Abstract
Leflunomide, used for the treatment of rheumatoid arthritis, has been reported to cause severe liver problems and liver failure; however, the underlying mechanisms are not clear. In this study, we used multiple approaches including genomic analysis to investigate and characterize the possible molecular mechanisms of the cytotoxicity of leflunomide in hepatic cells. We found that leflunomide caused endoplasmic reticulum (ER) stress and activated an unfolded protein response, as evidenced by increased expression of related genes including CHOP and GADD34; and elevated protein levels of typical ER stress markers including CHOP, ATF-4, p-eIF2α, and spliced XBP1. The secretion of Gaussia luciferase was suppressed in cells treated with leflunomide in an ER stress reporter assay. Inhibition of ER stress with an ER stress inhibitor 4-phenylbutyrate, and knockdown of ATF-4 and CHOP genes partially protected cells upon leflunomide exposure. In addition, both genomic and biochemical analyses revealed that JNK and ERK1/2 of MAPK signaling pathways were activated, and both contributed to the leflunomide-induced cytotoxicity. Inhibiting JNK activation using a JNK inhibitor attenuated the ER stress and cytotoxicity of leflunomide, whereas inhibiting ERK1/2 using an ERK1/2 inhibitor or ERK1/2 siRNA increased the adverse effect caused by leflunomide, suggesting opposite roles for the two pathways. In summary, our data indicate that both ER stress and the activation of JNK and ERK1/2 contribute to leflunomide-induced cytotoxicity.
Keywords: Leflunomide, Liver toxicity, ER stress, Gene expression, RNA-seq, MAPK pathway
1. Introduction
Leflunomide (originally branded as ARAVA®) is used to treat active moderate-to-severe rheumatoid arthritis and psoriatic arthritis. It is a pyrimidine synthesis inhibitor and belongs to immunosuppressive disease-modifying antirheumatic drug (DMARD) category (Sanders and Harisdangkul, 2002). During post approval usage, cases of liver injury and liver failure caused by leflunomide alone or in combination with other drugs have been reported. Forty-nine cases of severe liver injury, of which 14 resulted in fatal liver failure, were documented between 2002 and 2009 due to treatment of leflunomide (Alcorn et al., 2009). In 2010, the U.S. Food and Drug Administration (FDA) added a “black box” warning regarding severe liver injury for leflunomide, and the drug is contraindicated in certain patients with preexisting liver conditions. Moreover, due to reported abnormal elevation of liver enzymes caused by leflunomide (van Roon et al., 2004), the level of liver enzyme alanine transaminase in patients is recommended to be monitored during drug treatment. Despite these adverse effects, little information is available on the underlying mechanisms of the observed hepatotoxicity of leflunomide.
Multiple mechanisms such as mitochondrial dysfunction, chemically reactive metabolites, apoptotic and necrotic toxicity, lysosomal dysfunction, bile transport inhibition, and immune-mediated mechanisms make substantial contributions to the pathogenesis of drug-induced liver toxicity (Dragovic et al., 2016). Endoplasmic reticulum (ER) stress, which received less attention in the past, has been recently described as an important mechanism for drug-induced liver toxicity (Chen et al., 2014c, 2015; Ren et al., 2016; Uzi et al., 2013). Disruption of ER function by external stimuli can result in ER stress, a condition involving accumulation of unfolded proteins in the ER lumen. This perturbation activates an unfolded protein response (UPR), to re-establish the homeostasis in the ER (Chen et al., 2014b). UPR is mainly composed by three branches: PKR-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6); each of the branches regulates the expression of corresponding genes through various transcriptional factors. Upon the activation of PERK, eukaryotic initiation factor 2α (eIF2α) is phosphorylated and activated, which increases the translation of transcriptional factors, including transcription factor 4 (ATF4), and reduces overall protein synthesis to decrease the load on the ER. Activation of IRE1α by phosphorylation triggers the splicing of X-box binding protein 1 (XBP1) mRNA. Spliced XBP1 then enters the nucleus and regulates gene expression as a transcription factor. For the ATF6 branch, ER stress results in the cleavage of ATF6 in Golgi, and in turn promotes the expression of related downstream genes. Although UPR aims to promote cell survival, it can also result in cell death under excessive ER stress (Chen et al., 2014b; Iurlaro and Munoz-Pinedo 2016).
The mitogen-activated protein kinase (MAPK) signaling cascade is composed of a large network of kinases and regulates numerous cellular processes, including cell proliferation, survival, and death (Plotnikov et al., 2011). MAPK has been shown to have a critical role in drug-induced liver toxicities due to its broad involvement in cellular functions and its interaction with various signal transduction pathways (Darling and Cook, 2014). C-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase 1 and 2 (ERK1/2), and p38 are the three major pathways of the MAPK network. Generally, JNK and p38 mediate signal transduction leading to cell death, whereas ERK1/2 activation plays a protective role and promotes survival (Chang and Karin, 2001).
In the current study, we explored the mechanisms underlying the cytotoxicity of leflunomide. Using multiple approaches, we studied the role of ER stress in leflunomide-induced liver toxicity. We also investigated the role of MAPK signaling cascade, particularly, JNK and ERK1/2, in the side effect of leflunomide.
2. Material and methods
2.1. Chemicals and reagents
Williams’ Medium E, 4-phenylbutyrate acid (4-PBA), and dimethy-sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Antibiotic-antimycotic was obtained from Life Technologies (Grand Island, NY). PureCol Bovine Collagen Solution was from Advanced BioMatrix (San Diego, CA). Leflunomide was purchased from Enzo Life Sciences (Farmingdale, NY). SP600125 (JNK inhibitor) and PD184352 (ERK1/2 inhibitor) were from LC laboratories (Woburn, MA).
2.2. Cell culture and drug treatment
HepG2 human hepatic cells were cultured in Williams’ Medium E supplemented with 10% FBS and 1 × antibiotic-antimycotic, as described previously (Chen et al., 2014a, 2015). The passage number did not exceed 10. Depending on the specific assay, cells were either seeded at a density of 3 × 105 cells/ml in volumes of 100 µl in the wells of 96-well tissue culture plates, or in volumes of 5 ml in 60 mm plates. Unless otherwise specified, cells were maintained in growth medium for approximately 24 h before treatment with the indicated concentrations of leflunomide and/or inhibitors, or the vehicle DMSO control (the final concentration did not exceed 0.1%). For drug treatment, leflunomide was added at the final concentrations of 50 to 300 µM as indicated in the text, to cells in 96-well plates for toxicity assays or 60 mm plates for biochemical assays. The ER stress inhibitor 4-PBA (1 mM) was added 2 h prior to drug treatment and remained in the medium during the treatment. The JNK inhibitor SP600125 was added 2 h prior to drug treatment at a final concentration of 20 µM, and was reduced to 10 µM in the medium during the treatment. The ERK1/2 inhibitor PD184352 (2 µM) was added 2 h prior to the treatment and then removed before exposure to leflunomide. The stable cell line for the Gaussia Luciferase assay (HepG2-Fluc-Gluc) was established previously (Chen et al., 2014c) and grown in Williams’ Medium E. The preparation and drug treatment conditions for this cell line were the same as described for the HepG2 cells.
HepaRG cells (terminally differentiated hepatic cells) were obtained from Life Technologies. Terminally differentiated HepaRG cells were cultured in Williams’ Medium E supplemented with the Thaw, Plate, & General Purpose Medium Supplement (Life Technologies) for one day, and then maintained in Williams’ Medium E supplemented with the Maintenance/Metabolism medium supplement for additional 7 days. Differentiated cells were then seeded at a density of 3.5 × 105 cells/ml in volumes of 100 µl in the wells of 96-well tissue culture and incubated for another 2 days for drug treatment. Cells were exposed to leflunomide for 6 h at the concentrations from 50 to 300 µM, and the ATP contents were measured.
Three samples of primary human hepatocytes, HH1051 (single donor, 23 year-old male Caucasian), HH1083 (single donor, 45 year-old female Caucasian), and PHH8007A (pooled from 5 donors) (In Vitro ADMET Laboratories LLC, Columbia, MD) were thawed and recovered using Universal Cryopreserved Recovery Medium (UCRM, IVAL Inc., Columbia, MD). The cells were then suspended in hepatocyte induction medium (HIM, IVAL Inc) and plated in bovine collagen-coated 96-well tissue culture plates at a density of 3 × 104 cells/100 µl/per well. The cells were incubated in a humidified 95% balanced air/5% CO2 atmosphere in a 37 °C incubator for 16 h to facilitate attachment. After attachment, the medium was removed and the cells were washed once to remove unattached cells before the treatment.
2.3. Cellular ATP level measurement
Cellular ATP contents were quantified using a CellTiter-Glo Luminescent Cell Viability Assay (Promega Corporation, Madison, WI). After the specified length of drug exposure, the supernatants were aspirated and 10 µl ATP assay reagent with 90 µl of serum-free culture medium was added to each well of the 96-well plates. Luminescence was immediately measured with a Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT). The cellular ATP content of treated cells was calculated as the percentage of the DMSO controls.
2.4. MTS cell viability assay
Cell viability was quantified using a tetrazolium reduction-based assay (MTS, Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay, Promega) as previously described (Li et al., 2011).
2.5. Next-generation sequencing analysis (RNA-seq)
RNA-seq was performed at the Microarray Core Facility of the University of Texas Southwestern Medical Center (http://micro-array.swmed.edu). RNA-seq libraries were constructed and sequenced using the TruSeq protocol on an Illumina HiSeq 2000 platform. Data analysis was as described previously (Chen et al., 2017). The read counts for each sample were normalized as reads per million mapped reads (RPM) and transformed to log2 scale for further analysis. For each gene, a value of 1 was added to the read count values to avoid infinite values before log2 transformation. A differentially expressed gene was identified with a fold change (FC) greater than 1.5 (both up-and down-regulation) and a nonstringent p cutoff of 0.05 in comparison to the DMSO control group. Principal component analysis (PCA) was performed using Array Track software developed by NCTR/FDA (version 3.5.0) (http://www.fda.gov/nctr/science/centers/toxicoinformatics/ArrayTrack/).
2.6. RNA isolation and taqman quantitative real-time PCR
HepG2 cells were seeded on 60 mm plates approximately 24 h before exposure to various concentrations of leflunomide. Total RNA was isolated using a mini-RNeasy kit (Qiagen, Germantown, Maryland), and the yield of the RNA was measured by a NanoDrop 8000 spectrometer (Thermo Fisher Scientific, Wilmington, Delaware). cDNA was synthesized from 2 µg of total RNA with a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific) following the manufacturer’s protocol. The gene expression levels of CHOP, GADD34, and GAPDH were analyzed using Taqman real-time PCR as described previously (Chen et al., 2014c), with the following probes from Thermo Fisher Scientific: human CHOP (DDIT3, Hs99999172_m1), human GADD34 (PPP1R15A, Hs00169585_m1), and human GAPDH (Hs02758991_g1).
2.7. Luciferase activity
Secreted Gaussia luciferase (Gluc) activity was measured using a NanoFuel Glow Assay kit (Nanolight Technology, Pinetop, AZ). GLOW working solution was prepared by a 1–50 dilution of coelenterazine substrate with GLOW reagent. Five µl of medium was saved from each well of a 96-well plate; then 50 µl of GLOW working solution was added to the cell-free medium and bioluminescence was recorded immediately using a Synergy 2 Microplate Reader. For the measurement of firefly luciferase (Fluc) activity, 50 µl of firefly luciferase assay buffer (Nanolight Technology) was added to each well of cells in a 96-well plate. After 15 min incubation at room temperature, the bioluminescence was measured with a Synergy 2 Microplate Reader.
2.8. siRNA transfection
HepG2 cells were reverse transfected with SignalSilence® p44/42 MAPK (ERK1/2) siRNA, SignalSilence® Control siRNA (Cell Signaling Technology, Danvers, MA), Silencer® ATF4 siRNA, Silencer® CHOP siRNA, or Silencer® Negative Control #1 siRNA (Life Technologies) using Lipofectamine® RNAiMAX Transfection Reagent (Life Technologies). In brief, HepG2 cells were dissociated, and seeded to each 10 cm dish at a density of 3 × 106 cells/dish in 10 ml medium composed of 8 ml of antibiotic-free growth medium, 2 ml of Opti-MEM (Life Technologies), 20 µl of Lipofectamine RNAiMAX, and 200 pmol of desired siRNA. After 24 h of transfection, cells were changed to normal growth medium and plated into 96-well plates and/or 6 cm plates, as described before. Drug treatment started 48 h post siRNA transfection. The silencing efficiency of the siRNAs was determined by Western blotting.
2.9. Western blot analysis
HepG2 cells were grown and treated with leflunomide in 60 mm tissue culture plates. Standard Western blot analyses were performed using antibodies against CHOP (Thermo Fisher Scientific), phospho(p)-eIF2α (Ser 51), eIF2α, p-ERK1/2 (Thr202/Tyr204), ERK1/2, p-p38, p38, p-JNK (Thr183/Tyr185), JNK (Cell Signaling Technology, Danvers, MA), ATF-4, p-c-Jun, c-Jun, XBP1, GAPDH (as an internal control, Santa Cruz Biotechnology, Santa Cruz, CA), followed by a secondary antibody conjugated with horseradish peroxidase (HRP) (Santa Cruz Biotechnology). The bands were detected by FluorChem E and M Imagers (ProteinSimple, San Jose, CA) and quantified by ImageJ software (NIH, Bethesda, MD).
2.10. Statistical analysis
Data are presented as mean ± standard deviation (SD) of at least three independent experiments. Analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). Statistical significance was determined by two-way ANOVA followed by the Bonferroni post-test for multiple comparisons. The difference was considered statistically significant when p < 0.05.
3. Results
3.1. Leflunomide induces cellular damage in HepG2 cells, HepaRG cells, and primary human hepatocytes
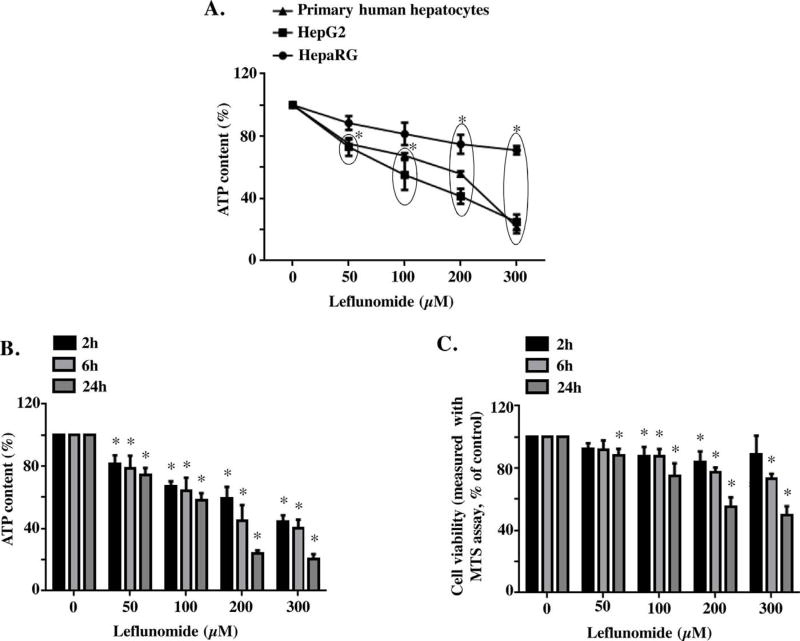
In order to obtain the cytotoxicity profile of leflunomide and determine the suitable cell model for mechanistic study, we exposed HepG2 cells, HepaRG cells, and primary human hepatocytes, the three liver cell model systems most commonly used to study drug-induced hepatotoxicity, to leflunomide for 6 h at concentrations from 50 to 300 µM. Cytotoxicity was assessed using ATP content. As shown in Fig. 1A, exposure to leflunomide resulted in decreased ATP content in all three tested cells and the differences in loss of cellular ATP levels in primary vs HepG2 are probably not significant. At 300 µM, the ATP content was 24.9, 21.7, and 71.0% of its corresponding DMSO control in HepG2 cells, primary human hepatocytes, and HepaRG cells, respectively. Overall, HepG2 cells showed similar response to primary human hepatocytes upon leflunomide treatment, so we chose HepG2 cells for further experiments; other rationales for using this cell line are detailed in the discussion section.
Fig. 1. Leflunomide induces cytotoxicity in hepatic cells.
(A) Primary human hepatocytes, HepG2 cells, and HepaRG cells were exposed to leflunomide for 6 h at concentrations from 50 to 300 µM, and cytotoxicity was measured by cellular ATP content. The data for primary human hepatocytes was the average of three experiments, two experiments were performed using cells from two individual donors, and the third experiment usedpooled cells from 5 donors. (B, C) HepG2 cells were exposed to leflunomide for 2, 6, and 24 h at concentrations from 50 to 300 µM, and cytotoxicity was measured by cellular ATP content (B) and MTS cell viability assay (C). DMSO served as control. The results shown are mean ± S.D. from 3 to 4 independent experiments. *p < 0.05.
Leflunomide-induced cytotoxicity was assessed in HepG2 cells at 2, 6, and 24 h using two different parameters, ATP content and MTS viability assay. As shown in Fig. 1B & C, the results from both ATP content measurement and MTS viability assay showed a concentration-and time-dependent cytotoxicity in HepG2 cells exposed to leflunomide; although the sensitivity is different, with ATP assay being more sensitive than MTS assay.
3.2. Leflunomide induces ER stress
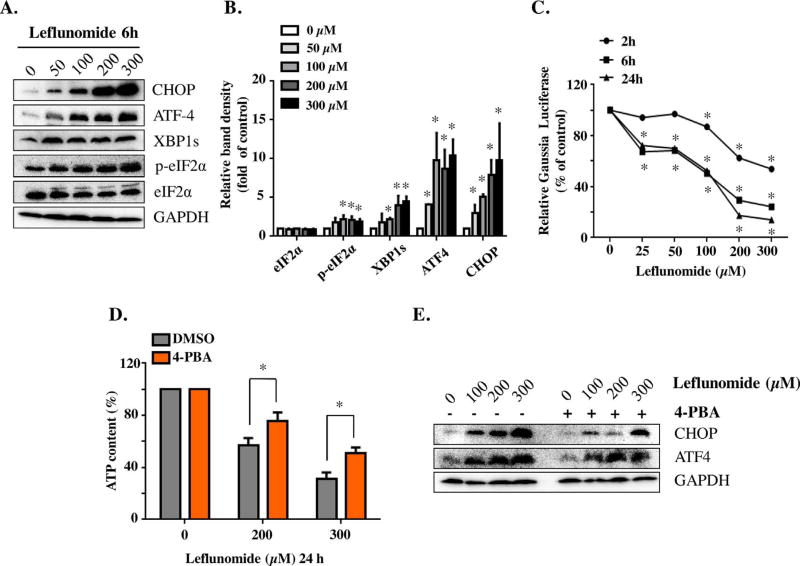
Multiple recent studies, including ours, have shown that ER stress contributes to drug induced toxicity. In this study, we first examined whether or not leflunomide causes ER stress in HepG2 cells by testing a battery of ER stress markers using Western blotting. CHOP, a hallmark of ER stress, showed a significant concentration-dependent increase upon 6 h leflunomide treatment (Fig. 2A & B). In addition to CHOP, the levels of other components of the UPR pathways, including ATF4, p-eIF2α, and spliced XBP1 (XBP1s), were also increased. The level of total eIF2α remained roughly constant.
Fig. 2. Leflunomide triggers ER stress and UPR in HepG2 cells.
(A, B) Total cellular proteins from HepG2 cells were extracted after 6 h leflunomide treatment at the concentrations indicated. Protein levels of CHOP, ATF-4, spliced XBP1 (XBP1s), p-eIF2α, total eIF2α, and GAPDH as internal control were assessed by Western blotting (A) and quantified (B). (C) HepG2-Gluc-Fluc cells were exposed to 25–300 µM of leflunomide for 2, 6, and 24 h, and the activity of Gluc was measured. (D) HepG2 cells were treated with 4-PBA for 2 h before exposure to leflunomide for additional 24 h. The protective effect of 4-PBA was assessed by cellular ATP content. (E) Representative Western blots showed that 4-PBA attenuated leflunomide-induced increase of CHOP and ATF-4. The results shown are mean ± S.D. from three independent experiments. *p < 0.05.
ER plays a critical role in protein processing, transport, and following secretion into extracellular space. Thus, decreased protein secretion, which can be monitored by secreted reporters such as Gaussia luciferase, is a distinct characteristic of functional disruption of ER. We have previously reported the construction of a stable cell line (HepG2-Fluc-Gluc) that expressed both firefly luciferase (Fluc) and secreted Gaussia luciferase (Gluc) (Chen et al., 2014c); Fluc is used as internal control of the total cell number in each well, whereas Gluc indicates the secretion activity. ER stress causes a decrease in secreted Gluc in the medium in this cell model, and hence enables easy detection of ER stress using a Gaussia luciferase reporter assay. In the current study, we exposed the HepG2-Fluc-Gluc cells to leflunomide at concentrations from 25 µM to 300 µM for 2, 6, and 24 h. As shown in Fig. 2C, leflunomide decreased the secretion of Gluc at as early as 2 h, starting from 100 µM. Treatment for 6 or 24 h also showed the concentration-dependent reduction of Gluc secretion and the significant secretion reduction was observed even at the lowest concentration tested. Taken together, these results indicated that leflunomide triggers ER stress in HepG2 cells.
3.3. Suppression of ER stress attenuates leflunomide induced cytotoxicity
4-Phenylbutyrate (4-PBA), a chemical chaperone, has been widely used as an ER stress inhibitor for in vitro studies. As shown in Fig. 2D, pre-treatment with 4-PBA partially alleviated the cytotoxicity induced by leflunomide compared with the treatment with leflunomide alone. At 200 µM of leflunomide, the presence of 4-PBA partially rescued cellular ATP level from 56% to 75% of control. Similar effects were observed at 300 µM of leflunomide. 4-PBA diminished the leflunomide-induced activation of CHOP and ATF4, as examined using Western blot (Fig. 2E), confirming its role as an ER stress inhibitor.
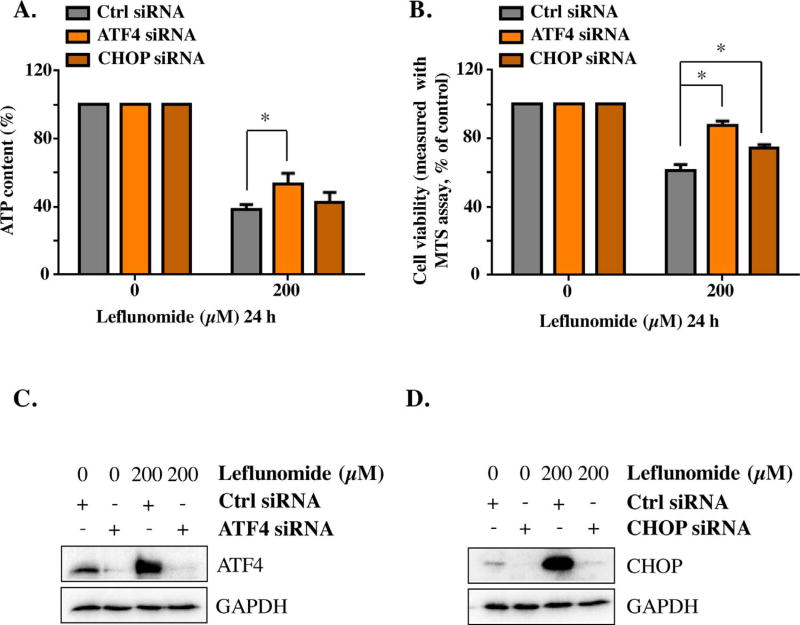
The lack of high specificity is one limitation of using chemical inhibitors in ER stress studies. Therefore, we took a direct approach to knockdown the genes that are critical in ER stress pathways and examined the resulting effects. In accordance with the results obtained using 4-PBA, knocking down ATF4 using siRNAs reduced leflunomide-induced cytotoxicity. Similarly, knocking down CHOP rescued the cell viability (Fig. 3A & B). The efficiency of suppressing gene expression using siRNAs was confirmed by Western blotting (Fig. 3C & D).
Fig. 3. Knockdown ATF-4 or CHOP gene attenuates the cytotoxicity of leflunomide.
HepG2 cells were transiently transfected with ATF-4 siRNA, CHOP siRNA, or negative control siRNA. Forty-eight hours post transfection, cells were exposed to 200 µM of leflunomide for 24 h, and the cellular ATP content was measured (A), together with a MTS viability assay (B). The knockdown efficiency of ATF4 siRNA (C) and CHOP siRNA (D) was assessed by Western blot. The results shown are mean ± S.D. from three independent experiments. *p < 0.05.
3.4. Leflunomide treatment alters the expression of genes related to MAPK pathway
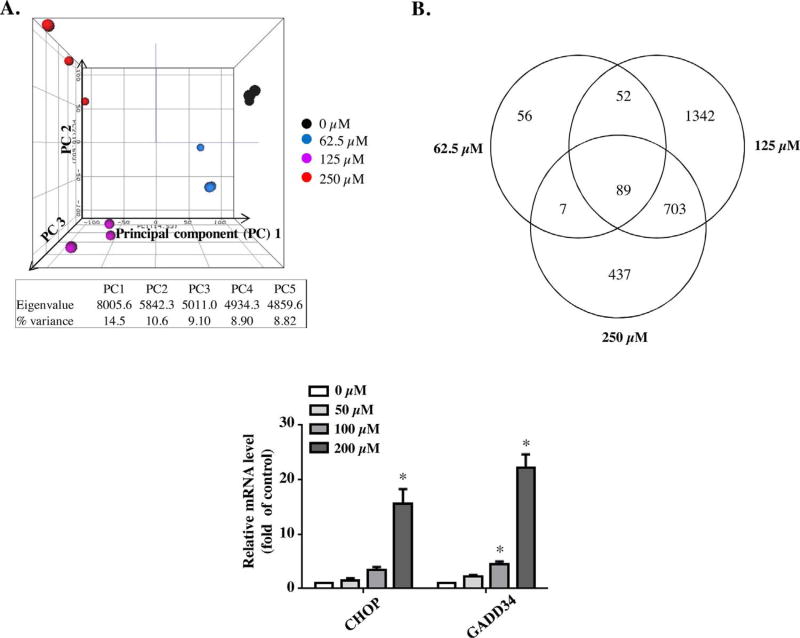
To study further the molecular mechanisms underlying the toxicity of leflunomide, we performed gene expression analysis on the RNA isolated from the cells treated with leflunomide for 6 h, using next-generation RNA-sequencing (RNA-seq). There was one DMSO control group and there were three leflunomide treatment groups (62.5, 125, and 250 µM), each group containing 3 biological replicates (Supplementary Table 1). Unsupervised principal component analysis (PCA) was performed to explore the effect of various leflunomide exposures on gene expression profiles, as shown in Fig. 4A. Samples displayed clear clustering patterns based on the treatment groups and higher leflunomide treatments (125 and 250 µM) resulted in more distinct gene expression profiles compared with the DMSO-treated group. A differentially expressed gene (DEG) was identified using the criteria of a fold change > 1.5 (up- or down-regulation) and a p-value < 0.05 in comparison to the DMSO control group. Based on these criteria, 204, 2186, and 1236 DEGs were identified in 62.5, 125, and 250 µM leflunomide treatment groups, respectively (Fig. 4B) (DEG lists are provided in Supplementary Table 2 a, b & c). In order to study more profoundly affected pathways, we mapped the DEGs to query the Kyoto Encyclopedia of Genes and Genomes (KEGG) database for pathways analysis. Table 1 lists the pathways significantly altered by leflunomide. As shown in Table 1, the MAPK signaling pathway is the most significantly affected pathway for all three leflunomide treatment groups, with DEGs of 6, 34, and 27; Fisher p values of 3.17 × 10−2, 3.33 × 10−3, and 2.09 × 10−5, for the 62.5, 125, and 250 µM leflunomide treatment groups, respectively. Of the DEGs involved in the MAPK signaling pathway, the c-Fos, c-Jun, JunB, JunD, and MAPK6 genes were upregulated by 70.0–, 7.8–, 11.84–, 3.41–, and 2.1–fold, respectively (Table 2).
Fig. 4. Gene expression profiles of different leflunomide treatment groups and confirmation of RNA-seq results by Taqman real-time PCR.
(A) Principal component analysis (PCA) of gene expression profiles. Unbiased PCA analysis was applied to the values of the log2 (RPM) of the entire gene set without specific cut off. (B) Numbers of differentially expression genes and overlapping of altered genes. A differentially expressed gene was identified if the fold change > 1.5 or<0.667 and a p value < 0.05 in comparison with the DMSO control group. (C) The expression of CHOP and GADD34 at mRNA level in HepG2 cells was examined using Taqman real-time PCR after 6 h exposure to 50–200 µM leflunomide. The results shown are mean ± S.D. from three independent experiments. *p < 0.05.
Table 1.
Pathways affected upon leflunomide exposure for 6 h.
| Leflunomide concentration | Pathway Name | Gene_Found | Gene_Pathway | Percentage a | p-value |
|---|---|---|---|---|---|
| 62.5 µM | Metabolic pathways | 19 | 1131 | 2% | 6.01E-03 |
| Cell cycle | 7 | 128 | 5% | 1.05E-04 | |
| MAPK signaling pathway | 6 | 268 | 2% | 3.17E-02 | |
| Steroid biosynthesis | 6 | 19 | 32% | 1.09E-09 | |
| p53 signaling pathway | 5 | 69 | 7% | 1.42E-04 | |
| 125 µM | Pathways in cancer | 43 | 327 | 13% | 4.62E-04 |
| MAPK signaling pathway | 34 | 268 | 13% | 3.33E-03 | |
| Cell cycle | 31 | 128 | 24% | 8.67E-09 | |
| Cytokine-cytokine receptor interaction | 29 | 265 | 11% | 3.98E-02 | |
| Focal adhesion | 26 | 200 | 13% | 6.88E-03 | |
| Oocyte meiosis | 21 | 114 | 18% | 1.48E-04 | |
| p53 signaling pathway | 21 | 69 | 3% | 1.97E-08 | |
| Jak-STAT signaling pathway | 20 | 155 | 13% | 1.65E-02 | |
| Gap junction | 19 | 90 | 21% | 3.93E-05 | |
| Wnt signaling pathway | 18 | 151 | 12% | 4.31E-02 | |
| Ubiquitin mediated proteolysis | 17 | 139 | 12% | 3.83E-02 | |
| Osteoclast differentiation | 17 | 128 | 13% | 1.86E-02 | |
| Tight junction | 16 | 133 | 12% | 4.84E-02 | |
| Progesterone-mediated oocyte maturation | 16 | 87 | 18% | 7.41E-04 | |
| Prostate cancer | 15 | 89 | 17% | 2.60E-03 | |
| TGF-beta signaling pathway | 15 | 85 | 18% | 1.60E-03 | |
| Fc gamma R-mediated phagocytosis | 14 | 95 | 15% | 1.18E-02 | |
| GnRH signaling pathway | 13 | 101 | 13% | 4.04E-02 | |
| Glioma | 13 | 65 | 20% | 8.12E-04 | |
| Apoptosis | 12 | 89 | 13% | 3.30E-02 | |
| Bile secretion | 12 | 71 | 17% | 5.54E-03 | |
| Adipocytokine signaling pathway | 12 | 68 | 18% | 3.79E-03 | |
| Pathogenic Escherichia coli infection | 12 | 58 | 21% | 8.41E-04 | |
| Small cell lung cancer | 11 | 85 | 13% | 4.97E-02 | |
| Adherens junction | 11 | 73 | 15% | 1.74E-02 | |
| Metabolism of xenobiotics by cytochrome P450 | 11 | 71 | 15% | 1.42E-02 | |
| Bladder cancer | 11 | 42 | 26% | 1.20E-04 | |
| Gastric acid secretion | 10 | 74 | 14% | 4.32E-02 | |
| Chronic myeloid leukemia | 10 | 73 | 14% | 3.97E-02 | |
| Long-term potentiation | 10 | 70 | 14% | 3.03E-02 | |
| Renal cell carcinoma | 10 | 70 | 14% | 3.03E-02 | |
| Colorectal cancer | 10 | 62 | 16% | 1.32E-02 | |
| Acute myeloid leukemia | 10 | 58 | 17% | 8.03E-03 | |
| Non-small cell lung cancer | 10 | 54 | 19% | 4.61E-03 | |
| Endometrial cancer | 10 | 52 | 19% | 3.40E-03 | |
| Vibrio cholerae infection | 9 | 54 | 17% | 1.33E-02 | |
| Vasopressin-regulated water reabsorption | 9 | 44 | 20% | 2.99E-03 | |
| Amyotrophic lateral sclerosis (ALS) | 8 | 54 | 15% | 3.46E-02 | |
| Nucleotide excision repair | 8 | 45 | 18% | 1.14E-02 | |
| DNA replication | 8 | 36 | 22% | 2.41E-03 | |
| Aldosterone-regulated sodium reabsorption | 7 | 42 | 17% | 2.21E-02 | |
| Base excision repair | 7 | 34 | 21% | 6.20E-03 | |
| Sphingolipid metabolism | 6 | 40 | 15% | 4.74E-02 | |
| Thyroid cancer | 6 | 29 | 21% | 8.92E-03 | |
| Mismatch repair | 6 | 23 | 26% | 2.20E-03 | |
| Dorso-ventral axis formation | 5 | 25 | 20% | 1.56E-02 | |
| 250 µM | MAPK signaling pathway | 27 | 268 | 10% | 2.09E-05 |
| Pathways in cancer | 26 | 327 | 8% | 1.22E-03 | |
| Cytokine-cytokine receptor interaction | 20 | 265 | 8% | 7.49E-03 | |
| Cell cycle | 17 | 128 | 13% | 1.79E-05 | |
| Osteoclast differentiation | 15 | 128 | 12% | 2.18E-04 | |
| p53 signaling pathway | 15 | 69 | 22% | 5.90E-08 | |
| Phagosome | 14 | 156 | 9% | 4.74E-03 | |
| TGF-beta signaling pathway | 13 | 85 | 15% | 2.79E-05 | |
| Jak-STAT signaling pathway | 12 | 155 | 8% | 2.39E-02 | |
| Wnt signaling pathway | 12 | 151 | 8% | 1.98E-02 | |
| Ubiquitin mediated proteolysis | 12 | 139 | 9% | 1.06E-02 | |
| Gap junction | 10 | 90 | 11% | 2.62E-03 | |
| Oocyte meiosis | 9 | 114 | 8% | 3.55E-02 | |
| Prostate cancer | 9 | 89 | 10% | 7.39E-03 | |
| Progesterone-mediated oocyte maturation | 9 | 87 | 10% | 6.32E-03 | |
| Chagas disease (American trypanosomiasis) | 8 | 104 | 8% | 4.86E-02 | |
| Rheumatoid arthritis | 8 | 92 | 9% | 2.49E-02 | |
| Small cell lung cancer | 8 | 85 | 9% | 1.57E-02 | |
| Adherens junction | 8 | 73 | 11% | 6.04E-03 | |
| Epithelial cell signaling in Helicobacter pylori infection | 8 | 68 | 12% | 3.77E-03 | |
| Adipocytokine signaling pathway | 8 | 68 | 12% | 3.77E-03 | |
| Chronic myeloid leukemia | 7 | 73 | 10% | 1.85E-02 | |
| Pathogenic Escherichia coli infection | 7 | 58 | 12% | 4.84E-03 | |
| Endometrial cancer | 7 | 52 | 13% | 2.43E-03 | |
| Metabolism of xenobiotics by cytochrome P450 | 6 | 71 | 8% | 4.43E-02 | |
| PPAR signaling pathway | 6 | 70 | 9% | 4.15E-02 | |
| Colorectal cancer | 6 | 62 | 10% | 2.33E-02 | |
| Acute myeloid leukemia | 6 | 58 | 10% | 1.67E-02 | |
| Non-small cell lung cancer | 6 | 54 | 11% | 1.16E-02 | |
| Vibrio cholerae infection | 6 | 54 | 11% | 1.16E-02 | |
| Bladder cancer | 6 | 42 | 14% | 2.85E-03 | |
| Basal cell carcinoma | 5 | 55 | 9% | 4.03E-02 |
Percentage stands for the percentage of genes found changed upon leflunomide treatment among all the genes in the designated pathway.
Table 2.
Significantly changed genes in selective pathways in HepG2 cells treated with 125 µM leflunomide for 6 h.
| Relate+G5:M22d functions |
Symbol | Accession # | Description | Folds of Change |
Adjust p-value |
|---|---|---|---|---|---|
| ER stress and UPR | PPP1R15A | NM_014330.3 | GADD34 | 14.44 | 0.002 |
| DDIT3 | NM_001195053.1 | DNA damage-inducible transcript 3 protein (DDIT3), also known as C/EBP-homologous protein (CHOP) | 9.78 | 0.004 | |
| ADM2 | NM_001253845.1 | Adrenomedullin 2 | 3.22 | 0.021 | |
| TRIB3 | NM_001301188.1 | Tribbles homolog 3 | 3.11 | 0.023 | |
| CEBPB | NM_001285878.1 | CCAAT/enhancer-binding protein beta (C/EBP beta) | 3.05 | 0.025 | |
| XBP1 | NM_001079539.1 | X-box-binding protein 1 | 1.89 | 0.003 | |
| HSPA4L | NM_001317381.1 | Heat shock 70 kDa protein 4L | 1.53 | 0.011 | |
| VCP | NM_007126.3 | Transitional endoplasmic reticulum ATPase (TER ATPase) | −1.61 | 0.025 | |
| HSPA1B | NM_005345.5 | Heat shock 70 kDa protein 1B | −14.36 | 0.007 | |
| MAPK signaling pathway | FOS | NM_005252.3 | Proto-oncogene c-Fos | 70.05 | 0.006 |
| JUN | NM_002228.3 | c-Jun | 7.77 | 0.007 | |
| JUNB | NM_002229.2 | JunB, member of Jun family | 11.84 | 0.027 | |
| JUND | NM_005354.5 | JunD, member of Jun family | 3.41 | 0.007 | |
| MYC | NM_002467.4 | c-Myc | 3.66 | 0.002 | |
| MAPK6 | NM_002748.3 | Mitogen-activated protein kinase 6 (MAPK6), also known as Extracellular signal-regulated kinase 3 (ERK3) | 2.10 | 0.001 | |
| BRAF | NM_004333.4 | Serine/threonine-protein kinase B-raf | 2.08 | 0.037 | |
| EGFR | NM_005228.3 | Epidermal growth factor receptor | 1.79 | 0.007 |
Besides MAPK-related genes, we also found that multiple genes involved in ER stress exhibited significant changes, as listed in Table 2. For example, gene DDIT3 (also known as CHOP, a hallmark of ER stress), was 9.8–fold up-regulated. The expression of the stress-related gene GADD34, which can be activated by ATF4 and CHOP and function as a mediator of ER stress-induced cell death, showed a 14.4–fold increase. Several other genes involved in or regulated by the UPR pathways displayed significant increases as well, including XBP1 (1.9–fold), TRIB3 (3.1–fold), ADM2 (3.2–fold), and C/EBPβ (3.1–fold). Chaperone heat shock protein 70 s plays a pivotal role in the folding of newly translated proteins and the stabilization of preexisting proteins in the ER. Our RNA-seq analysis indicated that at least two members of the heat shock protein family, HSPA4L (1.53–fold upregulation) and HSPA1 B (14.4–fold downregulation), were considerably modulated by leflunomide exposure, suggesting perturbation of chaperon function.
The results from RNA-seq were validated further with Taqman quantitative real-time PCR. The expression of CHOP and GADD34 mRNA levels was examined after 6 h exposure to 50–200 µM of leflunomide, as indicated in Fig. 4C. An elevated level of GADD34 mRNA was observed at 100 µM of leflunomide, and the effect was more drastic at 200 µM of leflunomide (22.2–fold upregulation compared with DMSO treated control). The mRNA level of CHOP also displayed a 15.5–fold increase upon exposure to 200 µM of leflunomide (Fig. 4C).
3.5. Leflunomide activates the MAPK pathway
The MAPK signaling network is involved in numerous cellular functions and an increasing body of literature has identified its critical role in drug-induced toxicity. Interestingly, RNA-seq data indicate that the MAPK signaling pathway is one of the most significantly affected pathways, prompting our hypotheses that the MAPK signaling pathway may contribute to the adverse effects of leflunomide.
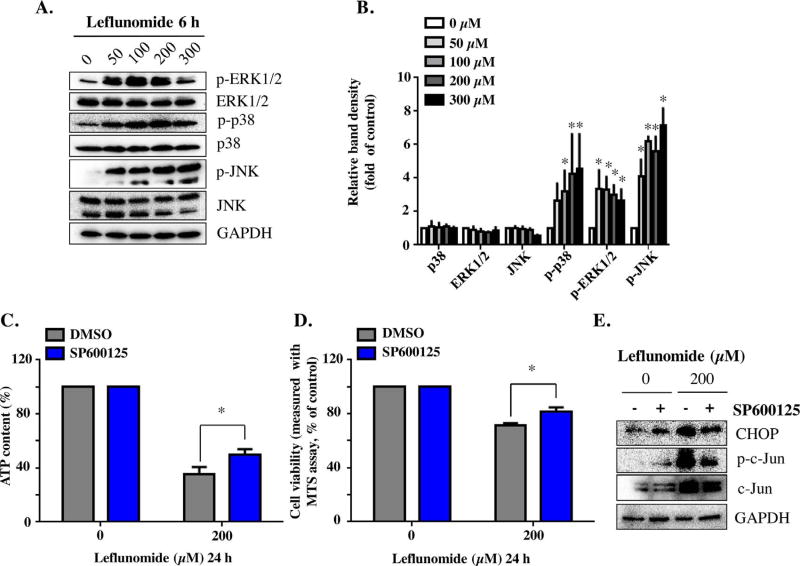
The activation of MAPK was confirmed by Western blotting. Upon treatment of leflunomide for 6 h, JNK, ERK1/2, and p38, the three major branches of the MAPK signaling pathway, were all activated in a concentration-dependent manner, as demonstrated by the increased phosphorylation of these proteins (Fig. 5A & B). Activation was observed at 50 µM of leflunomide, the lowest level tested. Among the three MAPK families, JNK displayed the most prominent changes in response to leflunomide treatment, with an approximately 5 fold increase in phosphorylation at 100–300 µM of leflunomide. Little change was observed for the total levels of these proteins.
Fig. 5. Leflunomide activates MAPK cascade, and JNK signaling pathway contributes to leflunomide’s toxicity.
(A, B) A 6-h treatment of leflunomide at concentration 50 to 300 µM induced a concentration-dependent increase in p-ERK1/2, p-p38, and p-JNK. No significant changes were observed for the total level of the proteins. (C, D) Pretreatment with the JNK inhibitor SP600125 (20 µM) partially protected the cells from leflunomide-induced damage, as measured by ATP content (C) and MTS assay (D). (E) Representative Western blots showed that SP600125 efficiently reduced the phosphorylation of c-Jun, and attenuated leflunomide-induced CHOP activation. The results shown are mean ± S.D. from three independent experiments. *p < 0.05.
3.6. JNK and ERK1/2 signaling pathway regulates the cytotoxicity of leflunomide
Using an MAPK inhibition approach, we investigated further the role of the MAPK signaling pathway in leflunomide-induced cytotoxicity. SP600125, a broad-spectrum JNK inhibitor that inhibits the phosphorylation of c-Jun, was applied to HepG2 cells together with leflunomide. The presence of SP600125 partially protected the cells from the damage caused by leflunomide (Fig. 5C & D). Western blotting confirmed that the phosphorylation of c-Jun was inhibited by SP600125, with no effect on the total level of c-Jun (Fig. 5E). Moreover, the protein level of CHOP was reduced in the SP600125-treated cells, consistent with attenuated ER stress (Fig. 5E).
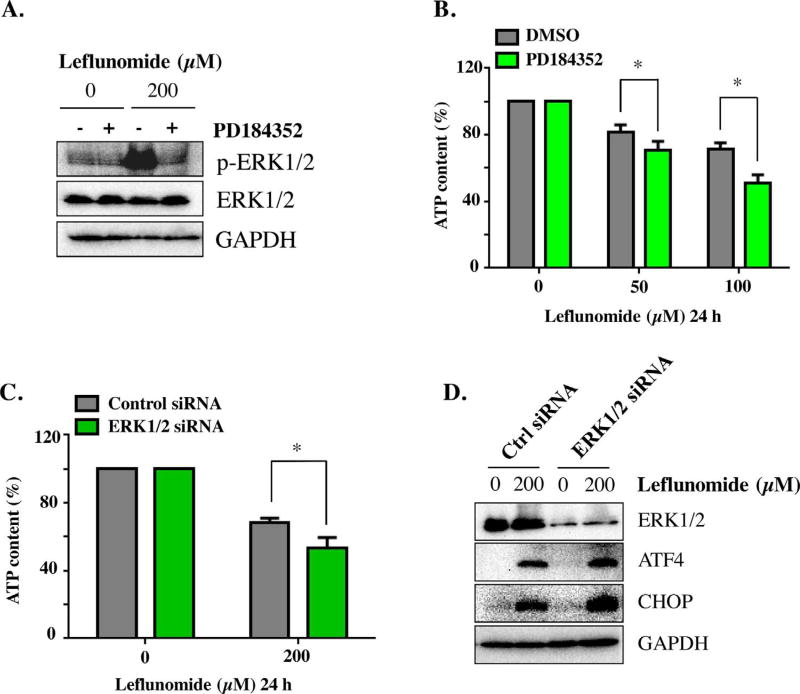
Inhibition of ERK1/2, on the contrary, exacerbated the cytotoxicity of leflunomide. Pretreatment for 2 h with PD184352, a highly selective inhibitor for ERK1/2, effectively inhibited the phosphorylation of ERK1/2 in HepG2 cells, as examined by Western blotting (Fig. 6A). Inhibiting ERK1/2 further decreased the ATP contents caused by leflunomide (Fig. 6B). Knocking down ERK1/2 by siRNA achieved similar results, as shown by the reduction of ATP content at 200 µM of leflunomide (Fig. 6C). Interestingly, knockdown of ERK1/2 resulted in increase of ATF4 and CHOP, indicating an amplification of ER stress (Fig. 6D). No significant effect was observed upon inhibition of p38 (data not shown). Therefore, our data suggest that both JNK and ERK1/ 2 signaling pathways are involved in the cytotoxicity and ER stress by leflunomide, but with distinctly different roles.
Fig. 6. ERK1/2 activation protects HepG2 cells during leflunomide exposure.
(A) Representative Western blots showed that PD184352 efficiently inhibited the activation of ERK1/2. (B) Pretreatment with the ERK1/2 inhibitor PD184352 (2 µM) for 2 h prior to leflunomide exposure increased leflunomide-induced cytotoxicity, as measured by ATP content. (C) Knocking down ERK1/2 gene by siRNA transfection resulted in further reduction of cellular ATP upon leflunomide treatment, in comparison with control siRNA. (D) Representative Western blots showed that ERK1/2 siRNA significantly reduced ERK1/2 and resulted in a more prominent induction of ATF4 and CHOP. The results shown are mean ± S.D. from three independent experiments. *p < 0.05.
4. Discussion
Leflunomide has been associated with abnormal liver enzyme levels and liver failure; however, the underlying mechanisms are not well defined. In the current study, we showed that ER stress contributes to the cytotoxicity of leflunomide. Also, our study showed that two branches of the MAPK signaling cascade, JNK and ERK1/2, participate in leflunomide-induced cytotoxicity, but they play different roles.
Upon oral administration, leflunomide rapidly undergoes non-enzymatic opening of the isoxazole ring to give its active derivative terisflunomide, also known as AA77 1726, in a non-enzymatic manner (Kalgutkar et al., 2003; Rozman 2002). Studies have shown that isoxazole ring opening is temperature and pH dependent and that it can occur rapidly in human and rat plasma or whole blood, or in the presence of human serum albumin (Kalgutkar et al., 2003). Our HPLC analysis revealed that a rapid ring-opening also occurred in HepG2 cells exposed to leflunomide, evidenced by the presence of teriflunomide from almost time 0 (Supplementary Table 3). The parent drug was below the detection limit of the assay within 24 h, suggesting a complete conversion. Moreover, under our experimental conditions, leflunomide conversion did not depend on cells, because a similar rate of conversion was observed in cell-free samples (Supplementary Table 3). Once converted to teriflunomide, the drug is highly protein bound in plasma, and has a very long half-life (18–19 days in healthy volunteers) (Rozman, 2002). The cytotoxicity of teriflunomide (A77 1726) in primary rat hepatocytes can be attenuated by CYP450 enzymes, suggesting that metabolites of A77 1726 are less toxic (Shi et al., 2011). In addition, we compared the toxicity of leflunomide to HepG2 cells, HepaRG cells, and primary human hepatocytes, which have greater metabolic capability (Guillouzo et al., 2007; Guo et al., 2011). Our results demonstrated that leflunomide was more toxic in HepG2 compared to HepaRG cells, and the response induced in HepG2 cells was similar to that in primary human hepatocytes (Fig. 1). Taken together, these results suggest that levels of CYP450 activity, which is a major difference between HepG2 cells and cells with higher CYP450 expression (e.g. primary hepatocytes and HepaRG), is of the less concern in the current study of leflunomide. Therefore, we took the advantage of the ready availability, higher sensitivity to leflunomide, and ease of manipulation of HepG2 cells in our study. Clinically, the mean steady-state plasma concentration of metabolite teriflunomide is estimated to be 63 mg/ L (233 µM), for 25 mg/day maintenance dosage (Rozman, 2002). Our HPLC analysis confirmed quantitative conversion of leflunomide to teriflunomide in the HepG2 cells (Supplementary Table 3) as reported in vivo (Dias et al., 1995). In the current study, the maximal concentration of leflunomide was up to 300 µM for our mechanism studies; thus, the concentrations we used are clinically-relevant.
The roles of ER stress in the pathogenesis of diseases such as diabetes, neurodegeneration, cancer, and inflammatory disease have been well recognized (Xu et al., 2005); however, the importance of this mechanism has been underestimated in drug-induced liver toxicity in the past. It has been receiving increased attention in recent years and a growing body of evidence now indicates that ER stress plays a pivotal role in drug associated liver toxicity (Apostolova et al., 2013; Auman et al., 2007; Chen et al., 2014c; Craig et al., 2006; Mohammad et al., 2012; Nagy et al., 2010; Naranmandura et al., 2012; Ren et al., 2016; Uzi et al., 2013). It is worth noting that the application of either chemical inhibitors or siRNAs only partially rescued the cells viability after leflunomide treatment (Figs. 2 and 3), indicating that additional mechanisms might be involved in the cytotoxicity of leflunomide. The ER stress response is often accompanied by other cellular effects relevant to cytotoxicity, such as oxidative stress, apoptosis, and mitochondrial dysfunction (Dara et al., 2011). In particular, mitochondria and the ER share direct contact via the mitochondrial-associated membranes (MAM) and this physical association enables a tight functional connection between the two organelles (Kornmann et al., 2009; Vance 1990). In the current study, we observed that upon leflunomide treatment, the decrease of cellular ATP content is faster and more prominent compared with the effects on cell viability (Fig. 1), which is often a characteristic of mitochondria damage. Currently we are exploring whether or not mitochondrial dysfunction is involved in leflunomide-induced toxicity, and hope to report it in another paper.
Previous studies demonstrated that several key mechanisms of drug induced liver toxicity such as mitochondrial dysfunction, apoptosis, and ER stress are mediated by the MAPK signaling pathway (Chen et al., 2017; Ren et al., 2016). In the current study, another important finding is that the activation of JNK and ERK1/2, two branches of the MAPK cascade, contributes to the toxicity of leflunomide. Generally, activation of JNK promotes cell death, whereas ERK1/2 facilitates cell survival (Chang and Karin, 2001). Our observations that JNK increases, while ERK1/2 attenuates, the cytotoxicity of leflunomide are in good agreement with these general findings (Figs. 5 and 6). It is well established that activation of IRE1 can lead to the phosphorylation of JNK, which plays critical roles in pro-apoptotic pathways (Tabas and Ron, 2011). Meanwhile, research also found that JNK signaling can reciprocally regulate ER stress through an unidentified mechanism (Li et al., 2013; Verma and Datta 2010). In our study, we demonstrated that blocking JNK activity using the inhibitor SP600125 could partially attenuate the increase in CHOP and recover the cell viability, further supported the complicated role of JNK signaling in ER stress (Fig. 5). P38 activation can be induced by ER stress, and has been shown to increase the activity of ATF6 and CHOP (Darling and Cook, 2014). We observed that 6 h leflunomide exposure significantly increased the phosphorylation of p38 (Fig. 5); however, application of p38 inhibitor together with leflunomide showed minimal rescue effect on cell viability, suggesting p38 activation may not play a pivotal role in leflunomide-induced cytotoxicity. In addition, it is worth noting that ERK1/2 activation plays a critical role in regulating the cell cycle (Chambard et al., 2007; Lu and Xu 2006; Mebratu and Tesfaigzi 2009). Previous research has shown that leflunomide and/or its metabolite teriflunomide (AA77 1726) cause cell cycle arrest in multiple cell lines (Baumann et al., 2009; Hail et al., 2010; Zhu et al., 2013). ERK1/2 signaling may be involved in cell cycle regulation by leflunomide; further mechanistic studies are warranted for identifying the signal pathways involved.
Finally, it should be noted that while this in vitro study was designed to elucidate the mechanisms of leflunomide-induced liver toxicity, it is important to identify the risk factors such as the altered activity of some CYPs in patients that may contribute to individual susceptibility in the clinical setting.
In summary, using a genomic approach together with molecular and biochemical analyses, our work suggests that and multiple mechanisms, including ER stress and MAPK signaling pathway activation, play a role in the cytotoxicity of leflunomide. These findings provide new insights into the mechanisms involved in leflunomide toxicity and improve our understanding of drug induced liver injury.
Supplementary Material
Acknowledgments
Z.R. and DK.Y. were supported by appointments to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. FDA.
This article is not an official guidance or policy statement of the U.S. Food and Drug Administration (FDA). No official support or endorsement by the U.S. FDA is intended or should be inferred.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tox.2017.10.002.
References
- Alcorn N, Saunders S, Madhok R. Benefit-risk assessment of leflunomide: an appraisal of leflunomide in rheumatoid arthritis 10 years after licensing. Drug Saf. 2009;32:1123–1134. doi: 10.2165/11316650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Gomez-Sucerquia LJ, Alegre F, Funes HA, Victor VM, Barrachina MD, Blas-Garcia A, Esplugues JV. ER stress in human hepatic cells treated with Efavirenz: mitochondria again. J. Hepatol. 2013 doi: 10.1016/j.jhep.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Auman JT, Chou J, Gerrish K, Huang Q, Jayadev S, Blanchard K, Paules RS. Identification of genes implicated in methapyrilene-induced hepatotoxicity by comparing differential gene expression in target and nontarget tissue. Environ. Health Perspect. 2007;115:572–578. doi: 10.1289/ehp.9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Mandl-Weber S, Volkl A, Adam C, Bumeder I, Oduncu F, Schmidmaier R. Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. Mol. Cancer Ther. 2009;8:366–375. doi: 10.1158/1535-7163.MCT-08-0664. [DOI] [PubMed] [Google Scholar]
- Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen S, Dobrovolsky VN, Liu F, Wu Y, Zhang Z, Mei N, Guo L. The role of autophagy in usnic acid-induced toxicity in hepatic cells. Toxicol. Sci. 2014a;142:33–44. doi: 10.1093/toxsci/kfu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Melchior WB, Jr, Guo L. Endoplasmic reticulum stress in drug- and environmental toxicant-induced liver toxicity. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2014b;32:83–104. doi: 10.1080/10590501.2014.881648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xuan J, Couch L, Iyer A, Wu Y, Li QZ, Guo L. Sertraline induces endoplasmic reticulum stress in hepatic cells. Toxicology. 2014c;322:78–88. doi: 10.1016/j.tox.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang Z, Wu Y, Shi Q, Yan H, Mei N, Tolleson WH, Guo L. Endoplasmic reticulum stress and store-operated calcium entry contribute to usnic acid-induced toxicity in hepatic cells. Toxicol. Sci. 2015;146:116–126. doi: 10.1093/toxsci/kfv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang Z, Qing T, Ren Z, Yu D, Couch L, Ning B, Mei N, Shi L, Tolleson WH, Guo L. Activation of the Nrf2 signaling pathway in usnic acid-induced toxicity in HepG2 cells. Arch. Toxicol. 2017;91:1293–1307. doi: 10.1007/s00204-016-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A, Sidaway J, Holmes E, Orton T, Jackson D, Rowlinson R, Nickson J, Tonge R, Wilson I, Nicholson J. Systems toxicology: integrated genomic, proteomic and metabonomic analysis of methapyrilene induced hepatotoxicity in the rat. J. Proteome Res. 2006;5:1586–1601. doi: 10.1021/pr0503376. [DOI] [PubMed] [Google Scholar]
- Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta. 2014;1843:2150–2163. doi: 10.1016/j.bbamcr.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Dias VC, Lucien J, LeGatt DF, Yatscoff RW. Measurement of the active leflunomide metabolite (A77 1726) by reverse-phase high-performance liquid chro-matography. Ther. Drug Monit. 1995;17:84–88. doi: 10.1097/00007691-199502000-00014. [DOI] [PubMed] [Google Scholar]
- Dragovic S, Vermeulen NP, Gerets HH, Hewitt PG, Ingelman-Sundberg M, Park BK, Juhila S, Snoeys J, Weaver RJ. Evidence-based selection of training compounds for use in the mechanism-based integrated prediction of drug-induced liver injury in man. Arch. Toxicol. 2016;90:2979–3003. doi: 10.1007/s00204-016-1845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem. Biol. Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab. Dispos. 2011;39:528–538. doi: 10.1124/dmd.110.035873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hail N, Jr, Chen P, Bushman LR. Teriflunomide (leflunomide) promotes cy-tostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia. 2010;12:464–475. doi: 10.1593/neo.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro R, Munoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- Kalgutkar AS, Nguyen HT, Vaz AD, Doan A, Dalvie DK, McLeod DG, Murray JC. In vitro metabolism studies on the isoxazole ring scission in the anti-inflammatory agent lefluonomide to its active alpha-cyanoenol metabolite A771726: mechanistic similarities with the cytochrome P450-catalyzed dehydration of aldox-imes. Drug Metab. Dispos. 2003;31:1240–1250. doi: 10.1124/dmd.31.10.1240. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mei H, Wu Q, Zhang S, Fang JL, Shi L, Guo L. Methysticin and 7,8-dihydromethysticin are two major kavalactones in kava extract to induce CYP1A1. Toxicol. Sci. 2011;124:388–399. doi: 10.1093/toxsci/kfr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu C, Yang P. c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes. 2013;62:599–608. doi: 10.2337/db12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? ABBV Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, Joshi-Barve S. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol. Appl. Pharmacol. 2012;265:73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Szarka A, Lotz G, Doczi J, Wunderlich L, Kiss A, Jemnitz K, Veres Z, Banhegyi G, Schaff Z, Sumegi B, Mandl J. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 2010;243:96–103. doi: 10.1016/j.taap.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Naranmandura H, Xu S, Koike S, Pan LQ, Chen B, Wang YW, Rehman K, Wu B, Chen Z, Suzuki N. The endoplasmic reticulum is a target organelle for tri-valent dimethylarsinic acid (DMAIII)-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2012;260:241–249. doi: 10.1016/j.taap.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Ren Z, Chen S, Zhang J, Doshi U, Li AP, Guo L. Endoplasmic reticulum stress induction and ERK1/2 activation contribute to nefazodone-Induced toxicity in hepatic cells. Toxicol. Sci. 2016;154:368–380. doi: 10.1093/toxsci/kfw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozman B. Clinical pharmacokinetics of leflunomide. Clin. Pharmacokinet. 2002;41:421–430. doi: 10.2165/00003088-200241060-00003. [DOI] [PubMed] [Google Scholar]
- Sanders S, Harisdangkul V. Leflunomide for the treatment of rheumatoid arthritis and autoimmunity. Am. J. Med. Sci. 2002;323:190–193. doi: 10.1097/00000441-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Shi Q, Yang X, Greenhaw J, Salminen WF. Hepatic cytochrome P450 s attenuate the cytotoxicity induced by leflunomide and its active metabolite A77 1726 in primary cultured rat hepatocytes. Toxicol. Sci. 2011;122:579–586. doi: 10.1093/toxsci/kfr106. [DOI] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, Valverde AM, Iwawaki T, Nahmias Y, Xavier R, Chung RT, Tirosh B, Shibolet O. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J. Hepatol. 2013;59:495–503. doi: 10.1016/j.jhep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Verma G, Datta M. IL-1beta induces ER stress in a JNK dependent manner that determines cell death in human pancreatic epithelial MIA PaCa-2 cells. Apoptosis. 2010;15:864–876. doi: 10.1007/s10495-010-0498-4. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Yan X, Xiang Z, Ding HF, Cui H. Leflunomide reduces proliferation and induces apoptosis in neuroblastoma cells in vitro and in vivo. PLoS One. 2013;8:e71555. doi: 10.1371/journal.pone.0071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon EN, Jansen TL, Houtman NM, Spoelstra P, Brouwers JR. Leflunomide for the treatment of rheumatoid arthritis in clinical practice: incidence and severity of hepatotoxicity. Drug Saf. 2004;27:345–352. doi: 10.2165/00002018-200427050-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.