Abstract
Quantification of T-cell receptor excision circles (TRECs) for newborn screening for SCID has advanced the diagnosis of severe combined immune deficiency (SCID). However, it has led to the identification of infants with T cell lymphopenia without known cause. The clinical characteristics, appropriate laboratory monitoring, and outcomes of patients remain unclear. We performed a retrospective review of clinical and laboratory studies for 26 infants collected from 7 New York State referral centers from 2010 to 2016 with low TRECs (mean, 70 copies/μl) and subnormal CD3 counts (mean, 1150/cubic mm). Over time absolute CD3 counts increased in 17 and decreased in 9; 22 (85%) have done well clinically regardless of absolute T cell values. Additional infants with TCL will continue to be identified in newborn screening panels. While most patients seem to do well clinically, parameters for diagnosis and monitoring have yet to be formalized, and additional information needs to be collected, causes and outcomes reported.
Keywords: Newborn screening, TREC level, Idiopathic T-cell lymphopenia, Severe combined immunodeficiency
1. Introduction
The method of evaluating T-cell receptor excision circles (TRECs) to universally screen infants for a profound deficiency in T cells in the newborn period was established in Wisconsin and Massachusetts in 2009, based on methodology developed by Puck [1–5]. This practice has now been extended to the majority of states and the success of this approach in identifying infants with severe combined immunodeficiency (SCID) and other forms of severe T cell impairment, has been proven [3,4,6–9]. As the method does not discriminate between the underlying causes of T cell reduction, prematurity, birth defects, and a variety of other medical reasons can also lead to abnormally low TREC values and T cell deficiency. For example, Wisconsin identified 33 infants with T cell lymphopenia of varying degrees through newborn screening between 2008 and 2011. Nineteen infants had secondary causes of T cell lymphopenia, such as anatomic, metabolic, and chromosomal abnormalities. Fourteen infants had primary causes of T cell lymphopenia, including 5 infants with SCID, 4 infants with the 22q11.2 deletion syndrome; however 5 other infants with T cell lymphopenia, not considered to have genetic or medical causes, were considered to have idiopathic T cell lymphopenia [10]. Comeau et al. in the Massachusetts study, noted that of 51 infants referred for additional testing, 9 infants had idiopathic T cell lymphopenia and remained under the care of an immunologist [3].
Newborn screening for SCID began in New York State in September 2010; infants with low TRECs were originally referred to one of 8 referral centers, based on location of birth: Albany Medical Center, Long Island Jewish Health System, Montefiore Medical Center, Mount Sinai Hospital, New York Medical College, University of Rochester Medical Center, Upstate Medical University, and Women’s & Children’s Hospital of Buffalo. At these tertiary centers, complete blood cell counts and flow cytometry were performed to identify infants with potentially severe T cell defects. As in other reports, infants in New York State were identified with SCID, DiGeorge syndrome or other genetic anomaly, or other clinically significant, non-SCID abnormalities leading to loss of T cells [8]. In this earlier report 30 infants were classified as having idiopathic T cell lymphopenia (TCL), defined as infants without SCID, other genetic defect, or another medical syndrome that required ongoing monitoring or treatment [8]. The laboratory parameters and clinical treatment were variable for the infants in this report; 6 (20%) were treated with antibiotics, and fewer than half (N = 11; 37.9%), were said to have resolution of the flow cytometry abnormalities by around 12 months of age. However, for 19 infants, the lymphopenia had not resolved in the two year period and no immunologic or follow-up data on these subjects was presented. In this report we provide demographic, clinical and laboratory information on 26 infants with CD3 lymphopenia identified between 2010 and 2016, 24 of them not included in the prior report. For all infants we collected longer term data, revealing additional medical complexities in some of the infants followed and persistence of lymphopenia in many after a number of months to years of follow-up.
2. Methods
Dried blood spots were collected at birth through standardized newborn screening practices. DNA was extracted and TREC levels were quantified using real-time quantitative polymerase chain reaction as described [8]. The initial cut off TREC value of <200 copies/μl was originally chosen in NYS based on parameters derived from both normal infants and infants with SCID; this was subsequently reduced to <125 copies/μl for referral to specialized centers. Infants were evaluated with complete blood counts and flow cytometry to assess T, B, and NK cell numbers. There was no uniform protocol for each center to follow for investigation of abnormal values. Naïve and memory T cells were not routinely collected. Infants with absolute lymphocyte counts <2500/cubic mm3 [9,11] without evidence of prematurity (<37 weeks gestation), birth defect, or other identifiable causes of lymphopenia are included in this report. Infants were followed for additional visits, at intervals defined by each center, for ongoing monitoring and/or diagnostic testing as requested by each center director. Statistical analysis was performed utilizing GraphPad Prism (Graphpad Software, La Jolla CA); all tests were performed using a level of significance of <0.05.
3. Results
Data on a group of 26 infants with abnormally low TRECs and CD3 T cell counts lower than 2500/cubic mm were collected from 6 of the NYS referral centers. These infants were assigned a case dispensation of “non SCID” and had no identified genetic defect or identifiable medical cause that could lead to lymphopenia (Tables 1 and 2). Aside from baseline counts for Cases #13 and #21, these data do not overlap with the prior report [8]. The median triplicate TREC value for these infants was 66.5 (range 0–171; 25 to 75% = 27.3–107.8). The mean absolute CD3 count for these infants was 1150/cubic mm (range 24 to 2439 cubic mm); three of the infants (patient #2, #10 and #26) also had low B cells counts and two of them, low NK cells at baseline. While the TREC level for referral was reduced from 200 to 125 copies/μl over time, the initial TREC values were positively correlated to the absolute CD3 counts from the first blood test (rs = 0.62, p < 0.0001) (Fig. 1). (Table 1) However, this was not always predictive, as a TREC triplicate average of 22 copies/μl correlated with an absolute CD3 count of 1125/cubic mm in one infant, while a TREC triplicate average of 46 copies/μl correlated with an absolute CD3 count of 254/cubic mm in another infant. While there was and still is, no consensus protocol for continuing to test or follow lymphopenic infants with no known genetic or medical reason for low levels, the 26 newborns discussed here had been recalled for clinical follow-up by the referral center, and had at least one, and in some cases, a number of additional flow cytometry tests performed over time. These showed that absolute CD3 counts had increased in 17 infants but decreased in 9, in a period of 6 months to 66 months of follow-up. (Table 1) Fig. 2 shows the most recent counts for these infants.
Table 1.
Infants with T cell lymphopenia identified by New York State Newborn Screening.
| Pt. # | Gender | Center | TREC Avg | CD3 (2500–5500)* | CD19 (300–2000) | CD16/56 (170–1100) | CD4 (1600–4000) | CD8 (560–1700) | CD4/CD8 | LAST CD3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | Buffalo | 143 | 741 | 1097 | 414 | 613 | 117 | 5.24 | 324 |
| 2 | Male | Buffalo | 46 | 254 | 53 | 101 | 124 | 116 | 1.07 | 2054 |
| 3 | Female | LIJ | 29 | 1360 | 1139 | 771 | 861 | 487 | 1.77 | 1434 |
| 4 | Female | LIJ | 58 | 1438 | 1525 | 1046 | 1222 | 255 | 4.79 | 1186 |
| 5 | Male | LIJ | 0 | 544 | 977 | 1046 | 444 | 89 | 4.99 | 558 |
| 6 | Male | LIJ | 44 | 2129 | 2392 | 195 | 1525 | 548 | 2.78 | 2145 |
| 7 | Female | LIJ | 171 | 1371 | 688 | 134 | 909 | 439 | 2.07 | 2715 |
| 8 | Male | Monte | 110 | 2439 | N/A | N/A | 1864 | 649 | 2.87 | 3392 |
| 9 | Male | Monte | 146 | 1846 | N/A | N/A | 1308 | 502 | 2.61 | 2232 |
| 10 | Male | Monte | 94 | 1991 | 298 | 578 | 2212 | 1533 | 1.44 | 2382 |
| 11 | Female | Monte | 143 | 1641 | 1778 | 546 | 1335 | 320 | 4.17 | 1453 |
| 12 | Female | Monte | 88 | 1215 | 303 | 786 | 818 | 339 | 2.41 | 2242 |
| 13 | Male | NYMC# | 118 | 2237 | 1785 | 387 | 1593 | 545 | 2.92 | 1564 |
| 14 | Male | NYMC | 58 | 1149 | 932 | 489 | 778 | 345 | 2.26 | 975 |
| 15 | Male | UPS | 0 | 377 | 816 | 942 | 289 | 78 | 3.71 | 742 |
| 16 | Male | UPS | 0 | 212 | 654 | 514 | 169 | 42 | 4.02 | 921 |
| 17 | Female | MSSM | 107 | 918 | 1742 | 557 | 455 | 347 | 1.31 | 1965 |
| 18 | Female | MSSM | 75 | 617 | 682 | 971 | 424 | 193 | 2.20 | 301 |
| 19 | Female | MSSM | 77 | 860 | 344 | 387 | 645 | 194 | 3.32 | 986 |
| 20 | Female | MSSM | 18 | 393 | 2058 | 349 | 292 | 68 | 4.29 | 559 |
| 21 | Male | MSSM# | 79 | 1166 | 1722 | 220 | 717 | 434 | 1.65 | 1509 |
| 22 | Male | MSSM | 103 | 1650 | 481 | 851 | 1032 | 607 | 1.70 | 1284 |
| 23 | Female | MSSM | 22 | 1125 | 770 | 146 | 769 | 314 | 2.45 | 1774 |
| 24 | Male | MSSM | 0 | 895 | 269 | 824 | 534 | 269 | 1.99 | 679 |
| 25 | Female | MSSM | 47 | 1170 | 456 | 376 | 781 | 347 | 2.25 | 1650 |
| 26 | Female | MSSM | 49 | 74 | 6 | 72 | 45 | 19 | 2.37 | 2800 |
Reference normals (9) # initial data included in Table VI in reference (6).
Table 2.
Infants followed at Mount Sinai Medical Center.
| Pt # | TREC avg | Ig levels (mg/dL) | Vaccine response | Mitogen response | Molecular studies | Clinical manifestations | Antibiotic prophylaxis | Current status; age |
|---|---|---|---|---|---|---|---|---|
| 17 | 107 | At 4 months: IgG 308, IgA 7, IgM 54 | At 9 months: Protective titers for Hemophilus, Diph + Tet; MMR given with no adverse problems | Low lymphocyte response to PHA at 2 months | None | Early watery diarrhea post rotavirus vaccine (rotavirus antigen negative); somewhat obese, currently no infections of note | TMP/SMX | Low muscle tone and sensory integration disorder; 5 years 6 months old |
| 18 | 75 | At 2 months: IgG 331, IgA 5, IgM 31 | At 1 year: Protective titers | Low lymphocyte response to ConA and PHA; at 3 months | IL7R, ADA = normal; chromosomal translocation of chromosome 3 and 14; same as her mother | None | TMP/SMX | Healthy; 5 years, 4 months |
| 19 | 77 | At 16 months: IgG 745, IgA 49, IgM 58 | Initial non responder for Hemophilus. Protective titers for Diphtheria, Tetanus and Pneumococcus; MMR given with no adverse reaction | Low lymphocyte response to ConA and PHA. Normal response to PWM | Chromosomal breakage study. Telomere length short. Metabolic panel | Failure to thrive; milk protein intolerance; macrocytic anemia | None | Mitochondrial dysfunction suspected; age 4 years |
| 20 | 18 | N/A | At 10 months: Protective titers for Tetanus. At 21 months, protective titers for Hemophilus; live viral vaccines withheld. | N/A | None | None | Prophylactic antibiotics were used for 6 months (CD3 < 500 mm3) | Healthy; 4 years 6 months |
| 21 | 79 | N/A | All vaccines, including MMR, up to date. No adverse effects. | N/A | None | At 6 months: Escherichia coli UTI treated with ceftriaxone (×2) and then cephalexin. At 8 months: acute otitis media treated with Amoxicillin. At 11 months: pneumonia treated with Azithromycin | None | Speech and cognitive delay; 5 years, 1 month |
| 22 | 103 | N/A | At 15 months: Protective titers for Hemophilus, Tetanus, Diphtheria and Pneumococcus | N/A | None | Coxsackie infection (self-resolved), also had a few viral respiratory infections | None | Healthy; 5 years |
| 23 | 22 | N/A | All vaccines, including MMR, up to date. Titers not done. | Normal ratio of CD 45 RO/RA cells | Amino acid panel | Possible autoimmune neutropenia of infancy | None | Healthy; Given live vaccines; 4 years 6 months |
| 24 | 0 | N/A | At 9 months: Protective titers for Diph and Tet: Non Protective for HiB. At 11 months: Protective titers for Pneumococcus | Normal mitogen proliferative response | FISH for DiGeorge | After the last Prevnar vaccination, developed a fever. URI without fevers and right hydronephrosis | None | Healthy; Given live vaccines with no problem; 18 months |
| 25 | 47 | At 8 months: IgG 334, IgA 5, IgG 18 | All vaccines, including MMR, up to date. | Normal mitogen response to PHA and Pokeweed. Low for ConA | Metabolic panel. Amino acid panel. CGH Array for DiGeorge negative | Failure to thrive. Poor feeding and high urine lactate. Macrocytoisis Pulmonic stenosis | None | Mitochondrial dysfunction is suspected; 17 months |
| 26 | 49 | N/A | At 3 months: Protective titers for Hep B and Tetanus | Normal mitogen proliferative response | ADA and PNP activity | Stooling 1–2 times per week; mild neutropenia | TMP/SMX | Normalized: lymphopenia due to utero 6-MP exposure; 2 years 6 months |
Fig. 1.
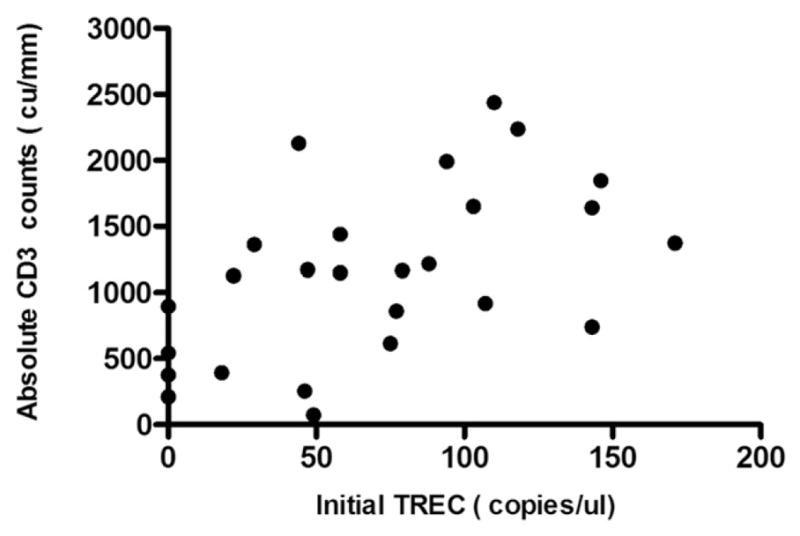
Correlation between the initial TREC value and the absolute CD3 T cell number for the 26 infants.
Fig. 2.
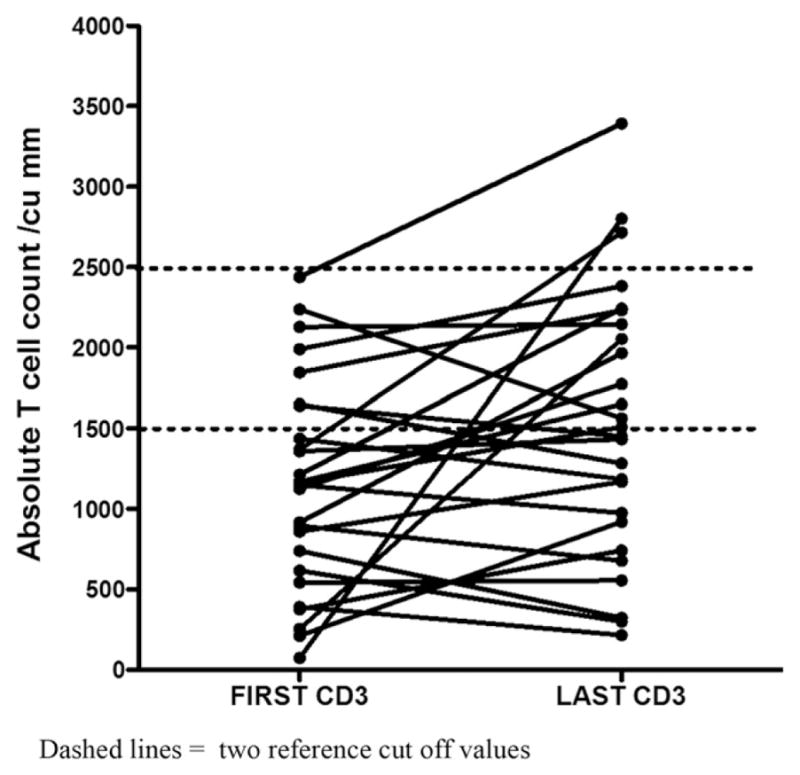
The first and more recent CD3T cell count for each infant. The normal CD3 T cell ranges 1,500 and 2,500 cu/mm used in newborn screening reports [9,11] are indicated with dotted lines. Dashed lines = two reference cut off values.
Interestingly, there were 4 infants, all males, with newborn TREC triplicate averages of “0” who are included in this cohort. Three had quite low absolute CD3 counts (212, 544, 377, 895/cubic mm). All are clinically stable (up to now age 5.5 years), and have had 2 to 7 subsequent flow cytometry examinations showing improvement, but still abnormally low counts in all (Table 3).
Table 3.
Follow-up on infants with zero TRECS.
| Pt number | TREC | Initial CD3 | Most recent CD3 | Age (years) |
|---|---|---|---|---|
| 5 | 0 | 544 | 588 | 3* |
| 15 | 0 | 377 | 1188 | 5.5 |
| 16 | 0 | 212 | 742 | 4.5 |
| 24 | 0 | 895 | 1077 | 2.4 |
Genetics negative for 22q11 and 10p13 deletions; possible DiGeorge secondary to maternal gestational diabetes.
Ten of the lymphopenic infants have been followed at Mount Sinai, and 2 to 11 additional flow cytometry tests have been performed, at varying months, up to a maximum of 66 months. Additional data on these infants, and 2 other infants followed at Upstate Medical University (identified by case numbers as from Tables 1 and 2), studied over this time are shown on Fig. 3. For this group, 8 infants had absolute CD3 counts higher than their baseline on follow-up, although 4 others had lower counts than their baseline. Only one (#26) (who had been exposed to 6-mercaptopurine, (see below) achieved levels over 2500 cu/mm within 5 months. Six other infants had levels of at least 1500 cu/mm but others were consistently under this value on follow-up.
Fig. 3.
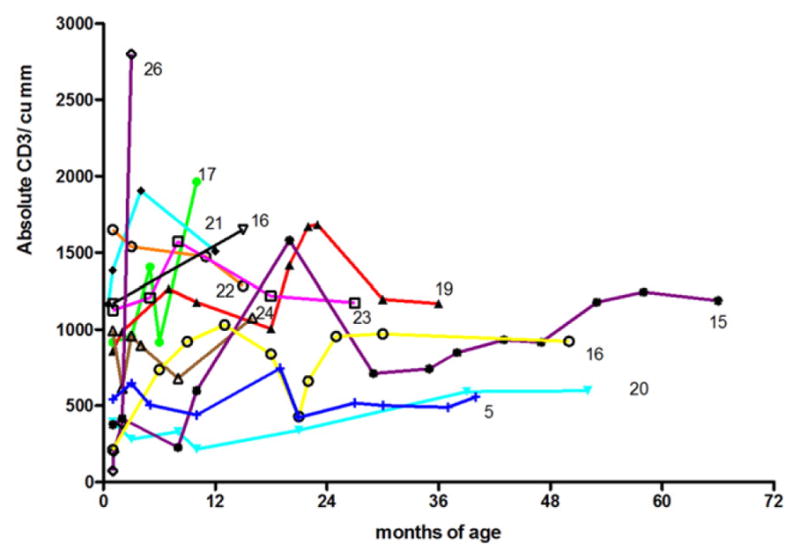
Serial CD3 counts in 11 infants; the number shown indicates the patient from Tables 1 and 2. CD3 T cell normal ranges are 1,500 and 2,500 cu/mm [9,11].
For ten infants followed at Mount Sinai (infants #17–26 on Table 1), additional data were collected, which is compiled on Table 2. These data included lymphocyte proliferation, CD45RO/RA markers, molecular testing in some, including adenosine deaminase (ADA), genetic testing for DiGeorge syndrome, and/or IL-7R gene sequencing), lymphocyte proliferation to mitogens, immunoglobulin levels and antibody titers after vaccinations, metabolic panels, telomere lengths). Infants with the lowest CD3 numbers were placed on trimethoprim sulfamethoxazole prophylaxis at the first encounter (#16, #17, #18 and #26); none of these 10 infants received live viral vaccines in the first year. While 6 infants are healthy and thriving, a variety of medical conditions have been identified in 4 of these children. Patient #17 had low muscle tone but no additional diagnosis by the age of 5, patient #21 had speech delay at age 3, and two sisters (patients #19 and #25) have had failure to thrive and lactic acidosis, leading to suspicion of mitochondrial dysfunction, but no defects have yet been verified. One of these sisters also has pulmonic stenosis. In addition to these infants, potential maternal causes of lymphopenia were also present: infant #17s mother was previously diagnosed with systemic lupus erythematosus, and while not being treated, was noted to have renal disease during pregnancy; infant #18 has the same chromosome translocation (3 and 14) previously identified in her healthy mother. While initial counts and lymphocyte proliferative response to ConA and PHA were diminished, her T cells counts rapidly increased, and she continues to be healthy and has developed protective titers to diphtheria and tetanus vaccines. Finally, infant #26 had remarkable lymphopenia at birth and a zero TREC average, but she had been exposed to 6-mercaptopurine in utero, taken by her mother for Crohn’s disease. However, over the following 4 months, her lymphocyte counts rapidly returned to normal, and she had normal mitogen proliferation and developed antibody responses to both tetanus and hepatitis B vaccines in the first 6 months of follow-up. Maternal immune suppression, as recently reported, may lead to profound neonatal lymphopenia, easily mistaken for SCID [12].
4. Discussion
The premise of newborn screening (NBS) for SCID is to detect severe T cell disorders at birth so that earlier therapeutic interventions can be applied. While infants with SCID and primary and secondary T cell defects can now be readily identified, referral centers are also identifying infants with idiopathic TCL the unexpected observation that infants with idiopathic TCL are also being identified [1,3,5] prompted longer term follow-up of such infants in this report. The true incidence of this is not clear, but the overall denominator reported for New York State, was 30 lymphopenic infants in 2 years, with 485,912 infants screened in this interval [8]. The causes of lymphopenia, and overall outcomes for these infants are still unclear; follow-up of some infants has shown resolution in some but not in others [8,10], While the normal range of CD3+ T cells at birth is broad (2500 to 5500 cu/mm) [11], some sources use >2500/cubic mm3 and other experts use >1500 mm3 [9]. New York State allowed the Specialty Care Centers to define T cell lymphopenia [8, 9]. As the TRECs values are not always positively correlated to the absolute CD3 counts, a cutoff of 2500 mm3 T cell numbers as noted previously as out of range for newborns, was used here [11]. However, for the infants in this study, the mean absolute CD3 count was 1150/cubic mm, and seven out of the 26 infants had counts >1500/cubic mm. Here we provide baseline and up to 5 years of follow-up for demographic, clinical and laboratory information on 26 infants with CD3 lymphopenia, revealing the medical complexities that developed in some and long term persistence of lymphopenia in many.
With the adoption of newborn screening for SCID, there continues to be wide variability in the criteria for recalling TCL infants for additional specimens, implementation of referral to specialists for follow-up, and the type of immunological investigations which should be performed [13,14]. Although there was no state-wide protocol in place for following these infants, referral centers in NYS commonly recalled these infants based on low CD3 T cell numbers, although they were not considered to have SCID or other medical reasons for lymphopenia, and no known T cell defects had been identified. As for all other centers doing newborn screening, how much testing and how long the follow-up should be for these lymphopenic infants, is not clear. Some experts recommend avoidance of live vaccines, protection from infectious exposures, transfusion precautions, and, in some cases, administration of prophylactic antibiotics or immunoglobulin infusions [10,15]. While infants with T cell counts of 300–1500 cells/cubic mm and demonstrable T cell functional impairment could be categorized as “variant SCID” according to the Clinical and Laboratory Standards Institute [16], genetic testing or testing of cellular immunity was generally not performed on the infants included in this study. As discussed here, most of these infants are thriving and have produced protective titers of antibodies. However, amongst these infants are two sisters with probable mitochondrial defects leading to lactic acidosis; both had low TRECS and modest lymphopenia. Most likely infants with additional metabolic defects will be found to have abnormal newborn TREC screens in time. Another child, now 5 and a half, is followed for low muscle tone and defects of sensory integration; signs of ataxia telangiectasia, a cause of low TRECS [17,18] have not appeared.
We also noted several maternal issues that could affect newborns: a maternally inherited chromosomal translocation in one, renal failure in another and the use of 6-mercaptopurine in a third, leading to very severe lymphopenia compatible with SCID. Maternal immune suppression, as recently reported, is now known to lead to profound neonatal lymphopenia, easily mistaken for a severe immune defect [12].
This study is limited to the data collected from Specialty Care Centers without full review of medical records, and it may be incomplete in its description of testing procedures and antibiotic use for individual patients. T cell lymphopenia in the context of newborn screening is a newly described diagnosis with little published data on management, outcomes, or anticipatory guidance. Whole exome sequencing may uncover new as yet undescribed reasons for lymphopenia in these infants and early efforts in this direction have been initiated in NYS. Learning the natural history of these infants and defining their underlying disorders is of potential interest for both immunology and clinical medicine [13,14].
Conclusions
As all states implement the TREC assay for newborn screening for SCID, more infants with TCL will undoubtedly be identified. While these patients overall appear to do well clinically, parameters for monitoring and care have yet to be standardized, and additional information will need to be collected and reported. Currently management is based on clinical experience and individualized patient considerations. Gene defects not previously known may impair T-cell development, providing an opportunity to further understand normal T cell development.
Acknowledgments
Funding sources
This work was supported by the National Institutes of Health, AI-101093, AI-086037, AI-48693, and the David S Gottesman Immunology Chair.
The authors would like to acknowledge Victoria Popson, Public Health Representative II at the New York State Department of Health, for her assistance in follow-up for screen positive newborns and especially we thank Artemio Jongco MD PhD and Wendy Holz NP for their help with collecting additional patient data.
Abbreviations
- TCL
T cell lymphopenia
- SCID
severe combined immunodeficiency
- TREC
T-cell receptor excision circle
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Routes JM, Grossman WJ, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, Baker MW. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302:2465–2470. doi: 10.1001/jama.2009.1806. [DOI] [PubMed] [Google Scholar]
- 2.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115:391–398. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Comeau AM, Hale JE, Pai SY, Bonilla FA, Notarangelo LD, Pasternack MS, Meissner HC, Cooper ER, DeMaria A, Sahai I, Eaton RB. Guidelines for implementation of population-based newborn screening for severe combined immunodeficiency. J Inherit Metab Dis. 2010;33:S273–S281. doi: 10.1007/s10545-010-9103-9. [DOI] [PubMed] [Google Scholar]
- 4.Gerstel-Thompson JL, Wilkey JF, Baptiste JC, Navas JS, Pai SY, Pass KA, Eaton RB, Comeau AM. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of severe combined immunodeficiency in population-based newborn screening. Clin Chem. 2010;56:1466–1474. doi: 10.1373/clinchem.2010.144915. [DOI] [PubMed] [Google Scholar]
- 5.Hale JE, Bonilla FA, Pai SY, Gerstel-Thompson JL, Notarangelo LD, Eaton RB, Comeau AM. Identification of an infant with severe combined immunodeficiency by newborn screening. J Allergy Clin Immunol. 2010;126:1073–1074. doi: 10.1016/j.jaci.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, Lewis DB, McGhee SA, Moore TB, Stiehm ER, Porteus M, Aznar CP, Currier R, Lorey F, Puck JM. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132:140–150. doi: 10.1016/j.jaci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwan A, Hu D, Song M, Gomes H, Brown DR, Bourque T, Gonzalez-Espinosa D, Lin Z, Cowan MJ, Puck JM. Successful newborn screening for SCID in the Navajo Nation. Clin Immunol. 2015;158:29–34. doi: 10.1016/j.clim.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel BH, Bonagura V, Weinberg GA, Ballow M, Isabelle J, DiAntonio L, Parker A, Young A, Cunningham-Rundles C, Fong CT, Celestin J, Lehman H, Rubinstein A, Siegel S, Weiner L, Saavedra-Matiz C, Kay DM, Caggana M. Newborn screening for SCID in New York State: experience from the first two years. J Clin Immunol. 2014;34:289–303. doi: 10.1007/s10875-014-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, Baker M, Ballow M, Bartoshesky LE, Bonilla FA, Brokopp C, Brooks E, Caggana M, Celestin J, Church JA, Comeau AM, Connelly JA, Cowan MJ, Cunningham-Rundles C, Dasu T, Dave N, De La Morena MT, Duffner U, Fong CT, Forbes L, Freedenberg D, Gelfand EW, Hale JE, Hanson IC, Hay BN, Hu D, Infante A, Johnson D, Kapoor N, Kay DM, Kohn DB, Lee R, Lehman H, Lin Z, Lorey F, Abdel-Mageed A, Manning A, McGhee S, Moore TB, Naides SJ, Notarangelo LD, Orange JS, Pai SY, Porteus M, Rodriguez R, Romberg N, Routes J, Ruehle M, Rubenstein A, Saavedra-Matiz CA, Scott G, Scott PM, Secord E, Seroogy C, Shearer WT, Siegel S, Silvers SK, Stiehm ER, Sugerman RW, Sullivan JL, Tanksley S, Tierce MLt, Verbsky J, Vogel B, Walker R, Walkovich K, Walter JE, Wasserman RL, Watson MS, Weinberg GA, Weiner LB, Wood H, Yates AB, Puck JM, Bonagura VR. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312:729–738. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, Reddy S, Margolis D, Casper J, Gries M, Desantes K, Hoffman GL, Brokopp CD, Seroogy CM, Routes JM. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008–2011) J Clin Immunol. 2012;32:82–88. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 11.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA, Pediatric ACTG. Lymphocyte subsets in healthy children from birth through 18 years of age: the pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Kuo CY, Garcia-Lloret MI, Slev P, Bohnsack JF, Chen K. Profound T-cell lymphopenia associated with prenatal exposure to purine antagonists detected by TREC newborn screening. J Allergy Clin Immunol Pract. 2017;5:198–200. doi: 10.1016/j.jaip.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buelow BJ, Verbsky JW, Routes JM. Newborn screening for SCID: lessons learned. Expert Rev Hematol. 2016;9:579–584. doi: 10.1080/17474086.2016.1180243. [DOI] [PubMed] [Google Scholar]
- 14.Kwan A, Puck JM. History and current status of newborn screening for severe combined immunodeficiency. Semin Perinatol. 2015;39:194–205. doi: 10.1053/j.semperi.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Spek J, Groenwold RH, van der Burg M, van Montfrans JM. TREC based newborn screening for severe combined immunodeficiency disease: a systematic review. J Clin Immunol. 2015;35:416–430. doi: 10.1007/s10875-015-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI. CLSI Document NBS06-A, Clinical and Laboratory Standards Institute Document NBS06-A. 2013. Newborn blood spot screening for severe combined immunodeficiency by measurement of T-cell receptor excision circles; approved guideline. [Google Scholar]
- 17.Lingman Framme J, Borte S, von Dobeln U, Hammarstrom L, Oskarsdottir S. Retrospective analysis of TREC based newborn screening results and clinical phenotypes in infants with the 22q11 deletion syndrome. J Clin Immunol. 2014;34:514–519. doi: 10.1007/s10875-014-0002-y. [DOI] [PubMed] [Google Scholar]
- 18.Mallott J, Kwan A, Church J, Gonzalez-Espinosa D, Lorey F, Tang LF, Sunderam U, Rana S, Srinivasan R, Brenner SE, Puck J. Newborn screening for SCID identifies patients with ataxia telangiectasia. J Clin Immunol. 2013;33:540–549. doi: 10.1007/s10875-012-9846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


