ABSTRACT
Checkpoint 1 (Chk1), as an important member of DNA replication checkpoint and DNA damage response, has an important role during the G2/M stage of mitosis. In this study, we used porcine oocyte as a model to investigate the function of Chk1 during porcine oocyte maturation. Chk1 was expressed from germinal vesicle (GV) to metaphase II (MII) stages, mainly localized in the cytoplasm at GV stage and moved to the spindle after germinal vesicle breakdown (GVBD). Chk1 depletion not only induced oocytes to be arrested at MI stage with abnormal chromosomes arrangement, but also inhibited the degradation of Cyclin B1 and decreased the expression of Mitotic Arrest Deficient 2-Like 1 (Mad2L1), one of spindle assembly checkpoint (SAC) proteins, and cadherin 1 (Cdh1), one of coactivation for anaphase-promoting complex/cyclosome (APC/C). Moreover, Chk1 overexpression delayed GVBD. These results demonstrated that Chk1 facilitated the timely degradation of Cyclin B1 at anaphase I (AI) and maintained the expression of Mad2L1 and Cdh1, which ensured that all chromosomes were accurately located in a line, and then oocytes passed metaphase I (MI) and AI and exited from the first meiotic division successfully. In addition, we proved that Chk1 had not function on GVBD of porcine oocytes, which suggested that maturation of porcine oocytes did not need the DNA damage checkpoint, which was different from the mouse oocyte maturation.
KEYWORDS: checkpoint kinase 1 (Chk1), oocyte, metaphase I (MI), spindle assembly checkpoint, chromosomes, pig
Introduction
Chk1 plays important roles on cellular events. As a key protein in DNA replication checkpoint and DNA damage response, it inhibits cell division cycle 25A/C (Cdc25A/C),1 which maintains Cdk1 and Cdk2 in deactivation,2,3 and then DNA cannot be replicated. Furthermore, it facilitates activation of Wee1. Therefore, both inhibited Cdc25A/C and activated Wee1 block phosphorylation and activation of promoting mature factor (MPF) and then cells are arrested in G2 stage or GV (germinal vesicle) stage.4 In addition, Chk1 negatively regulates Treslin, a TopBP1-binding protein, to inhibit initiating DNA replication.5 Chk1 is also involved in regulation of gene expression.6 Chk1 binds to chromatin and phosphorylates histone H3 at T11 site, which results in acetylation of histone H3K9. However, once Chk1 is dissociated from chromatin, histone H3K9 is deacetylated and transcriptions of genes are decreased. In addition, apoptosis is associated with Chk1, and activated Chk1 facilitates apoptosis by inhibiting Casp2.7
Chk1 protein has four structural domains: N-terminal domain, middle linking domain, domain rich of Ser/Thr-Glu (SQ/TQ) sites and C-terminal domain. N-terminal domain, as a kinase domain, has the ability to catalyze and regulate downstream targets; Conservative SQ/TQ is a recognition site at which ataxia telangiectasia mutated (ATM)/ATM Rad3 related (ATR) catalyzes substrates; C-terminal domain is a self-regulating domain.8,9 The regulation of Chk1 activity is involved in recruitment of a series of proteins and interaction with these proteins:10 First, to activate Chk1, Claspin, Rad9-Rad1-Hus1 (9:1:1) complex and topology isomerase binding protein 1 (TopBP1) are recruited to single-stranded DNA induced by DNA damage. TopBP1 activates ATR. ATR phosphorylates not only Ser345 in SQ/TQ site of Chk1 but also the Chk1 kinase area (CKA) of Claspin. The phosphorylated CKA plays a role on the kinase domain of Chk1. Second, to transport Chk1, the phosphorylated SQ/TQ site of Chk1 meanwhile is a binding site for 14-3-3 protein. Active Chk1 dissociates from chromatins and interacts with 14-3-3 protein, which maintains Chk1 in nucleoplasm. In addition, CRM1 can mediate nuclear export of Chk1. Third, to degradate and inactivate Chk1, Plk at G2 stage of cell cycle has the ability to ubiquitinate and degrade Chk1 and Claspin by phosphorylating SCFβ-TrcP ubiquitin ligase. Degradation of Chk1 needs cullin-RING ligase (CRL), but the degradation is in different ways. CRL1-SKP1-Fbx6 in cytoplasm or CRL4-DDB1-CDT2 in nucleus mediates this process.
During mitosis, activated Chk1 is involved with the regulation of SAC. It can phosphorylate Mad2 at some sites, especially S185 and T187.11 Cdc20, BubR1 and Mad2 cannot be located at kinetochores and the expressions of Cdc20 and Mad2 decrease because of Chk1 knockdown.12 Chk1 has an effect on the connection of kinetochores with microtubes and cytoplasmic division by phosphorylating Aurora B at Ser311.13-17 Onset of anaphase requires APC/C activated by Cdc20, which facilitates degradation of Pds1, so Esp1 can clean up cohesin.18,19 When DNA damage occurs, Chk1 activated by Mec1 (a PI3K-like sensor kinase, homology to ATR) phosphorylates and activates Pds1 which prevents Esp1 from degrading cohesin subunit Scc1.20,21
There are limited studies about Chk1 in oocytes meiosis. At the early stage of the first meiotic prophase, although, γ-H2AX (a variant of histone H2A, the DNA damage maker22) and ATM/ATR are phosphorylated and activated, it does not seem to be Chk1 but Checkpoint 2 (Chk2) that is activated and taken roles in mouse or other animals oocytes.23-26 Furuno et al.27 classified Xenopus GV oocytes into six stages according to its diameters and traced relevant regulator proteins. Their conclusion shows that the increased expression of negative regulator proteins (Wee1, Chk1 and Cds) is earlier than the expression of positive regulator proteins (MEK, MAPK and Cdc25C). There is a path of Chk1-Cdc25C-Cdc2 in Xenopus GV oocytes, and in this path, Chk1 can phosphorylate Cdc25C at Ser287 and inhibit the function of Cdc25C.28,29 However, ATM/ATR cannot regulate Chk1 in Xenopus GV oocytes, which is different from somatic cells.29 In mouse oocytes, the complete G2/M DNA damage checkpoint can be activated by etoposide which is an inhibitor for topoisomerase II, but Chk1 is not enough sensitive to etoposide and only activated by high concentration of etoposide.30 Moreover, the substrate of Chk1 is not Cdc25A but Cdc25B, and Chk1 phosphorylates and inhibits Cdc25B at Ser323.30 However, overexpression of Chk1 results in decreasing Cdc25A and increasing Cdh1 in mouse GV oocytes.31 In porcine oocytes, although BLM or etoposide induces increasing γH2AX and active ATM, they do not work on Chk1 because ATM cannot activate Chk1, and there is no DNA damage checkpoint to regulate porcine GV oocytes.32,33 It is unknown whether Chk1 independently functions during porcine oocytes maturation at present. During oocytes meiotic maturation, some proteins involved in GV oocytes are related with Chk1, such as SUN1,34 γH2AX,22,35 malignant brain tumor domain-containing protein 1 (MBTD1) and Pr-Set7.36 SUN1 is involved in the regulation of nuclear export of mRNA.37 MBTD1 interacting with Pr-Set7 stabilizes H4K20me1 and then regulates gene transcription36 Therefore, we test whether Chk1 is associated with gene expression during porcine oocyte maturation in this study.
It is possible that Chk1 has effects on oocytes after GVBD. Neocarzinostatin, etoposide and ultraviolet light which induce DNA damage response cause the arrest at MI, and the arrest is related with Mps1 and Mad2 in mouse oocytes.38,39 Despite the arrest, DNA repair seems to be more efficient in mouse oocytes than in somatic cells.40,41 Mouse oocytes exposed to bleomycin have similar phenotypes, and further study shows that double strands break (DSB) caused by bleomycin decreases the degradation of Cyclin B1 at the transition from MI to AI.42 These results are in accordance with the study about Chk1 during mouse oocytes maturation.31 DNA damage also causes similar abnormities in porcine oocytes, but the arrest at MI does not recover and it is not associated with Chk1.32 We hypothesize that Chk1 plays roles in porcine oocyte maturation; however, it is absolutely not dependent on DNA damage checkpoint.
Results
Expression and localization of Chk1 during porcine oocyte meiotic maturation
Cumulus oocyte complexes (COCs) from porcine ovaries were cultured for 0 h, 24 h, 28 h or 44 h, which respectively corresponded to GV, GVBD, MI and MII stage. To determine the expression and localization of Chk1 during porcine oocyte meiotic maturation, the denuded oocytes at each time point were tested for quantificational real-time polymerase chain reaction (qRT-PCR), western blot and Chk1-eGFP overexpression. The results of qRT-PCR showed that Chk1 was highly expressed at MI stage during maturation (Fig. 1A). The results of western blot showed that Chk1 was stable after GVBD and slightly reduced at MII (Fig. 1B). To observe the location of Chk1 during maturation, we tried several Chk1 antibodies; however, all of them never worked for immunofluorescent staining. Therefore, we performed Chk1-eGFP overexpression to localize Chk1. It was shown that Chk1-eGFP was mainly localized in the cytoplasm of porcine oocytes at GV stage. After GVBD, Chk1-eGFP moved to the spindle (Fig. 1C). This location suggested that Chk1 might take effects by affecting spindle formation during porcine oocytes maturation.
Figure 1.
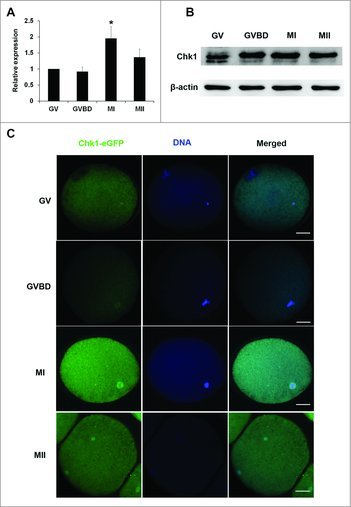
Expression and subcellular localization of Chk1 during porcine oocytes meiotic maturation. (A) Samples were collected after oocytes had been cultured for 0, 24 h, 28 h and 44 h, corresponding to GV, GVBD, MI and MII stages, respectively. Expression of Chk1 mRNA in different stage oocytes relative to that in GV oocytes. N = 3. (B) Proteins from a total of 300 oocytes were loaded for each sample. The molecular mass of Chk1 is 54 kDa and that of β-actin is 42 kDa. (C) Subcellular localization of Chk1-eGFP protein during porcine oocytes maturation. Bar = 25 μm.
Chk1 knockdown declined the rate of porcine oocytes maturation and had an effect on MI chromosomes alignment
When Chk1 knockdown or overexpression was performed, oocytes needed time to degrade the endogenous mRNA which Chk1 siRNA targeted or translate exogenous mRNA to proteins, so maturation inhibition was needed. Porcine GV oocytes with cumulus cells were exposed in different concentration of dbcAMP for 24 h, and we found that 1 mM dbcAMP significantly declined the rate of GVBD (70.10 ± 3.08 vs 32.50 ± 3.76, P < 0.05, Fig. S1), and 2 mM dbcAMP highly significantly declined the rate of GVBD (70.10 ± 3.08 vs 7.23 ± 2.47, P < 0.01, Fig. S1). To prevent oocytes from starting GVBD as far as possible, we used 2 mM dbcAMP to block GVBD after Chk1 siRNA or mRNA microinjection. Porcine COCs were injected with Chk1 siRNA and inhibited with dbcAMP for 20 h. Then the level of Chk1 mRNA or protein was analyzed by qRT-PCR or western blot. The results of both qRT-PCR and western blot showed that Chk1 RNAi successfully decreased Chk1 mRNA and protein at a highly significant level (1 vs 0.008 ± 0.003, P < 0.01, Fig. 2A) (1 vs 0.51 ± 0.11, P < 0.05, Fig. 2B and C).
Figure 2.
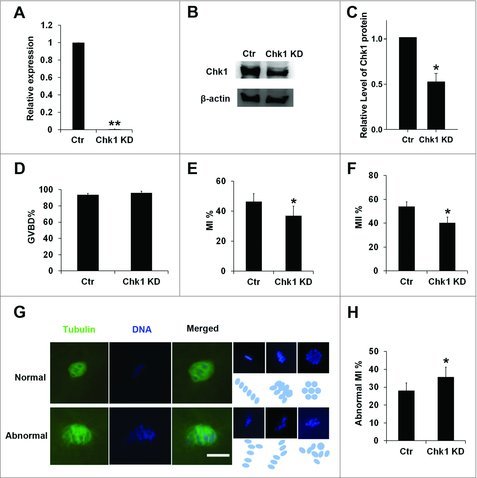
Chk1 depletion induced oocytes to be arrested at the MI stage and impaired the spindle organization and chromosome alignment. (A) Expression of Chk1 mRNA in the siRNA-injected oocytes. Porcine COCs were microinjected with the control and Chk1-specific siRNA, respectively. After injection, porcine COCs were incubated with dbcAMP for 20 h and followed by qRT-PCR. (B) Expression of Chk1 protein in the siRNA-injected oocytes. Porcine COCs were injected with siRNA and incubated with dbcAMP for 20 h, followed by Western blotting. The molecular mass of Chk1 is 54 kDa and that of β-actin is 42 kDa. (C) Relative levels of Chk1 protein between control and Chk1 knockdown groups. (D) Percentage of GVBD oocytes in the control group and Chk1 siRNA microinjected group. (E) Percentage of MI oocytes in the control group and Chk1 siRNA microinjected group. (F) Percentage of MII oocytes in the control group and Chk1 siRNA microinjected group. (G) Chk1 depletion triggered abnormal chromosomes arrangement; (Blue ovals or circulars showed chromosomes arrangement. The macroaxis of ovals referred to the co-orientation of chromosomes. Circulars also showed that the co-orientation was perpendicular to viewing plane. Bar = 10 μm. (H) Percentage of abnormal MI oocytes in the control group and Chk1 siRNA microinjected group. All graphs show as mean ± SE. *p<0.05. (KD, knockdown).
After being inhibited by dbcAMP, the oocytes injected with Chk1 siRNA and control oocytes were cultured further with dbcAMP-free medium, and determined at different time points. When they were cultured further for 24 h, we found there was no difference between control group and Chk1 knockdown group for GVBD (96.00 ± 2.10 vs 93.67 ± 1.58, Fig. 2D). However, when they were cultured for 28 h, the rate of oocytes at MI stage in Chk1 knockdown group were significant lower than it in control group (46.45 ± 5.28 vs 37.05±6.24, P < 0.05, Fig. 2E). Moreover, the maturation rate of oocytes in knockdown group was decreased significantly compared to control (54.03 ± 3.78 vs 40.37 ± 4.85, P < 0.05, Fig. 2F) if cultured further for 44 h. We checked the spindle morphology in these oocytes by immunofluorescent staining after GVBD. For MI oocytes injected with Chk1 siRNA, many chromosomes of oocytes were in misalignment with abnormal spindle (28.00 ± 4.43 vs 35.53 ± 5.69, P < 0.05, Fig. 2G and H). These abnormal chromosomes showed partial delaying, multiple equatorial plane or all in a mess. To confirm these findings, we also cultured porcine oocytes in different concentrations of LY260361, a Chk1 inhibitor. When oocytes were cultured in medium containing 5 μM LY260361 for 44 h, the rate of maturation declined significantly compared to control (60.23 ± 3.55 vs 43.4 ± 1.52, P < 0.05, Fig. S2). However, there were no significantly difference between the rate of oocytes through GVBD in medium with 10 μM LY260361 and control (90.40 ± 2.51 vs 88.20 ± 3.40, P < 0.05, Fig. S3) when they were cultured for 24 h. These results suggested that depletion of Chk1 resulted in abnormal MI and meiotic arrest, and Chk1 was required for porcine oocytes maturation.
Chk1 overexpression declined the rate of GVBD oocytes but had no effect on subsequent maturation progression
To further study the function of Chk1 during porcine oocyte maturation, we overexpressed Chk1 by injecting Chk1 mRNA synthesized in vitro into GV oocytes and cultured them with 2 mM dbcAMP for 2 h. The results showed that oocytes with Chk1 overexpression had a higher level of Chk1 protein than control (1 vs 1.53 ± 0.04, P < 0.05, Fig. 3A). When these oocytes were transferred into dbcAMP-free medium and further cultured for 24 h, the rate of GVBD oocytes in Chk1 overexpression group was declined in a great extent compared to control (90.77 ± 3.64 vs 37.17 ± 2.77, P < 0.01, Fig. 3B). When these oocytes were further cultured in dbcAMP-free medium for 44 h, the rate of MII oocytes in Chk1 overexpression group was declined much more than control (66.57 ± 0.93 vs 50.13 ± 2.53, P < 0.05, Fig. 3C). To make it clear whether Chk1 overexpression had an effect on maturation progression after GVBD or not, we got rid of oocytes that did not undergo GVBD with DAPI staining and only counted the matured oocytes after cultured for 44 h. We found that there was no significant difference between Chk1 overexpression group and control group in the rate of MII oocytes if oocytes underwent GVBD (65.60 ± 1.90 vs 57.53 ± 2.24, Fig. 3D). It could be concluded that Chk1 overexpression inhibited meiotic recovery of porcine oocytes from GV stage and had no significant effects on subsequent maturation progression.
Figure 3.
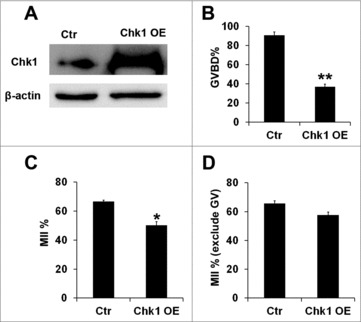
The effects of Chk1 overexpression (OE) on the porcine oocytes meiotic maturation. (A) Samples from control and the overexpression group were collected to test the expression of Chk1 by Western blotting. The molecular mass of Chk1 is 54 kDa and that of β-actin is 42 kDa. (B) Percentage of GVBD oocytes in the control group and Chk1 overexpression group. (C) Percentage of oocytes with first polar bodies (PB1) in the control group and Chk1 overexpression group. (D) Percentage of MII oocytes in the control group and Chk1 overexpression group after GVBD. All graphs show as mean ± SE. **p<0.01. *p<0.05.
Depletion of Chk1 inhibited the degradation of Cyclin B1
Oocytes recovered meiosis from GV stage, because MPF was activated.43 However, CCNB1 started to be degraded once oocytes developed to MI stage, which resulted in the deactivation of MPF.44 Therefore, we determined the level of CCNB1 after Chk1 knockdown. When COCs were injected with CCNB1-eGFP mRNA or the mixture of Chk1 siRNA and CCNB1-eGFP mRNA and cultured for 28 h, we found that CCNB1-eGFP was distributed not only in the cytoplasm but also at the spindle. To avoid the heterochrony of oocytes maturation, we got rid of oocytes at other stage with fluorescence microscope and then we found that the average relative intensity of fluorescent CCNB1-eGFP in each abnormal oocytes was significantly higher than it in normal oocytes (1 vs 1.35 ± 0.07, P < 0.05, Fig. 4A, B), which suggested that depletion of Chk1 inhibited the degradation of CCNB1.
Figure 4.
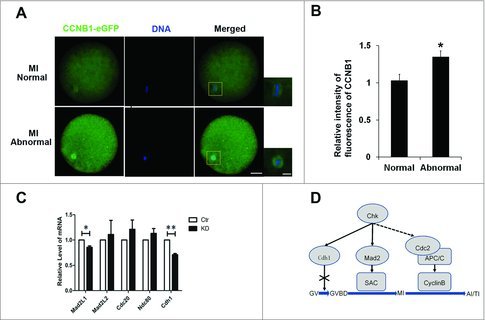
Chk1 depletion inhibited Cyclin B1 degrading in oocytes at MI-AI. (A) The effects of Chk1 depletion on the Cyclin B1 degradation in normal and abnormal MI oocytes. Red box is the area of amplificatory figure on the right. Left bar = 25 μm. Right bar = 10 μm. (B) Quantification of fluorescence intensity of CCNB1 in normal and abnormal MI oocytes after Chk1 depletion. (C) The effects of Chk1 depletion on the expression of genes related to SAC or APC/C (including Mad2L1, Mad2L2, Cdc20, Ndc80 and Cdh1). (D) Schematic illustrating function of Chk1 during porcine oocytes maturation. Chk1 functions at MI-AI stage rather than GV stage. Chk1 facilitated the timely degradation of Cyclin B1 at anaphase I (AI) and maintained the expression of Mad2L1 and Cdh1 to activate SAC and APC-Cdh1 and degrade MPF. All graphs show as mean ± SE. *p<0.05.
Depletion of Chk1 resulted in the decrease of Mad2L1 and Cdh1
SAC and APC/C took important roles before MI stage and in late MI stage respectively. We tested whether the depletion of Chk1 had effects on SAC or APC/C. When oocytes injected with Chk1 siRNA were further cultured for 28 h, we found that expressions of Mad2L1, one of SAC proteins, and Cdh1, one of coactivators for APC/C, were decreased significantly compared to control. However, Chk1 depletion had no effects on the expression of Ndc80, one of kinetochore proteins, and Cdc20, the other one of coactivators for APC/C (Fig. 4C).
Discussion
In this study, we used porcine oocytes as a model to investigate the function of Chk1 during porcine oocyte maturation. As we know, the signal transduction of DNA damage checkpoint is blocked between ATM and Chk1 during porcine oocytes maturation. Our results demonstrated that Chk1 regulated the degradation of Cyclin B1 at anaphase I (AI) and maintained the expression of Mad2L1 and Cdh1 to activate SAC and APC-Cdh1 and degrade MPF, which ensured that all chromosomes were accurately located in a line, and then oocytes passed MI, entered AI and exited from the first meiotic division successfully (Fig. 4D). Our observation is important to indicate the mechanism of chromosomes separation during porcine meiotic maturation.
DNA damage checkpoint, consisting of ATM, ATR, Chk1 or Chk2, Cdc25 and other related downstream proteins,45 has extensive roles on cells growth by the signal pathway ATM/ATR-Chk1/Chk2-Cdc25.46-48 Chk1 participates in numerous molecular signal transductions.13,49 In recent studies, it was found that the activation of DNA damage checkpoint contributed to function of SAC,50 and the depletion of Chk1 resulted in abnormal mitotic progression because of consecutively inactive SAC.51 Compare to the blastocyst cells, the ability of ATM to be activated was limited in oocytes and phosphorylated ATM was maintained at a basal level in mouse oocytes.30 It was reported that porcine oocytes lacked DNA damage checkpoint.32 It can be concluded that ATM could be activated but it was not easy in oocytes. Even if ATM was active, it could not regulate Chk1 in porcine oocytes as same as it in somatic cells. However, our result of Chk1 overexpression showed porcine oocytes were arrested at GV stage. We speculated that Chk1 might inhibit Cdc25C and pre-MPF could not be activated.
Cyclin B1 is one of composition of MPF. Once oocytes enter the stage of AI, Cyclin B1 starts to be degraded by active APC/C.52 Because oocytes with Chk1 depletion were arrested at MI and could not enter AI stage, we first classified normal and abnormal MI oocytes, and then tested Cyclin B1 respectively. Our results showed that Chk1 knockdown reduced the degradation of Cyclin B1 in MI oocytes. Moreover, the similar locations of Chk1 and Cyclin B1 indicated that there was a close relation between them. We also found that Chk1 was continuously expressed during porcine oocytes maturation and this was similar to that in mouse oocytes, however, the difference is that porcine oocytes had more expression of Chk1 protein in MI stage rather than pre-MI and Cyclin B1 was not localized at the mouse oocyte spindle.31
In MI oocytes, all chromosomes are uniformly arranged in the equatorial plane and their co-orientations are perpendicular to the plane because each pair of homologous chromosomes are under the forces to opposite directions.53 Once this process is finished, SAC loses its activity and oocytes start to enter AI stage.54 In our studies, we found the oocytes with Chk1 depletion were arrested at MI stage and chromosomes were in misalignment. As a key composition of SAC, Mad2 in the form of closed conformation (C-Mad2) is located at kinetochores unconnected with microtubes and combines with other proteins to active SAC.55 In addition, it can combine with Cdc20 and recruit other proteins to form meiosis checkpoint compounds (MCC)56 which inhibit the activity of APC/C.54 NDC80 is a component of kinetochore proteins which establish a connection between chromosomal centromeres and microtubes of spindle. NDC80 bridge is involved with the reductional division in meiosis I.57 Related studies have shown that Chk1 could interact with Mad2.11 So we detected the expression of Mad2L1, Mad2L2, and Ndc80 in Chk1 depleted oocytes. Our results showed that Chk1 depletion decreased the expression of Mad2L1, which was consistent with the studies in somatic cells.12
Whether oocytes enter AI stage depends on the activity of APC/C.52 In somatic cells, Cdh1 and Cdc20 are co-activators of APC/C.58 APC/C-Cdc20 recognizes the D box of substrates, and then degrades substrates by ubiquitination, for example Cyclin B1. APC/C-Cdh1 degrades not only substrates containing D box but also substrates containing KEN box, for example Pds1.59 Some studies showed that Cyclin B1 was degraded at AI, but both Pds1 in which D box functional mutated and Cyclin B1 without D box were not degraded by APC/C.60,61 We checked expressions of Cdc20 and Cdh1 after Chk1 knockdown and found that the level of Cdc20 was not changed; however, the expression of Cdh1 was reduced significantly. APC/C-Cdh1 takes roles at G2 stage or prophase I in somatic cells or mouse oocytes.62,63 Therefore, these results suggested that Chk1 might be important in subsequent progress and early embryo development because Cdh1 was involved in mitosis and exit from meiosis and it was normally not activated at MI or AI stage in oocytes.64
Taken together, DNA damage checkpoint has been studied in somatic cells; however, there are limited studies in oocytes, such as Xenopus and mouse oocytes. We further verified that the maturation of porcine oocytes did not need the DNA damage checkpoint, which was different from the mouse oocyte maturation. Moreover, we first reported that Chk1 regulated the timely degradation of Cyclin B1 at AI and ensued that porcine oocytes could pass MI, enter AI and exit from the first meiotic division successfully. These results provide important information that could potentially be used to culture porcine oocytes in vitro and provide more oocytes with high quality for research or embryo production.
Materials and methods
Porcine oocytes collection and culture
Porcine ovaries were obtained from a slaughterhouse, put into the normal saline at 38.5°C and transported to the laboratory in about 40 min. Follicular fluid from 3–6 mm antral follicles was aspirated with an injection syringe the volume of which is 10 ml. COCs were selected and culture as reported.65 The denuded oocytes obtained from COCs were used for subsequent experiments.
Immunofluorescent staining and confocal microscope
For the staining of spindle, oocytes were fixed in 4% (W/V) paraformaldehyde, washed in washing buffer (PBS with 0.01% Triton X-100) for three times. The lipid droplets of oocytes were extracted by placing oocytes into 50% methanol for 5 min, 100% methanol for 5 min, and 100% acetone for 5 min in turn. Then oocytes were washed three times (5 min each time), permeablized in permeabilization solution (PBS with 1% Triton X-100 (V/V), 20 mM Hepes, pH 7.4, 3 mM MgCl2, 50 mM NaCl, 300 mM sucrose, and 0.02% NaN3) for least 8 h at RT. After oocytes had been blocked with 3% BSA for 1 h at RT, they were stained with FITC-anti-α-tubulin antibody (Sigma, F2168) at a dilution of 1:200 for 1 h at RT, washed five times (10 min each time) and counterstained with 1 mg/ml DAPI in vectashield mounting medium (Vector Laboratories, Burlingame, CA) to stain DNA. For the observation of CCNB1, oocytes were fixed in 4% (W/V) paraformaldehyde, washed three times and stained with 1 mg/ml DAPI. Finally, all these oocytes were mounted on glass slides. Slides were scanned by using a Zeiss confocal microscope (Zeiss LSM 510 UV). At least 30 samples in each group were analyzed in three repeated experiments.
RNA isolation, reverse transcription and qRT-PCR
Total RNA of each sample was isolated from 50 porcine oocytes using an Arcturus Pico Pure kit (Life Technologies, Grand Island, NY). Enhanced GFP (eGFP) cRNA was transcribed in vitro from pIVT-eGFP66 and 1 ng was added to each sample prior to RNA isolation as an internal control. The cDNA of each sample was obtained using a Fast Quant RT kit (Tiangen). Quantitative RT-PCR was performed according to the instruction in Super Real PreMix Plus kit (Tiangen), using cDNA of each sample. Relative gene expressions were calculated using the Δ Cq method67 with eGFP expression for normalization. Primers were listed in Table S1.
Vector construction and transcription in vitro
The cDNA of porcine nephridial tissue and oocyte, as the templates of Chk1 and CCNB1 genes cloning respectively, were obtained with the method of RNA isolation and reverse transcription as previously mentioned. Chk1 and CCNB1 genes were respectively cloned with DNA polymerase (Takara, Prime STAR Mix, DP214-02). The products were used for A-tailing and TA cloning with DNA A-Tailing kit (Takara, 6109), then transformed into competent Escherichia coli cells. Positive colonies were selected and plasmids were extracted with plasmid extraction kit (AxyGen, AP-MN-P-50). Chk1 and CCNB1 were inserted into the pIVT vector and the pIVT-C-eGFP vector. After linearization, these vectors were used for in vitro transcription and tailing with mMESSAGE mMACHINE T7 kit (Thermo, AM1344). The mRNA was purified with RNAeasy kit (Tiangen) and stored at −80°C.
Microinjection
Microinjections were performed using Nikon Ti-S inverted microscope (Nikon, Japan). COCs were held by holding pipette which had an outside diameter of about 120 μm, an inner diameter of about 40 μm and an angle of about 30 at the tip. Injection pipette which had an inner diameter of about 1 μm was stuck into oocytes at depth of about 65 μm. SiRNA or mRNA was injected by FemtoJet® 4i (Eppendorf, Germany). To make sure that injection was successful, the expanded oocyte cytoplasm should be as big as nucleus of GV oocyte by adjusting the injection pressure of FemtoJet. For the Chk1 knockdown experiment, 5–10 pl Chk1 small interfering RNAs (siRNAs, 50 μM) or negative control siRNA (Gene Pharma) solution was injected into the cytoplasm of oocytes. For subcellular localization of Chk1, 0.5 mg/mL Chk1-eGFP mRNA was injected into the cytoplasm of GV oocytes. To observe CCNB1, the mixture of 0.5 mg/mL CCNB1-eGFP mRNA and 50 μM Chk1 siRNA was co-injected into the cytoplasm of GV oocytes; as the control, 0.5 mg/mL CCNB1-eGFP mRNA was co-injected into the cytoplasm. These microinjections were finished within 30 minutes. These COCs were exposed in 2 mM dbcAMP for 20 h31 and then cultured in vitro. For the Chk1 overexpression experiment, 1.5 mg/mL Chk1 mRNA was co-injected into the cytoplasm of oocytes. After exposed in 2 mM dbcAMP for 2 h,31 the COCs were cultured in vitro.
Western blot
300 oocytes were lysed in RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% (V/V) NP40, 0.5% (W/V) Sodium deoxycholate, 0.1% (W/V) SDS, 1mM EDTA, 1mM NaF, 1mM Na3VO4, 1mM PMSF and 1mM Aprotinin), heated at 98°C for 10 min at SDS sample buffer. Proteins of the sample were separated by SDS-PAGE and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Membranes were blocked in 5% skim milk for 1 h, and then incubated with rabbit anti-CHK1 antibody (Sigma, PLA0085, 1:1000) or rabbit anti-β-actin antibody (abcam, ab8227, 1:2000) overnight at 4°C. After washing in TBST for 3 times (10 min each time), membranes were incubated with HRP-conjugated goat anti-rabbit IgG (Beijing Biodragon Immunotechnologies Co., Ltd, BF03008, 1:1000) at RT for 1 h, and detected by chemiluminescence reagent ECL (Thermo Fisher Scientific, 32106).
Statistical analysis
For fluorescence intensity analysis, the control and treated oocytes were mounted on the same glass slide and used the same parameters to normalize across the replicates. Using Image J, for each figure with fluorescent oocytes, background was subtracted. Each oocyte was circled and selected, then cropped. For each oocyte, a value which represented fluorescence intensity was got. We omitted the abnormal oocytes with strong or weak fluorescence intensity. Average intensities of control and treated oocytes were made after testing all of the measurements. For qRT-PCR results, the level of mRNA in control or at GV stage was set to 1. For western blot results, the band intensity was measured by Image J and control band intensity was set to 1. Each analysis used at least three replicates and each replicate was finished by an independent experiment. Results were endowed as means ± SEM. Statistical evaluation of the data was performed with a 2-tailed Student's t test in prism. A p-value of < 0.05 was considered significant which was showed as “*”. A p-value of < 0.01 was considered great significant which was showed as “**”.
Author contributions
Z. W. N., L. C., Q. S. J., Y. Y. G., T. W. conducted the experiments; Z. W. N. and Y. L. M. analyzed the data, designed the experiments and wrote the manuscript. All authors reviewed the manuscript.
Additional information
Competing financial interests: The authors declare no competing financial interests.
Supplementary Material
Funding Statement
National Major Project for Breeding of Transgenic Pig (2016ZX08006-002), “The Recruitment Program for Young Professionals” of “The Thousand Talents Plan” (Grant No.159905 and 169903) and Starting Fund for New Recruitment of Huazhong Agricultural University (Grant No.14009)
Acknowledgments
This research was supported by the National Major Project for Breeding of Transgenic Pig (2016ZX08006-002), “The Recruitment Program for Young Professionals” of “The Thousand Talents Plan" (Grant No.159905 and 169903) and Starting Fund for New Recruitment of Huazhong Agricultural University (Grant No.14009).
References
- [1].Raleigh JM, O'Connell MJ. The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. Journal of cell science 2000;113(Pt 10):1727-36. PMID:10769204 [DOI] [PubMed] [Google Scholar]
- [2].Pollok S, Stoepel J, Bauerschmidt C, Kremmer E, Nasheuer HP. Regulation of eukaryotic DNA replication at the initiation step. Biochemical Society transactions 2003;31:266-9. doi: 10.1042/bst0310266. PMID:12546699 [DOI] [PubMed] [Google Scholar]
- [3].Takeda DY, Dutta A. DNA replication and progression through S phase. Oncogene 2005;24:2827-43. doi: 10.1038/sj.onc.1208616. PMID:15838518 [DOI] [PubMed] [Google Scholar]
- [4].Oh JS, Han SJ, Conti M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. The Journal of cell biology 2010;188:199-207. doi: 10.1083/jcb.200907161. PMID:20083600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hassan BH, Lindsey-Boltz LA, Kemp MG, Sancar A. Direct role for the replication protein treslin (Ticrr) in the ATR kinase-mediated checkpoint response. The Journal of biological chemistry 2013;288:18903-10. doi: 10.1074/jbc.M113.475517. PMID:23696651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito H, Nakanishi M. Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell 2008;132:221-32. doi: 10.1016/j.cell.2007.12.013. PMID:18243098 [DOI] [PubMed] [Google Scholar]
- [7].Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, Pascual J, Imamura S, Kishi S, Amatruda JF, et al.. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell 2008;133:864-77. doi: 10.1016/j.cell.2008.03.037. PMID:18510930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kosoy A, O'Connell MJ. Regulation of Chk1 by its C-terminal domain. Molecular biology of the cell 2008;19:4546-53. doi: 10.1091/mbc.E08-04-0444. PMID:18716058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Walker M, Black EJ, Oehler V, Gillespie DA, Scott MT. Chk1 C-terminal regulatory phosphorylation mediates checkpoint activation by de-repression of Chk1 catalytic activity. Oncogene 2009;28:2314-23. doi: 10.1038/onc.2009.102. PMID:19421147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Smits VA, Gillespie DA. DNA damage control: regulation and functions of checkpoint kinase 1. The FEBS journal 2015;282:3681-92. doi: 10.1111/febs.13387. PMID:26216057 [DOI] [PubMed] [Google Scholar]
- [11].Chila R, Celenza C, Lupi M, Damia G, Carrassa L. Chk1-Mad2 interaction: a crosslink between the DNA damage checkpoint and the mitotic spindle checkpoint. Cell cycle (Georgetown, Tex) 2013;12:1083-90. doi: 10.4161/cc.24090. PMID:23454898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang X, Xu W, Hu Z, Zhang Y, Xu N. Chk1 is required for the metaphase-anaphase transition via regulating the expression and localization of Cdc20 and Mad2. Life sciences 2014;106:12-8. doi: 10.1016/j.lfs.2014.04.011. PMID:24747134 [DOI] [PubMed] [Google Scholar]
- [13].Nahse V, Christ L, Stenmark H, Campsteijn C. The Abscission Checkpoint: Making It to the Final Cut. Trends in cell biology 2017;27:1-11. doi: 10.1016/j.tcb.2016.10.001. PMID:27810282 [DOI] [PubMed] [Google Scholar]
- [14].Peddibhotla S, Lam MH, Gonzalez-Rimbau M, Rosen JM. The DNA-damage effector checkpoint kinase 1 is essential for chromosome segregation and cytokinesis. Proceedings of the National Academy of Sciences of the United States of America 2009;106:5159-64. doi: 10.1073/pnas.0806671106. PMID:19289837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Petrovic A, Keller J, Liu Y, Overlack K, John J, Dimitrova YN, Jenni S, van Gerwen S, Stege P, Wohlgemuth S, et al.. Structure of the MIS12 Complex and Molecular Basis of Its Interaction with CENP-C at Human Kinetochores. Cell 2016;167:1028-40.e15. doi: 10.1016/j.cell.2016.10.005. PMID:27881301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Petsalaki E, Akoumianaki T, Black EJ, Gillespie DA, Zachos G. Phosphorylation at serine 331 is required for Aurora B activation. The Journal of cell biology 2011;195:449-66. doi: 10.1083/jcb.201104023. PMID:22024163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Petsalaki E, Zachos G. Chk1 and Mps1 jointly regulate correction of merotelic kinetochore attachments. Journal of cell science 2013;126:1235-46. doi: 10.1242/jcs.119677. PMID:23321637 [DOI] [PubMed] [Google Scholar]
- [18].Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 1998;93:1067-76. doi: 10.1016/S0092-8674(00)81211-8. PMID:9635435 [DOI] [PubMed] [Google Scholar]
- [19].Haering CH, Nasmyth K. Building and breaking bridges between sister chromatids. BioEssays : news and reviews in molecular, cellular and developmental biology 2003;25:1178-91. doi: 10.1002/bies.10361. PMID:14635253 [DOI] [PubMed] [Google Scholar]
- [20].Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, Elledge SJ. Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science (New York, NY) 1999;286:1166-71. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- [21].Wang H, Liu D, Wang Y, Qin J, Elledge SJ. Pds1 phosphorylation in response to DNA damage is essential for its DNA damage checkpoint function. Genes & development 2001;15:1361-72. doi: 10.1101/gad.893201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The Journal of biological chemistry 1998;273:5858-68. doi: 10.1074/jbc.273.10.5858. PMID:9488723 [DOI] [PubMed] [Google Scholar]
- [23].Bakken AH, McClanahan M. Patterns of RNA synthesis in early meiotic prophase oocytes from fetal mouse ovaries. Chromosoma 1978;67:21-40. doi: 10.1007/BF00285645. PMID:688843 [DOI] [PubMed] [Google Scholar]
- [24].Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science (New York, NY) 2014;343:533-6. doi: 10.1126/science.1247671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Experimental cell research 1961;24:495-507. doi: 10.1016/0014-4827(61)90449-9. [DOI] [PubMed] [Google Scholar]
- [26].Miles DC, van den Bergen JA, Sinclair AH, Western PS. Regulation of the female mouse germ cell cycle during entry into meiosis. Cell cycle (Georgetown, Tex) 2010;9:408-18. doi: 10.4161/cc.9.2.10691. PMID:20023406 [DOI] [PubMed] [Google Scholar]
- [27].Furuno N, Kawasaki A, Sagata N. Expression of cell-cycle regulators during Xenopus oogenesis. Gene expression patterns : GEP 2003;3:165-8. doi: 10.1016/S1567-133X(03)00037-1. PMID:12711544 [DOI] [PubMed] [Google Scholar]
- [28].Nakajo N, Oe T, Uto K, Sagata N. Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. Developmental biology 1999;207:432-44. doi: 10.1006/dbio.1998.9178. PMID:10068474 [DOI] [PubMed] [Google Scholar]
- [29].Oe T, Nakajo N, Katsuragi Y, Okazaki K, Sagata N. Cytoplasmic occurrence of the Chk1/Cdc25 pathway and regulation of Chk1 in Xenopus oocytes. Developmental biology 2001;229:250-61. doi: 10.1006/dbio.2000.9968. PMID:11133168 [DOI] [PubMed] [Google Scholar]
- [30].Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Current biology : CB 2012;22:989-94. doi: 10.1016/j.cub.2012.03.063. PMID:22578416 [DOI] [PubMed] [Google Scholar]
- [31].Chen L, Chao SB, Wang ZB, Qi ST, Zhu XL, Yang SW, Yang CR, Zhang QH, Ouyang YC, Hou Y, et al.. Checkpoint kinase 1 is essential for meiotic cell cycle regulation in mouse oocytes. Cell cycle (Georgetown, Tex) 2012;11:1948-55. doi: 10.4161/cc.20279. PMID:22544319 [DOI] [PubMed] [Google Scholar]
- [32].Wang H, Luo Y, Zhao MH, Lin Z, Kwon J, Cui XS, Kim NH. DNA double-strand breaks disrupted the spindle assembly in porcine oocytes. Molecular reproduction and development 2016;83:132-43. doi: 10.1002/mrd.22602. PMID:26642846 [DOI] [PubMed] [Google Scholar]
- [33].Zhang T, Zhang GL, Ma JY, Qi ST, Wang ZB, Wang ZW, Luo YB, Jiang ZZ, Schatten H, Sun QY. Effects of DNA damage and short-term spindle disruption on oocyte meiotic maturation. Histochemistry and cell biology 2014;142:185-94. doi: 10.1007/s00418-014-1182-5. PMID:24477549 [DOI] [PubMed] [Google Scholar]
- [34].Luo Y, Lee IW, Jo YJ, Namgoong S, Kim NH. Depletion of the LINC complex disrupts cytoskeleton dynamics and meiotic resumption in mouse oocytes. Scientific reports 2016;6:20408. doi: 10.1038/srep20408. PMID:26842404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurz EU, Douglas P, Lees-Miller SP. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. The Journal of biological chemistry 2004;279:53272-81. doi: 10.1074/jbc.M406879200. PMID:15489221 [DOI] [PubMed] [Google Scholar]
- [36].Luo YB, Ma JY, Zhang QH, Lin F, Wang ZW, Huang L, Schatten H, Sun QY. MBTD1 is associated with Pr-Set7 to stabilize H4K20me1 in mouse oocyte meiotic maturation. Cell cycle (Georgetown, Tex) 2013;12:1142-50. doi: 10.4161/cc.24216. PMID:23475131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li P, Noegel AA. Inner nuclear envelope protein SUN1 plays a prominent role in mammalian mRNA export. Nucleic acids research 2015;43:9874-88. PMID:26476453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Collins JK, Lane SI, Merriman JA, Jones KT. DNA damage induces a meiotic arrest in mouse oocytes mediated by the spindle assembly checkpoint. 2015;6:8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jamsai D, O'Connor AE, Deboer KD, Clark BJ, Smith SJ, Browne CM, Bensley JG, Merriman JA, Yuen WS, Koopman P, et al.. Loss of GGN leads to pre-implantation embryonic lethality and compromised male meiotic DNA double strand break repair in the mouse. PloS one 2013;8:e56955. doi: 10.1371/journal.pone.0056955. PMID:23451117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Heijink AM, Krajewska M, van Vugt MA. The DNA damage response during mitosis. Mutation research 2013;750:45-55. doi: 10.1016/j.mrfmmm.2013.07.003. PMID:23880065 [DOI] [PubMed] [Google Scholar]
- [41].Mayer A, Baran V, Sakakibara Y, Brzakova A, Ferencova I, Motlik J, Kitajima TS, Schultz RM, Solc P. DNA damage response during mouse oocyte maturation. Cell cycle (Georgetown, Tex) 2016;15:546-58. doi: 10.1080/15384101.2015.1128592. PMID:26745237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ma JY, Ou Yang YC, Wang ZW, Wang ZB, Jiang ZZ, Luo SM, Hou Y, Liu ZH, Schatten H, Sun QY. The effects of DNA double-strand breaks on mouse oocyte meiotic maturation. Cell cycle (Georgetown, Tex) 2013;12:1233-41. doi: 10.4161/cc.24311. PMID:23518501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Adhikari D, Liu K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes[J]. Molecular and cellular endocrinology, 2014, 382(1): 480-487. doi: 10.1016/j.mce.2013.07.027. PMID:23916417 [DOI] [PubMed] [Google Scholar]
- [44].Huo L J, Fan H Y, Zhong Z S, et al.. Ubiquitin–proteasome pathway modulates mouse oocyte meiotic maturation and fertilization via regulation of MAPK cascade and cyclin B1 degradation[J]. Mechanisms of development, 2004, 121(10): 1275-1287. doi: 10.1016/j.mod.2004.05.007. PMID:15327787 [DOI] [PubMed] [Google Scholar]
- [45].Karlsson-Rosenthal C, Millar JB. Cdc25: mechanisms of checkpoint inhibition and recovery. Trends in cell biology 2006;16:285-92. doi: 10.1016/j.tcb.2006.04.002. PMID:16682204 [DOI] [PubMed] [Google Scholar]
- [46].Palou R, Palou G, Quintana DG. A role for the spindle assembly checkpoint in the DNA damage response. Current genetics 2017;63:275-80. doi: 10.1007/s00294-016-0634-y. PMID:27488803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schmitt E, Paquet C, Beauchemin M, Bertrand R. DNA-damage response network at the crossroads of cell-cycle checkpoints, cellular senescence and apoptosis. Journal of Zhejiang University Science B 2007;8:377-97. doi: 10.1631/jzus.2007.B0377. PMID:17565509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Selvarajah J, Elia A, Carroll VA, Moumen A. DNA damage-induced S and G2/M cell cycle arrest requires mTORC2-dependent regulation of Chk1. Oncotarget 2015;6:427-40. doi: 10.18632/oncotarget.2813. PMID:25460505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Peralta-Sastre A, Manguan-Garcia C, de Luis A, Belda-Iniesta C, Moreno S, Perona R, Sanchez-Perez I. Checkpoint kinase 1 modulates sensitivity to cisplatin after spindle checkpoint activation in SW620 cells. The international journal of biochemistry & cell biology 2010;42:318-28. doi: 10.1016/j.biocel.2009.11.011. [DOI] [PubMed] [Google Scholar]
- [50].Kim EM, Burke DJ. DNA damage activates the SAC in an ATM/ATR-dependent manner, independently of the kinetochore. PLoS genetics 2008;4:e1000015. doi: 10.1371/journal.pgen.1000015. PMID:18454191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Carrassa L, Sanchez Y, Erba E, Damia G. U2OS cells lacking Chk1 undergo aberrant mitosis and fail to activate the spindle checkpoint. Journal of cellular and molecular medicine 2009;13:1565-76. doi: 10.1111/j.1582-4934.2008.00362.x. PMID:19778378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zeng X, King RW. An APC/C inhibitor stabilizes cyclin B1 by prematurely terminating ubiquitination. Nature chemical biology 2012;8:383-92. doi: 10.1038/nchembio.801. PMID:22366722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 2011;146:568-81. doi: 10.1016/j.cell.2011.07.031. PMID:21854982 [DOI] [PubMed] [Google Scholar]
- [54].Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Current biology : CB 2012;22:R966-80. doi: 10.1016/j.cub.2012.10.006. PMID:23174302 [DOI] [PubMed] [Google Scholar]
- [55].Tipton AR, Wang K, Link L, Bellizzi JJ, Huang H, Yen T, Liu ST. BUBR1 and closed MAD2 (C-MAD2) interact directly to assemble a functional mitotic checkpoint complex. The Journal of biological chemistry 2011;286:21173-9. doi: 10.1074/jbc.M111.238543. PMID:21525009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tipton AR, Tipton M, Yen T, Liu ST. Closed MAD2 (C-MAD2) is selectively incorporated into the mitotic checkpoint complex (MCC). Cell cycle (Georgetown, Tex) 2011;10:3740-50. doi: 10.4161/cc.10.21.17919. PMID:22037211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li X, Dawe R K. Fused sister kinetochores initiate the reductional division in meiosis I[J]. Nature cell biology, 2009, 11(9): 1103. doi: 10.1038/ncb1923. PMID:19684578 [DOI] [PubMed] [Google Scholar]
- [58].de Boer HR, Llobet SG, van Vugt MA. Erratum to: Controlling the response to DNA damage by the APC/C-Cdh1. Cellular and molecular life sciences : CMLS 2016;73:2985-98. doi: 10.1007/s00018-016-2279-x. PMID:27251328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes & development 2000;14:655-65. [PMC free article] [PubMed] [Google Scholar]
- [60].Chang HY, Levasseur M, Jones KT. Degradation of APCcdc20 and APCcdh1 substrates during the second meiotic division in mouse eggs. Journal of cell science 2004;117:6289-96. doi: 10.1242/jcs.01567. PMID:15561765 [DOI] [PubMed] [Google Scholar]
- [61].Herbert M, Levasseur M, Homer H, Yallop K, Murdoch A, McDougall A. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nature cell biology 2003;5:1023-5. doi: 10.1038/ncb1062. PMID:14593421 [DOI] [PubMed] [Google Scholar]
- [62].Peters J M. The anaphase promoting complex/cyclosome: a machine designed to destroy[J]. Nature reviews. Molecular cell biology, 2006, 7(9): 644. doi: 10.1038/nrm1988. PMID:16896351 [DOI] [PubMed] [Google Scholar]
- [63].Solc P, Schultz R M, Motlik J. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells[J]. Molecular human reproduction, 2010, 16(9): 654-664. doi: 10.1093/molehr/gaq034. PMID:20453035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Homer H. The APC/C in female mammalian meiosis I. Reproduction (Cambridge, England) 2013;146:R61-71. doi: 10.1530/REP-13-0163. [DOI] [PubMed] [Google Scholar]
- [65].Gao YY, Chen L, Wang T, Nie ZW, Zhang X, Miao YL. Oocyte aging-induced Neuronatin (NNAT) hypermethylation affects oocyte quality by impairing glucose transport in porcine. Scientific reports 2016;6:36008. doi: 10.1038/srep36008. PMID:27782163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Igarashi H, Knott JG, Schultz RM, Williams CJ. Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Developmental biology 2007;312:321-30. doi: 10.1016/j.ydbio.2007.09.028. PMID:17961538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 2001;29:e45. doi: 10.1093/nar/29.9.e45. PMID:11328886 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


