of SOCs (Parekh and Putney, 2005).
It is known that the distribution in ER Ca2+ homeostasis leads to ER stress (Selvaraj et al., 2012). We suspected that depletion of ER Ca2+ stores provides one mechanism for the toxicity of usnic acid. For this reason, we attempted to measure ER Ca2+; however, due to the difficulty in experimentally measuring the changes in ER Ca2+, we were not able to obtain direct evidence for Ca2+ depletion in the ER due to usnic acid treatment. Nonetheless, the increased intracellular Ca2+ influx (Fig. 3), the increased expression of SOC proteins (Fig. 4A), and the direct involvement of ORAI1 in usnic acid-induced ER stress (Figs. 5A and 6D) provide a link between the depletion of ER Ca2+ and the ER stress caused by usnic acid.
SOCE is thought to have an essential role in regulating cell proliferation and death (Parekh, 2010). Studies have reported that SOCE is closely involved in drug- and chemical-induced toxicity (Chen et al., 2013a; Chiu et al., 2009; Li et al., 2014a; Liu et al., 2012a). A recent study reported that 6-hydroxydopamine, a neurotoxic synthetic compound that causes Parkinsonism in laboratory animals, induced ROS in a Ca2+-dependent manner in undifferentiated PC12 cell lines. Down-regulation of STIM1 by siRNA prevented ROS production and apoptotic cell death. Moreover, inhibition of STIM1 attenuated mitochondrial dysfunction and decreased ER stress, indicating that SOCE interferes with multiple signaling pathways involved in cytotoxicity and STIM1 may be responsible for neuronal toxicity initiated by ER stress and mitochondrial dysfunction (Li et al., 2014a). Another study using HepG2 cells demonstrated that increased expression of ORAI1 and STIM1 is involved in the elevation of intracellular Ca2+ and consequent hepatic cellular damage induced by ethanol (Liu et al., 2012a). Inhibiting SOCE with either SOCE antagonist 2-APB or siRNA silencing STIM1 attenuated the cell damaging effect. All of these results suggest that activation of SOCE is responsible for the pathogenesis of cell death.
In agreement with these studies, we also found that SOCE participated in usnic acid-induced cytotoxicity in HepG2 cells because STIM1 and ORAI1 were increased as early as 2h (Fig. 4A). Although both STIM1 and ORAI1 are up-regulated by usnic acid, usnic acid-associated cytotoxicity is more likely dependent on ORAI1-mediated SOCE than STIM1-mediated SOCE because overexpression of ORAI1 significantly enhanced usnic acid-induced ATP depletion, whereas overexpression of STIM1 had a minimal effect on usnic acid-induced ATP depletion (Fig. 4D). The role of ORAI1 in usnic acid’s toxicity was confirmed further by inhibition studies; that is, suppression of ORAI1 by ORAI1 inhibitor 2-APB or by sh-RNA silencing attenuated usnic acid-induced cytotoxicity (Figs. 5C and 6C) and also inhibited the induction of CHOP (Figs. 5A and 6D). It is of a note that the reversal effect on ATP depletion is not completely identical when using 2-APB or sh-RNA silencing to inhibit ORAI1 (Figs. 5C and 6C). The reason of this discrepancy is not clear; could due to complex of 2-APB. Although generally described as ORAI1 inhibitor, the specificity of 2-APB is considered to be relative because other calcium regulators such as IP3 receptor can be effected by 2-APB (Putney, 2010). It is possible that 2-APB exerts a complicated mechanism in protecting usnic acid-induced ATP depletion. In general, a cross validation study (sh-RNA silencing in our present study) is needed for an inhibitory study especially when highly specific inhibitors are not available.
It is recognized that ER Ca2+ homeostasis and the ER stress interact. Disturbance of ER Ca2+ leads to the accumulation of unfolded proteins in the ER lumen that in turn causes ER stress (Selvaraj et al., 2012), whereas prolonged ER stress results in a sustained increase of Ca2+ influx (Li et al., 2014a). In our present study, we also studied the interrelationship between SOCE and ER stress. We demonstrated that SOCE influences usnic acid-induced ER stress because overexpression of ORAI1 increased ER stress and the inhibition of ORAI1 by either sh-ORAI1 or inhibitor 2-APB attenuated usnic acid-induced ER stress (Figs. 5A and 6D). In our case, ER stress does not seem to regulate usnic acid-induced Ca2+ influx because a specific inhibitor of ER stress failed to either promote or diminish Ca2+ influx (data not shown).
In summary, our work demonstrates that in HepG2 cells, usnic acid induces ER stress and intracellular Ca2+ influx. SOCE, especially ORAI1, is involved in Ca2+ influx and usnic acid-induced ER stress and hepatotoxicity.
Supplementary Material
Acknowledgments
S.C., Y.W., and ZH.Z. were supported by appointments to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. FDA. We thank Drs James Fuscoe and Baitang Ning for their critical review of this manuscript. This work was supported by U.S. FDA’s intramural grant.
Footnotes
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
Disclaimer: This article is not an official guidance or policy statement of the U.S. Food and Drug Administration (FDA). No official support or endorsement by the U.S. FDA is intended or should be inferred.
References
- Abo-Khatwa AN, al-Robai AA, al-Jawhari DA. Lichen acids as uncouplers of oxidative phosphorylation of mouse-liver mitochondria. Nat. Toxins. 1996;4:96–102. doi: 10.1002/19960402nt7. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Gomez-Sucerquia LJ, Gortat A, Blas-Garcia A, Esplugues JV. Compromising mitochondrial function with the antiretroviral drug efavirenz induces cell survival-promoting autophagy. Hepatology. 2011;54:1009–1019. doi: 10.1002/hep.24459. [DOI] [PubMed] [Google Scholar]
- Barritt GJ, Litjens TL, Castro J, Aromataris E, Rychkov GY. Store-operated Ca2+ channels and microdomains of Ca2+ in liver cells. Clin. Exp. Pharmacol. Physiol. 2009;36:77–83. doi: 10.1111/j.1440-1681.2008.05095.x. [DOI] [PubMed] [Google Scholar]
- CFSAN. [Accessed April 23, 2015];FDA Warns Consumers Not to Use the Dietary Supplement LipoKinetix. 2001 http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHuman-MedicalProducts/ucm172824.htm.
- Chen S, Dobrovolsky VN, Liu F, Wu Y, Zhang Z, Mei N, Guo L. The role of autophagy in usnic acid-induced toxicity in hepatic cells. Toxicol. Sci. 2014a;142:33–44. doi: 10.1093/toxsci/kfu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Melchior WB, Jr, Guo L. Endoplasmic reticulum stress in drug- and environmental toxicant-induced liver toxicity. J. Environ. Sci. Health C. 2014b;32:83–104. doi: 10.1080/10590501.2014.881648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Melchior WB, Jr, Wu Y, Guo L. Autophagy in drug-induced liver toxicity. J. Food Drug Anal. 2014c;22:161–138. doi: 10.1016/j.jfda.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xuan J, Couch L, Iyer A, Wu Y, Li QZ, Guo L. Sertraline induces endoplasmic reticulum stress in hepatic cells. Toxicology. 2014d;322C:78–88. doi: 10.1016/j.tox.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xuan J, Wan L, Lin H, Couch L, Mei N, Dobrovolsky VN, Guo L. Sertraline, an antidepressant, induces apoptosis in hepatic cells through the mitogen-activated protein kinase pathway. Toxicol. Sci. 2014e;137:404–415. doi: 10.1093/toxsci/kft254. [DOI] [PubMed] [Google Scholar]
- Chen T, Zhu J, Zhang C, Huo K, Fei Z, Jiang XF. Protective effects of SKF-96365, a non-specific inhibitor of SOCE, against MPP+-induced cytotoxicity in PC12 cells: potential role of Homer1. PLoS One. 2013a;8:e55601. doi: 10.1371/journal.pone.0055601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca(2)(+) sensor STIM1 regulates actomyosin contractility of migratory cells. J. Cell Sci. 2013b;126:1260–1267. doi: 10.1242/jcs.121129. [DOI] [PubMed] [Google Scholar]
- Chiu TY, Teng HC, Huang PC, Kao FJ, Yang DM. Dominant role of Orai1 with STIM1 on the cytosolic entry and cytotoxicity of lead ions. Toxicol. Sci. 2009;110:353–362. doi: 10.1093/toxsci/kfp099. [DOI] [PubMed] [Google Scholar]
- Durazo FA, Lassman C, Han SH, Saab S, Lee NP, Kawano M, Saggi B, Gordon S, Farmer DG, Yersiz H, et al. Fulminant liver failure due to usnic acid for weight loss. Am. J. Gastroenterol. 2004;99:950–952. doi: 10.1111/j.1572-0241.2004.04165.x. [DOI] [PubMed] [Google Scholar]
- Favreau JT, Ryu ML, Braunstein G, Orshansky G, Park SS, Coody GL, Love LA, Fong TL. Severe hepatotoxicity associated with the dietary supplement LipoKinetix. Ann. Intern. Med. 2002;136:590–595. doi: 10.7326/0003-4819-136-8-200204160-00008. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Tomita T, Janoshazi A, Putney JW. Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities. J. Cell Sci. 2012;125:4354–4361. doi: 10.1242/jcs.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Shi Q, Fang JL, Mei N, Ali AA, Lewis SM, Leakey JE, Frankos VH. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Health C. 2008;26:317–338. doi: 10.1080/10590500802533392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Matsumaru K, Rettori D, Kaplowitz N. Usnic acid-induced necrosis of cultured mouse hepatocytes: inhibition of mitochondrial function and oxidative stress. Biochem. Pharmacol. 2004;67:439–451. doi: 10.1016/j.bcp.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, DiCicco-Bloom E, Richardson JR. Hippocampal ER Stress and Learning Deficits Following Repeated Pyrethroid Exposure. Toxicol. Sci. 2015;143:220–228. doi: 10.1093/toxsci/kfu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin. Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Gee KR, Archer EA, Regehr WG. Monitoring presynaptic calcium dynamics in projection fibers by in vivo loading of a novel calcium indicator. Neuron. 2000;27:25–32. doi: 10.1016/s0896-6273(00)00006-4. [DOI] [PubMed] [Google Scholar]
- Li B, Xiao L, Wang ZY, Zheng PS. Knockdown of STIM1 inhibits 6-hydroxydopamine-induced oxidative stress through attenuating calcium-dependent ER stress and mitochondrial dysfunction in undifferentiated PC12 cells. Free Radic. Res. 2014a;48:758–768. doi: 10.3109/10715762.2014.905687. [DOI] [PubMed] [Google Scholar]
- Li S, Izumi T, Hu J, Jin HH, Siddiqui AA, Jacobson SG, Bok D, Jin M. Rescue of enzymatic function for disease-associated RPE65 proteins containing various missense mutations in non-active sites. J. Biol. Chem. 2014b;289:18943–18956. doi: 10.1074/jbc.M114.552117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Couch L, Higuchi M, Fang JL, Guo L. Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol. Sci. 2012;127:582–591. doi: 10.1093/toxsci/kfs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jia X, Luo Z, Guan H, Jiang H, Li X, Yan M. Inhibition of store-operated Ca(2+) channels prevent ethanol-induced intracellular Ca(2+) increase and cell injury in a human hepatoma cell line. Toxicol. Lett. 2012a;208:254–261. doi: 10.1016/j.toxlet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhao X, Lu X, Fan X, Wang Y. Proteomic study on usnic acid-induced hepatotoxicity in rats. J. Agric. Food Chem. 2012b;60:7312–7317. doi: 10.1021/jf2046834. [DOI] [PubMed] [Google Scholar]
- Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011;3:1–30. doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Lynch J, Groenendyk J, Guo L, Robert Parker JM, Opas M. Calreticulin in cardiac development and pathology. Biochim. Biophys. Acta. 2002;1600:32–37. doi: 10.1016/s1570-9639(02)00441-7. [DOI] [PubMed] [Google Scholar]
- Nagy G, Kardon T, Wunderlich L, Szarka A, Kiss A, Schaff Z, Banhegyi G, Mandl J. Acetaminophen induces ER dependent signaling in mouse liver. Arch. Biochem. Biophys. 2007;459:273–279. doi: 10.1016/j.abb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Naranmandura H, Xu S, Koike S, Pan LQ, Chen B, Wang YW, Rehman K, Wu B, Chen Z, Suzuki N. The endoplasmic reticulum is a target organelle for trivalent dimethylarsinic acid (DMAIII)-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2012;260:241–249. doi: 10.1016/j.taap.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Neff GW, Reddy KR, Durazo FA, Meyer D, Marrero R, Kaplowitz N. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J. Hepatol. 2004;41:1062–1064. doi: 10.1016/j.jhep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Numaga-Tomita T, Putney JW. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J. Cell Sci. 2013;126:605–612. doi: 10.1242/jcs.115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. Store-operated CRAC channels: function in health and disease. Nat. Rev. Drug Discov. 2010;9:399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Paschen W, Doutheil J. Disturbances of the functioning of endoplasmic reticulum: a key mechanism underlying neuronal cell injury? J. Cereb. Blood Flow Metab. 1999;19:1–18. doi: 10.1097/00004647-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Pramyothin P, Janthasoot W, Pongnimitprasert N, Phrukudom S, Ruangrungsi N. Hepatotoxic effect of (+)usnic acid from Usnea siamensis Wainio in rats, isolated rat hepatocytes and isolated rat liver mitochondria. J. Ethnopharmacol. 2004;90:381–387. doi: 10.1016/j.jep.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Putney JW. Pharmacology of store-operated calcium channels. Mol. Interv. 2010;10:209–218. doi: 10.1124/mi.10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM) Biochim. Biophys. Acta. 2013;1833:213–224. doi: 10.1016/j.bbamcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Sahu SC, Amankwa-Sakyi M, O’Donnell MW, Jr, Sprando RL. Effects of usnic acid exposure on human hepatoblastoma HepG2 cells in culture. J. Appl. Toxicol. 2012;32:722–730. doi: 10.1002/jat.1721. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflam. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J. Clin. Invest. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Yang X, Greenhaw J, Salminen WF. Hepatic cytochrome P450s attenuate the cytotoxicity induced by leflunomide and its active metabolite A77 1726 in primary cultured rat hepatocytes. Toxicol. Sci. 2011;122:579–586. doi: 10.1093/toxsci/kfr106. [DOI] [PubMed] [Google Scholar]
- Smaili SS, Pereira GJ, Costa MM, Rocha KK, Rodrigues L, do Carmo LG, Hirata H, Hsu YT. The role of calcium stores in apoptosis and autophagy. Curr. Mol. Med. 2013;13:252–265. doi: 10.2174/156652413804810772. [DOI] [PubMed] [Google Scholar]
- Sonko BJ, Schmitt TC, Guo L, Shi Q, Boros LG, Leakey JE, Beger RD. Assessment of usnic acid toxicity in rat primary hepatocytes using (1)(3)C isotopomer distribution analysis of lactate, glutamate and glucose. Food Chem. Toxicol. 2011;49:2968–2974. doi: 10.1016/j.fct.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targos B, Baranska J, Pomorski P. Store-operated calcium entry in physiology and pathology of mammalian cells. Acta Biochim. Pol. 2005;52:397–409. doi: 10.18388/abp.2005_3452. [DOI] [PubMed] [Google Scholar]
- Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, Valverde AM, Iwawaki T, Nahmias Y, Xavier R, et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J. Hepatol. 2013;59:495–503. doi: 10.1016/j.jhep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellapu RK, Mittal V, Grewal P, Fiel M, Schiano T. Acute liver failure caused by ‘fat burners’ and dietary supplements: a case report and literature review. Can. J. Gastroenterol. 2011;25:157–160. doi: 10.1155/2011/174978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.