Abstract
Cancer inpatients commonly suffer from impairments that can prohibit safe discharge home from the acute care inpatient medical service and thus require transfer to a post-acute inpatient rehabilitation facility. It has been demonstrated in multiple studies that cancer rehabilitation inpatients are able to make statistically significant functional improvements and at a similar pace as their non-cancer counterparts. Medical fragility and reimbursement regulations are concerns that affect acceptance and triage of cancer rehabilitation inpatients. Strategies to rehabilitate these challenging patients include considering risk factors for medical complications, consult based inpatient rehabilitation and improved communication and coordination with oncology teams.
INTRODUCTION
The majority of cancer physiatry has been in the outpatient setting primarily due to the increasing number of long term cancer survivors without evidence of disease. However, inpatient rehabilitation is necessary for many patients with advanced cancer undergoing active treatment. In 2009, there were 4.7 million adult cancer related hospitalizations in the United States of which 1.2 million had cancer as the principal diagnosis.1 An estimated 27% of direct medical costs for cancer patients in 2014 were due to inpatient hospital stays.2 Oncology inpatients can suffer from a number of debilitating impairments from systemic/generalized weakness and more focal sources including the central nervous system, peripheral nervous system, and musculoskeletal system.2 These impairments can have functional implications that make discharge home from acute care unsafe. Table 1 lists impairments that may require post-acute inpatient rehabilitation admission.
Table 1.
Impairments That May Require Post-Acute Inpatient Rehabilitation Admission
1) Systemic:
|
3) Musculoskeletal:
|
2) Neurologic:
|
Rehabilitation consults during the acute care stay can help improve function and minimize debility. Despite the fact that hospitalized oncology service patients frequently have impairments, research has shown an under-referral of these individuals to rehabilitation. 4,5 Inpatient physiatry and rehabilitation services consults often occur when the primary acute care medical team realizes that the patient is unsafe to go home. A common scenario is that acute care medical treatment has finished and the attending oncology physician informs the patient (and his/her family) that it’s time for discharge; however, the patient/family express concerns regarding readiness for discharge. In these cases, it is likely that a physiatry and/or other rehabilitation consultation that is provided earlier in the course of the hospitalization could improve discharge planning and reduce anxiety and/or prevent the need for transfer to an inpatient rehabilitation facility or an unplanned acute care readmission shortly after discharge.
Inpatient cancer rehabilitation occurs in a number of settings. During an acute care hospitalization, patients can receive physical, occupational, and speech therapy sometimes with or without the support of a physiatrist, while they are receiving medical treatment. In the United States, for patients that require post-acute care inpatient rehabilitation, there are three types of inpatient rehabilitation facilities: 1. acute inpatient rehabilitation facilities (IRF) and sub-acute rehabilitation facilities that are divided into: 2. skilled nursing facilities (SNF); and, 3. long term acute care facilities (LTAC).
Post-acute inpatient rehabilitation physiatrists may be reluctant to accept cancer patients for a number of reasons: 1. Many cancer patients continue to receive radiation treatment or chemotherapies which can be expensive and result in reduced margins in a Medicare Prospective Payment System environment; 2. Medicare requirements regarding IRF admission composition may present significant challenges for IRF admission reimbursement which are discussed in the next section; 3. Physiatrists may be hesitant due to concerns about the medical stability of cancer patients. The challenges of rehabilitating cancer patients in an inpatient setting has stimulated research to improve rehabilitation triage and creative ways to rehabilitate them.
REGULATORY CONSIDERATIONS
Within the United States, acute inpatient rehabilitation is delivered within IRFs. Those IRFs that are certified by the Centers for Medicare & Medicaid Services (CMS) have regulations and requirements regarding the admission and continued inpatient stay for patients requiring rehabilitation services.
Admissions to inpatient rehabilitation must be deemed both reasonable and necessary, and must generally meet the following criteria on admission:
Requirement for multiple therapy disciplines (physical, occupational, speech-language pathology, or prosthetics/orthotics), of which one must be physical or occupational therapy
Delivery of therapy services for at least 3 hours of therapy per day at least 5 days per week (Intensity may also be demonstrated by the provision of 15 hours in a 7-consecutive day period starting from the date of admission, in certain well-documented cases6)
Active participation and significant benefit for patients from an intensive rehabilitation therapy program
Supervision by a rehabilitation physician for at least 3 days per week to assess and treat medical and functional issues
Multidisciplinary and intensive coordinated team approach to delivery of care
As a requirement for participation in the Medicare reimbursement program (called the Prospective Payment System), IRFs are also required to maintain a minimum percentage of their total inpatient population within one of 13 diagnostic categories. Although the percentage has varied in the past, the current threshold for compliance is 60%, and hence this requirement is termed the 60% rule (Table 2).7 This rule has provided challenges regarding the admission of cancer survivors, as none of the diagnoses listed are explicitly cancer. This does not necessarily indicate that cancer patients will not benefit from comprehensive rehabilitation services at IRF.
Table 2.
60% Rule as Established by the Centers for Medicare & Medicaid Services for IRF7
| 13 Defined Medical Conditions | |
|---|---|
| Amputation | Brain injury |
| Burns | Congenital deformity |
| Fracture of femur (hip fracture) | Major multiple trauma |
| Spinal cord injury | Stroke |
| Active polyarticular rheumatoid arthritis, psoriatic arthritis, and seronegative arthropathies resulting in significant functional impairment of ambulation and other activities of daily living | Systemic vasculitides with joint inflammation resulting in significant functional impairment of ambulation and other activities of daily living |
Severe or advanced osteoarthritis (osteoarthrosis or degenerative joint disease) involving two or more weight bearing joints (elbow, shoulders, hips, or knees but not counting a joint with a prosthesis) with the following characteristics:
|
Knee or hip joint replacement (or both) during an acute care hospitalization immediately preceding the inpatient rehabilitation stay which meet at least one of the following criteria:
|
Neurological disorders, including:
| |
IRF=Acute Inpatient Rehabilitation Facility
The difficulty for many institutions is how to maintain 60% compliance and provide access to care for the cancer population. Interestingly, several cancer diagnoses can be coded as compliant within the 60% rule. For example, brain tumors (both primary and metastatic) may be considered brain injuries. Sarcoma resections with resultant amputation of the affected extremity are appropriately diagnosed as an amputation. Primary and metastatic spinal tumors with neurological impairment may be considered spinal cord injuries. Pathological lesions in the femur can be categorized as a femur fracture. Polyneuropathy secondary to chemotherapy or myopathy due to corticosteroids may also fall under the 60% rule. Several other examples may exist either due to the primary effects from tumor, or secondary effects of treatment. Studies have shown that non-60% rule compliant diagnoses are able to make significant functional improvements in an IRF. For example, Guo et al. was able to demonstrate that asthenic (a non-60% rule compliant diagnosis) cancer patients are able to make statistically significant functional improvements on inpatient rehabilitation.8 A study by Sliwa et al. revealed no significant differences in functional gains made by cancer patients with diagnoses that were 60% rule compliant versus 60% rule non-compliant.9 However, institutions must also be aware of the ability to use the remaining 40% of admissions for “non-compliant” diagnoses. Several patients that have primary cancer or consequences from oncological treatment may not be “60% compliant” but can still be admitted to an IRF so long as they meet the reasonable and necessary criteria. Careful management of admissions and coding of diagnoses by the utilization department of an IRF can provide clinicians guidance as to how many “non-compliant” patients with cancer can be admitted on an annual basis. Physician documentation of the impairment diagnosis in the patient history and physical examination is also critical.
However, there is a lack of clarity of what defines reasonable and necessary criteria for admission. CMS has outlined several of these items (Table 3).7 Many of the requirements surround documentation. From a clinical perspective one of the primary requirements is that a patient is medically stable enough to benefit from IRF services, but medically complex enough that close physician supervision is required for managing medical conditions. Several challenges exist in trying to maintain an appropriate balance of medical complexity with stability, and this dynamic is often difficult to balance for many institutions. It also causes inconsistency when attempting to standardize medical necessity criteria, as required by Medicare. For these reasons, denials have become more frequent for all aspects of inpatient rehabilitation care. Claims data from July 2007 showed that approximately 85% of the programs affected by Medicare’s Recovery Audit Contractor (RAC) are inpatient rehabilitation based.10 Fortunately, it was also found that 63% of appeals for denials were overturned when reviewed at the administrative law judge level. Due to changes in medical necessity definitions in 2010, the frequency of RAC audits may continue to increase.11
Table 3.
Reasonable and Necessary Criteria for IRF Admission6
Reasonable and Necessary Criteria
|
IRF=Acute Inpatient Rehabilitation Facility
Many of the regulations surrounding inpatient rehabilitation encompass both managing cost of care and optimizing outcomes. All rehabilitation facilities participating in the Medicare program are required to participate in the IRF Quality Reporting Program (QRP), with the intention of providing higher quality and more efficient healthcare for Medicare beneficiaries.12 These quality measures must be evaluated on all patients, regardless of payor mix and diagnosis. By 2018, 13 measures will be used to measure the performance and outcomes (Table 4).13 Many of these measures have been established to ensure all settings in the post-acute care (PAC) sector are comparable. With the development of the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act), patient assessment data will be standardized across the entire PAC spectrum, including Home Health Agencies, LTACs, SNFs, and IRFs. This standardization then allows for comparison of quality, resource use, and other metrics relevant to healthcare delivery and outcomes.14 Hence, it is important to understand how the admission of cancer survivors may have affect these metrics for IRFs, and thus potentially augment or diminish reimbursement rates based on institutional performance.
Table 4.
IRF Quality Reporting Program Measures Active by Fiscal Year 201812
| IRF Quality Reporting Program | |
|---|---|
| NQF #0138 NHSN Catheter-Associated Urinary Tract Infection Outcome |
NQF #0674 Application of Percent of Residents Experiencing One or More Falls with Major Injury (Long Stay) Application of Percent of Long-Term Care |
| NQF #0678 Percent of Residents or Patients with Pressure Ulcers |
NQF #2631 Hospital Patients with an Admission and Discharge Functional Assessment and a Care Plan That addresses Function |
| NQF #0431 Influenza Vaccination Coverage among Healthcare Personnel |
NQF #2633 IRF Functional Outcome Measure: Change in Self-Care for Medical Rehabilitation Patients |
| NQF #0680 Percent of Residents or Patients Who Were Assessed and Appropriately Given the Seasonal Influenza Vaccine (Short-Stay) |
NQF #2634 IRF Functional Outcome Measure: Change in Mobility Score for Medical Rehabilitation |
| NQF #2502 All-Cause Unplanned Readmission Measure for 30 Days Post Discharge from an IRF |
NQF #2635 IRF Functional Outcome Measure: Discharge Self-Care Score for Medical Rehabilitation Patients |
| NQF #1716 NHSN Facility-Wide Inpatient Hospital-Onset Methicillin-Resistant Staphylococcus aureus Bacteremia Outcome Measure |
NQF #2636 IRF Functional Outcome Measure: Discharge Mobility Score for Medical Rehabilitation Patients |
| NQF #1717 NHSN Facility – Wide Inpatient Hospital-Onset Clostridium difficile Infection Outcome Measure | |
IRF=Acute Inpatient Rehabilitation Facility, NQF=National Quality Forum, NHSN=National Health Safety Network
To moderate cost and improve outcomes, CMS has initiated the Bundled Payments for Care Improvement (BPCI) Initiative. The BPCI was developed by the Medicare and Medicaid Innovation Center to help reduce expenditures while preserving or enhancing the quality of care for beneficiaries. The current model proposes four pilots with different models of reimbursement based on episodes of care, and may extend beyond institutional reimbursement to also include services such as physician services. Several diagnoses have been considered for BPCI, but most notably major joint replacement of the lower extremity and stroke have already had significant impact as they relate to patient utilization of IRF.15 It has been noted for both orthopedic and cardiovascular surgery, there was a statistically significant shift from more expensive institutional PAC to less expensive home health care for beneficiaries discharging to any PAC setting when utilizing the Model 2 paradigm. However, limitations still exist regarding the impact of these initiatives due to insufficient sample size and limited time for which the BPCI has been conducted.16
The aging baby boomer population is a significant area of focus for cancer care, given that an estimated 62% of cancer survivors were over the age of 65 in 2016.17 To address the needs of Medicare beneficiaries, who are often 65 years of age or older, CMS has developed the Oncology Care Model (OCM). Its goal is to provide higher quality, more coordinated oncology care at the same or lower cost to Medicare. The OCM incorporates a two-part payment system, which creates incentives to improve the quality of care and furnish enhanced services. Participating physician practices are encouraged to address complex care needs, in a comprehensive and appropriate manner, for beneficiaries receiving chemotherapy for improved patient experience or health outcomes. Several commercial payers are also participating in the program to align financial incentives for better outcomes and cost savings.18 Given that the episode of care is defined from initiation of outpatient chemotherapy, and continues for six months, there may be several situations where inpatient hospitalization would be necessary due to either continued chemotherapy needs, decreased functional status, or new-onset medical co-morbidities. Several of these factors may be opportunities for IRF admission, which could provide inpatient level care for medically complex patients at a lower cost compared to acute care services. Further work is necessary to understand how to integrate PAC into the OCM for improved patient outcomes and cost benefit.
EFFECTS OF ACUTE CANCER INPATIENT REHABILITATION
Functional Improvement
Acute inpatient rehabilitation (IRF) has been shown in a number of studies to demonstrate statistically significant improvements in functional scores including general cancer, 19,20,21,22,23,24,25,26,27 asthenia/deconditioning,8 brain tumor, 28,29,30,31,32 hemipelvectomy for sarcoma,33 oncologic spinal cord injury,34,35,36 and paraneoplastic populations.37
In the IRF setting, the mean length of stay of cancer patients has been found to be shorter than their non-cancer cohorts including spinal cord injury38,39 and brain injury40,41,42 comparison studies. There are likely a number of factors that contribute to lower length of stays including poorer prognosis and the need to resume cancer treatment.43 IRF cancer patients improve at similar rates to their non-cancer counterparts. A number of studies have shown similar functional improvement rates (demonstrated by Functional Independence Measure efficiencies) in cancer populations versus non-cancer populations including comparisons of pediatric cancer vs. non-cancer patients,44 brain tumor vs. traumatic brain injury,45 brain tumor vs. stroke,41,42 and neoplastic vs. traumatic spinal cord injury.39 Because cancer patients improve at a similar rate to non-cancer patients, but stay a shorter time, overall changes in FIM score from admission to discharges are less. A recent study using national Uniform Data System for Medical Rehabilitation data of American cancer IRF patients revealed that admission total FIM scores have been decreasing and total FIM change has been increasing from 2002 to 2011.19
Survival and Symptoms
There has been an increasing body of evidence that exercise and physical activity may benefit cancer patient survival. There are two studies that suggest inpatient rehabilitation as an intervention may lead to increased survival.46,47 Patients with a higher functional gain may also live longer.48 However, more research is needed.
Cancer rehabilitation inpatients, like many advanced cancer patients, can suffer from significant symptom burdens that can affect activity and function.49 Common cancer related symptoms encountered by rehabilitation professionals include fatigue, pain, nausea, cognitive dysfunction and cachexia. Symptom severity can negatively impact function while improving symptom burden can predict better functional status and less disability.50,51,52 Reducing cancer symptom severity is a priority for inpatient rehabilitation physiatrists. Not only can it impact functional improvement but it can impact a patient’s ability to tolerate therapy. This is particularly important in an IRF where participation in three hours of therapy per day is mandatory. Inpatient rehabilitation patients have also shown to demonstrate statistically significant improvements in their cancer related symptoms from IRF admission to discharge possibly due to pharmacologic management by the physiatry team or due to physical activity.53,54 Furthermore, a study has shown that symptomatic improvements were maintained six months after inpatient rehabilitation discharge.55
MEDICAL FRAGILITY
Frailty has been defined as the condition that results in an increased risk of adverse outcomes following hospital admission.56 The Cardiovascular Health Study identified frailty as a syndrome with three or more of five criteria that include unintentional weight loss (10 pounds in the past year), self-reported exhaustion, weakness, slow walking speed and low physical activity. A patient with three or more criteria would be considered “frail”.57 All five criteria would be common in an inpatient cancer rehabilitation setting and illustrates the fragility of this patient population. Transfer back to the primary acute care service of general deconditioned IRF patients is higher than other impairment groups and has been reported between 11–14%.58,59,60,61 Deconditioning, while not a 60% rule diagnosis, is a common impairment among cancer inpatients.3
Beyond the issue of frailty as a contributor to increased medical fragility is that cancer patients also have additional risk factors compared to the general inpatient population. Leukopenia from chemotherapy or during hematopoietic stem cell transplant engraftment and the use of immunosuppressant agents such as steroids or anti-graft versus host disease medications can contribute to an increased likelihood of infection. Infection has been reported in a number of cancer IRF studies as the most common reason for transfer to the primary acute care service.62,63,64,65,66 Thrombocytopenia from chemotherapy can lead to an increased risk of bleeding complications. Because many IRF cancer rehabilitation inpatients will have active disease, progression of disease requiring transfer for further treatment can also occur.
Alam et al. reported a statistically significant difference of unplanned transfers back to the primary acute care service of 21% of cancer IRF patients versus 9.7% of non-cancer matched controls.62 Other studies have reported return to the primary acute care service rates of general cancer IRF patients between 16.5 and 35%9,20,,21,67,68,69 In most cases, an uninterrupted acute inpatient rehabilitation course with discharge home is considered a successful IRF admission.70,71 The higher frequency of medical complications causing return to the primary acute care service can be problematic. This has spurred research in exploring predictors of return to the primary acute care service events.
Predictors of Return to the Primary Acute Care Service of Cancer Rehabilitation Inpatients
A number of risk factors have been implicated in general cancer IRF patient populations with return to the primary acute care service. A lower functional status, elevated creatinine, reduced albumin and the presence of indwelling tubes including feeding tubes and Foley catheters at the time of inpatient rehabilitation admission have been identified.70,71
Cancer inpatients are a heterogeneous group which may make generalizing the results of prior studies on general cancer populations uncertain. Compared to solid tumor patients, liquid tumor, also known as hematologic malignancy, patients are among the most fragile cancer populations. The primary hematologic malignancies are leukemia, lymphoma and multiple myeloma. Hematologic stem cell transplant is a common treatment for some liquid tumor patients. Neutropenia and thrombocytopenia are particularly profound in these patients due to their disease and treatment. In a series of studies by Fu et al., frequencies and risk factors for return to the primary acute care service of different hematologic malignancy populations were explored. Return to the primary acute care service rates were 41% of hematopoietic stem cell transplant (with 38% of those who transferred back dying in the hospital),65 37% of leukemia,64 27% of lymphoma(unplanned only),66 and 26% of multiple myeloma patients (unplanned only).63 Table 5 summarizes the results of statistically significant or near significant variables associated with return to the primary acute care service of various cancer IRF population studies.
Table 5.
Significant Risk Factors Associated with Return to the Primary Acute Care Service in Different Cancer Populations
| General Cancer (n=184) Asher et al.68 No score |
General Cancer (n=98) Guo et al.67 No score |
Bone Marrow Transplant (n=147) Fu et al.65 RTP BMT Score (up to 67.88% probability) |
Leukemia (n=255) Fu et al.64 RTP Leukemia Score (up to 49.52% probability) |
Lymphoma (n=127) Fu et al.66 No score |
Multiple Myeloma (n=122) Fu et al.63 No score |
|---|---|---|---|---|---|
| Platelet count < 43,000 (p<.01) | Platelet count < 140,000 (p=.0132) | Platelet count < 140,000 (p=.008) | |||
| Creatinine > 1.3 (p=.01) | Creatinine level > 0.9 (p<.01) | Creatinine > 1.3 (p=.0008) | |||
| Presence of antifungal agent (p<.05) | Presence of antifungal (p=.1065) | Presence of IV antifungal (p=.0878) | |||
| Presence of antiviral agent (p=.0501) | Presence of antiviral (p=.0615) | ||||
| Male Sex (p=.0003) | Male Sex (p=.005) | ||||
| Presence of Feed Tube/Modified Diet (p=.004) | Presence of Tube Feeding (p=.03) | ||||
| Motor FIM score < 35 (p=.001) | Albumin < 3.5 (p=.04) Presence of Foley Catheter (p=.02) |
Presence of antibacterial agent (p=.0519) Leukemia, lymphoma or multiple myeloma diagnosis (p<.05) |
Peripheral blast > 0% (p=.0096) | History of BMT (p=0.0473) Presence of CVC (p=0.0573) |
FIM=Functional Independence Measure, RTP=Return to Primary, BMT=Bone Marrow Transplant, IV=intravenous, CVC=Central Venous Catheter
Acute Care Hospital Readmission – Can Inpatient Cancer Rehabilitation Have An Impact?
A major contributor to high-cost medical care is unplanned medical admissions and post-admission events.72 Improving a patient’s functional status, reducing symptom burden, building strength reserves and observing patients in a medical environment during the transition from acute care to discharge home could potentially reduce hospital readmission from the community. This also could result in better medical outcomes and reduced cost. Does the higher therapy intensity and greater physician involvement in an IRF compared to a SNF reduce the risk of hospital readmission of cancer patients? Due to the natural history of oncological disease, particularly in advanced cases, as well as the side-effects of treatment and associated co-morbid conditions, many cancer patients are at risk for unanticipated hospital readmissions. There are no published studies on the possible impact of different inpatient rehabilitation settings on the readmission rate of cancer patients. Research is needed.
There has been some limited research in the general population on this topic which could shed some light on this question. A shorter IRF stay, lower change in motor FIM scores, and lower motor discharge FIM scores were associated with a higher risk of acute care hospital readmission after inpatient rehabilitation discharge.73 One-third of Medicare patients with debility were readmitted to the hospital within 90 days of discharge from acute inpatient rehabilitation.74 The impact of a transitional care stage from acute care to home is being studied.75
Inpatient Rehabilitation Triage
A major role of consult cancer physiatrists is to assist in inpatient rehabilitation setting triage. This includes educating the patient and family regarding the different types of inpatient rehabilitation facilities and regulations. The process of inpatient rehabilitation triage must take into account a number of issues. First, the patient’s current and expected functional status (after inpatient rehabilitation) is considered. Second, therapy tolerance must be taken into account. IRF patients must be able to consistently tolerate 3 hours of therapy/day. If a patient is demonstrating an inability to tolerate much therapy while on acute care, an alternative setting should be considered including sub-acute rehabilitation. Third, the patient’s discharge disposition including physical home situation (e.g. stairs, wheelchair ramp) and available caregiver supervision (e.g. assistance in home, transportation) are also factors. Safe discharge home is one of the main goals of inpatient rehabilitation. Rehabilitation inpatients with an expected prolonged length of stay due to a low functional level or home situation are often triaged to sub-acute facilities. Fourth, payer/insurance limitations are issues. While the approval rate of IRF rehabilitation can vary significantly by insurer, it has been reported that 87% of private insurance authorization requests for acute inpatient cancer rehabilitation are approved.76
Lastly, their ongoing medical/nursing needs may dictate rehabilitation setting. Many free-standing IRFs and SNFs are unable to provide blood transfusions or more complicated medical nursing needs. Physician and/or midlevel (i.e. physician assistant or nurse practitioner) visits are typically more frequent in an IRF than in a SNF setting.77 It has been reported that some oncology clinicians suspect that there is a higher risk of acute care hospital readmission in a SNF versus an IRF and refuse to transfer their most fragile patients to SNFs.3 According to a report by the American Medical Rehabilitation Providers Association, when compared to SNFs, IRF clinical outcomes are better but come at a higher cost.78 Patients with a higher probability of medical complications and return to the primary acute care service may require more communication with oncologists, a rehabilitation facility with more physician involvement (such as an IRF over a SNF) or closer proximity to intensive care units and supporting medical specialists (e.g. rehabilitation within the acute care hospital versus a freestanding rehabilitation hospital). Triage of higher risk cancer rehabilitation inpatients to settings that provide more suitable medical care could potentially help save lives and reduce costs.
Inpatient Hospitalist Care while on Inpatient Rehabilitation
Support from a hospitalist to treat medical issues while on inpatient rehabilitation perhaps may reduce transfers to the primary acute care service. One study demonstrated a reduction in transfers to the primary acute care service of leukemia patients under the supervised care of an internal medicine hospitalist.64 However, the change was not statistically significant.
Consult Based Inpatient Cancer Rehabilitation
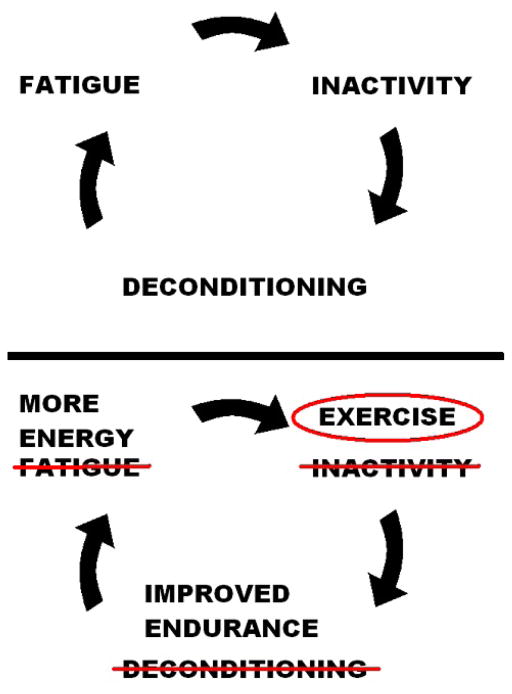
Besides cancer rehabilitation triage, consult cancer physiatrists are able to assist cancer inpatients in a number of ways. First, they help direct rehabilitation among the therapists on the acute care floor. Second, they may be able to prescribe medications and offer procedures/injections to promote functional improvement. Third, cancer physiatrists provide education to patients to help improve their performance status. Most cancer inpatients do not meet the recommended guidelines for physical activity and diet.79 Resuming physical activity after a prolonged period of bed rest during a complicated hospitalization can be difficult for advanced cancer inpatients. Exercise and physical activity are one of the most effective treatments for reducing cancer symptoms.80,81 However, the constellation of cancer symptoms including fatigue, pain, cachexia and depression discourage patients from participating in physical activity, therapy and exercise programs. Figure 1 illustrates the cycle of inactivity, leading to more weakness and fatigue, that consult physiatrists and therapists often need to disrupt. Educating patients that although their body is telling them to lay in bed, it is better for most to get up and make an effort to remain physically active if possible and safe. Frequently, sitting in a chair is the first step to improving their endurance and strength.
Figure 1.
Early physiatry consultation while on the acute care service, enables physiatrists and therapists to minimize patient functional declines while on medical treatment and improve function, perhaps enough, to avoid needing transfer to an inpatient rehabilitation facility. The Mayo Clinic in Rochester, Minnesota has used a model of consult based inpatient cancer rehabilitation for over 30 years called the Cancer Adaptation Team (CAT). The CAT consists of a physiatrist, physical therapist, occupational therapist, nurse coordinator, social worker, nutritionist, and chaplain. They meet weekly with the primary medical service and floor nurses to facilitate coordination and to provide more intensive rehabilitation while under the care of the oncology medical service. In a uncontrolled descriptive study, Sabers et al was able to demonstrate substantial improvements in Karnofsky and Barthel Mobility scores of patients seen by the CAT.82 The Mayo Clinic -Rochester acute inpatient rehabilitation unit (at the Saint Mary’s Campus) is located 1.2 miles away from the Hematology/Oncology inpatient wards (at the Methodist Campus). By exposing cancer inpatients to a more organized and intensive rehabilitation program during their acute care stay, they may be able to minimize functional declines, begin to improve function earlier and reduce transfers to their acute inpatient rehabilitation unit over a mile away from the oncology team. In 2013, only 11 liquid and 15 solid tumor cancer patients were transferred to the Mayo Clinic Acute Inpatient Rehabilitation Unit.83 A similar model of consult based rehabilitation while on the primary oncology service, called the mobile team, was used at MD Anderson Cancer Center. The mobile team was thought of as mobile IRF with physiatry oversight where patients could receive up to an hour of physical therapy, an hour of occupational therapy and an hour of speech therapy (if needed) daily. The advantage was that patients could receive intense therapy while their medical issues were being addressed by the acute care medical service. Like an acute inpatient rehabilitation interdisciplinary team, the mobile team also met weekly to discuss patient cases among team members. Providing acute care inpatients additional therapy may be financially difficult under the Medicare Prospective Payment System. However, upcoming bundled care and reimbursement based on patient outcomes, may encourage these type of rehabilitation programs in the future.
CONCLUSION
Inpatient cancer rehabilitation can be challenging due to medical fragility and regulatory constraints. An understanding of these issues can guide inpatient rehabilitation triage. Changes in medical reimbursement aimed at providing more emphasis on care quality and outcomes may encourage earlier involvement of physiatrists and other rehabilitation professionals during the acute care stays of cancer patients. It is possible that inpatient cancer rehabilitation can reduce hospital readmission, improve care quality and reduce cost; however, additional high quality evidence is necessary to support the growth of the field of cancer rehabilitation.
Acknowledgments
Supported in part by the M.D. Anderson Cancer Center Support Grant CA 016672.
Footnotes
Disclosures: Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Price RA, Stranges E, Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb–2012 Feb. Cancer Hospitalizations for Adults, 2009: Statistical Brief #125. [PubMed] [Google Scholar]
- 2.Cancer Facts & Figures. American Cancer Society; 2017. [Accessed March 22, 2017]. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. [Google Scholar]
- 3.Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011 May;90(5 Suppl 1):S63–8. doi: 10.1097/PHM.0b013e31820be1a4. [DOI] [PubMed] [Google Scholar]
- 4.Lin HF, Wu YT, Tsauo JY. Utilization of rehabilitation services for inpatient with cancer in Taiwan: a descriptive analysis from national health insurance database. BMC Health Serv Res. 2012 Aug 16;12:255. doi: 10.1186/1472-6963-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Movsas SB, Chang VT, Tunkel RS, Shah VV, Ryan LS, Millis SR. Rehabilitation needs of an inpatient medical oncology unit. Arch Phys Med Rehabil. 2003 Nov;84(11):1642–6. doi: 10.1053/s0003-9993(03)00345-9. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare & Medicaid Services, Department of Health and Human Services. [Accessed January 11, 2017];Inpatient rehabilitation therapy services: complying with documentation requirements. Available at https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Inpatient_Rehab_Fact_Sheet_ICN905643.pdf.
- 7.Centers for Medicare & Medicaid Services, Department of Health and Human Services. [Accessed January 12, 2017];Inpatient Rehabilitation Facility Prospective Payment System. Available at https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/InpatRehabPaymtfctsht09-508.pdf.
- 8.Guo Y, Shin KY, Hainley S, Bruera E, Palmer JL. Inpatient rehabilitation improved functional status in asthenic patients with solid and hematologic malignancies. Am J Phys Med Rehabil. 2011 Apr;90(4):265–71. doi: 10.1097/PHM.0b013e3182063ba6. [DOI] [PubMed] [Google Scholar]
- 9.Sliwa JA, Shahpar S, Huang ME, Spill G, Semik P. Cancer Rehabilitation: Do Functional Gains Relate to 60 Percent Rule Classification or to the Presence of Metastasis? PM R. 2016 Feb;8(2):131–7. doi: 10.1016/j.pmrj.2015.06.440. [DOI] [PubMed] [Google Scholar]
- 10.Granger CV, Carlin M, Riggs RV, et al. Medicare’s recovery audit contractor program: inpatient rehabilitation facilities are taking back takebacks, but enough? Am J Phys Med Rehabil. 2011;90(5):426–31. doi: 10.1097/PHM.0b013e318214ec54. [DOI] [PubMed] [Google Scholar]
- 11.American Hospital Association. [Accessed January 12, 2017];Limiting Access to Inpatient Medical Rehabilitation. 2007 Oct; Available at http://www.aha.org/content/00-10/071003rehablcd.pdf.
- 12.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; inpatient rehabilitation facility prospective payment system for federal fiscal year 2017. Final rule. Fed Regist. 2016;81(151):52055–141. [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. [Accessed January 13, 2017];IRF Quality Reporting Measures Information. Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/IRF-Quality-Reporting/IRF-Quality-Reporting-Program-Measures-Information-.html.
- 14.Centers for Medicare and Medicaid Services. [Accessed January 13, 2017];Post-Acute Care Quality Initiatives. Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014-and-Cross-Setting-Measures.html.
- 15.Centers for Medicare and Medicaid Services. [Accessed January 16, 2017];Bundled Payments for Care Improvement (BPCI) Initiative: General Information. Available at https://innovation.cms.gov/initiatives/bundled-payments/
- 16.The Lewin Group (Prepared for CMS) [Accessed January 16, 2017];CMS Bundled Payments for Care Improvement Initiative Models 2–4: Year 2 Evaluation and Monitoring Annual Report. 2016 Aug; Available at https://innovation.cms.gov/Files/reports/bpci-models2-4-yr2evalrpt.pdf.
- 17.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–36. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. [Accessed January 16, 2017];Oncology Care Model. Available at https://innovation.cms.gov/initiatives/oncology-care.
- 19.Gallegos-Kearin V, Mix J, Knowlton S, Schneider JC, Zafonte R, Goldstein R. Outcome Trends of Adult Cancer Patients Receiving Inpatient Rehabilitation: A 10-year Review of the Uniform Data System for Medical Rehabilitation. Arch Phys Med Rehabil. 2016 Oct;97(10):e46. doi: 10.1097/PHM.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 20.Mix JM, Granger CV, LaMonte MJ, Niewczyk P, DiVita M, Goldstein R, Yates J, Freudenheim JL. A Characterization of Cancer Patients in Inpatient Rehabilitation Facilities: A Retrospective Cohort Study. Arch Phys Med Rehabil. 2017 Feb 1; doi: 10.1016/j.apmr.2016.12.023. pii: S0003-9993(17)30063-1 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991 Oct;155(4):384–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996 Jan;77(1):54–7. doi: 10.1016/s0003-9993(96)90220-8. [DOI] [PubMed] [Google Scholar]
- 23.Cole RP, Scialla SJ, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000 May;81(5):623–7. doi: 10.1016/s0003-9993(00)90046-7. [DOI] [PubMed] [Google Scholar]
- 24.Tay SS, Ng YS, Lim PA. Functional outcomes of cancer patients in an inpatient rehabilitation setting. Ann Acad Med Singapore. 2009 Mar;38(3):197–201. [PubMed] [Google Scholar]
- 25.Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013 Jun;32(4):443–56. doi: 10.1177/0733464811432632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang ME, Sliwa JA. Inpatient rehabilitation of patients with cancer: efficacy and treatment considerations. PM R. 2011 Aug;3(8):746–57. doi: 10.1016/j.pmrj.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 27.McEwen SE, Elmi S, Waldman M, Bishev M. Inpatient oncology rehabilitation in Toronto: a descriptive 18-month retrospective record review. Support Care Cancer. 2012 Jul;20(7):1541–7. doi: 10.1007/s00520-011-1243-4. [DOI] [PubMed] [Google Scholar]
- 28.Huang M, Wartella J, Kreutzer J, et al. Review of subject: Functional outcomes and quality of life in patients with brain tumours: A review of the literature. Brain Injury. 2005;15:843–56. doi: 10.1080/02699050010013653. [DOI] [PubMed] [Google Scholar]
- 29.O’Dell MW, Barr K, Spanier D, et al. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79:1530–4. doi: 10.1016/s0003-9993(98)90414-2. [DOI] [PubMed] [Google Scholar]
- 30.Marciniak CM, Sliwa JA, Heinemann AW, et al. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82:457–63. doi: 10.1053/apmr.2001.21862. [DOI] [PubMed] [Google Scholar]
- 31.Fu JB, Parsons HA, Shin KY, Guo Y, Konzen BS, Yadav RR, Smith DW. Comparison of functional outcomes in low- and high-grade astrocytoma rehabilitation inpatients. Am J Phys Med Rehabil. 2010 Mar;89(3):205–12. doi: 10.1097/PHM.0b013e3181ca2306. [DOI] [PubMed] [Google Scholar]
- 32.Formica V, Del Monte G, Giacchetti I, Grenga I, Giaquinto S, Fini M, Roselli M. Rehabilitation in neuro-oncology: a meta-analysis of published data and a mono-institutional experience. Integr Cancer Ther. 2011 Jun;10(2):119–26. doi: 10.1177/1534735410392575. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Fu J, Palmer JL, Hanohano J, Cote C, Bruera E. Comparison of postoperative rehabilitation in cancer patients undergoing internal and external hemipelvectomy. Arch Phys Med Rehabil. 2011 Apr;92(4):620–5. doi: 10.1016/j.apmr.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Tan M, New PW. Retrospective study of rehabilitation outcomes following spinal cord injury due to tumour. Spinal Cord. 2012 Feb;50(2):127–31. doi: 10.1038/sc.2011.103. [DOI] [PubMed] [Google Scholar]
- 35.Garrard P, Farnham C, Thompson AJ, Playford ED. Rehabilitation of the cancer patient: experience in a neurological unit. Neurorehabil Neural Repair. 2004 Jun;18(2):76–9. doi: 10.1177/0888439004266306. [DOI] [PubMed] [Google Scholar]
- 36.McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs. traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000 Mar-Apr;79(2):138–44. doi: 10.1097/00002060-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Fu JB, Raj VS, Asher A, Lee J, Guo Y, Konzen BS, Bruera E. Inpatient rehabilitation performance of patients with paraneoplastic cerebellar degeneration. Arch Phys Med Rehabil. 2014 Dec;:9. doi: 10.1016/j.apmr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.New PW, Reeves RK, Smith É, Eriks-Hoogland I, Gupta A, Scivoletto G, Townson A, Maurizio B, Post MW. International Retrospective Comparison of Inpatient Rehabilitation for Patients With Spinal Cord Dysfunction: Differences According to Etiology. Arch Phys Med Rehabil. 2016 Mar;97(3):380–5. doi: 10.1016/j.apmr.2015.10.107. [DOI] [PubMed] [Google Scholar]
- 39.McKinley WO, Huang ME, Brunsvold KT. Neoplastic versus traumatic spinal cord injury: an outcome comparison after inpatient rehabilitation. Arch Phys Med Rehabil. 1999 Oct;80(10):1253–7. doi: 10.1016/s0003-9993(99)90025-4. [DOI] [PubMed] [Google Scholar]; 5(12):2496–9. [Google Scholar]
- 40.Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000 Jul-Aug;79(4):327–35. doi: 10.1097/00002060-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998 Nov;79(11):1386–90. doi: 10.1016/s0003-9993(98)90232-5. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006 Jul;85(7):568–73. doi: 10.1097/01.phm.0000223218.38152.53. [DOI] [PubMed] [Google Scholar]
- 43.Tan M, New P. Survival after rehabilitation for spinal cord injury due to tumor: a 12-year retrospective study. J Neurooncol. 2011 Aug;104(1):233–8. doi: 10.1007/s11060-010-0464-6. [DOI] [PubMed] [Google Scholar]
- 44.Tsao E, Bjornson K, Christensen A, Apkon S. Functional Outcomes and Unplanned Transfers of Pediatric Patients With Central Neurological Impairments Receiving Inpatient Rehabilitation Care With Cancer and Noncancer Diagnoses. PM R. 2016 Jun;8(6):529–35. doi: 10.1016/j.pmrj.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: Comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79:327–35. doi: 10.1097/00002060-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Saotome T, Klein L, Faux S. Cancer rehabilitation: a barometer for survival? Support Care Cancer. 2015 Oct;23(10):3033–41. doi: 10.1007/s00520-015-2673-1. [DOI] [PubMed] [Google Scholar]
- 47.Roberts PS, Nuño M, Sherman D, Asher A, Wertheimer J, Riggs RV, Patil CG. The impact of inpatient rehabilitation on function and survival of newly diagnosed patients with glioblastoma. PM R. 2014 Jun;6(6):514–21. doi: 10.1016/j.pmrj.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008 Jun;255(6):820–7. doi: 10.1007/s00415-008-0695-z. [DOI] [PubMed] [Google Scholar]
- 49.Kummer F, Catuogno S, Perseus JM, Bloch W, Baumann FT. Relationship between cancer-related fatigue and physical activity in inpatient cancer rehabilitation. Anticancer Res. 2013 Aug;33(8):3415–22. [PubMed] [Google Scholar]
- 50.Kroenke K, Johns SA, Theobald D, Wu J, Tu W. Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Support Care Cancer. 2013 Mar;21(3):765–73. doi: 10.1007/s00520-012-1578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang HL, Kroenke K, Wu J, Tu W, Theobald D, Rawl SM. Cancer-related pain and disability: a longitudinal study. J Pain Symptom Manage. 2011 Dec;42(6):813–21. doi: 10.1016/j.jpainsymman.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroenke K, Zhong X, Theobald D, Wu J, Tu W, Carpenter JS. Somatic symptoms in patients with cancer experiencing pain or depression: prevalence, disability, and health care use. Arch Intern Med. 2010 Oct 11;170(18):1686–94. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu JB, Lee J, Tran KB, Siangco CM, Ng AH, Smith DW, Bruera E. Symptom Burden and Functional Gains in a Cancer Rehabilitation Unit. Int J Ther Rehabil. 2015 Nov;22(11):517–523. doi: 10.12968/ijtr.2015.22.11.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Y, Young BL, Hainley S, Palmer JL, Bruera E. Evaluation and pharmacologic management of symptoms in cancer patients undergoing acute rehabilitation in a comprehensive cancer center. Arch Phys Med Rehabil. 2007 Jul;88(7):891–5. doi: 10.1016/j.apmr.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Bertheussen GF, Kaasa S, Hokstad A, Sandmæl JA, Helbostad JL, Salvesen Ø, Oldervoll LM. Feasibility and changes in symptoms and functioning following inpatient cancer rehabilitation. Acta Oncol. 2012 Nov;51(8):1070–80. doi: 10.3109/0284186X.2012.699684. [DOI] [PubMed] [Google Scholar]
- 56.Illsley A, Clegg A. Assessment of frailty in the inpatient setting. Br J Hosp Med (Lond) 2016 Jan;77(1):29–32. doi: 10.12968/hmed.2016.77.1.29. [DOI] [PubMed] [Google Scholar]
- 57.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 58.Haley R, Sullivan DH, Granger CV, Kortebein P. Inpatient rehabilitation outcomes for older adults with nondebility generalized weakness. Am J Phys Med Rehabil. 2011;90:791–797. doi: 10.1097/PHM.0b013e31822deaf4. [DOI] [PubMed] [Google Scholar]
- 59.Galloway RV, Granger CV, Karmarkar AM, et al. The Uniform Data System for Medical Rehabilitation report of patients with debility discharged from inpatient rehabilitation programs in 2000–2010. Am J Phys Med Rehabil. 2013;92:14–27. doi: 10.1097/PHM.0b013e31827441bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kortebein P, Bopp MM, Granger CV, Sullivan DH. Outcomes of inpatient rehabilitation for older adults with debility. Am J Phys Med Rehabil. 2008;87:118–125. doi: 10.1097/PHM.0b013e3181588429. [DOI] [PubMed] [Google Scholar]
- 61.Kortebein P, Granger CV, Sullivan DH. A comparative evaluation of inpatient rehabilitation for older adults with debility, hip fracture, and myopathy. Arch Phys Med Rehabil. 2009;90:934–938. doi: 10.1016/j.apmr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Alam E, Wilson RD, Vargo MM. Inpatient cancer rehabilitation: a retrospective comparison of transfer back to acute care between patients with neoplasm and other rehabilitation patients. Arch Phys Med Rehabil. 2008 Jul;89(7):1284–9. doi: 10.1016/j.apmr.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Fu JB, Lee J, Shin BC, Silver JK, Smith DW, Shah JJ, Bruera E. Return to the Primary Acute Care Service Among Patients with Multiple Myeloma on an Acute Inpatient Rehabilitation Unit. PM R. 2017 Jan 8; doi: 10.1016/j.pmrj.2016.12.007. pii: S1934-1482(17)30021-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Fu JB, Lee J, Smith DW, Bruera E. Frequency and Reasons for Return to the Primary Acute Care Service Among Leukemia Patients Undergoing Inpatient Rehabilitation. Am J Phys Med Rehabil. 2013 Mar;92(3):215–22. doi: 10.1097/PHM.0b013e3182744151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu JB, Lee J, Smith DW, Guo Y, Bruera E. Return to Primary Service Among Bone Marrow Transplant Rehabilitation Inpatients: An Index for Predicting Outcomes. Arch Phys Med Rehab. 2013 Feb;94(2):356–61. doi: 10.1016/j.apmr.2012.08.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu JB, Lee J, Smith DW, Shin K, Guo Y, Bruera E. Frequency and Reasons for Return to the Primary Acute Care Service among Lymphoma Patients Undergoing Inpatient Rehabilitation. PM R. 2014 Jul;6(7):629–34. doi: 10.1016/j.pmrj.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo Y, Persyn L, Palmer JL, Bruera E. Incidence of and risk factors for transferring cancer patients from rehabilitation to acute care units. Am J Phys Med Rehabil. 2008 Aug;87(8):647–53. doi: 10.1097/PHM.0b013e31817fb94e. [DOI] [PubMed] [Google Scholar]
- 68.Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC) PM R. 2014 Sep;6(9):808–13. doi: 10.1016/j.pmrj.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Chen C, Koh GC, Naidoo N, Cheong A, Fong NP, Tan YV, Chan KM, Tan BY, Menon E, Ee CH, Lee KK, Ng YS, Koh D, Chia KS, Teo YY. Trends in length of stay, functional outcomes, and discharge destination stratified by disease type for inpatient rehabilitation in Singapore community hospitals from 1996 to 2005. Arch Phys Med Rehabil. 2013 Jul;94(7):1342–1351. e4. doi: 10.1016/j.apmr.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Kus S, Muller M, Strobl R, Grill E. Patient goals in post-acute geriatric rehabilitation--goal attainment is an indicator for improved functioning. J Rehabil Med. 2011;43(2):156–61. doi: 10.2340/16501977-0636. [DOI] [PubMed] [Google Scholar]
- 71.Graham JE, Chang PF, Berges IM, Granger CV, Ottenbacher KJ. Race/ethnicity and outcomes following inpatient rehabilitation for hip fracture. J Gerontol A Biol Sci Med Sci. 2008;63(8):860–6. doi: 10.1093/gerona/63.8.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guilcher SJ, Bronskill SE, Guan J, Wodchis WP. Who Are the High-Cost Users? A Method for Person-Centred Attribution of Health Care Spending. PLoS One. 2016 Mar 3;11(3):e0149179. doi: 10.1371/journal.pone.0149179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisher SR, Graham JE, Krishnan S, Ottenbacher KJ. Predictors of 30-Day Readmission Following Inpatient Rehabilitation for Patients at High Risk for Hospital Readmission. Phys Ther. 2016 Jan;96(1):62–70. doi: 10.2522/ptj.20150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galloway RV, Karmarkar AM, Graham JE, Tan A, Raji M, Granger CV, Ottenbacher KJ. Hospital Readmission Following Discharge From Inpatient Rehabilitation for Older Adults With Debility. Phys Ther. 2016 Feb;96(2):241–51. doi: 10.2522/ptj.20150030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Occelli P, Touzet S, Rabilloud M, Ganne C, Poupon Bourdy S, Galamand B, Debray M, Dartiguepeyrou A, Chuzeville M, Comte B, Turkie B, Tardy M, Luiggi JS, Jacquet-Francillon T, Gilbert T, Bonnefoy M. Impact of a transition nurse program on the prevention of thirty-day hospital readmissions of elderly patients discharged from short-stay units: study protocol of the PROUST stepped-wedge cluster randomised trial. BMC Geriatr. 2016 Mar 3;16:57. doi: 10.1186/s12877-016-0233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu JB, Bianty JR, Wu J, Ngo-Huang A, Shin KY, Bruera E. An Analysis of Inpatient Rehabilitation Approval Among Private Insurance Carriers at a Cancer Center. PM R. 2016 Jul;8(7):635–9. doi: 10.1016/j.pmrj.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dy SM, List DJ, Barbe C, Knight L. A quality improvement initiative for improving appropriateness of referrals from a cancer center to subacute rehabilitation. J Pain Symptom Manage. 2014 Jul;48(1):127–31. doi: 10.1016/j.jpainsymman.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Dobson DaVanzo & Associates. [Accessed January 13, 2017];Assessment of Patient Outcomes of Rehabilitative Care Provided in Inpatient Rehabilitation Facilities (IRFs) and After Discharge. 2014 Jul; Available at: http://www.amrpa.org/newsroom/Dobson%20DaVanzo%20Final%20Report%20-%20Patient%20Outcomes%20of%20IRF%20v%20%20SNF%20-%207%2010%2014%20redated.pdf.
- 79.Gjerset GM, Loge JH, Gudbergsson SB, Bye A, Fosså SD, Oldervoll LM, Kiserud CE, Demark-Wahnefried W, Thorsen L. Lifestyles of cancer survivors attending an inpatient educational program-a cross-sectional study. Support Care Cancer. 2016 Apr;24(4):1527–36. doi: 10.1007/s00520-015-2936-x. [DOI] [PubMed] [Google Scholar]
- 80.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012 Nov 14;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012 Jul-Aug;62(4):243–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 82.Sabers SR, Kokal JE, Girardi JC, Philpott CL, Basford JR, Therneau TM, Schmidt KD, Gamble GL. Evaluation of consultation-based rehabilitation for hospitalized cancer patients with functional impairment. Mayo Clin Proc. 1999 Sep;74(9):855–61. doi: 10.4065/74.9.855. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt K. Cancer Rehabilitation Inpatient Team at Mayo Clinic – Rochester. 91st American Congress of Rehabilitation Annual Meeting; October 11, 2014. [Google Scholar]